Abstract
We investigated in a retrospective multicenter study the impact of chromosome arm 13q deletion (13q-) as detected by fluorescence in situ hybridization (FISH) on outcome after dose-reduced allografting in patients with multiple myeloma. In 68 of 140 patients, data on chromosome 13q status were available. Most patients included had advanced myeloma. At 2 years, patients with 13q deletion (n = 31) had a shorter event-free (18% vs 42%; P = .05) and overall survival (18% vs 67%; P = .03) than patients without 13q- (n = 37). Patients with 13q- experienced a higher relapse rate (77% vs 44%; P < .001) but a similar incidence of transplantation-related mortality at one year (24% vs 18%). In a multivariate analysis, 13q- remained a significant risk factor for a higher relapse rate (hazard ratio [HR], 3.28; 95% confidence interval [CI], 1.31-8.24; P = .01) and a shorter event-free survival (HR, 1.94; 95% CI, 1.03-3.67; P = .04). Concerning overall survival, 2 or more cycles of prior high-dose chemotherapy were associated with a significantly higher probability of death (HR, 2.48; 95% CI, 1.19-5.17; P = .02), while patients with deletion 13q had a nearly 2 times higher risk of death (HR, 1.94; 95% CI, 0.95-3.98; P = .07) after dose-reduced allogeneic stem cell transplantation.
Introduction
Allogeneic stem cell transplantation may cure patients with multiple myeloma (MM). Despite high treatment-related mortality of 17% to 40%,1-4 lower relapse rates in comparison to autologous transplantation have been reported, probably due to a graft-versus-myeloma effect mediated by immunocompetent donor lymphocytes.5,6 Recently, reduced-intensity conditioning (RIC) allograft, using nonmyeloablative regimens, has been successfully undertaken in a variety of hematologic malignancies including myeloma.6-14 In myeloma patients, the transplantation-related mortality (TRM) was lower than after standard conditioning, allowing the successful investigation of this approach, even in elderly patients and in patients with unrelated donors.9,15-18 In the reported trials the major prognostic factors for better survival were chemosensitivity at time of transplantation,11,16 chronic graft-versus-host disease (GVHD),16,18 and absence of relapse to a prior autograft.11,15 Conventional cytogenetic analysis, especially for patients with deletion of the long arm of chromosome 13 (13q-), has been reported to predict an unfavorable outcome following high-dose or conventional chemotherapy.19-21 However, due to the low proliferation rate of myeloma cells, chromosome banding analysis is only able to reveal genetic changes in about 30% to 40% of the patients,22,23 while fluorescence in situ hybridization (FISH) allows detection of specific abnormalities in virtually all myeloma patients. With an incidence of 33% to 88%, 13q- by FISH has been shown to be the most frequent chromosomal loss in multiple myeloma24-29 and is regarded as one of the most powerful independent risk factors for conventional30 and high-dose chemotherapy.24,27,31,32 Therefore it has been proposed that patients with 13q- will not have a substantial benefit from autologous high-dose chemotherapy and that these patients should be treated with innovative treatment approaches among which dose-reduced allogeneic stem cell transplantation should be considered. However, the impact of 13q- on outcome after allogeneic stem cell transplantation is currently unknown. Therefore, the aim of this international multicenter study was to evaluate the impact of 13q- detected by FISH on outcome after allogeneic dose-reduced conditioning in patients with multiple myeloma.
Patients, materials, and methods
Patient characteristics
One hundred forty patients with advanced multiple myeloma were enrolled in 4 international studies between July 1998 and December 2002 investigating a dose-reduced conditioning consisting either of melphalan/fludarabine (n = 120) or total body irradiation (TBI; 2 Gy)/cyclophosphamide/fludarabine (n = 20) followed by allogeneic stem cell transplantation in multiple myeloma. In 68 of 140 patients, data on chromosome 13q status were available: 37 patients had no 13q deletion, while 31 patients displayed this aberration. Of these patients, 55 patients received dose-reduced allogeneic stem cell transplantation from related or unrelated donors after conditioning with melphalan (70-140 mg/m2) and fludarabine (150-180 mg/m2), and 13 patients received TBI (2 Gy), cyclophosphamide (40 mg/kg), and fludarabine (150 mg/m2).
To be included, patients were required to have a sufficient cardiac function (ejection fraction > 30%), a creatinine clearance level of more than 30 mL/minute, a lung diffusion capacity of at least 50%, and liver transaminases lower than 3 times the upper limit of normal. The median age of the patients was 53 years (range, 32-65 years). There were 43 male and 25 female patients. The median β2-microglobulin level at diagnosis was 3.2 mg/dL (range, 2-14 mg/dL). Forty patients had received prior radiation therapy, and the median number of previous chemotherapy cycles was 5 (range, 2-13 cycles). Sixty-five patients had received at least one previous cycle of high-dose chemotherapy followed by autologous stem cell transplantation. Fourteen patients had received 2 cycles and 3 patients had received 3 cycles of previous high-dose chemotherapy. Thirty-eight patients experienced relapse after autologous transplantation, while 31 patients received allogeneic transplantation as consolidation therapy after an autograft. Four patients received dose-reduced allograft without preceding autologous transplantation. The disease status prior to allogeneic transplantation was complete remission (CR; n = 3), partial remission (PR; n = 31), minor remission (MR; n = 6), no change (NC; n = 6), and progressive disease (PD; n = 22). Patients received transplants from related (n = 38) and unrelated donors (n = 30). Two patients received transplants from mismatched unrelated donors (HLA-DRB,1 n = 1; and HLA-DQB1, n = 2, respectively). The median time from diagnosis to transplantation was 23 months (range, 6-105 months). The cytomegalovirus (CMV) serostatus was positive in 51 patients and negative in 16 patients. The source of stem cells was peripheral blood in 63 patients and bone marrow in 5 patients. No manipulation of the graft was performed. The median follow-up of the surviving patients was 20 months (range, 5-55 months).
As shown in Table 1, the patients in both groups were well balanced regarding age, β2-microglobulin level, relapse to a prior autograft, donor sex, related and unrelated donor, graft source, CD34+ cell dose, and chemosensitivity at time of transplantation. There were slightly more patients in the deletion 13q group with 2 or more high-dose regimens (10 vs 7), which received slightly more chemotherapy regimens (median 5 vs 4; P = .06). There were less patients seropositive for CMV in the deletion 13 group (65% vs 86%; P = .03). A trend for a shorter interval between diagnosis and transplantation was seen in the del 13q group (25 vs 35 months; P = .08).
Patient characteristics
. | Deletion 13q; n = 31 . | No deletion 13q; n = 37 . | P . |
|---|---|---|---|
| Median age, y (range) | 53 (35-65) | 53 (32-65) | .4 |
| Mean β2-microglobulin level, mg/dL (n) | 3.1 (25) | 3.4 (24) | .8 |
| Median no. of prior chemotherapy regimens (range) | 5 (2-13) | 4 (2-10) | .06 |
| Prior high-dose regimen, n (%) | .2 | ||
| None | 3 (10) | 0 | |
| 1 autologous transplantation | 18 (58) | 30 (81) | |
| At least 2 autologous transplantations | 10 (32) | 7 (19) | |
| Relapse to prior high-dose regimen, n (%) | 17 (55) | 21 (57) | .9 |
| Chemotherapy-resistant prior allo-SCT, NC/PD, n (%) | 16 (52) | 18 (49) | .8 |
| No relapse to conventional or high-dose chemotherapy, n (%) | 10 (32) | 13 (35) | .8 |
| Donor, n (%) | .1 | ||
| Unrelated | 17 (55) | 13 (35) | |
| Related | 14 (45) | 24 (65) | |
| Donor sex, n (%) | .5 | ||
| Female | 10 (35) | 15 (41) | |
| Male | 21 (65) | 22 (59) | |
| Sex mismatch, n (%) | 13 (42) | 18 (47) | .6 |
| Patient sex, n (%) | .7 | ||
| Female | 10 (32) | 15 (41) | |
| Male | 21 (68) | 22 (59) | |
| CMV-seropositive recipient, n (%) | 20 (65) | 32 (86) | .03 |
| Graft source, n (%) | .2 | ||
| PBSC | 30 (97) | 33 (89) | |
| BM | 1 (3) | 4 (11) | |
| Median CD34+ cell dose, × 106/kg BW (range) | 5.4 (1.3-13.2) | 5.8 (1.4-13.7) | .3 |
| Median time from diagnosis to allograft, mo (range) | 16 (3-86) | 27 (8-171) | .02 |
| Melphalan/fludarabine, n (%) | 22 (71) | 33 (89) | .8 |
| TBI (2 Gy)/fludarabine/cyclophosphamide | 9 (29) | 4 (11) | .1 |
. | Deletion 13q; n = 31 . | No deletion 13q; n = 37 . | P . |
|---|---|---|---|
| Median age, y (range) | 53 (35-65) | 53 (32-65) | .4 |
| Mean β2-microglobulin level, mg/dL (n) | 3.1 (25) | 3.4 (24) | .8 |
| Median no. of prior chemotherapy regimens (range) | 5 (2-13) | 4 (2-10) | .06 |
| Prior high-dose regimen, n (%) | .2 | ||
| None | 3 (10) | 0 | |
| 1 autologous transplantation | 18 (58) | 30 (81) | |
| At least 2 autologous transplantations | 10 (32) | 7 (19) | |
| Relapse to prior high-dose regimen, n (%) | 17 (55) | 21 (57) | .9 |
| Chemotherapy-resistant prior allo-SCT, NC/PD, n (%) | 16 (52) | 18 (49) | .8 |
| No relapse to conventional or high-dose chemotherapy, n (%) | 10 (32) | 13 (35) | .8 |
| Donor, n (%) | .1 | ||
| Unrelated | 17 (55) | 13 (35) | |
| Related | 14 (45) | 24 (65) | |
| Donor sex, n (%) | .5 | ||
| Female | 10 (35) | 15 (41) | |
| Male | 21 (65) | 22 (59) | |
| Sex mismatch, n (%) | 13 (42) | 18 (47) | .6 |
| Patient sex, n (%) | .7 | ||
| Female | 10 (32) | 15 (41) | |
| Male | 21 (68) | 22 (59) | |
| CMV-seropositive recipient, n (%) | 20 (65) | 32 (86) | .03 |
| Graft source, n (%) | .2 | ||
| PBSC | 30 (97) | 33 (89) | |
| BM | 1 (3) | 4 (11) | |
| Median CD34+ cell dose, × 106/kg BW (range) | 5.4 (1.3-13.2) | 5.8 (1.4-13.7) | .3 |
| Median time from diagnosis to allograft, mo (range) | 16 (3-86) | 27 (8-171) | .02 |
| Melphalan/fludarabine, n (%) | 22 (71) | 33 (89) | .8 |
| TBI (2 Gy)/fludarabine/cyclophosphamide | 9 (29) | 4 (11) | .1 |
PBSC indicates peripheral blood stem cells; SCT, stem cell transplantation; BM, bone marrow; and BW, body weight.
Methods
Engraftment was defined as a leukocyte count higher than 1 × 109/L for 3 consecutive days and a nontransfused platelet count higher than 20 × 109/L. HLA-A and -B antigens were typed by serologic methods; HLA-DRB1 alleles were typed using sequence-specific oligonucleotide probes.
The standard criteria were used for grading of acute and chronic GVHD.33,34 Acute GVHD was treated with high-dose steroids, and extensive chronic GVHD was treated with cyclosporine A and steroids. Chronic GVHD was evaluated in patients who survived at least 100 days with sustained engraftment. Antibiotic, antiviral, and antifungal prophylaxis were performed according to the centers' policy. GVHD prophylaxis consisted of cyclosporine A (CSA) plus short-course methotrexate in 64 patients and CSA plus mycophenolat-mofetil (MMF) in 4 patients. Response to treatment was defined according to the European Group for Blood and Marrow Transplantation/International Bone Marrow Transplant Registry (EBMT/IBMTR) criteria.35 A written informed consent was received from each patient, and the treatment study was approved by each participating center.
Fluorescence in situ hybridization and cytoplasm immunoglobulin staining (cIg-FISH). Bone marrow samples were centrifuged on a ficoll-hypaque gradient to enrich for mononuclear cells. Cytospin slides were prepared and dried overnight at room temperature. The slides were then dehydrated in a series of ethanol (70%, 90%, and 100%) and stored at -20° C after air drying. Slide pretreatment was performed by 5 minutes shaking in 1 × phosphate-buffered saline (PBS) and then the slides were digested for 2.5 minutes in pepsin solution at 37° C, washed in 1 × PBS for 5 minutes, fixed in 2% paraformaldehyde solution, and finally washed in 1 × PBS for 5 minutes before dehydrating in a series of ethanol. The FISH probe D13S25 for chromosome band 13q14 (obtained from Vysis, Downer's Grove, IL) directly labeled in red was prepared as recommended by the manufacturer's instructions. Five microliters of each probe was placed on a pretreated slide, cover-slipped and sealed with rubber cement, denatured on a hot plate (75° C) for 7 minutes, and allowed to hybridize in a 37° C humidified chamber overnight. Following hybridization, the slides were washed in 0.5 × standard saline citrate (SSC) for 2 minutes at 72° C then in 1 × 5 minutes at room temperature. Excessive liquid was dropped off and slides were then stained with goat antihuman κ and λ light-chain antibodies conjugated with AMCA (7-amino-4-methylcoumarin-3-acetic acid; Vector Lab, Burlingame, CA), cover-slipped, sealed with rubber cement, and incubated overnight in a humidified chamber at 37° C. After the incubation, the slides were washed in 1 × PBS for 5 minutes. Finally Vectashield (Vector Labs) was applied and the slides were cover-slipped. For each patient at least 50 AMCA-positive plasma cells were scored.
Statistics. Event-free and overall survivals were calculated from start of allotransplantation to the respective event. Death from whatever cause and relapse/progression were counted as an event in case of event-free survival (EFS). All time-to-event curves were estimated according the method of Kaplan and Meier and compared univariately using the log-rank test. TRM and relapse/progression were calculated using cumulative incidence estimates. Multivariate analyses of mortality-rated and event-type data were performed with the logistic and Cox proportional hazard models, respectively, initially including all factors with a P value of no more than 0.1 in the univariate analysis and using a stepwise backward approach for model reduction. Wald test was 2-sided. A P value of less than .05 was considered to be significant.
Results
Engraftment and chimerism
Three patients died of treatment-related causes prior to engraftment. All other patients engrafted. The median time until achieving a leukocyte count greater than 1 × 109/L was 13 days in both groups, while platelet engraftment was slightly faster in patients without 13q- (16 vs 19 days; P = 0.3; Table 2).
Comparison between patients with or without deletion 13 after dose-reduced allograft
. | Deletion 13; n = 31 . | No deletion 13; n = 37 . | P . |
|---|---|---|---|
| Median days of leukocyte count higher than 1.0 × 109/L | 13 | 13 | .4 |
| Median days of platelet count higher than 20 × 109/L | 19 | 16 | .3 |
| Platelet substitution, mean package | 11 | 9 | .2 |
| Acute GVHD grades II-IV, % | 43 | 36 | .5 |
| Chronic GVHD, % | 26 | 27 | .9 |
| CR after allograft, n (%) | 9 (31) | 11 (35) | .7 |
| No response after allograft, n (%) | 8 (28) | 4 (13) | .3 |
| 2 years overall survival, % (95% CI) | 18 (0-38) | 67 (51-83) | .03 |
| 2 years event-free survival, % (95% CI) | 18 (1-35) | 42 (24-60) | .05 |
| Cumulative incidence of relapse, % (95% CI) | 77 (57-97) | 44 (22-66) | < .001 |
| 1 year treatment-related mortality, % (95% CI) | 18 (4-32) | 24 (10-38) | .4 |
. | Deletion 13; n = 31 . | No deletion 13; n = 37 . | P . |
|---|---|---|---|
| Median days of leukocyte count higher than 1.0 × 109/L | 13 | 13 | .4 |
| Median days of platelet count higher than 20 × 109/L | 19 | 16 | .3 |
| Platelet substitution, mean package | 11 | 9 | .2 |
| Acute GVHD grades II-IV, % | 43 | 36 | .5 |
| Chronic GVHD, % | 26 | 27 | .9 |
| CR after allograft, n (%) | 9 (31) | 11 (35) | .7 |
| No response after allograft, n (%) | 8 (28) | 4 (13) | .3 |
| 2 years overall survival, % (95% CI) | 18 (0-38) | 67 (51-83) | .03 |
| 2 years event-free survival, % (95% CI) | 18 (1-35) | 42 (24-60) | .05 |
| Cumulative incidence of relapse, % (95% CI) | 77 (57-97) | 44 (22-66) | < .001 |
| 1 year treatment-related mortality, % (95% CI) | 18 (4-32) | 24 (10-38) | .4 |
Graft-versus-host disease
Acute GVHD grades II-IV was noted in 43% of the patients with 13q- and in 37% of the patients without 13q- (P = .5). Chronic GVHD was seen in 26% in the 13q- group and in 27% in the non-13q- group (P = .9).
Response
Response was similar in both groups. Complete remission was seen in 35% of 13q- group and in 34% of the patients without 13q-. Patients without response to allografting (NC or PD) were slightly higher in the 13q- group but without reaching statistical significance (28% vs 13%; P = .3).
TRM
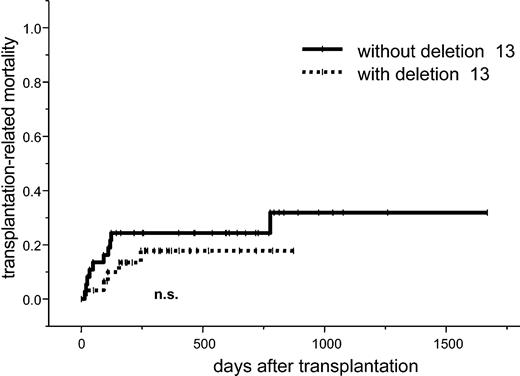
The cumulative risk at one year for TRM was 18% (95% confidence interval [CI], 4%-32%) for patients with 13q- and 24% (95% CI, 10%-38%) with normal 13q status (P = .4; Figure 1). In a univariate analysis, the TRM at one year was higher in patients with prior radiotherapy (43% vs 15%; P = .04), in patients older than 50 years of age (29% vs 5%; P = .02), and in patients receiving stem cell graft from a female donor (35% vs 12%; P = .05). There was a trend toward a higher TRM in patients with β2-microglobulin level of more than 3 mg/dL (39% vs 14%; P = .06). In a multivariate analysis, age younger than 50 years (hazard ratio [HR], 0.13; 95% CI, 0.02-1.00; P = .05) remained an independent favorable factor, while prior radiotherapy was an independent unfavorable factor for TRM (HR, 2.76; 95% CI, 0.96-7.91; P = .06; Table 3).
Univariate analysis
. | OS, 2 years, % (95% CI) . | P . | EFS, 2 years, % (95% CI) . | P . | Relapse, 2 years, % (95% CI) . | P . | TRM, 1 year, % (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| Age | .6 | .3 | .7 | .02 | ||||
| Younger than 50 years | 48 (24-72) | 43 (11-65) | 55 (31-79) | 5 (0-14) | ||||
| Older than 50 years | 45 (28-62) | 27 (11-43) | 62 (42-82) | 29 (16-42) | ||||
| Patient sex | .3 | .4 | .3 | .9 | ||||
| Female | 63 (44-82) | 37 (15-59) | 52 (27-77) | 20 (4-36) | ||||
| Male | 36 (18-54) | 28 (12-44) | 64 (45-83) | 22 (9-35) | ||||
| Status prior allograft | .07 | .07 | .05 | .9 | ||||
| Chemosensitive | 51 (31-71) | 37 (18-56) | 53 (29-77) | 24 (10-38) | ||||
| Chemorefractory | 41 (22-60) | 25 (7-43) | 69 (47-91) | 18 (5-31) | ||||
| Relapse after prior HD therapy | .09 | .06 | .02 | .9 | ||||
| Yes | 38 (21-55) | 21 (5-37) | 73 (53-93) | 23 (9-37) | ||||
| No | 57 (35-79) | 44 (24-64) | 45 (21-69) | 19 (5-33) | ||||
| Prior HD therapy | .005 | .04 | .03 | .2 | ||||
| At least 2 | 26 (4-48) | 14 (0-38) | 60 (33-87) | 35 (10-60) | ||||
| Fewer than 2 | 56 (40-72) | 35 (19-51) | 57 (34-75) | 18 (7-29) | ||||
| Prior radiotherapy | .2 | .4 | .9 | .04 | ||||
| Yes | 39 (21-57) | 33 (9-57) | 46 (15-77) | 43 (19-67) | ||||
| No | 53 (35-71) | 31 (13-49) | 63 (41-95) | 15 (4-26) | ||||
| β2-microglobulin level | .1 | .1 | .6 | .06 | ||||
| Less than 3 mg/dL | 46 (17-63) | 45 (21-69) | 47 (20-74) | 14 (0-28) | ||||
| Greater than 3 mg/dL | 36 (17-55) | 29 (11-47) | 47 (21-68) | 39 (20-58) | ||||
| Deletion 13q14 | .03 | .05 | <.001 | .4 | ||||
| Yes | 18 (0-38) | 18 (1-32) | 77 (57-97 | 18 (4-32) | ||||
| No | 67 (51-83) | 42 (24-60) | 44 (22-66) | 24 (10-38) | ||||
| IgA | 56 (27-85) | .8 | 24 (0-61) | .9 | 60 (13-100) | .9 | 29 (2-56) | .9 |
| Others | 46 (31-61) | 32 (16-48) | 60 (43-77) | 20 (9-31) | ||||
| Patient's CMV serostatus | .4 | .1 | .01 | .3 | ||||
| Positive | 54 (39-69) | 36 (20-52) | 53 (34-72) | 24 (12-36) | ||||
| Negative | 16 (0-43) | 13 (0-33) | 16 (0-41) | 16 (0-36) | ||||
| Donor's age | .08 | .2 | .4 | .4 | ||||
| Younger than 50 y | 28 (6-50) | 38 (20-56) | 48 (28-68) | 27 (11-43) | ||||
| Older than 50 y | 61 (35-87) | 38 (5-65) | 53 (22-84) | 17 (0-35) | ||||
| Donor's sex | .3 | .3 | .9 | .05 | ||||
| Female | 41 (19-63) | 21 (2-40) | 69 (44-94) | 35 (15-55) | ||||
| Male | 51 (34-68) | 38 (21-55) | 56 (36-76) | 12 (2-22) | ||||
| Transplantation | .1 | .4 | .9 | .3 | ||||
| MUD | 37 (15-59) | 16 (0-42) | 48 (21-75) | 27 (11-43) | ||||
| Related | 57 (41-73) | 33 (17-49) | 61 (43-79) | 17 (5-29) | ||||
| Cell dose CD34+ cells/kg BW | .6 | .9 | .5 | .2 | ||||
| Less than 5 × 106 cells | 49 (22-71) | 21 (0-45) | 75 (46-100) | 17 (0-35) | ||||
| Greater than 5 × 106 cells | 55 (39-71) | 38 (20-56) | 49 (27-71) | 28 (13-43) | ||||
| Acute GVHD grades II-IV | .7 | .8 | .6 | .3 | ||||
| Yes | 49 (27-71) | 37 (17-57) | 50 (25-75) | 24 (7-41) | ||||
| No | 48 (30-66) | 30 (12-48) | 66 (46-86) | 15 (4-26) | ||||
| Interval DX-TX | .7 | .5 | .6 | .7 | ||||
| Shorter than 2 y | 51 (31-71) | 32 (14-50) | 60 (38-82) | 19 (5-33) | ||||
| Longer than 2 y | 36 (17-55) | 22 (3-41) | 70 (46-94) | 24 (9-39) | ||||
| Chronic GVHD | .8 | .7 | .8 | .3 | ||||
| Yes | 58 (32-84) | 32 (17-47) | 63 (36-90) | 20 (10-30) | ||||
| No | 52 (33-71) | 40 (0-83) | 52 (33-71) | 40 (0-83) |
. | OS, 2 years, % (95% CI) . | P . | EFS, 2 years, % (95% CI) . | P . | Relapse, 2 years, % (95% CI) . | P . | TRM, 1 year, % (95% CI) . | P . |
|---|---|---|---|---|---|---|---|---|
| Age | .6 | .3 | .7 | .02 | ||||
| Younger than 50 years | 48 (24-72) | 43 (11-65) | 55 (31-79) | 5 (0-14) | ||||
| Older than 50 years | 45 (28-62) | 27 (11-43) | 62 (42-82) | 29 (16-42) | ||||
| Patient sex | .3 | .4 | .3 | .9 | ||||
| Female | 63 (44-82) | 37 (15-59) | 52 (27-77) | 20 (4-36) | ||||
| Male | 36 (18-54) | 28 (12-44) | 64 (45-83) | 22 (9-35) | ||||
| Status prior allograft | .07 | .07 | .05 | .9 | ||||
| Chemosensitive | 51 (31-71) | 37 (18-56) | 53 (29-77) | 24 (10-38) | ||||
| Chemorefractory | 41 (22-60) | 25 (7-43) | 69 (47-91) | 18 (5-31) | ||||
| Relapse after prior HD therapy | .09 | .06 | .02 | .9 | ||||
| Yes | 38 (21-55) | 21 (5-37) | 73 (53-93) | 23 (9-37) | ||||
| No | 57 (35-79) | 44 (24-64) | 45 (21-69) | 19 (5-33) | ||||
| Prior HD therapy | .005 | .04 | .03 | .2 | ||||
| At least 2 | 26 (4-48) | 14 (0-38) | 60 (33-87) | 35 (10-60) | ||||
| Fewer than 2 | 56 (40-72) | 35 (19-51) | 57 (34-75) | 18 (7-29) | ||||
| Prior radiotherapy | .2 | .4 | .9 | .04 | ||||
| Yes | 39 (21-57) | 33 (9-57) | 46 (15-77) | 43 (19-67) | ||||
| No | 53 (35-71) | 31 (13-49) | 63 (41-95) | 15 (4-26) | ||||
| β2-microglobulin level | .1 | .1 | .6 | .06 | ||||
| Less than 3 mg/dL | 46 (17-63) | 45 (21-69) | 47 (20-74) | 14 (0-28) | ||||
| Greater than 3 mg/dL | 36 (17-55) | 29 (11-47) | 47 (21-68) | 39 (20-58) | ||||
| Deletion 13q14 | .03 | .05 | <.001 | .4 | ||||
| Yes | 18 (0-38) | 18 (1-32) | 77 (57-97 | 18 (4-32) | ||||
| No | 67 (51-83) | 42 (24-60) | 44 (22-66) | 24 (10-38) | ||||
| IgA | 56 (27-85) | .8 | 24 (0-61) | .9 | 60 (13-100) | .9 | 29 (2-56) | .9 |
| Others | 46 (31-61) | 32 (16-48) | 60 (43-77) | 20 (9-31) | ||||
| Patient's CMV serostatus | .4 | .1 | .01 | .3 | ||||
| Positive | 54 (39-69) | 36 (20-52) | 53 (34-72) | 24 (12-36) | ||||
| Negative | 16 (0-43) | 13 (0-33) | 16 (0-41) | 16 (0-36) | ||||
| Donor's age | .08 | .2 | .4 | .4 | ||||
| Younger than 50 y | 28 (6-50) | 38 (20-56) | 48 (28-68) | 27 (11-43) | ||||
| Older than 50 y | 61 (35-87) | 38 (5-65) | 53 (22-84) | 17 (0-35) | ||||
| Donor's sex | .3 | .3 | .9 | .05 | ||||
| Female | 41 (19-63) | 21 (2-40) | 69 (44-94) | 35 (15-55) | ||||
| Male | 51 (34-68) | 38 (21-55) | 56 (36-76) | 12 (2-22) | ||||
| Transplantation | .1 | .4 | .9 | .3 | ||||
| MUD | 37 (15-59) | 16 (0-42) | 48 (21-75) | 27 (11-43) | ||||
| Related | 57 (41-73) | 33 (17-49) | 61 (43-79) | 17 (5-29) | ||||
| Cell dose CD34+ cells/kg BW | .6 | .9 | .5 | .2 | ||||
| Less than 5 × 106 cells | 49 (22-71) | 21 (0-45) | 75 (46-100) | 17 (0-35) | ||||
| Greater than 5 × 106 cells | 55 (39-71) | 38 (20-56) | 49 (27-71) | 28 (13-43) | ||||
| Acute GVHD grades II-IV | .7 | .8 | .6 | .3 | ||||
| Yes | 49 (27-71) | 37 (17-57) | 50 (25-75) | 24 (7-41) | ||||
| No | 48 (30-66) | 30 (12-48) | 66 (46-86) | 15 (4-26) | ||||
| Interval DX-TX | .7 | .5 | .6 | .7 | ||||
| Shorter than 2 y | 51 (31-71) | 32 (14-50) | 60 (38-82) | 19 (5-33) | ||||
| Longer than 2 y | 36 (17-55) | 22 (3-41) | 70 (46-94) | 24 (9-39) | ||||
| Chronic GVHD | .8 | .7 | .8 | .3 | ||||
| Yes | 58 (32-84) | 32 (17-47) | 63 (36-90) | 20 (10-30) | ||||
| No | 52 (33-71) | 40 (0-83) | 52 (33-71) | 40 (0-83) |
OS indicates overall survival; EFS, event-free survival; HD, high dose; MUD, matched unrelated donor; and DX-TX, time from diagnosis to transplantation.
Relapse
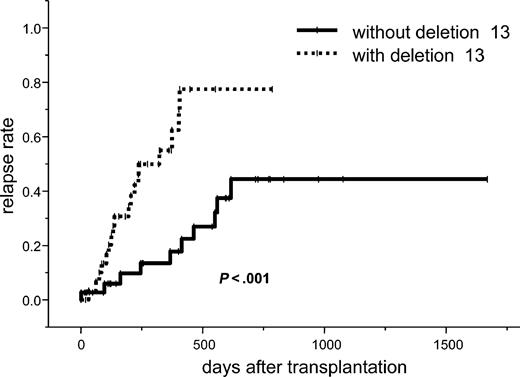
The incidence of relapse at 2 years for all patients was 60% (95% CI, 44%-76%). In the univariate analysis, 2 or more prior high-dose chemotherapies (73% vs 45%; P = .02), 13q- (77% vs 44%; P < .001), chemorefractory at time of transplantation (69% vs 53%; P = .05), CMV negativity of the recipient (53% vs 16%; P = .01), and relapse to a prior high-dose regimen (73% vs 45%; P = .02) were the significant factors for a higher relapse rate. In a multivariate analysis, 13q- was an independent factor for a higher relapse rate (HR, 3.28; 95% CI, 1.31-8.24; P = .01; Tables 3, 4; Figure 2).
Multivariate analysis of overall and event-free survival, relapse, and treatment-related mortality, only significant factors
. | Overall survival . | . | Event-free survival . | . | Relapse probability . | . | TRM . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | ||||
| At least 2 prior high-dose therapies | 2.48 (1.19-5.17) | .02 | - | - | - | - | - | - | ||||
| Deletion 13 | - | - | 1.94 (1.03-3.67) | .04 | 3.28 (1.31-8.24) | .01 | - | - | ||||
| Younger than 50 years of age | - | - | - | - | - | - | 0.13 (0.02-1.00) | .05 | ||||
. | Overall survival . | . | Event-free survival . | . | Relapse probability . | . | TRM . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | ||||
| At least 2 prior high-dose therapies | 2.48 (1.19-5.17) | .02 | - | - | - | - | - | - | ||||
| Deletion 13 | - | - | 1.94 (1.03-3.67) | .04 | 3.28 (1.31-8.24) | .01 | - | - | ||||
| Younger than 50 years of age | - | - | - | - | - | - | 0.13 (0.02-1.00) | .05 | ||||
-indicates not significant.
Overall survival
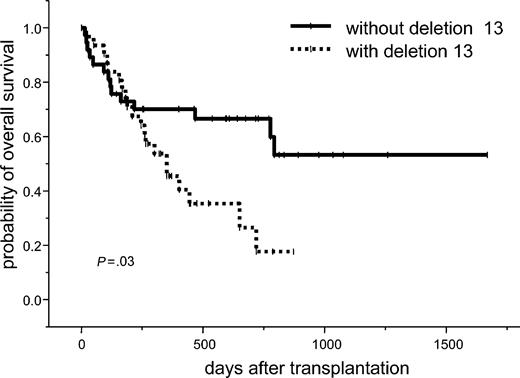
The estimated 2-year overall survival for all patients was 47% (95% CI, 33%-61%). In the univariate analysis, 2 or more prior high-dose chemotherapies (38% vs 57%; P = .005) and 13q- (18% vs 67%; P = .03) were significant unfavorable factors for overall survival. A trend toward unfavorable outcome was seen in patients with no chemosensitivity at time of transplantation (41% vs 51%; P = .07), relapse to prior high-dose chemotherapy (38% vs 57%; P = .09), and donor age older than 50 years (28% vs 61%; P = .08). In a multivariate analysis, 2 or more prior high-dose chemotherapies were associated with a significantly higher probability of death (HR, 2.48; 95% CI, 1.19-5.17; P = .02). Patients with deletion 13q had a nearly 2 times higher risk for death after treatment (HR, 1.94; 95% CI, 0.95-3.98; P = .07; Table 3; Figure 3).
Event-free survival
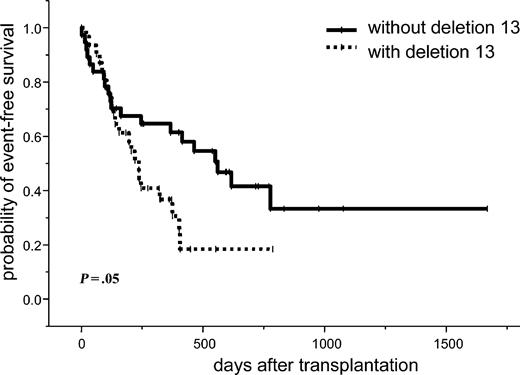
The estimated 2-year event-free survival for all patients was 33% (95% CI, 19%-47%). In the univariate analysis, 2 or more prior high-dose chemotherapies (14% vs 35%; P = .04) and 13q- (18% vs 42%; P = .05) were significant factors for an unfavorable event-free survival. A trend toward a lower relapse rate was seen in chemosensitive patients (P = .07) and those who did not experience relapse to a prior high-dose chemotherapy (P = .06). In a multivariate analysis, 13q- was the only independent significant factor for an unfavorable event-free survival (HR, 1.94; 95% CI, 1.03-3.67; P = .04; Tables 3, 4; Figure 4).
Discussion
Allogeneic stem cell transplantation after dose-reduced conditioning exerts a powerful graft-versus-myeloma effect and is associated with a relatively low treatment-related mortality in comparison to standard conditioning. Several strategies to incorporate the dose-reduced allo-approach within the treatment of multiple myeloma have been performed, such as a tandem auto-allo transplantation10,12 or salvage therapy after failure to a prior autograft.7-9 Risk factors for unfavorable outcome reported in smaller series of patients are no chemosensitivity at time of allografting,9,15,16 relapse to a prior autograft,11,15 or no experience of chronic GVHD.16,18 In a retrospective analysis of 256 patients that received transplants of various conditioning regimens, the EBMT reported an unfavorable overall survival in male patients receiving grafts from female donors and in patients who were chemorefractory at time of transplantation.36 In a recent multicenter international analysis involving 120 patients after melphalan/fludarabine conditioning, the most important factor for better survival was no relapse to a prior autograft, and also patients without chronic GVHD had a significantly higher probability of relapse than those with chronic GVHD.37 Chromosomal abnormalities, especially chromosome 13 losses, have become an important prognostic factor in multiple myeloma. Recent data clearly showed that 13q- is associated with a worse outcome after standard chemotherapy and after high-dose chemotherapy followed by autologous stem cell transplantation.18,19,21,24,27,32 So far, no data regarding the impact on distinct genomic changes on the outcome of patients after dose-reduced or standard conditioning followed by allografting are available. One report on 2 patients with 13q- suggests a possible benefit of allogeneic stem cell transplantation in those patients.38 The present international study showed that deletion 13q detected by FISH remains a prognostic negative factor for the outcome after dose-reduced allografting. It should be noted that the worse outcome is not due to higher treatment-related mortality but to a nearly 2 times higher probability for relapse than in those patients lacking deletion 13q. Because in the present study mainly advanced myeloma patients were included, it cannot be stated that deletion 13q is also a worse prognostic factor for untreated or chemosensitive patients. In our analysis only 10 patients with and 13 patients without del 13q did not experience relapse to conventional or high-dose chemotherapy at time of allografting. In this small group of patients only a trend for a worse 1-year overall and event-free survival (43% vs 72%; P = .1; and 28% vs 59%; P = .2) was seen for the del 13q patients, which did not reach statistical significance. Since the rate of complete remission after allografting was similar in patients with or without 13q-, in order to decrease relapse patients with 13q- should receive posttransplantation therapies to maintain remission, such as prophylactic donor lymphocyte infusion, thalidomide, vaccination strategies or specific cytotoxic T cells against myeloma-specific targets, or minor histocompatibility antigen.39 From the presented data it is likely that del 13q is also a worse prognostic factor for dose-reduced allograft, which has been shown for conventional and high-dose chemotherapy followed by autologous transplantation. A comparison between allo-mini and autologous transplantation for newly diagnosed high-risk myeloma patients is needed. Currently, randomized studies comparing tandem autologous transplantation with a single autologous transplantation followed by a dose-reduced allograft in high-risk patients with elevated β2-microglobulin level and/or 13q- are under investigation and the expected data will show whether or not patients with 13q- will benefit more from dose-reduced allografting than from autologous transplantation.
Prepublished online as Blood First Edition Paper, February 24, 2004; DOI 10.1182/blood-2003-12-4435.
Supported by a grant of the Deutsche Krebshilfe (NK-70-3133-Kr I).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staff of the bone marrow transplantation (BMT) units for providing excellent care of our patients and the medical technicians for their excellent work in the laboratories.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal