Abstract
Whites have a more favorable prognosis than African Americans for a number of cancers. The relationship between race and outcome is less clear in acute myeloid leukemia (AML). Using data from 7 Cancer and Leukemia Group B studies initiated from 1985 to 1997, we conducted a retrospective cross-sectional analysis of 2570 patients (270 African American and 2300 white) with de novo AML who received induction chemotherapy. African Americans were younger than whites (48 versus 54 years, P < .001). African Americans also had different cytogenetic risk group distributions than whites (P < .001): they were more commonly classified in the favorable (23% versus 14%) and unfavorable (31% versus 23%) groups, and less commonly classified in the intermediate group (47% versus 63%). African American men had a lower complete remission (CR) rate (54%, compared with 64% for white men, 65% for white women, and 70% for African American women, P = .001) and a worse overall survival compared with all other patients (P = .004), when known risk factors are taken into account. African Americans and whites with AML differ with respect to important prognostic factors. African American men have worse CR rates and overall survival than whites and African American women, and should be considered a poor-risk group.
The CALGB is chaired by Richard L. Schilsky.
Institution . | Principal investigator . |
|---|---|
| Main member institutions | |
| Dana-Farber Partners Cancer Care | George Canellos |
| Dartmouth Medical School - Norris Cotton Cancer Center | Marc Ernstoff |
| Duke University Medical Center | Jeffrey Crawford |
| Georgetown University Medical Center, Lombardi Cancer Center | Edward P. Gelmann |
| Memorial Sloan-Kettering Cancer Center | Clifford A. Hudis |
| Mount Sinai School of Medicine | Lewis Silverman |
| North Shore - Long Island Jewish Health System | Daniel Budman |
| The Ohio State University Medical Center | Clara D. Bloomfield |
| Rhode Island Hospital | William Sikov |
| Roswell Park Cancer Institute | Ellis Levine |
| SUNY Upstate Medical University | Stephen L. Graziano |
| University of California at San Diego | Stephen Seagren |
| University of California at San Francisco | Alan Venook |
| University of Chicago Medical Center | Gini Fleming |
| University of Illinois at Chicago | Thomas Lad |
| University of Iowa Hospitals | Gerald Clamon |
| University of Maryland Cancer Center | Martin Edelman |
| University of Massachusetts Medical Center | Pankaj Bhargava |
| University of Minnesota | Bruce Peterson |
| University of Missouri/Ellis Fischel Cancer Center | Michael Perry |
| University of Nebraska Medical Center | Anne Kessinger |
| University of North Carolina at Chapel Hill | Thomas Shea |
| Vermont Cancer Center | Hyman Muss |
| Wake Forest University School of Medicine | David D. Hurd |
| Walter Reed Army Medical Center | Joseph J. Drabeck |
| Washington University School of Medicine | Nancy Bartlett |
| Weill Medical College of Cornell University | Scott Wadler |
| At large institutions/CCOPs | |
| Cedars-Sinai Medical Center | Alan T. Lefor |
| Christiana Care Health Services, Inc | Stephen Grubbs |
| McGill University | Gerald Batist |
| Missouri Baptist Medical Center | Alan P. Lyss |
| Mount Sinai Medical Center - Miami | Rogerio Lilenbaum |
| Northern Indiana Cancer Research CCOP | Rafat Ansari |
| Southern Nevada Cancer Research Foundation | John Ellerton |
| Southeast Cancer Control Consortium, Inc | James N. Atkins |
| Syracuse Hematology-Oncology Associates | Jeffrey Kirshner |
| University of Texas Southwestern Medical Center | Debasish Tripathy |
| Van Andel Research Institute | George F. Vande Woude |
| Western Pennsylvania Cancer Institute | Richard K. Shadduck |
| Baptist Cancer Institute CCOP | Lee S. Schwartzberg |
| Evanston Northwestern Healthcare CCOP | Gershon Y. Locker |
| Grand Rapids Clincal Oncology Program CCOP | Kathleen J. Yost |
| Greenville CCOP | Jeffrey K. Giguere |
| Illinois Oncology Research Association CCOP | John W. Kugler |
| Kansas City Community Clincal Oncology Program CCOP | Jorge C. Paradelo |
| Massey Cancer Center MBCCOP | John D. Roberts |
Institution . | Principal investigator . |
|---|---|
| Main member institutions | |
| Dana-Farber Partners Cancer Care | George Canellos |
| Dartmouth Medical School - Norris Cotton Cancer Center | Marc Ernstoff |
| Duke University Medical Center | Jeffrey Crawford |
| Georgetown University Medical Center, Lombardi Cancer Center | Edward P. Gelmann |
| Memorial Sloan-Kettering Cancer Center | Clifford A. Hudis |
| Mount Sinai School of Medicine | Lewis Silverman |
| North Shore - Long Island Jewish Health System | Daniel Budman |
| The Ohio State University Medical Center | Clara D. Bloomfield |
| Rhode Island Hospital | William Sikov |
| Roswell Park Cancer Institute | Ellis Levine |
| SUNY Upstate Medical University | Stephen L. Graziano |
| University of California at San Diego | Stephen Seagren |
| University of California at San Francisco | Alan Venook |
| University of Chicago Medical Center | Gini Fleming |
| University of Illinois at Chicago | Thomas Lad |
| University of Iowa Hospitals | Gerald Clamon |
| University of Maryland Cancer Center | Martin Edelman |
| University of Massachusetts Medical Center | Pankaj Bhargava |
| University of Minnesota | Bruce Peterson |
| University of Missouri/Ellis Fischel Cancer Center | Michael Perry |
| University of Nebraska Medical Center | Anne Kessinger |
| University of North Carolina at Chapel Hill | Thomas Shea |
| Vermont Cancer Center | Hyman Muss |
| Wake Forest University School of Medicine | David D. Hurd |
| Walter Reed Army Medical Center | Joseph J. Drabeck |
| Washington University School of Medicine | Nancy Bartlett |
| Weill Medical College of Cornell University | Scott Wadler |
| At large institutions/CCOPs | |
| Cedars-Sinai Medical Center | Alan T. Lefor |
| Christiana Care Health Services, Inc | Stephen Grubbs |
| McGill University | Gerald Batist |
| Missouri Baptist Medical Center | Alan P. Lyss |
| Mount Sinai Medical Center - Miami | Rogerio Lilenbaum |
| Northern Indiana Cancer Research CCOP | Rafat Ansari |
| Southern Nevada Cancer Research Foundation | John Ellerton |
| Southeast Cancer Control Consortium, Inc | James N. Atkins |
| Syracuse Hematology-Oncology Associates | Jeffrey Kirshner |
| University of Texas Southwestern Medical Center | Debasish Tripathy |
| Van Andel Research Institute | George F. Vande Woude |
| Western Pennsylvania Cancer Institute | Richard K. Shadduck |
| Baptist Cancer Institute CCOP | Lee S. Schwartzberg |
| Evanston Northwestern Healthcare CCOP | Gershon Y. Locker |
| Grand Rapids Clincal Oncology Program CCOP | Kathleen J. Yost |
| Greenville CCOP | Jeffrey K. Giguere |
| Illinois Oncology Research Association CCOP | John W. Kugler |
| Kansas City Community Clincal Oncology Program CCOP | Jorge C. Paradelo |
| Massey Cancer Center MBCCOP | John D. Roberts |
Introduction
Acute myeloid leukemia (AML) is a malignant disorder arising through the acquisition of genetic mutations in hematopoietic stem or progenitor cells,2 resulting in impairment of hematopoiesis and unrestrained proliferation of an immature clone. In the United States, the annual population incidence of AML in whites is 3.7 cases per 100 000 people, whereas in African Americans it is 2.9 cases per 100 000 people.3
For a number of cancers, particularly cancers of the colon, breast, and prostate, whites have a more favorable prognosis than African Americans in the United States.4-11 The relationship between race and outcome is less clear in leukemia. African American children with acute lymphoblastic leukemia (ALL) are less likely to achieve remission and have an inferior disease-free and overall survival compared with white children with the same disease.12,13 One recent study from the Children's Cancer Group examined racial and ethnic differences in children with ALL and found that black children were overrepresented in high-risk characteristic groups and had a worse outcome compared with white children.14 Although data concerning incidence and outcome in AML according to race or ethnic group are sparse, Latinos appear to have a higher prevalence of one of the better-risk subtypes, acute promyelocytic leukemia (APL) (French-American-British [FAB] classification M3).15-17 It is unclear whether racial differences in subtypes and outcome in leukemia result from environmental or cultural influences,17 differences in genetics, or variability in reporting at diagnosis and/or follow-up.14
Surveillance, Epidemiology, and End Result (SEER) Program data indicate that the age-adjusted mortality rate for AML is slightly higher in whites compared with African Americans (2.5 versus 2.0 per 100 000 US population).3 The age adjustment is important, as whites are diagnosed at a median of 67 years, while the median for African Americans is 60 years. It is not known whether AML-specific mortality differences between the 2 races exist nationwide.18 In addition, racespecific death rates do not control for other prognostic factors, including cytogenetics and AML subtype.
Disparate cancer outcomes between races often are attributed to differential access to care, varying treatment aggressiveness and compliance, and biologic variability.5,6,8,11,13,19-21 Even if differential access to care and treatment aggressiveness are eliminated as potential confounders in describing racial differences in outcome for AML (as should occur within a cooperative group study), biologic variability may still play a role.22,23
Using data from Cancer and Leukemia Group B (CALGB) AML studies initiated from 1985 to 1997, we compared baseline prognostic factors, treatment-related complications, and outcome in African American and white patients receiving similar therapy for AML.
Patients, materials, and methods
Study population
We conducted a retrospective cross-sectional study of 2570 patients with de novo AML who were eligible to receive intensive chemotherapy as part of 1 of 7 CALGB studies: CALGB 8525, 8923, 9022, 9222, 9420, and 9621, and the initial 120 patients of CALGB 9720, the only study that allowed patients with prior myelodysplastic syndrome (MDS) or patients who had received prior cytotoxic chemotherapy to be included.24-31 Patients identified their own race, and those who were classified as being neither white nor African American (221 patients) were excluded from our analyses. Patients with APL were included in CALGB 8525, 9022, 9222, and the majority of the enrollment period for 8923, but excluded from the other, more recent studies.
Treatment and clinical data
Patients were treated with the use of protocol guidelines for study entry, treatment compliance, and therapy. Induction therapy included cytarabine (Ara-C) and daunorubicin; patients in some studies also received etoposide (VP-16) as a third agent. Postremission therapy varied by protocol (Table 1). Clinical data collected at the CALGB Statistical Center (Durham, NC) on most patients included age; sex; CALGB performance status; evidence of extramedullary leukemia; baseline complete blood counts; FAB type; immunophenotype (available on 554 patients [22%]); number of induction courses; response to induction; complete remission (CR) rates (which followed accepted criteria)32 ; disease-free survival (DFS) (defined as the interval from attainment of a CR or randomization to postremission therapy to the date of relapse, the date of death from any cause, or the date the patient was last known to be in remission); and overall survival (OS), which included patients who did not attain a CR. Median follow-up time for patients was 7.3 years (interquartile range, 3.0-10.4 years).
Summary of included trials
. | Treatment protocol . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8525a . | 8923b . | 9022c . | 9222d . | 9420e . | 9621f . | 9720g . | ||||||
| Years of patient accrual | 10/85-10/90 | 2/90-11/93 | 10/90-3/92 | 7/92-12/95 | 1/95-7/97 | 2/97-3/00 | 1/98-3/99 | ||||||
| Trial phase | 3 | 3 | 2 | 3 | 1 | 1/2 | 3 | ||||||
| Patientsh | |||||||||||||
| Total | 1036 | 360 | 213 | 407 | 103 | 340 | 111 | ||||||
| White, no. (%) | 920 (89) | 334 (93) | 192 (90) | 354 (87) | 94 (91) | 301 (89) | 105 (95) | ||||||
| African American, no. (%) | 116 (11) | 26 (7) | 21 (10) | 53 (13) | 9 (9) | 39 (11) | 6 (5) | ||||||
| Cytogenetics available, no. (%) | 563 (54) | 210 (58) | 124 (58) | 269 (66) | 62 (60) | 270 (79) | 82 (74) | ||||||
| Induction 1l | Yes | Yesi | Yes | Yes | Yesj | Yesj | Yesj | ||||||
| Etoposide used in inductions 1 (and 2, if needed) | No | No | No | No | Yes: 60 or 100 mg/m2/d × 3 days | Yes: 60-150 mg/m2/d × 3 days | Yes: 60 or 100 mg/m2/d × 3 days | ||||||
| Induction 2, if needed | Yes | Yes | Yes | Yes | Yesk | Yesk | Yesk | ||||||
| Intensification or consolidation therapy randomized, by arm | Yes, arms A, B, C | Yes, arms A, B | No | Yes, arms A, B | No | Yes, risk-adapted, arms A, B | No | ||||||
| Intensification or consolidation therapy 1, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: M + IDAC | HDAC | A: HDAC; B: HDAC | 5 + 2 + 2 | A:HDAC; B: HDAC + E | 5 + 2 + 2 | ||||||
| Intensification or consolidation therapy 2, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: M + IDAC | E + C | A: HDAC; B: E + C | - | A: HDAC; B: SCT | - | ||||||
| Intensification or consolidation therapy 3, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: None | M + D | A: HDAC; B: M + D | - | A: HDAC; B: None | - | ||||||
| Intensification or consolidation therapy 4, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: None | - | - | - | - | - | ||||||
| Maintenance therapy | Yes | No | No | No | Yes | Yes | Yes | ||||||
. | Treatment protocol . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8525a . | 8923b . | 9022c . | 9222d . | 9420e . | 9621f . | 9720g . | ||||||
| Years of patient accrual | 10/85-10/90 | 2/90-11/93 | 10/90-3/92 | 7/92-12/95 | 1/95-7/97 | 2/97-3/00 | 1/98-3/99 | ||||||
| Trial phase | 3 | 3 | 2 | 3 | 1 | 1/2 | 3 | ||||||
| Patientsh | |||||||||||||
| Total | 1036 | 360 | 213 | 407 | 103 | 340 | 111 | ||||||
| White, no. (%) | 920 (89) | 334 (93) | 192 (90) | 354 (87) | 94 (91) | 301 (89) | 105 (95) | ||||||
| African American, no. (%) | 116 (11) | 26 (7) | 21 (10) | 53 (13) | 9 (9) | 39 (11) | 6 (5) | ||||||
| Cytogenetics available, no. (%) | 563 (54) | 210 (58) | 124 (58) | 269 (66) | 62 (60) | 270 (79) | 82 (74) | ||||||
| Induction 1l | Yes | Yesi | Yes | Yes | Yesj | Yesj | Yesj | ||||||
| Etoposide used in inductions 1 (and 2, if needed) | No | No | No | No | Yes: 60 or 100 mg/m2/d × 3 days | Yes: 60-150 mg/m2/d × 3 days | Yes: 60 or 100 mg/m2/d × 3 days | ||||||
| Induction 2, if needed | Yes | Yes | Yes | Yes | Yesk | Yesk | Yesk | ||||||
| Intensification or consolidation therapy randomized, by arm | Yes, arms A, B, C | Yes, arms A, B | No | Yes, arms A, B | No | Yes, risk-adapted, arms A, B | No | ||||||
| Intensification or consolidation therapy 1, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: M + IDAC | HDAC | A: HDAC; B: HDAC | 5 + 2 + 2 | A:HDAC; B: HDAC + E | 5 + 2 + 2 | ||||||
| Intensification or consolidation therapy 2, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: M + IDAC | E + C | A: HDAC; B: E + C | - | A: HDAC; B: SCT | - | ||||||
| Intensification or consolidation therapy 3, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: None | M + D | A: HDAC; B: M + D | - | A: HDAC; B: None | - | ||||||
| Intensification or consolidation therapy 4, by arm | A: SDAC; B: IDAC; C: HDAC | A: SDAC; B: None | - | - | - | - | - | ||||||
| Maintenance therapy | Yes | No | No | No | Yes | Yes | Yes | ||||||
SDAC indicates 100 mg/m2 cytarabine by ci every day × 5 days; IDAC, 400 mg/m2 cytarabine by ci every day × 5 days; HDAC, 3 g/m2 cytarabine by intravenous bolus (ivb) over 3 hours every 12 hours on days 1, 3 and 5; HDAC + E, 2 mg/m2 cytarabine by ivb over 2 hours every 12 hours on days 1-4 and 40 mg/kg etoposide by ci every day for 4 days; M + IDAC, 5 mg/m2 mitoxantrone every 12 hours and 500 mg/m2 cytarabine every 12 hours for 6 doses each; E + C, 1800 mg/m2 etoposide by ci day 1 plus 50 mg/kg cyclophosphamide ivb on days 2 and 3; M + D, 12 mg/m2 mitoxantrone ivb plus 24 mg/m2 diaziquone by ci days 1, 2, and 3 plus 5 μg/kg filgrastim subcutaneously days 4 through 28; 5 + 2 + 2, 100 mg/m2 cytarabine by continuous infusion every day × 5 days plus 2 days of daunorubicin and etoposide at doses equivalent to those received in induction 1, ± PSC 833. Maintenance therapy for study 8525 consisted of 45 mg/m2 daunorubicin ivb on day 1 plus 100 mg/m2 cytarabine subcutaneously every 12 hours on days 1 through 5 repeated monthly × 4; for studies 9420, 9621, and 9720, maintenance therapy consisted of interleukin-2 as specified by Farag et al30 and Baer et al.31 ivp indicates intravenous push; SCT, stem cell transplantation; and -, not applicable.
Mayer et al.24
Moore et al.26
Moore et al.27
Lee et al.28
Kolitz et al.29
Baer et al.31
Patient population data are as follows: total, N = 2570; white, n = 2300 (89.5% of total); African American, n = 270 (10.5% of total); and cytogenetics available, n = 1580 (61% of total)
50% of enrolled patients received granulocyte-macrophage colony-stimulating factor (GM-CSF) during induction
Patients in 9420 received daunorubicin at doses ranging from 30 to 60 mg/m2/d × 3 days and also received the multidrug resistance modulator PSC 833 at a loading dose of 1.5 mg/kg and a continuous infusion of 10 mg/kg/d for 3 days; patients in 9621 received daunorubicin at doses ranging from 40 to 90 mg/m2/d × 3 days depending on their randomization to PSC 833 at a loading dose of 2.8 mg/kg and a continuous infusion of 10 mg/kg/d for 3 days; patients in 9720 received daunorubicin at doses of 40 or 60 mg/m2/d × 3 days depending on their randomization to PSC 833 at a loading dose of 2.8 mg/kg and a continuous infusion of 10 mg/kg/d for 3 days
Doses were equivalent to those received in induction 1. Patients receiving PSC 833 for induction 1 received it for induction 2 over 2 days
Induction 1 consisted of daunorubicin 45 mg/m2 ivp × 3 days and cytarabine 100 or 200 mg/m2 by continuous infusion (ci) × 7 days. Induction 2, which was administered if needed, consisted of daunorubicin 45 mg/m2 ivp × 2 days and cytarabine 100 or 200 mg/m2 by ci × 5 days
Cytogenetic data
Centrally reviewed cytogenetic data were available on 1580 patients (61%) in the study sample who were enrolled on CALGB 8461, a prospective cytogenetics study initiated in 1984.33 Specimens were obtained at diagnosis from all patients and were processed with the use of unstimulated short-term cultures, a direct method, or both. G-banding was usually done, although Q-banding was also acceptable. At least 20 marrow metaphase cells were analyzed in patients designated as having a normal karyotype.
Cytogenetic risk groups were classified by the schema developed by CALGB for overall survival,33 as follows: favorable risk cytogenetics included t(8;21), inv(16), or t(16;16); intermediate risk cytogenetics included a normal karyotype, del (9q), -Y, del(5q), loss of 7q, t(9;11), +11, del(11q), abn(12p), +13, del(20q), and +21. Unfavorable cytogenetics included complex (3 or more abnormalities) karyotypes, inv(3) or t(3;3), t(6;9), t(6;11), -7, +8, and t(11;19)(q23;p13.1). Of the 1580 patients, 89 individuals with t(15;17) (indicative of APL) or t(9;22) (suggestive of the blastic phase of chronic myelogenous leukemia) were analyzed separately, as these were thought to represent distinct AML subtypes. An additional 108 patients with karyotypes that included single nonrecurring structural abnormalities, or cytogenetic abnormalities that were found in fewer than 5 patients, were also excluded from the cytogenetic risk stratification, leaving 1383 patients stratified into 1 of the 3 cytogenetic risk groups. Immunophenotyping was performed by multiparameter flow cytometry, as previously described.34
Statistical analysis
Descriptive statistics, including proportions, medians, and 5-year time-to-event probabilities, were used to summarize the distribution of variables within subgroups. The Fisher exact test was used to describe the associations in 2 × 2 and 2 × 3 contingency tables. Kaplan-Meier plots35 and the log-rank test were used to examine difference in overall survival and DFS. The proportional hazards model was used to model both OS and DFS as a function of demographic, clinical, and cytogenetic risk group (ie, the 3-level variable described in Table 4). Likewise, the logistic regression model was used to model CR rate as a function of this same set of variables.
Percentage of whites and African Americans with each cytogenetic abnormality
Risk group . | Whites, n (%) . | African Americans, n (%) . | Total, n (%) . | P . |
|---|---|---|---|---|
| Favorable | ||||
| t(8;21) | 72 (5.8) | 24 (17.0) | 96 (7.0) | < .0001 |
| inv(16) or t(16;16) | 101 (8.1) | 8 (5.7) | 109 (7.9) | .40 |
| Intermediate | ||||
| Normal | 644 (51.9) | 53 (37.5) | 697 (50.6) | .0014 |
| del(9q) | 34 (2.7) | 5 (3.5) | 39 (2.8) | .59 |
| t(9;11) | 31 (2.5) | 3 (2.1) | 34 (2.5) | 1.0 |
| del(5q) | 51 (4.1) | 8 (5.7) | 59 (4.3) | .38 |
| loss of 7q | 23 (1.9) | 5 (3.5) | 28 (2.0) | .20 |
| +11 | 25 (2.0) | 3 (2.1) | 28 (2.0) | .76 |
| del(11q) | 9 (0.7) | 1 (0.7) | 10 (0.7) | 1.0 |
| abn(12p) | 38 (3.1) | 6 (4.3) | 44 (3.2) | .44 |
| +13 | 32 (2.6) | 5 (3.5) | 37 (2.7) | .42 |
| del(20q) | 14 (1.1) | 2 (1.4) | 16 (1.2) | .67 |
| +21 | 36 (2.9) | 1 (0.7) | 37 (2.7) | .17 |
| -Y | 44 (3.5) | 7 (5.0) | 51 (3.7) | .35 |
| Unfavorable | ||||
| Complex, 3 or more abn | 131 (10.5) | 18 (12.8) | 149 (10.8) | .30 |
| inv(3) or t(3;3) | 18 (1.5) | 1 (0.7) | 19 (1.4) | .71 |
| t(6;9) | 7 (0.6) | 3 (2.1) | 10 (0.7) | .07 |
| t(6;11) | 7 (0.6) | 4 (2.8) | 11 (0.8) | .02 |
| -7 | 37 (3.0) | 5 (3.6) | 42 (3.0) | .61 |
| +8 | 139 (11.2) | 22 (15.6) | 161 (11.7) | .13 |
| t(11;19) (q23;p13.1) | 9 (0.7) | 0 (0) | 9 (0.7) | .61 |
Risk group . | Whites, n (%) . | African Americans, n (%) . | Total, n (%) . | P . |
|---|---|---|---|---|
| Favorable | ||||
| t(8;21) | 72 (5.8) | 24 (17.0) | 96 (7.0) | < .0001 |
| inv(16) or t(16;16) | 101 (8.1) | 8 (5.7) | 109 (7.9) | .40 |
| Intermediate | ||||
| Normal | 644 (51.9) | 53 (37.5) | 697 (50.6) | .0014 |
| del(9q) | 34 (2.7) | 5 (3.5) | 39 (2.8) | .59 |
| t(9;11) | 31 (2.5) | 3 (2.1) | 34 (2.5) | 1.0 |
| del(5q) | 51 (4.1) | 8 (5.7) | 59 (4.3) | .38 |
| loss of 7q | 23 (1.9) | 5 (3.5) | 28 (2.0) | .20 |
| +11 | 25 (2.0) | 3 (2.1) | 28 (2.0) | .76 |
| del(11q) | 9 (0.7) | 1 (0.7) | 10 (0.7) | 1.0 |
| abn(12p) | 38 (3.1) | 6 (4.3) | 44 (3.2) | .44 |
| +13 | 32 (2.6) | 5 (3.5) | 37 (2.7) | .42 |
| del(20q) | 14 (1.1) | 2 (1.4) | 16 (1.2) | .67 |
| +21 | 36 (2.9) | 1 (0.7) | 37 (2.7) | .17 |
| -Y | 44 (3.5) | 7 (5.0) | 51 (3.7) | .35 |
| Unfavorable | ||||
| Complex, 3 or more abn | 131 (10.5) | 18 (12.8) | 149 (10.8) | .30 |
| inv(3) or t(3;3) | 18 (1.5) | 1 (0.7) | 19 (1.4) | .71 |
| t(6;9) | 7 (0.6) | 3 (2.1) | 10 (0.7) | .07 |
| t(6;11) | 7 (0.6) | 4 (2.8) | 11 (0.8) | .02 |
| -7 | 37 (3.0) | 5 (3.6) | 42 (3.0) | .61 |
| +8 | 139 (11.2) | 22 (15.6) | 161 (11.7) | .13 |
| t(11;19) (q23;p13.1) | 9 (0.7) | 0 (0) | 9 (0.7) | .61 |
Number of patients tested were as follows: whites, n = 1242; African Americans, n = 141; total, n = 1383. Abnormalities are ordered in this table according to the way in which they were used to form cytogenetic risk groups. Percentages do not sum up to 100% because patients could have more than one abnormality.
Our major interest was race differences in prognostic factors and clinical outcomes; this focus was narrowed when large clinical differences between African American men and others emerged. The covariates used in the tests of African American male effects included age, M3 status, white blood cell (WBC) count, and cytogenetic risk group. As slightly more than 1000 patients did not have cytogenetic information, we first controlled only for age, M3 status, and WBC count. Then cytogenetic risk group was added to the model, and we observed if there was any change in the race or African American male regression coefficient. As the addition of cytogenetic risk group had minimal impact on the regression coefficient, or the regression coefficient became even larger, covariate-adjusted results are reported for the control of only age, M3, and WBC count. Controlling for individual cytogenetic abnormalities had no impact on the association between race/sex and the clinical endpoints. A 2-sided alpha level of 0.05 was used for all tests; as a large number of tests were performed, those of borderline significance should be interpreted with caution. Statistical analyses were performed at the CALGB Statistical Center.
Results
Clinical and biologic characteristics at study entry
Of the 2570 patients included in our analysis, 270 (10.5%) were African American and 2300 (89.5%) were white. The distribution of patients by study is summarized in Table 1. There were no significant differences in the percentage of African American men and women represented in each study.
Overall, baseline characteristics were similar in the 2 groups (Table 2). However, African Americans were younger, with a median age of 48 years compared with 54 years for whites (P < .001), and were more likely to be female (58% versus 46%, P < .001). African Americans also had more leukemic involvement of the central nervous system (CNS) at diagnosis (2% versus 1%, P = .04), but were less likely to have skin involvement (4% versus 8%, P = .02) than whites.
Baseline characteristics
Characteristic . | Whites, N = 2300 . | African Americans, N = 270 . | P . |
|---|---|---|---|
| Median age, y | 54 | 48 | < .001 |
| Minimum | 16 | 17 | |
| Maximum | 86 | 86 | |
| Sex, n (%) | < .001 | ||
| Men | 1233 (54) | 114 (42) | |
| Women | 1067 (46) | 156 (58) | |
| Performance status, n (%) | .21 | ||
| 0 | 673 (30) | 67 (25) | |
| 1 | 1015 (45) | 117 (44) | |
| 2 | 431 (19) | 59 (22) | |
| 3 | 111 (5) | 16 (6) | |
| 4 | 28 (1) | 6 (2) | |
| Extramedullary leukemia, n (%) | |||
| Liver | .91 | ||
| No | 2041 (90) | 239 (91) | |
| Yes | 215 (10) | 24 (9) | |
| Spleen | .73 | ||
| No | 2051 (91) | 244 (92) | |
| Yes | 203 (9) | 22 (8) | |
| CNS | .04 | ||
| No | 2236 (99) | 261 (98) | |
| Yes | 14 (≤ 1) | 5 (2) | |
| PNS | .72 | ||
| No | 2255 (99) | 267 (> 99) | |
| Yes | 14 (≤ 1) | 1 (< 1) | |
| Nodes | .44 | ||
| No | 1968 (87) | 226 (85) | |
| Yes | 291 (13) | 39 (15) | |
| Skin | .02 | ||
| No | 2086 (92) | 258 (96) | |
| Yes | 170 (8) | 10 (4) | |
| Gum | .76 | ||
| No | 2002 (89) | 235 (88) | |
| Yes | 255 (11) | 32 (12) | |
| Mass | .73 | ||
| No | 2237 (99) | 265 (99) | |
| Yes | 20 (1) | 3 (1) | |
| Median WBC count, × 109/L | 13 700 | 16 300 | .39 |
| Minimum | 100 | 300 | |
| Maximum | 560 000 | 301 000 | |
| Median platelet level, × 109/L | 54 000 | 57 000 | .52 |
| Minimum | 4000 | 2000 | |
| Maximum | 1 200 000 | 426 000 | |
| Median Hgb level, g/L | 92 | 90 | .59 |
| Minimum | 28 | 35 | |
| Maximum | 149 | 150 | |
| Histology, FAB, n (%) | .04 | ||
| M0 | 49 (2) | 9 (3) | |
| M1 | 497 (22) | 52 (19) | |
| M2 | 678 (29) | 84 (31) | |
| M3 | 144 (6) | 31 (11) | |
| M4 | 539 (23) | 61 (23) | |
| M5 | 251 (11) | 19 (7) | |
| M6 | 70 (3) | 6 (2) | |
| M7 | 13 (1) | 1 (< 1) | |
| Other | 24 (1) | 3 (1) | |
| Missing | 35 (2) | 4 (1) |
Characteristic . | Whites, N = 2300 . | African Americans, N = 270 . | P . |
|---|---|---|---|
| Median age, y | 54 | 48 | < .001 |
| Minimum | 16 | 17 | |
| Maximum | 86 | 86 | |
| Sex, n (%) | < .001 | ||
| Men | 1233 (54) | 114 (42) | |
| Women | 1067 (46) | 156 (58) | |
| Performance status, n (%) | .21 | ||
| 0 | 673 (30) | 67 (25) | |
| 1 | 1015 (45) | 117 (44) | |
| 2 | 431 (19) | 59 (22) | |
| 3 | 111 (5) | 16 (6) | |
| 4 | 28 (1) | 6 (2) | |
| Extramedullary leukemia, n (%) | |||
| Liver | .91 | ||
| No | 2041 (90) | 239 (91) | |
| Yes | 215 (10) | 24 (9) | |
| Spleen | .73 | ||
| No | 2051 (91) | 244 (92) | |
| Yes | 203 (9) | 22 (8) | |
| CNS | .04 | ||
| No | 2236 (99) | 261 (98) | |
| Yes | 14 (≤ 1) | 5 (2) | |
| PNS | .72 | ||
| No | 2255 (99) | 267 (> 99) | |
| Yes | 14 (≤ 1) | 1 (< 1) | |
| Nodes | .44 | ||
| No | 1968 (87) | 226 (85) | |
| Yes | 291 (13) | 39 (15) | |
| Skin | .02 | ||
| No | 2086 (92) | 258 (96) | |
| Yes | 170 (8) | 10 (4) | |
| Gum | .76 | ||
| No | 2002 (89) | 235 (88) | |
| Yes | 255 (11) | 32 (12) | |
| Mass | .73 | ||
| No | 2237 (99) | 265 (99) | |
| Yes | 20 (1) | 3 (1) | |
| Median WBC count, × 109/L | 13 700 | 16 300 | .39 |
| Minimum | 100 | 300 | |
| Maximum | 560 000 | 301 000 | |
| Median platelet level, × 109/L | 54 000 | 57 000 | .52 |
| Minimum | 4000 | 2000 | |
| Maximum | 1 200 000 | 426 000 | |
| Median Hgb level, g/L | 92 | 90 | .59 |
| Minimum | 28 | 35 | |
| Maximum | 149 | 150 | |
| Histology, FAB, n (%) | .04 | ||
| M0 | 49 (2) | 9 (3) | |
| M1 | 497 (22) | 52 (19) | |
| M2 | 678 (29) | 84 (31) | |
| M3 | 144 (6) | 31 (11) | |
| M4 | 539 (23) | 61 (23) | |
| M5 | 251 (11) | 19 (7) | |
| M6 | 70 (3) | 6 (2) | |
| M7 | 13 (1) | 1 (< 1) | |
| Other | 24 (1) | 3 (1) | |
| Missing | 35 (2) | 4 (1) |
CNS indicates central nervous system; PNS, peripheral nervous system; and Hgb, hemoglobin.
The FAB classification at study entry also differed between races (P = .04). African Americans were more likely than whites to have a diagnosis of M3 (acute promyelocytic) leukemia (11% versus 6%, P = .003), but less likely to have a diagnosis of M5 (monocytic) leukemia, with a trend toward significance (7% versus 11%, P = .06). These differences persisted when age was controlled for.
Flow cytometry data were available for 62 African Americans (23%) and 492 whites (21%). Human leukocyte antigen DR (HLA-DR) was expressed on myeloblasts from African Americans less frequently than on blasts from whites (73% versus 85%, P = .02), as would be expected from the FAB classification data. Other cell surface antigen expression was similar in the 2 groups.
Baseline cytogenetic data were available for 168 African Americans (62%) and 1412 whites (61%) (Table 3). African Americans were more likely than whites to have a t(15;17), the translocation associated with APL (8.3% versus 4.7%, respectively, P = .06) and a t(9;22) (1.8% versus 0.4%, P = .06), though numbers were small. There were 1383 patients (141 African Americans and 1242 whites) who were classifiable into favorable, intermediate, or unfavorable cytogenetic risk score groups. African Americans had significantly different cytogenetic risk group distributions than whites (P < .001): they were more commonly classified in the favorable (23% versus 14%) and unfavorable (31% versus 23%) risk groups, and less commonly classified in the intermediate group (47% versus 63%).
Cytogenetic characteristics among 1580 patients with adequate karyotypes
Cytogenetic classification . | Whites, n (%) . | African Americans, n (%) . | Total, n (%) . |
|---|---|---|---|
| t(15;17)* | 66 (4.7) | 14 (8.3) | 80 (5.1) |
| t(9;22)* | 6 (0.4) | 3 (1.8) | 9 (0.6) |
| Not included in risk group | 98 (7) | 10 (6) | 108 (6.8) |
| Included in risk group | 1242 (88.0) | 141 (83.9) | 1383 (87.5) |
| Total | 1412 (100) | 168 (100) | 1580 (100) |
| Risk group† | |||
| Favorable | 173 (14) | 32 (23) | 205 (15) |
| Intermediate | 786 (63) | 66 (47) | 852 (62) |
| Unfavorable | 283 (23) | 43 (31) | 326 (24) |
| Total | 1242 (100) | 141 (100) | 1383 (100) |
Cytogenetic classification . | Whites, n (%) . | African Americans, n (%) . | Total, n (%) . |
|---|---|---|---|
| t(15;17)* | 66 (4.7) | 14 (8.3) | 80 (5.1) |
| t(9;22)* | 6 (0.4) | 3 (1.8) | 9 (0.6) |
| Not included in risk group | 98 (7) | 10 (6) | 108 (6.8) |
| Included in risk group | 1242 (88.0) | 141 (83.9) | 1383 (87.5) |
| Total | 1412 (100) | 168 (100) | 1580 (100) |
| Risk group† | |||
| Favorable | 173 (14) | 32 (23) | 205 (15) |
| Intermediate | 786 (63) | 66 (47) | 852 (62) |
| Unfavorable | 283 (23) | 43 (31) | 326 (24) |
| Total | 1242 (100) | 141 (100) | 1383 (100) |
P = .06
P = < .001. Two-degrees-of-freedom test of difference between races in risk group distribution
Table 4 shows cytogenetic abnormalities for the 1383 patients classified into 1 of the 3 risk groups. African Americans were more likely than whites to have the favorable t(8;21) (17.0% versus 5.8%, P < .0001). Whites, on the other hand, were more likely to have a normal karyotype (51.9% versus 37.6%, P = .0014). African Americans were more likely to have t(6;9) (2.1% versus 0.6%, P = .07) and t(6;11) (2.8% versus 0.6%, P = .02), both unfavorable cytogenetic abnormalities. There was no difference in the distribution of cytogenetic abnormalities between African American men and women.
Clinical outcome
African American and white AML patients received a similar number of courses of remission induction therapy (Table 5) and had similar treatment-related mortality rates (18% for whites and 16% for African Americans), which did not differ by sex. Induction toxicity did not differ in the 2 races. Whites were more likely to have grade 3 or 4 anemia, however (P = .03).
Overall clinical outcome
. | Whites . | . | . | African Americans . | . | . | P unadjusted (adjusted)* . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Men . | Women . | Total . | Men . | Women . | Total . | Comparing races† . | For AA men‡ . | |||||
| Induction courses, n (%) | |||||||||||||
| 1 | 867 (70) | 788 (74) | 1655 (72) | 87 (76) | 111 (71) | 198 (73) | .67 | .34 | |||||
| 2 | 366 (30) | 279 (26) | 645 (28) | 27 (24) | 45 (29) | 72 (27) | (.35) | (.24) | |||||
| Response to induction, n (%) | |||||||||||||
| CR | 783 (64) | 698 (65) | 1481 (64) | 61 (54) | 109 (70) | 170 (63) | .64 | .01 | |||||
| NR/death | 450 (37) | 369 (35) | 819 (36) | 53 (46) | 47 (30) | 100 (37) | (.10) | (.001) | |||||
| Disease-free survival | |||||||||||||
| 5-year probability | 0.26 | 0.27 | 0.26 | 0.27 | 0.21 | 0.21 | .09 | .21 | |||||
| Standard error | 0.016 | 0.107 | 0.123 | 0.058 | 0.041 | 0.033 | (.02) | (.08) | |||||
| Overall survival | |||||||||||||
| 5-year probability | 0.24 | 0.26 | 0.25 | 0.16 | 0.26 | 0.22 | .19 | .04 | |||||
| Standard error | 0.013 | 0.014 | 0.009 | 0.036 | 0.036 | 0.026 | (.009) | (.004) | |||||
. | Whites . | . | . | African Americans . | . | . | P unadjusted (adjusted)* . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | Men . | Women . | Total . | Men . | Women . | Total . | Comparing races† . | For AA men‡ . | |||||
| Induction courses, n (%) | |||||||||||||
| 1 | 867 (70) | 788 (74) | 1655 (72) | 87 (76) | 111 (71) | 198 (73) | .67 | .34 | |||||
| 2 | 366 (30) | 279 (26) | 645 (28) | 27 (24) | 45 (29) | 72 (27) | (.35) | (.24) | |||||
| Response to induction, n (%) | |||||||||||||
| CR | 783 (64) | 698 (65) | 1481 (64) | 61 (54) | 109 (70) | 170 (63) | .64 | .01 | |||||
| NR/death | 450 (37) | 369 (35) | 819 (36) | 53 (46) | 47 (30) | 100 (37) | (.10) | (.001) | |||||
| Disease-free survival | |||||||||||||
| 5-year probability | 0.26 | 0.27 | 0.26 | 0.27 | 0.21 | 0.21 | .09 | .21 | |||||
| Standard error | 0.016 | 0.107 | 0.123 | 0.058 | 0.041 | 0.033 | (.02) | (.08) | |||||
| Overall survival | |||||||||||||
| 5-year probability | 0.24 | 0.26 | 0.25 | 0.16 | 0.26 | 0.22 | .19 | .04 | |||||
| Standard error | 0.013 | 0.014 | 0.009 | 0.036 | 0.036 | 0.026 | (.009) | (.004) | |||||
Patient populations are as follows: white men, N = 1233; white women, N = 1067; total whites, N = 2300; African American men, N = 114; African American women, N = 156; and total African Americans, N = 270.
Adjusted P values control for age, M3 status, and white blood cell count
P values for comparison of African Americans to whites
P values for comparison of African American men to others
African American men had a significantly lower CR rate than all other patients when age, M3, and WBC count are controlled for (P = .001). African American men had a CR rate of 54% (n = 114) as compared with the much higher CR rates of 64% (n = 1233) for white men, 65% (n = 1067) for white women, and 70% (n = 156) for African American women. Differences persisted when cytogenetic risk group is controlled for.
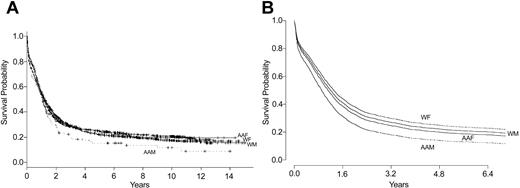
While there was a significant difference between the races in OS when age, M3, and WBC count are controlled for (P = .009), this difference was due almost entirely to the poorer survival of African American men compared with all other patients (P = .004). Figure 1 shows Kaplan-Meier curves demonstrating survival by race and sex, and predicted survival curves after age, M3, and WBC count are controlled for. There was also a small, but significant difference between the races in DFS (P = .02). Controlling for cytogenetic risk group had negligible impact on OS or DFS.
Survival by race and sex. (A) Unadjusted results (Kaplan-Meier plot). P = .04 for unadjusted comparison of survival of African American men with all other groups. (B) Adjusted results (log-rank test). P = .004 for comparison of survival of African American men with all other groups. AAF indicates African American women (n = 156); AAM, African American men (n = 114); WF, white women (n = 1067); and WM, white men (n = 1233).
Survival by race and sex. (A) Unadjusted results (Kaplan-Meier plot). P = .04 for unadjusted comparison of survival of African American men with all other groups. (B) Adjusted results (log-rank test). P = .004 for comparison of survival of African American men with all other groups. AAF indicates African American women (n = 156); AAM, African American men (n = 114); WF, white women (n = 1067); and WM, white men (n = 1233).
Survival within subgroups favored whites. Within FAB subgroups, this survival difference was statistically significant only within the M4 subtype of AML, for which African Americans had a median survival of 0.8 years, compared with the 1.3 year median survival for whites (P = .004). Within cytogenetic risk groups, African Americans with favorable cytogenetics had a median survival of 2.1 years, compared with 5.6 years for whites (P = .42). Similar patterns were found in those with intermediate (1.0 versus 1.3 years, P = .10) and unfavorable (0.3 versus 0.6 years, P = .96) risk cytogenetic cohorts. Looking at specific cytogenetic abnormalities within cytogenetic subgroups shows that African Americans with a t(15;17) lived a median of 2.1 years, compared with 2.4 years for whites (P = .73), while those with a t(8;21) survived a median of 3.1 years, compared with 5.1 years for whites (P = .89). African Americans with complex karyotypes had a dismal survival (a median of 0.2 years), but survival was also quite poor for whites (a median of 0.5 years, P = .31).
Discussion
Patients with AML receive similar treatment regimens consisting of an anthracycline or anthracenedione and cytarabine, regardless of race. In general, modifications to this therapy are introduced under the following circumstances: (1) patients with an advanced age or poor health or multiple comorbidities may choose nonintensive or palliative therapy; (2) patients with the FAB subtype M3 AML, associated with the t(15;17), now receive additional all-trans retinoic acid; or (3) in patients with a particularly poor prognosis, a hematopoietic stem cell transplantation (SCT) may be considered, age and health permitting, once remission is achieved. The results of our study indicate that African Americans, particularly African American men, are more likely than whites to fall into the latter category and that African Americans have worse overall survival than whites when the 2 groups were given similar initial therapies.
We pooled data from 7 CALGB AML treatment studies conducted over 14 years to compare African American and white patients with respect to prognostic variables and outcome. African Americans represented 10.5% of study patients, a number that approaches the proportion of African Americans 18 years or older in the United States, 11.4%, as determined by US Census Bureau data.37
Prognostic variables differed in the 2 groups. The percentage of African Americans with the favorable FAB subtype M3 AML (APL) was almost twice that of whites (and correlated to the percentage with a t(15;17)). African Americans also were 3 times as likely to have the favorable-risk t(8;21). Whites, on the other hand, were more likely to have a normal karyotype (51.9% of cytogenetic findings, compared with only 37.5% of findings in African Americans). The explanation for this difference is not apparent. Patients with APL were excluded from the more contemporary studies (as the use of differentiation agents became standard practice) and may have been overrepresented in earlier studies (which excluded patients with antecedent MDS, which rarely precedes APL). Thus, the prevalence of APL in this study (6.8%) cannot be thought of as representative of the population, and rates in each race may have been skewed. It is also possible that African Americans with favorable morphology, such as the FAB subtype M3 AML, were preferentially referred to CALGB study sites for therapy. African Americans with “nonnormal” cytogenetics may have more environmental exposures that predispose them to cancer and bone marrow stem cell disorders compared with whites.38,39 This may account for the increased incidence of unfavorable cytogenetics in this group, which occurs in pediatric ALL.40 There may also be a genetic predisposition to certain favorable chromosomal rearrangements.
Perhaps more importantly, a new prognostic variable emerged. In both unadjusted and adjusted analyses, African American men had lower rates of complete remission and overall survival than any other group. This difference cannot be explained by other prognostic factors, which were controlled for; by treatment-related toxicities, which were similar in both ethnic groups; or by differences in therapies, as all patients were treated on CALGB trials and received a similar number of remission induction courses. While it is possible that African American men did not receive similar postremission therapy (eg, they may not have undergone an SCT owing to lack of a donor, as unrelated donors are less common in this population),41,42 their CR rates also were significantly lower than those in any other group, which would predict for a worse survival regardless of postremission therapy. Information regarding SCT following CR1 or in first relapse was not routinely collected. However, the number of patients who underwent an SCT in CR1 was small, as few patients were removed from these treatment trials to undergo an SCT, and this procedure would have been highly unlikely in studies focusing on AML in older adults (CALGB studies 8923, 9420, and 9720). Moreover, where such SCT information was collected, we would be concerned about systematic information bias. This finding of a worse outcome in African American men with AML is similar to what has been found in studies of men with colon cancer43,44 and may be due to biologic heterogeneity and/or differential drug metabolism and variable efficacy, as would occur with myeloblast expression of MDR1 or an FLT3 internal tandem duplication, and merits further study.
This study has several potential limitations. It is a cooperative group study involving patients who qualified for clinical trials, who were on average younger than the median age at AML diagnosis in the United States, and were less likely to have antecedent MDS or to have therapy-related AML. Thus, these results may not be generalizable to the entire population of patients with AML. One key advantage of this design is the assurance that virtually every patient received identical initial therapy; thus, the study controls for therapy intensity as a potential confounder. In addition, we did not have data regarding socioeconomic status, and thus could not evaluate this known risk factor for poor outcome in patients with cancer.5,45 One future direction would involve performing a population-based study, enabling prospective comparisons of patients of different races, complete follow-up, and testing the generalizability of information about patients enrolled in cooperative group studies. This would allow enhanced examination of racial differences in older patients, and in patients with antecedent stem cell disorders or treatment-related AML.
In conclusion, African Americans and whites with AML differ with respect to some important prognostic factors. African American men have worse CR rates and overall survival than whites and African American women. These findings support more research concerning the specific biologic features as well as the development of novel therapeutic approaches in African American men, who should be considered a poor risk group. In addition, it should be recognized that African Americans are more likely than whites to present with good-risk AML (including APL or AML with a t(8;21)), and appropriate therapy should be initiated early. Future population-based studies should try to define these racial differences further by prospectively focusing on differential environmental exposure rates; differences in drug metabolism and bioavailability; and rates of therapy compliance, toxicities, and access to remission induction and postremission therapy, including SCT.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-09-3118.
A complete list of the members of the Cancer and Leukemia Group B appears in the “Appendix.”
The research for CALGB 10401 was supported, in part, by grants from the National Cancer institute (CA3946) to the Cancer and Leukemia Group B. The CALGB Statistical Office, Durham, NC, is supported by CA33601. Support for cytogenetics data and for C.D.B. comes from National Institutes of Health grants CA16058, CA101140, CA31946, and CA77658, and from the Coleman Leukemia Research Fund.
Richard K. Dodge died on August 24, 2002.
Presented in part at the 44th annual meeting of the American Society of Hematology, Philadelphia, PA, December 9, 2002.1
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the patients, investigators, nurses, and data managers who participated in these clinical trials. The authors also wish to thank Brian J. Bolwell, MD, for critical review of the manuscript, and A. David McCollum, MD, for assistance with study design. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the views of the National Cancer Institute.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal