Abstract
Barth syndrome (BTHS) is a rare X-linked disease characterized by a triad of dilated cardiomyopathy, skeletal myopathy, and neutropenia. The disease is associated with mutations of the TAZ gene, resulting in defective cardiolipin (CL), an important inner mitochondrial membrane component. Untreated boys die in infancy or early childhood from septicemia or cardiac failure. To date, neutrophil function has never been studied. Directed motility and killing activity of neutrophils was investigated in 7 BTHS patients and found normal in those tested. The circulating neutrophils and eosinophils (but not monocytes or lymphocytes) showed annexin-V binding, suggesting phosphatidylserine (PS) exposure due to apoptosis. However, caspase activity was absent in fresh BTHS cells. Unexpectedly, the near absence of CL impacted neither the mitochondrial mass and shape in fresh BTHS neutrophils nor mitochondrial clustering and Bax translocation upon apoptosis. Annexin-V binding to BTHS neutrophils was not caused by phospholipid scrambling. Moreover, freshly purified BTHS neutrophils were not phagocytosed by macrophages. In sum, a massive number of circulating annexin-V–binding neutrophils in the absence of apoptosis can be demonstrated in BTHS. These neutrophils expose an alternative substrate for annexin-V different from PS and not recognized by macrophages, excluding early clearance as an explanation for the neutropenia.
Introduction
Barth syndrome (BTHS; Mendelian Inheritance in Man [MIM] 302060) is an X-linked recessive disorder comprising dilated cardiomyopathy, muscular hypotonia, and neutropenia. Affected children usually die during infancy as a consequence of septicemia, cardiac failure, or both.1 In the first description from 1983 by Barth et al,2 ultrastructural abnormalities were reported in mitochondria in cardiac muscle cells, myeloid progenitor cells, and, to a lesser extent (up to 10%), skeletal muscle cells. Apart from this observation, an explanation for the isolated neutropenia in BTHS is still lacking.
Neutrophils provide the essential first line of defense against bacterial and fungal pathogens.3 Although they are the predominant cell type among leukocytes, neutrophils have the shortest life span of all. Within a few days after leaving the bone marrow, apoptosis prevents the cytotoxic contents from the neutrophil granules from being released into the surrounding tissues and facilitates the elimination of cells by tissue macrophages.4-6 The exact molecular mechanisms underlying neutrophil apoptosis are unknown, although members of the B-cell lymphoma 2 (Bcl-2) protein family as well as caspases are involved.7-10 We have recently demonstrated that neutrophils also contain numerous mitochondria that cluster around the condensed nuclei during apoptosis. The proapoptotic Bcl-2 family member Bax translocates from the cytoplasm to these clustered mitochondria.11
The mutations causing BTHS are localized to a very gene-rich region in the distal portion of Xq28.12 The identification of unique mutations in one of the genes in this region, termed G4.5 and renamed TAZ, expressed at high level in cardiac and skeletal muscle, subsequently proved to be linked to the disease.13 Different mRNAs can be produced by alternative splicing of the primary G4.5 transcript, encoding proteins that vary at their N-terminus and in the central region. The BTHS mutations introduce stop codons in the open reading frame, interrupting translation of most of the putative proteins, designated “tafazzins.” A clear phenotype-genotype pattern seems to be absent.14,15
Sequence homology of the G4.5 or TAZ gene to a highly conserved superclass of acyltransferases (the Neuwald hypothesis16 ) predicts a glycerolphospholipid as the missing end product. Studies in cultured BTHS skin fibroblasts have indeed suggested a primary defect in cardiolipin (CL) and phosphatidylglycerol remodeling.17 CL is exclusively found in the inner mitochondrial membrane and is required for optimal function of many of the respiratory and adenosine triphosphate (ATP)–synthesizing enzymes. Recently, we and others confirmed that tetralinoleoyl-cardiolipin was lacking in various tissue cells from BTHS patients.18-20
Under normal conditions, cytochrome c is localized in the mitochondrial intermembrane space, attached to the inner membrane by its association with CL.21 Once cytochrome c is solubilized, permeabilization of the outer mitochondrial membrane (eg, by Bax) is sufficient to allow the extrusion of cytochrome c into the cytosol, starting a proapoptotic cascade through the activation of various executioner caspases.7-10
BTHS patients may die early from infection and cardiac decompensation. We investigated granulocyte functions in BTHS. Neutrophil function was largely undisturbed, but the outer plasma membrane of BTHS neutrophils showed exposure of a substrate for annexin-V without other signs of ensuing cell death. Human macrophages did not phagocytose these fresh annexin-V+ BTHS neutrophils, in contrast to cultured control (or BTHS) neutrophils. Normal findings in colony formation and myeloid differentiation in vitro argue against a bone marrow (BM) failure or maturational arrest in vivo. Although we have previously shown the presence of apoptotic neutrophils in the circulation of glycogen storage disease type 1b (GSD1b),22 another syndrome with coinciding neutropenia, we now show that in BTHS the granulocytes in the bloodstream strongly bind annexin-V in the absence of imminent death or macrophage-defined clearance.
Patients, materials, and methods
Neutrophil purification, functional testing, and culturing
Heparinized venous blood was collected from healthy donors and patients with BTHS after obtaining informed consent and with approval of the medical ethical committee of the Academic Medical Centre (Amsterdam, the Netherlands). Neutrophils were isolated by density gradient centrifugation over isotonic Percoll with subsequent erythrocyte lysis in an ice-cold isotonic NH4Cl solution.11,22 The remaining neutrophils were washed once in phosphate-buffered saline (PBS) and used for further manipulations.
Neutrophil migration was assessed by means of Fluoroblok inserts (Falcon; Becton Dickinson, San Jose, CA). Cells (5 × 106/mL) were labeled with calcein-AM (1 μM final concentration; Molecular Probes, Leiden, the Netherlands) for 30 minutes at 37° C, washed twice, and resuspended in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer at a concentration of 2 × 106/mL. Chemoattractant solution (C5a, interleukin 8 [IL-8], platelet-activating factor [PAF]; all at 10 nM) or medium alone (0.8 mL/well) was placed in a 24-well plate, and 0.3 mL of cell suspension was delivered to the inserts (3-μm pore size) and placed in the 24-well plate. Cell migration was assessed by measuring fluorescence in the lower compartment at 2.5-minute intervals for 45 minutes with the HTS7000+ plate reader (Perkin Elmer, Norwalk, CT). Maximal slope of migration was estimated over a 10-minute interval.
Nicotinamide adenine dinucleotide phosphate (NADPH)–oxidase activity was assessed as hydrogen peroxide production determined by an Amplex Red kit (Molecular Probes). Neutrophils (1 × 106/mL) were stimulated with 1 mg/mL serum-treated zymosan (STZ), PAF + fMLP (formyl-methionyl-leucylphenylalanine; both 1 μM, added simultaneously), or 100 ng/mL phorbol myristate acetate (PMA), in the presence of Amplex Red (0.5 μM) and horseradish peroxidase (1 U/mL). Fluorescence was measured at 30-second intervals for 20 minutes with the HTS7000+ plate reader. Maximal slope of H2O2 release was assessed over a 2-minute interval.
Neutrophil culturing was performed as described.11 Briefly, purified cells were resuspended at a final concentration of 2 × 106 cell/mL in Iscoves modified Dulbecco medium (IMDM; BioWhittaker, Brussels, Belgium) supplemented with 10% heat-inactivated fetal calf serum (FCS) and were incubated for 15 to 18 hours in a 5% CO2 incubator at 37° C with or without 100 ng/mL granulocyte colony-stimulating factor (G-CSF; Neupogen, Amgen, Breda, the Netherlands).
Annexin-V staining, morphology, and caspase activity
Annexin-V staining was performed as described,11 using annexin-V–fluorescein isothiocyanate (FITC; 1:250; Bender Med Systems, Vienna, Austria), which specifically binds phosphatidyl serine (PS) residues on the cell membrane, and propidium iodide (PI; Bender Med Systems) at 1 μg/mL to detect binding to DNA once the cell membrane has become permeable. Cells were analyzed by FACScan (Becton Dickinson).
Morphology was determined after Giemsa staining of cytospin preparations. Apoptotic morphology was defined as the presence of condensed nuclei and simultaneous loss of the poly-segmented nuclear appearance.
Total caspase activity was fluorimetrically assessed in neutrophil lysates (0.5 × 106 cells) as the release of 7-amino-4-methyl-coumarin (AMC) from 50 μM DEVD (acetyl-Asp-Glu-Val-Asp)–AMC (Alexis Biochemicals, San Diego, CA) after 90 minutes incubation at 37° C by means of the HTS7000+ plate reader.22
Surface expression of CD16 and CD62L
Surface expression of CD16 (FcγRIIIb) or CD62L (L-selectin) was determined by labeling the cells with monoclonal antibodies (mAbs) conjugated to phycoerythrin (PE; 10 μg/mL final concentration; CD16 mAb 5D2, Sanquin, Amsterdam, the Netherlands; CD62L mAb Leu-8, Becton Dickinson) and subsequent flow cytometry. In some experiments a counterstaining was performed by simultaneous addition of annexin-V–FITC.
Mitochondrial stainings
To estimate mitochondrial morphology, neutrophils were stained with 100 nM MitoTracker GreenFM (Molecular Probes) and analyzed by a confocal laser scanning microscope (LSM510; Carl Zeiss, Heidelberg, Germany) as described.11 Mitochondrial mass was assessed by 10N-nonyl acridine orange (NAO; Molecular Probes), reported to measure the inner mitochondrial CL content.23 Cells were stained with 0.1 μM NAO in IMDM medium for 15 minutes at 37° C, washed, and analyzed by flow cytometry.
Uptake of NBD-PS
Measurement of uptake of the fluorescent PS analog palmitoyl-C6-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-phosphatidylserine (NBD-PS; Avanti Polar Lipids, Birmingham, AL) was performed by flow cytometry as described24 with modifications. To initiate uptake, NBD-PS (final concentration 20 nM) was added to 200 μL of neutrophil cell suspension (2 × 106/mL) in PBS containing 1 mM CaCl2, and the mixture was placed at 37° C. To stop labeling, after a 15-minute incubation the mixture was split into 2 equal portions, which were put on ice and diluted by 100 μL of ice-cold PBS with 4% defatted bovine serum albumin (+BSA) to extract label remaining on the outer leaflet of the membrane or without BSA (–BSA) for measuring total fluorescence. After 5 minutes extraction time, samples were analyzed by FACScan. The fraction of the probe incorporated was determined by dividing the geometric mean NBD fluorescence of the sample population after BSA treatment (internalized probe) by the geometric mean of the NBD fluorescence of the same population in the absence of BSA (total bound probe) and was expressed as a +BSA/–BSA ratio.
Soluble FcγRIII in plasma or serum
Plasma or serum concentrations of soluble FcγRIII (sFcγRIII) were measured by an enzyme-linked immunosorbent assay (ELISA) system, as described before.25
Colony-forming units for granulocytes and macrophages/monocytes (CFU-GMs)
Peripheral blood mononuclear cells (PBMCs) were used in semisolid cultures for CFU-GMs. PBMCs were separated by Ficoll-Hypaque density centrifugation (density 1.077 g/cm3; Pharmacia, Uppsala, Sweden). The CD34+ fraction was enriched with an immunomagnetic method (Mini-MACS; Miltenyi-Biotec GmbH, Bergisch Gladbach, Germany), according to the manufacturer's instructions. After 14 days, colonies (> 40 cells per cluster) were determined and counted by microscopy for nuclear features of neutrophilic or monocytic origin. Colony formation was expressed as number of colony-forming units per 105 nucleated blood cells.
Mass spectrometry of cardiolipin
Cell pellets of 107 freshly purified neutrophils or neutrophils after overnight culturing were suspended in 1 mL of water and sonicated twice for 10 seconds; 50 μL was then removed and used for protein measurement. Fifty microliters of a 7.8 μM solution of internal standard (C14:0)4-CL was then added as CL tracer lipid in chloroform-methanol (2:1 by volume) and 3 mL of a chloroform-methanol solution (2:1 by volume) was added to the remaining cell suspension. Further separation was performed essentially as described before,19,20 using an HP1100 series binary-gradient pump, a vacuum degasser, a column temperature controller (all from Hewlett-Packard, Palo Alto, CA), a 231 XL autosampler (Gilson, Middleton, WI), and a Quattro II triple-quadruple mass spectrometer in the negative electrospray ionization (ESI) mode (Micromass, Cary, NC).
Phagocytosis of apoptotic neutrophils by human macrophages
Human monocytes were isolated on a Percoll gradient as described previously,26 resuspended in RPMI plus 10% heat-inactivated pooled human serum (RPMI+HPS) to a final concentration of 2 × 106 cells/mL, and allowed to adhere in 4-chamber polystyrene vessel tissue-culture–treated glass slides (Falcon) for 1 hour. Thereafter, nonadherent cells were removed by washes, and adherent cells were allowed to mature into macrophages over a 7- to 10-day period in RPMI+HPS.
Fresh or cultured (> 70% apoptotic by annexin-V staining and morphology) neutrophils were added to the macrophages and incubated at 37° C for 30 minutes. After 5 washes to remove attached noningested neutrophils, the preparations were fixed in formaldehyde vapors for 5 minutes and treated with dimethoxybenzidine for 5 to 10 minutes to stain myeloperoxidase (MPO) as a neutrophil marker. Macrophages were counterstained by hematoxylin solution for another 5 minutes. The results are expressed as a percentage of macrophages that have ingested neutrophils ([number of macrophages with MPO-positive inclusions/total number of macrophages scored by light microscopy] × 100).
Results
Patients
Clinical symptoms were compatible with BTHS in all 7 patients analyzed here. From the patients, the TAZ gene was sequenced, and gene mutation analysis confirmed classical BTHS in all but one (no. 4), in whom a mutation has not yet been determined. The justification of the diagnosis of BTHS was further indicated by the biochemical analysis of urine and platelet CL alterations (Table 1). The accompanying 3-methylglutaconic aciduria (type 2), as originally described as part of the BTHS in 1983 by Barth et al,2 is not a consistent or obligatory finding.1 Nonetheless, its presence represents additional support for the diagnosis.
Patient characteristics
Patient . | Age, y . | Dilated cardiomyopathy . | 3-Methyl glutaconic aciduria . | TAZ gene mutation . | Cardiolipin deficiency . | Infections . | ANC, no. per μL* . | Eos/neutro, % . | Plasma sFcγRIII, %† . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.7 | Yes | Yes | Codon 57, exon 2 | Fibroblasts, thrombocytes | No | 1410 ± 120 | 20-25 | 210 |
| CGA > CTA missense mutation | |||||||||
| Arg → Leu | |||||||||
| 2 | 2.1 | Yes, ventricular noncompaction | Yes | Intron 1, exon 2 | Fibroblasts, thrombocytes | No | 567 | 1-2 | 51 |
| AG → splice acceptor site | |||||||||
| 3 | 11 | Yes | Yes | Codon 197, exon 8 | Thrombocytes | Skin infections | 890 | 20 | 71 |
| GGG > GTG missense mutation | |||||||||
| Gly → Val | |||||||||
| 4 | 12 | Yes, mild, no treatment | Yes | Not known | Fibroblasts, thrombocytes | Neonatal infection | 989 ± 128 | 13-17 | 78 |
| 5 | 15 | Yes | Yes | Codon 51, exon 2 | Fibroblasts, thrombocytes | No | 807 ± 262 | 15-19 | 64 |
| TAC > TAG stop codon | |||||||||
| 6 | Adult | Yes | Yes | Codon 216, exon 8 | Fibroblasts, thrombocytes | No | 2080 | 4 | 114 |
| GGA > AGA missense mutation | |||||||||
| Gly → Arg | |||||||||
| 7 | Adult | Yes, symptomatic during infancy, spontaneous improvement | Not known | Codon 57, exon 2 | Fibroblasts, thrombocytes | No | 2990 | 2 | 120 |
| CGA > CTA missense mutation | |||||||||
| Arg → Leu |
Patient . | Age, y . | Dilated cardiomyopathy . | 3-Methyl glutaconic aciduria . | TAZ gene mutation . | Cardiolipin deficiency . | Infections . | ANC, no. per μL* . | Eos/neutro, % . | Plasma sFcγRIII, %† . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.7 | Yes | Yes | Codon 57, exon 2 | Fibroblasts, thrombocytes | No | 1410 ± 120 | 20-25 | 210 |
| CGA > CTA missense mutation | |||||||||
| Arg → Leu | |||||||||
| 2 | 2.1 | Yes, ventricular noncompaction | Yes | Intron 1, exon 2 | Fibroblasts, thrombocytes | No | 567 | 1-2 | 51 |
| AG → splice acceptor site | |||||||||
| 3 | 11 | Yes | Yes | Codon 197, exon 8 | Thrombocytes | Skin infections | 890 | 20 | 71 |
| GGG > GTG missense mutation | |||||||||
| Gly → Val | |||||||||
| 4 | 12 | Yes, mild, no treatment | Yes | Not known | Fibroblasts, thrombocytes | Neonatal infection | 989 ± 128 | 13-17 | 78 |
| 5 | 15 | Yes | Yes | Codon 51, exon 2 | Fibroblasts, thrombocytes | No | 807 ± 262 | 15-19 | 64 |
| TAC > TAG stop codon | |||||||||
| 6 | Adult | Yes | Yes | Codon 216, exon 8 | Fibroblasts, thrombocytes | No | 2080 | 4 | 114 |
| GGA > AGA missense mutation | |||||||||
| Gly → Arg | |||||||||
| 7 | Adult | Yes, symptomatic during infancy, spontaneous improvement | Not known | Codon 57, exon 2 | Fibroblasts, thrombocytes | No | 2990 | 2 | 120 |
| CGA > CTA missense mutation | |||||||||
| Arg → Leu |
Eos indicates eosinophils; and neutro, neutrophils.
ANC, normal range; more than 1500 per μL.
Soluble FcγRIII levels, normal range: 50% to 150% compared with pooled plasma.
Neutrophil counts and function
We tested neutrophil numbers in 7 and function in 6 patients with BTHS. In 4 patients, a mild-to-severe neutropenia was present (Table 1). The number of purified neutrophils was mostly low, although depending on the volume of blood drawn. Experiments were thus limited in most patients and repeated and expanded over time on several different occasions. In this way, some follow-up was possible. During one year of follow-up the absolute neutrophil counts (ANCs) remained rather constant (data not shown).
We focused on 2 major functional aspects of neutrophils. First, the production of hydrogen peroxide by neutrophils is a good reflection of respiratory burst activity (ie, NADPH-oxidase activity) upon cellular activation. Different stimuli were tested for different activation and signaling routes: viz, STZ for uptake via complement receptor type 3 (CR3); PMA for activation of protein kinase-C; and PAF-primed fMLP for activation via surface receptors.3,27 Second, we measured directed cell motility (chemotaxis) toward neutrophil-specific stimuli (ie, C5a, IL-8, or PAF) acting via different members of the aforementioned chemotaxin receptor superfamily.28,29 With both tests, the BTHS neutrophils did not show abnormalities (Table 2). Standard Escherichia coli killing assays were found normal in 3 BTHS patients tested (not shown).
Neutrophil functions in Barth syndrome
. | NADPH oxidase activity . | . | . | Chemotaxis . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | STZ . | PMA . | PAF + fMLP . | C5a . | IL-8 . | PAF . | ||||
| 1 | 0.52 (0.60) | NT | NT | 98 | 100 | 95 | ||||
| 2 | NT | NT | NT | 95 | NT | 68 | ||||
| 4 | 0.67 (0.66) | 2.21 (2.08) | 1.99 (1.92) | 96 | NT | 99 | ||||
| 5 | 0.98 (0.66) | 2.45 (1.98) | 2.15 (1.71) | 103 | NT | 77 | ||||
| 6 | 0.77 (0.62) | 2.14 (2.01) | 1.86 (1.90) | 115 | 114 | 97 | ||||
| 7 | 0.82 (0.71) | NT | NT | NT | NT | NT | ||||
. | NADPH oxidase activity . | . | . | Chemotaxis . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | STZ . | PMA . | PAF + fMLP . | C5a . | IL-8 . | PAF . | ||||
| 1 | 0.52 (0.60) | NT | NT | 98 | 100 | 95 | ||||
| 2 | NT | NT | NT | 95 | NT | 68 | ||||
| 4 | 0.67 (0.66) | 2.21 (2.08) | 1.99 (1.92) | 96 | NT | 99 | ||||
| 5 | 0.98 (0.66) | 2.45 (1.98) | 2.15 (1.71) | 103 | NT | 77 | ||||
| 6 | 0.77 (0.62) | 2.14 (2.01) | 1.86 (1.90) | 115 | 114 | 97 | ||||
| 7 | 0.82 (0.71) | NT | NT | NT | NT | NT | ||||
Neutrophil functions were tested in freshly purified cells from 6 BTHS patients. NADPH-oxidase activity is expressed as maximal slope of H2O2 release in nmol H2O2/minute per 106 cells. The mean of 2 age-matched controls measured on the same day is given in parentheses beneath the corresponding patient value. Chemotaxis is expressed as percentage of mean maximal slope of 2 age-matched controls measured on the same day.
NT indicates not tested.
Neutrophil development
Neutropenia can result from diminished bone marrow production and/or shortened circulation time or half-life in the blood stream, and, once extravascular, from enhanced clearance in the peripheral tissues.27 A bone marrow smear had previously been analyzed in the past in one patient only, showing slight hypocellularity of the myeloid lineage (R. S. Weening; unpublished observation, March 1983). Further bone marrow samples were not available. To obtain an idea about myeloid development, CFU-GM formation from PBMCs was tested. In BTHS, 15 ± 4 myeloid colonies were counted versus 16 ± 5 colonies in controls (mean ± SD; n = 3).
To test total neutrophil body mass and turnover in vivo, we determined the plasma levels of sFcγRIII, a neutrophil surface protein derived from cleavage upon activation or apoptosis in the tissues.25,27,30 As shown in Table 1, the levels of sFcγRIII were at the lower end of the normal values, except for 3 patients of whom 2 were related BTHS patients (no. 1 and no. 7). These 3 patients had relatively normal ANCs (Table 1). None of the patients ever received G-CSF (or prophylactic antibiotic treatment).
Annexin-V–FITC binding
Another aspect of cell turnover is programmed cell death or apoptosis. Neutrophils are different from the other leukocytes in showing a dramatic spontaneous apoptosis within 24 to 36 hours of culture, unless the process is retarded by growth factors such as G-CSF, inflammatory cytokines, or endotoxin.11,31,32
In all experiments with freshly isolated granulocytes from BTHS patients, these cells displayed a strongly increased binding of annexin-V in comparison to granulocytes from age-matched controls (Figure 1A-B). Neutrophils as well as eosinophils also displayed this phenomenon (Figure 1A). In contrast, the monocytes and lymphocytes in freshly obtained preparations did not bind any annexin-V. Erythrocytes and platelets were negative as well, and spontaneous platelet aggregation was also not observed. Moreover, neither prothrombin cleavage fragment (F1+2) nor thrombin-antithrombin (TAT) complexes was detectable (data not shown).
Freshly isolated granulocytes from patients with BTHS display increased annexin-V binding. Freshly purified granulocytes from 7 patients with BTHS and age-matched control individuals (n = 14) were stained by annexin-V–FITC and propidium iodide (PI) and analyzed by flow cytometry. (A) Representative plots for a patient and an age-matched control of the same day; numbers indicate the percentage of annexin-V+ cells among total cell population. (B) Summarized data of all measurements performed. Values represent mean ± SD. *P < .05 by the Student t test.
Freshly isolated granulocytes from patients with BTHS display increased annexin-V binding. Freshly purified granulocytes from 7 patients with BTHS and age-matched control individuals (n = 14) were stained by annexin-V–FITC and propidium iodide (PI) and analyzed by flow cytometry. (A) Representative plots for a patient and an age-matched control of the same day; numbers indicate the percentage of annexin-V+ cells among total cell population. (B) Summarized data of all measurements performed. Values represent mean ± SD. *P < .05 by the Student t test.
Since annexin-V binding is generally assumed to be an early event during apoptosis, other experiments were performed to determine whether programmed cell death was indeed detectable in the neutrophils obtained from the circulating granulocyte pool.
Absence of apoptosis
First, microscopic examination of cytospins prepared from freshly isolated neutrophils revealed no apoptotic changes in cellular morphology. Just as in control preparations, more than 98% of the neutrophils from the BTHS patients presented the usual poly-segmented nuclei, unless cultured overnight (Figure 2A-B).
Freshly isolated granulocytes from patients with BTHS display normal morphology and mitochondrial structures. Granulocytes from patients with BTHS were tested for internal signs of apoptosis in a freshly purified preparation (A,C) and upon overnight culture (B,D). (A-B) Neutrophil morphology by light microscopy. (C-D) Mitochondrial staining with MitoTracker GreenFM assessed by confocal laser scanning microscopy. All bars are 5 μm. Results shown are representative of 10 experiments performed on different occasions with cells from 6 patients in total. For comparison with normal neutrophils, see Maianski et al.11
Freshly isolated granulocytes from patients with BTHS display normal morphology and mitochondrial structures. Granulocytes from patients with BTHS were tested for internal signs of apoptosis in a freshly purified preparation (A,C) and upon overnight culture (B,D). (A-B) Neutrophil morphology by light microscopy. (C-D) Mitochondrial staining with MitoTracker GreenFM assessed by confocal laser scanning microscopy. All bars are 5 μm. Results shown are representative of 10 experiments performed on different occasions with cells from 6 patients in total. For comparison with normal neutrophils, see Maianski et al.11
Second, clustering of mitochondria, as another apoptotic marker occurring early during apoptosis of neutrophils, was also absent in the fresh neutrophils of BTHS patients (Figure 2C). The majority of these cells demonstrated normal tubular mitochondria after specific mitochondrial fluorescent staining,11 as did control neutrophils. The mitochondrial clustering became visible only during spontaneous apoptosis in overnight cultures (Figure 2D). One patient was monitored 4 times with 1-month intervals. In all tested samples from this patient, the annexin-V+ neutrophils were impermeable for PI (indicative of an intact plasma membrane), had a normal morphology, and had normal mitochondrial structures.
Third, the absence of fluorescent substrate cleavage used to quantify endogenous caspase activity also excluded the presence of activated caspases in the BTHS neutrophils (Table 3). Bax staining of fresh neutrophils additionally demonstrated the absence of Bax translocation or clustering, as normally seen during neutrophil apoptosis (not shown; see Maianski et al11 ).
Apoptosis and caspase activity in control and BTHS neutrophils
. | Control, n = 5 . | . | . | BTHS, n = 5 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil preparation . | Annexin-V+, %* . | Apoptotic morphology, %* . | Caspase activity, RFU† . | Annexin-V+, %* . | Apoptotic morphology, %* . | Caspase activity, RFU† . | ||||
| Fresh | 3 ± 2 | < 2 | 0 ± 13 | 56 ± 3 | < 2 | 0 ± 15‡ | ||||
| Overnight cultured | 75 ± 8 | 86 ± 12 | 527 ± 70 | 98 ± 2 | 92 ± 6 | NT | ||||
| Overnight + G-CSF | 52 ± 12 | 46 ± 17 | NT | 78§ | 50§ | NT | ||||
. | Control, n = 5 . | . | . | BTHS, n = 5 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil preparation . | Annexin-V+, %* . | Apoptotic morphology, %* . | Caspase activity, RFU† . | Annexin-V+, %* . | Apoptotic morphology, %* . | Caspase activity, RFU† . | ||||
| Fresh | 3 ± 2 | < 2 | 0 ± 13 | 56 ± 3 | < 2 | 0 ± 15‡ | ||||
| Overnight cultured | 75 ± 8 | 86 ± 12 | 527 ± 70 | 98 ± 2 | 92 ± 6 | NT | ||||
| Overnight + G-CSF | 52 ± 12 | 46 ± 17 | NT | 78§ | 50§ | NT | ||||
RFU indicates relative fluorescence unit (mean ± SD); and NT, not tested.
Fresh neutrophils or neutrophils cultured overnight with or without G-CSF (100 ng/mL) were labeled with annexin-V-FITC or stained with Giemsa solution, and apoptosis was assessed. Values represent mean ± SD of annexin-V+ cells or cells with apoptotic morphology in percentage of total number of neutrophils.
Overall caspase proteolytic activity in cell lysates prepared from freshly isolated or overnight cultured control neutrophils and fresh neutrophils of 3 BTHS patients, measured by the cleavage of the fluorogenic caspase substrate DEVD-AMC. The amount of released AMC was used as a measure of caspase activity.
n = 3.
n = 2.
Membrane expression of apoptosis-sensitive proteins and membrane lipid scrambling in BTHS neutrophils
Under normal conditions of early apoptosis, both FcγRIII (CD16) and L-selectin (CD62L) are shed rapidly from the plasma membrane of neutrophils.4,5 We tested the expression of these surface markers on neutrophils to confirm the nonactivated and nonapoptotic state of these cells. Whereas freshly isolated BTHS neutrophils were largely annexin-V+, surface expression of CD16 was as high as in control neutrophils (Figure 3 top). The same was true for L-selectin (not shown). In fact, the normal surface expression of these proteins also excluded early neutrophil activation as a possibility of membrane perturbation or early flip-flop of PS during isolation and purification procedures of the neutrophils. The CD16–/dull cells in the granulocyte preparations (Figure 3 top, within circles) were recognized as eosinophils (as confirmed by separate CD9 staining and morphology on cytospin preparations; not shown). Their numbers were considerably increased compared with the control cell preparations (Table 1).
Normal surface expression of CD16 and 10N-nonyl acridine orange (NAO) staining in freshly isolated granulocytes from patients with BHTS. Fresh granulocytes from a control individual (left) and from a BTHS patient (right) were colabeled with annexin-V–FITC and anti-CD16–PE mAb (top; representative for 7 controls and patients tested) or stained with NAO (bottom; representative for 3 controls and patients tested) and analyzed by flow cytometry. In the top panels, CD16–/dull cells (circles) were identified as eosinophils by separate staining with anti-CD9 mAb (a specific eosinophil marker) and morphology of cytospin preparations (data not shown). In the bottom panels, values indicate mean fluorescence intensity (MFI) of NAO staining.
Normal surface expression of CD16 and 10N-nonyl acridine orange (NAO) staining in freshly isolated granulocytes from patients with BHTS. Fresh granulocytes from a control individual (left) and from a BTHS patient (right) were colabeled with annexin-V–FITC and anti-CD16–PE mAb (top; representative for 7 controls and patients tested) or stained with NAO (bottom; representative for 3 controls and patients tested) and analyzed by flow cytometry. In the top panels, CD16–/dull cells (circles) were identified as eosinophils by separate staining with anti-CD9 mAb (a specific eosinophil marker) and morphology of cytospin preparations (data not shown). In the bottom panels, values indicate mean fluorescence intensity (MFI) of NAO staining.
The mechanism of PS exposure on the cell surface involves a coordinate inhibition of aminophospholipid translocase activity, which translocates PS from the outer to the inner leaflet of the plasma membrane, and activation of lipid scramblase, which catalyzes the randomization of membrane phospholipids.24 To determine whether these processes were operative in BTHS neutrophils, we measured uptake of fluorescently labeled PS (NBD-PS). After 15 minutes of incubation, both freshly purified control and BTHS neutrophils took up NBD-PS into the inner layer of the plasma membrane, since internalized phospholipids could not be extracted by BSA treatment. The ratio of fluorescence +BSA/–BSA was close to 1 (mean ratio ± SD of control 0.97 ± 0.03, n = 3; and of BTHS patients 0.99 ± 0.01, n = 3). In contrast, in apoptotic neutrophils the +BSA/–BSA fluorescence ratio was 0.54 ± 0.1 (n = 3), indicative for a disturbed transbilayer lipid movement, which can be explained by either inhibition of translocase or increased scramblase activity or both.24 Thus, an inherently abnormal lipid scrambling in the membrane of the BTHS neutrophils is not the reason for the enhanced annexin-V binding.
Spontaneous apoptosis and G-CSF–induced survival in BTHS neutrophils
From 5 patients, the number of neutrophils after purification was sufficient for further apoptosis studies. These BTHS neutrophils were subjected to overnight culturing. During spontaneous apoptosis, the proportion of annexin-V+ cells further increased, with typical apoptotic morphology (Table 3). G-CSF, an agent known to delay neutrophil spontaneous apoptosis, provided some antiapoptotic effect in the patients' neutrophils, decreasing the proportion of annexin-V+ cells after overnight culturing from 95% to 98% to 74% to 82%, which is still higher than the values observed with freshly isolated neutrophils. Also, the morphology was rescued from apoptotic features in approximately 50% of the BTHS cells, similar to control neutrophils (Table 3).
Mitochondrial CL in BTHS neutrophils
The acridine orange derivative NAO has been demonstrated to bind with high affinity to the major lipid of the inner mitochondrial membrane in intact whole cells (ie, CL).23 A linear relationship has been suggested between CL content of model membranes and the binding of this dye. In this way, the mitochondrial mass can be determined in neutrophils.
The possibility of a disturbed NAO staining in the BTHS neutrophils was therefore tested. As shown in the bottom panels of Figure 3, NAO staining was identical to the pattern obtained with neutrophils from the age-matched healthy controls. Thus, NAO is not suitable as a readout for the changed lipid remodeling of the mitochondrial inner membrane. In support of the apoptosis studies in BTHS neutrophils, the NAO experiments confirm that the actual number of mitochondria in BTHS seems unchanged, but the form and function of the mitochondria cannot be distinguished by NAO staining from those in normal neutrophils.
CL fractions in platelets were routinely determined in the blood samples of the BTHS patients.19 Total CL and CL subspecies were decreased in platelets and showed a specific decrease of various CL molecular species (not shown). Although NAO staining was unaffected in the BTHS neutrophils, we considered the possibility that the CL lipid alterations could be present in these cells as well.
We therefore analyzed CL molecular species by high-performance liquid chromatography–mass spectrometry (HPLC-MS). Various double-negatively charged CL molecular species were detected in fresh control neutrophils (Figure 4A). The peak at mass-charge ratio (m/z) 723.6 belongs to [(C18:2)4-2H]2–-CL, which is the most prevalent CL molecule. The peak at m/z 724.6 corresponds with the second isotope peak of m/z 723.6 (ie, with [(C18:2)3/(C18:1)1-2H]2–-CL). The peak at m/z 725.6 corresponds with the second isotope peak of m/z 724.6 (ie, with [(C18:2)2/(C18:1)2-2H]2–-CL). No significant differences were observed between freshly isolated neutrophils and neutrophils cultured overnight (with 76% to 82% annexin-V positivity; Figure 4A-B). In BTHS neutrophils, however, a significant decrease of the most abundant CL species (ie, (C18:2)4-CL, (C18:2)3(C18:1)1-CL and (C18:2)2(C18:1)2-CL) was present compared with control neutrophils (Figure 4C), although the phosphatidylinositol, phosphatidylcholine, and sphingomyelin species were identical in BHTS and control neutrophils (Figure 4D-G). These findings confirm that the alterations in CL most likely occur in any cell type in BTHS, including neutrophils (Figure 4) and PBMCs (data not shown), as was previously shown for fibroblasts and platelets19,20 as well as for skeletal and cardiac muscle.18 CL was studied in mitochondria-free plasma membrane fractions of control granulocytes and found, as expected, to be completely absent (data not shown). The CL changes may thus affect the mitochondrial lipid composition without changing its apoptotic program.
Cardiolipin composition and spectra of some other phospholipids determined by HPLC-MS in freshly isolated BTHS granulocytes. (A,D,F) Freshly purified control neutrophils; (B) neutrophils from the same donor after overnight culture with more than 80% annexin-V binding; (C,E,G) freshly purified neutrophils from a BTHS patient with more than 60% annexin-V binding. CL molecular species are shown in panels A-C. Arrows indicate the most abundant CL molecular species and their fatty acid composition. BTHS neutrophils did not contain CL species either in fresh cells (C) or in apoptotic cells (not shown). Phosphatidyl inositol molecular species are shown in panels D-E. Phosphatidyl choline and sphingomyelin molecular species are shown in panels F-G. Representative results from 3 BTHS patients and 3 control individuals.
Cardiolipin composition and spectra of some other phospholipids determined by HPLC-MS in freshly isolated BTHS granulocytes. (A,D,F) Freshly purified control neutrophils; (B) neutrophils from the same donor after overnight culture with more than 80% annexin-V binding; (C,E,G) freshly purified neutrophils from a BTHS patient with more than 60% annexin-V binding. CL molecular species are shown in panels A-C. Arrows indicate the most abundant CL molecular species and their fatty acid composition. BTHS neutrophils did not contain CL species either in fresh cells (C) or in apoptotic cells (not shown). Phosphatidyl inositol molecular species are shown in panels D-E. Phosphatidyl choline and sphingomyelin molecular species are shown in panels F-G. Representative results from 3 BTHS patients and 3 control individuals.
BTHS neutrophil uptake by human macrophages
To study the meaning of the increased annexin-V binding to BTHS neutrophils for recognition and uptake by scavenger cells, we used macrophages derived from monocytes of healthy control donors. For comparison, neutrophils from controls and BTHS patients directly after isolation and after overnight culture were coincubated with these macrophages. Freshly isolated normal neutrophils (< 5% annexin-V+ cells) were hardly taken up. Fresh BTHS neutrophils, despite being more than 60% annexin-V+, were not ingested either (Figure 5). Only after culture (when nuclear morphology, mitochondrial clustering, and annexin-V staining together indicated that more than 70% of the neutrophils were in apoptosis), both control (Figure 5) and BTHS neutrophils (not shown) were ingested by macrophages.
Uptake of BTHS neutrophils by human monocyte-derived macrophages. Freshly purified control granulocytes (fresh) and granulocytes from the same donor after overnight culture with more than 70% annexin-V binding (cultured) were incubated with human monocyte–derived macrophages for 30 minutes, and the percentage of phagocytizing macrophages was estimated as described in “Patients, materials, and methods” (▪). Freshly purified granulocytes from a BTHS patient with more than 60% annexin-V binding were used to compare with the control granulocytes (□). Uptake of cultured apoptotic BTHS granulocytes was similar compared with cultured control cells (not shown). Results shown (mean ± SD) are representative of experiments performed on different occasions with cells from 4 control individuals and 3 patients in total.
Uptake of BTHS neutrophils by human monocyte-derived macrophages. Freshly purified control granulocytes (fresh) and granulocytes from the same donor after overnight culture with more than 70% annexin-V binding (cultured) were incubated with human monocyte–derived macrophages for 30 minutes, and the percentage of phagocytizing macrophages was estimated as described in “Patients, materials, and methods” (▪). Freshly purified granulocytes from a BTHS patient with more than 60% annexin-V binding were used to compare with the control granulocytes (□). Uptake of cultured apoptotic BTHS granulocytes was similar compared with cultured control cells (not shown). Results shown (mean ± SD) are representative of experiments performed on different occasions with cells from 4 control individuals and 3 patients in total.
Neutrophils from obligate carriers and increased annexin-V binding
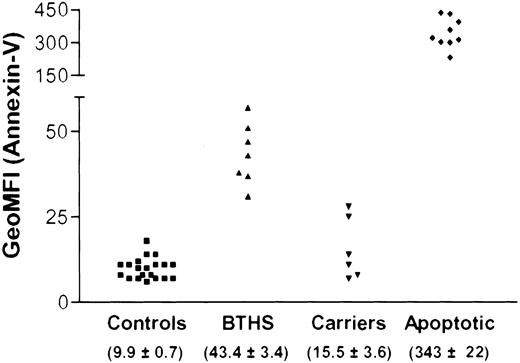
The presence of annexin-V binding was further studied in obligate carriers (n = 6), 3 of which had lost a child due to BTHS. We detected slightly increased levels of annexin-V binding to the granulocytes. This was not a very strong and constant finding (Figure 6), which is in accordance with the imperfect X-inactivation and selection against cells with the X-chromosome carrying the mutant allele demonstrated before.33 None of these carriers were neutropenic (ANC, 2.38 × 109/L ± 0.357 × 109/L [2380 ± 357 cells/μL]). Due to the broad distribution in annexin-V binding in the total population of neutrophils from BTHS carriers, it was impossible to discern 2 distinct subpopulations (ie, one with and another without annexin-V binding). In line herewith, comparison of the geometric mean fluorescence intensities (geo-MFIs) of annexin-V binding of the healthy adult controls and their apoptotic cells upon culture with the fresh neutrophils from carriers or BTHS patients indicated low sensitivity (Figure 6). Distinction of 2 subpopulations of cells in the carriers will not be successful without further RNA analysis in neutrophils.
Annexin binding of purified neutrophils from controls, obligate carriers, and BTHS patients in comparison with apoptotic neutrophils. Freshly purified granulocytes from healthy donors (Controls) and granulocytes from the same donor after overnight culture with more than 80% annexin-V binding (Apoptotic) were compared with freshly purified neutrophils from obligate carriers and BTHS patients. The geometric mean fluorescence intensity (geo-MFI) of annexin-V–FITC was determined by flow cytometry; the mean ± SEM is given per experimental cohort of individuals in parentheses.
Annexin binding of purified neutrophils from controls, obligate carriers, and BTHS patients in comparison with apoptotic neutrophils. Freshly purified granulocytes from healthy donors (Controls) and granulocytes from the same donor after overnight culture with more than 80% annexin-V binding (Apoptotic) were compared with freshly purified neutrophils from obligate carriers and BTHS patients. The geometric mean fluorescence intensity (geo-MFI) of annexin-V–FITC was determined by flow cytometry; the mean ± SEM is given per experimental cohort of individuals in parentheses.
We measured CL levels in the neutrophils and platelets of these carriers to see whether CL levels were more discriminating than the annexin-V binding. However, CL levels were all within the range of normal control cells, similar to the results previously obtained with fibroblasts of carriers19,20 (data not shown).
Discussion
Annexin-V binds to the plasma membrane of circulating BTHS neutrophils and eosinophils (but not other blood cells), yet apoptosis cannot explain this finding. We found a normal nuclear and mitochondrial morphology, the usual mitochondrial mass and cellular distribution, as well as the absence of enzymatic caspase activity in fresh BTHS neutrophils.
Aging of neutrophils is accompanied by a progressive loss of functions, such as adherence, chemotaxis, phagocytosis, and respiratory burst.34 Our recent observations in GSD1b have demonstrated the presence of circulating neutrophils that are truly apoptotic (ie, positive for annexin-V binding with nuclear condensation, Bax translocation to clustered mitochondria, and caspase activity).22 In BTHS, we did not observe these features. The patients' neutrophils had normal functional capacities, further excluding the possibility of a death-prone cell phenotype.
In overnight cultures of BTHS neutrophils, the usual mitochondrial clustering and Bax translocation were observed during spontaneous apoptosis, as in normal neutrophils.11 Targeting of Bax to the mitochondria has been suggested to involve CL, both at the inner membrane as well as in small contact points at the mitochondrial outer membrane.35-38 Although CL seems to be strongly decreased and differently acylated in BTHS neutrophils, this results neither in a changed Bax relocalization upon apoptosis nor in significant changes in NAO staining, both believed to be CL dependent.23,35,37 CL is integral to the normal functioning of mitochondrial electron transport and energy metabolism; loss of CL will lead to reduced cytochrome c–oxidase activity and destabilization of cytochrome c.38 However, neutrophil mitochondria neither show respiration nor participate in ATP synthesis.39 Moreover, cytochrome c, in contrast to many other apoptosis-related mitochondrial proteins, is hardly expressed in neutrophils,39,40 and neutrophils may therefore not depend on CL. On the other hand, reactions of BTHS lymphocytes toward various mitogens as well as activation-induced cell death in CD3-activated BTHS lymphocytes or Fas-induced lymphocyte apoptosis were normal (not shown). Thus, the concentration and modifications of CL in BTHS do not necessarily impose an altered apoptotic response on BTHS neutrophils or lymphocytes.
Annexin-V shows considerable affinity for PS. PS functions to enhance the procoagulatory cascade41-47 ; procoagulatory parameters of thrombin activation by cellular PS exposure were undetectable in BTHS patients. In addition, PS exposure on the neutrophils could theoretically contribute to the neutropenia in BTHS by enhanced clearance through the receptor for PS (PSR) on macrophages.48 However, the neutropenia was variable and considered moderate to mild in hematologic terms. Moreover, the percentage of annexin-V–binding cells did not correlate with absolute neutrophil counts and human macrophages did not phagocytose freshly isolated BTHS neutrophils.
Annexin-V binding to BTHS neutrophils can be explained in several ways. First, PS is the ligand for annexin-V on the cell membrane but the stereometry of PS on BTHS neutrophils might be different, leaving recognition by annexin-V intact while rendering the PSR ineffective. However, because the stereo-specificity of annexin-V is thought to be similar and uptake of apoptotic BTHS cells is intact, this explanation seems less plausible. Second, the ligand may still be PS, but PSR-mediated uptake by macrophages of apoptotic cells depends on cofactors not expressed on BTHS neutrophils, which can certainly be of relevance because the apoptotic machinery of caspase cleavage is not active in BTHS neutrophils.49 In both explanations, the reason of the PS flip-flop in BTHS neutrophils remains to be explained. Finally, annexin-V might bind to an alternative ligand or surface-expressed membrane molecule on granulocytes from BTHS patients that is by itself not recognized by the PSR. Observations on annexin-V binding without signs of apoptosis in murine B cells and mouse myotube formation are reminiscent to our observations in BTHS.50,51 In these reports, annexin-V binding was, without proof though assumed to be, strictly PS dependent.
In support of the possibility of an alternative ligand for annexin-V is the fact that abnormal membrane lipid scrambling in BTHS neutrophils seemed absent. Membrane lipid asymmetry is normally maintained as a concerted action of an inward-directed pump specific for PS and an outward-acting lipid pump, called aminophospholipid translocase and floppase, respectively. During the apoptotic process, activation of scramblase, which mediates Ca2+-dependent bidirectional movement of phospholipids across the membrane, and concomitant inhibition of the aminophospholipid translocase cause a collapse of the membrane phospholipid asymmetry, manifested by exposure of PS on the cell surface.24,41,45 Furthermore, annexin-V is able to bind to alternative substrates, as has been shown in studies with artificial lipid membranes or lipid peroxidation.52,53 Although HPLC-MS was sensitive enough to define and quantify the altered mitochondrial CLs in BTHS neutrophils, lipid aldehyde adducts resulting from peroxidation at the plasma membrane cannot be identified in this way.
Alternative splicing of the primary G4.5 transcript has been described, encoding proteins that vary at their N-terminus and in the central region. In BTHS, the mutations introduce stop codons in the open reading frame, interrupting translation of the so-called tafazzins.14,15 Recent findings in yeast indicate that only one transcript may be held responsible for the changes in CL metabolism.54 However, when certain transcripts still have acyltransferase activity toward lipids at the plasma membrane in neutrophils and eosinophils, preventing these lipids from annexin-V binding to these cells, the difference in annexin-V binding to the various blood cells in BTHS could be explained. Moreover, our data demonstrate that the relation between constitutive annexin-V binding and decreased CL levels is not strict. First, not all BTHS cells bind annexin-V while being CL depleted. Second, normal CL levels were measured in blood samples from 2 female carriers with clearly positive annexin-V binding to their neutrophils. Although there was considerable variability in annexin-V binding to the neutrophils among obligate carriers, none had even the smallest indication of low ANCs. These findings in carriers are further support against a direct causal relationship between the neutropenia in BTHS patients and the annexin-V binding, per se.
The neutropenia in BTHS cannot be explained by early clearance through phagocytosis of neutrophils. The absence of defective CFU-GM formation further excludes a failure in growth and differentiation of myeloid cells from CD34+ hematopoietic stem cells in BTHS. Most colonies in the BTHS cultures consisted of neutrophils, similar to the results with control cells. The size of the colonies in the BTHS CFU-GM cultures seemed even larger instead of smaller. More extensive studies in long-term BM cultures are needed to better understand the phenomenon of neutropenia.
In sum, we observed annexin-V staining of circulating BTHS neutrophils in the complete absence of detectable cell death. This result suggests that an increased binding of annexin-V (whatever its ligand is) does not necessarily reflect an apoptotic state of the cell. Thus, to get an accurate estimation of cell death, the use of several detection methods is required. The alterations in CL were present in BTHS neutrophils and other blood cells but did not result in perturbed responses. The annexin-V–binding ligand on BTHS neutrophils did not result in uptake by macrophages and thus cannot explain the variable neutropenia in BTHS patients. Further studies are ongoing to identify the annexin-V–binding surface molecule in order to appreciate the in vivo impact of the modifications at the plasma membrane of neutrophils in BTHS patients.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-11-3940.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr C. Voermans for help with CFU-GM and S. Scheij for assistance in macrophage culturing.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal