Abstract
Plasma cells (PCs) represent the final stage of B-cell differentiation and are devoted to the production of immunoglobulin (Ig). Perturbations to their development can result in human disorders characterized by PC expansion and hypergammaglobulinemia. Ig-secreting cells (ISCs) have been identified in secondary lymphoid tissues and bone marrow (BM). Most ISCs in lymphoid tissue are short-lived; in contrast, ISCs that migrate to the BM become long-lived PCs and continue to secrete immunoglobulin for extended periods. However, a small population of long-lived PCs has been identified in rodent spleen, suggesting that PCs may persist in secondary lymphoid tissue and that the spleen, as well as the BM, plays an important role in maintaining long-term humoral immunity. For these reasons, we examined ISCs in human spleen and identified a population that appears analogous to long-lived rodent splenic PCs. Human splenic ISCs shared morphologic, cellular, molecular, and functional characteristics with long-lived PCs in BM, demonstrating their commitment to the PC lineage. Furthermore, the detection of highly mutated immunoglobulin V region genes in splenic ISCs suggested they are likely to be antigen-selected and to secrete high-affinity immunoglobulin. Thus, our results suggest that splenic ISCs have an important role in humoral immunity and may represent the affected cell type in some B-cell dyscrasias.
Introduction
Plasma cells (PCs) are terminally differentiated immunoglobulin (Ig)–secreting cells (ISCs) responsible for humoral immunity.1-3 ISCs can arise from several differentiation pathways. During the extrafollicular reaction, some antigen (Ag)–specific naive B cells undergo rapid clonal expansion and differentiate into ISCs.1,4,5 Many extrafollicular ISCs are short-lived and secrete either IgM or downstream isotypes.6,7 The VH genes of extrafollicular ISCs typically remain in their germline configuration,4,8 indicating they have not undergone significant somatic mutation. Naive B cells can also give rise to ISCs by way of the germinal center (GC) reaction. It is within GC that antigen-specific naive B cells undergo proliferation, somatic mutation of Ig V region genes, Ig isotype switching, and affinity maturation.1,4,5 Antigen-selected GC B cells can differentiate into ISCs,5,9,10 most of which migrate to the bone marrow (BM) and become long-lived PCs, whereas a small proportion remains in the spleen.2,3,7,9,11,12 At the same time, some GC B cells develop into memory B cells,4,5,10 which can subsequently yield ISCs directly after repeated exposure to an immunizing antigen.13,14 Thus, the GC reaction serves to expand and diversify the B-cell repertoire, producing high-affinity variants for the long-term maintenance of protective immunity.
Most studies examining PCs have been performed using spleens and BM from immunized rodents. However, for a complete understanding of human PC differentiation, and, more important, human diseases characterized by the expansion or transformation of ISCs, it is necessary to examine the human counterpart of these cells directly. Human ISCs can be identified by an up-regulation in expression of CD38 and a concomitant down-regulation of CD20.15 Based on this phenotype, significant numbers have been detected in BM and peripheral blood (PB)15,16 and in mucosa-associated lymphoid tissue such as tonsils and gut,17-19 where they usually constitute a very small proportion of mononuclear cells (MNCs; less than 0.1%-1.0%).15,17,20,21 Although ISCs in human tonsils, PB, and BM have been extensively characterized,16,17,20-25 ISCs in human spleen have not been reported. Given the identification of a population of long-lived murine splenic PCs, we hypothesized that an analogous population would exist in humans and that this population could contribute to the maintenance of long-term humoral immunity. In this paper we report the identification of a population of human splenic CD38++CD20± cells that exhibited many characteristics of BM PCs, and we propose that these cells in human spleen represent an important population of ISCs. Identifying human splenic ISCs will contribute to our understanding of the role played by these cells in normal humoral immunity and of human diseases characterized by B-cell hyperactivity or deficiency.
Materials and methods
Reagents
The following monoclonal antibodies (mAbs) were used in this study: fluorescein isothiocyanate (FITC)–anti-CD20 and anti-CD27; phycoerythrin (PE)–anti-CD19, anti-CD28, and anti–bcl-2 mAb (Becton Dickinson, San Jose, CA); FITC- and PE-immunoglobulin G1 (PE-IgG1) isotype control mAb; PE-antimouse IgG, anti-CD21, anti-CD27, anti-CD45, anti-CD80, anti-CD84, anti-CD86, anti-CD95, anti-IgM, anti-IgG, anti-Igκ light chain mAb; and streptavidin (SA) conjugated to peridin chlorophyll protein (PerCP; BD PharMingen, San Diego, CA); PE-conjugated anti-CD22, anti-CD23, anti-CD38, anti-CD138, anti–HLA-DR mAb; biotinylated IgG1 isotype control mAb; biotinylated anti-CD38 mAb, allophycocyanin (APC)–isotype control mAb; anti-CD20 and anti-CD38 mAb; SA-APC, and SA-Texas red (Caltag, Burlingame, CA). PE–anti-CD40 (mAb89) and anti-CD39 (A1) have been previously described.26 Recombinant human interleukin-4 (IL-4) and IL-10 were provided by Dr Rene de Waal Malefyt (DNAX, Palo Alto, CA); IL-6 was from Peprotech (Rocky Hill, NJ).
Isolation of splenic B-cell subsets
Normal human spleens were obtained from organ donors (Australian Red Cross Blood Service, Sydney, Australia). MNCs were prepared as previously described and were cryopreserved in liquid nitrogen until required.27,28 Total human B cells (more than 98% CD19+) were isolated from splenic MNCs using CD19 DYNAbeads (Dynal, Oslo, Norway). These cells were occasionally enriched for CD27+ B cells using CD27 MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany).28 Naive, memory, and CD38++CD20± B cells were isolated by cell sorting using FACStarPlus (BD Biosciences, San Jose, CA) after labeling purified total or CD27+ B cells with either anti-CD20–FITC and anti-CD38–PE mAb to identify CD38++CD20± B cells15,29 or anti-CD20–FITC and anti-CD27–PE mAb to identify naive (CD20+CD27–) and memory (CD20+CD27+) B cells.26 Bone marrow aspirates from healthy donors were obtained from the Department of Haematology, Royal Prince Alfred Hospital, Sydney, Australia.
Immunofluorescence staining
Cells were incubated with a cocktail of mAbs containing CD27-FITC, CD38-biotin/SA-PerCP and CD20-APC, or CD27-FITC, CD38-PE, and CD20-APC to allow the resolution of naive, memory, and CD38++CD20± B cells. PE or biotinylated mAbs were also added, followed by SA-PerCp, and the cells were then incubated on ice for 30 minutes. To determine the expression of intracellular immunoglobulin and bcl-2, cells were initially labeled with the above-described mAb cocktails, fixed in 4% formaldehyde, washed twice with phosphate-buffered saline (PBS), and resuspended in PBS containing 0.5% saponin to permeabilize the cell membrane. PE or biotinylated Ab, followed by SA-PerCp (all prepared in PBS containing 0.5% saponin), was added to the cells and incubated on ice for 30 minutes. Flow cytometric acquisition was performed on a FACS Calibur (Becton Dickinson Immunohistochemistry Systems, San Jose, CA) and was analyzed using CELLQuest (Becton Dickinson) or FlowJo (Tree Star, San Carlos, CA) software. Fluorescence was measured on a log scale.
Morphologic analysis of B-cell subsets
Sort-purified naive, memory, and CD38++CD20± B cells were centrifuged onto polylysine slides (Menzel-Glaser, Braunschweig, Germany), fixed in methanol, and stained with Giemsa (0.4% wt/vol in methanol, pH 6.9; Sigma, St Louis, MO). After staining, slides were washed with water, dried at room temperature, and visualized using an Axiovision microscope and camera (Carl Zeiss, Camperdown, NSW, Australia).
Immunohistology
Human spleens were snap-frozen in liquid nitrogen, and 5-μm sections were acetone-fixed and air-dried before staining. Sections were first blocked with 30% horse serum and then labeled with FITC–anti-CD20 and biotinylated anti-IgM mAb followed by SA–Texas red for 30 to 45 minutes at 37° C in a humidified chamber. Images were visualized using a Leica immunofluorescence microscope.
Analysis of Ig secretion
ELISPOT assays were performed to determine the frequency of ISC within populations of naive, memory, and CD38++CD20± B cells.28 To determine the amount of Ig secreted, sort-purified CD38++CD20± B cells were cultured (approximately 5 × 103 cells/well) in duplicate in round-bottomed, 96-well plates (Becton Dickinson Labware, Franklin Lakes, NJ) in medium alone28 or with IL-4 (400 U/mL), IL-6 (10 ng/mL), or IL-10 (100 U/mL). After 5 days, supernatants were collected, and the level of secreted immunoglobulin was determined using enzyme-linked immunosorbent assay (ELISA).28
Molecular analysis of gene expression
Semiquantitative polymerase chain reaction (sqPCR) was used to examine gene expression in human splenic B cells. B-cell subsets were isolated by cell sorting. Total RNA was then extracted (RNeasy Kit; Qiagen, Clifton Hill, VIC, Australia) and transcribed into cDNA using random hexamers (Gibco BRL) as primer and Superscript II RNase H– reverse transcriptase (Gibco BRL). Resultant cDNA was then normalized for expression of the constitutively expressed housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH:5′ CCA CCC ATG GCAAAT TCC ATG GCA, 3′ TCT AGA CGG CAG GTC AGG TCC ACC), and was used as a template for PCR. The following primers were used (Sigma-Aldrich, Poole, United Kingdom): Bcl-6, 5′ CTG ACA GCT GTA TCC AGT TCA CC; Bcl-6, 3′ TCT TGG GGC ATC AGC ATC; BSAP, 5′ GCA TAG TGT CCA CTG GCT CC; BSAP, 3′ CCA GGA GTC GTT GTA CGA GG; BLIMP-1, 5′ GAT GCG GAT ATG ACT CTG TGG; BLIMP-1, 3′ CTC GGT TGC TTT AGA CTG CTC; XBP-1, 5′ GCT CAG ACT GCC AGA GAT CG; and XBP-1, 3′ GTC CAG AAT GCC CAA CAG G. All reactions were performed using REDTaq (Sigma). Samples were removed after 28, 30, 32, and 34 cycles; each cycle consisted of 1-minute denaturation (94° C), 1 minute annealing (55° C), and 1 minute extension (72° C) in a Thermal Cycler (MJ Research, Waltham, MA).
Sequence analysis of immunoglobulin VH genes
The VH5 genes were amplified from cDNA by nested PCR using Pfu DNA polymerase (Perkin Elmer, Foster City, CA) and 100 ng of 5′ and 3′ primers.26,27 Primers for the initial PCR corresponded to the 5′ region of the VH5 leader sequence (ATG GGG TCA ACC GCC ATC CT) and the 3′ Cμ constant region (GTC CTG TGC GAG GCA GCC AA). Primers for the second PCR were 5′ CTC CTG GCT GTT CTC CAA GG and 3′ AGG AGA CGG TGA CCA GGG TT. Each PCR consisted of 35 cycles under the conditions described in the previous paragraph. Amplified PCR products were cloned and transformed into TOP10F' bacteria (Invitrogen). Individual clones were selected, and plasmid DNA was recovered and sequenced (SUPAMAC; Royal Prince Alfred Hospital, Sydney, Australia). Nucleotide sequences were analyzed using the Sequencher program, and comparisons were performed using the GenBank database.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) with Prism software (GraphPad Software, San Diego, CA).
Results
Identifying potential ISCs in the human spleen
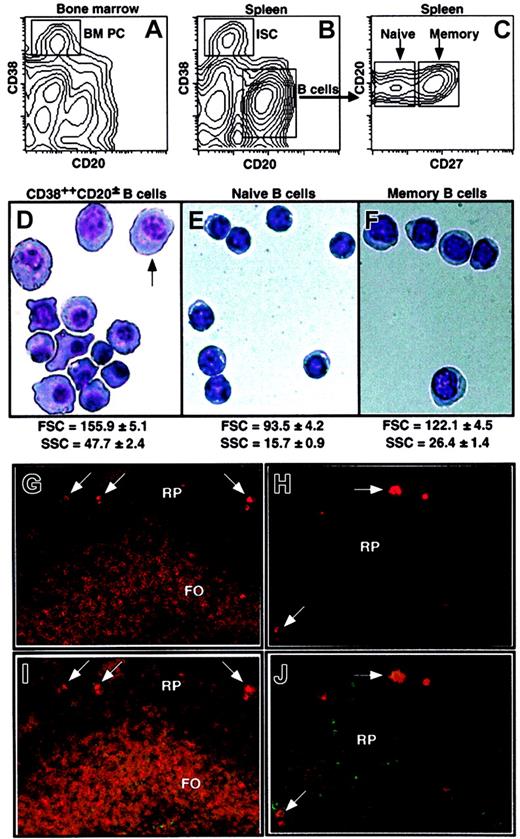
A hallmark of human PCs is the elevated surface expression of CD38 and the reduced expression of CD20 compared with mature B cells.15 This phenotype allows the resolution of a small population of cells in human BM (Figure 1A). A distinct population of CD38++CD20± cells was also detectable in every human spleen examined (Figure 1B) and was found to comprise 0.62% ± 0.42% (mean ± SD; range, 0.05%-1.8%; n = 31) of all lymphoid cells, a frequency similar to that of CD38++CD20± cells in normal human BM (approximately 0.6%).15,16 The CD38++CD20± cells were distinguishable from CD20+ mature splenic (Figure 1B) and BM (data not shown) B cells, which could be resolved into naive and memory populations on the basis of CD27 expression (Figure 1C).26,27
Identification of naive, memory, and CD38++CD20± B cells. Human BM (A) and spleen MNCs (B-C) labeled with anti-CD20, anti-CD27, and anti-CD38 mAb. (A-B) ISCs were identified as CD38++CD20±; total B cells were CD38dimCD20+. (C) The total B-cell population was further resolved into naive and memory subsets by the differential expression of CD27. Splenic CD38++CD20± (D), naive (E), and memory (F) B cells were isolated by sorting and were Giemsa stained. The arrow in panel D indicates an example of a cell with a clear perinuclear zone and condensed chromatin. Original magnification, × 100. Values represent the mean FSC and SSC (± SEM; n = 28) of the 3 B-cell populations. (G-J) Immunofluorescence staining was performed on spleen sections obtained from a healthy donor using anti-IgM (red; G-H) or in combination with anti-CD20 mAb (green; I-J). Follicular (FO) and red pulp (RP) areas, as well as ISCs (arrows), are indicated. Original magnifications: × 5 (G,I), × 20 (H,J).
Identification of naive, memory, and CD38++CD20± B cells. Human BM (A) and spleen MNCs (B-C) labeled with anti-CD20, anti-CD27, and anti-CD38 mAb. (A-B) ISCs were identified as CD38++CD20±; total B cells were CD38dimCD20+. (C) The total B-cell population was further resolved into naive and memory subsets by the differential expression of CD27. Splenic CD38++CD20± (D), naive (E), and memory (F) B cells were isolated by sorting and were Giemsa stained. The arrow in panel D indicates an example of a cell with a clear perinuclear zone and condensed chromatin. Original magnification, × 100. Values represent the mean FSC and SSC (± SEM; n = 28) of the 3 B-cell populations. (G-J) Immunofluorescence staining was performed on spleen sections obtained from a healthy donor using anti-IgM (red; G-H) or in combination with anti-CD20 mAb (green; I-J). Follicular (FO) and red pulp (RP) areas, as well as ISCs (arrows), are indicated. Original magnifications: × 5 (G,I), × 20 (H,J).
Cellular characterization of splenic CD38++CD20± B cells
The classification of B cells as PCs has traditionally been based on histologic analysis. Thus, the initial step in characterizing splenic CD38++CD20± B cells was to examine their morphology. Giemsa stains of sort-purified populations of splenic B cells revealed CD38++CD20± B cells were heterogeneous with respect to size (Figure 1D). Despite this, they were significantly larger than naive and memory B cells (P < .001), as revealed by flow cytometric analysis of forward light scatter (FSC) and side light scatter (SSC) characteristics, indicative of cell size and granularity, respectively (Figure 1D-F). The size and morphology of splenic CD38++CD20± B cells resembled those of BM PCs, which were also 2- to 3-fold larger and more granular than naive and memory B cells in human BM (FSC of naive, memory, and plasma cells in BM: 91.0 ± 5.5, 111.6 ± 6.3, 167.1 ± 9.4; SSC: 19.5 ± 0.4, 30.1 ± 1.6, 62.6 ± 5.2, respectively; n = 6). Furthermore, whereas splenic naive and memory B cells contained only a small rim of basophilic cytoplasm and central nuclei (Figure 1E-F), most CD38++CD20± B cells clearly displayed increased cytoplasmic content and eccentrically placed nuclei (Figure 1D). Some cells also appeared to display clear perinuclear zones consistent with increased Golgi membranes and condensed nuclear chromatin (indicated by arrow in Figure 1D). Thus, splenic CD38++CD20± B cells exhibited morphologic hallmarks of terminally differentiated PCs typically found in human BM.15,16
CD38++CD20± cells localize to the red pulp of human spleen
To determine the localization of CD38++CD20± cells, serial sections of human spleen were labeled with mAb specific for IgM and CD20. Mature B cells (surface IgM+CD20+) were detectable in the follicle in the white pulp (Figure 1G, I). In contrast, CD38++CD20± cells (identified as CD20loIgMhi cells; see next section) were positioned outside the follicles and in the red pulp (Figure 1G-J). Mature B cells displayed predominantly surface expression of IgM, whereas IgM is concentrated in the cytoplasm of CD38++CD20± cells (Figure 1G-J). Thus, CD38++CD20± cells reside in the red pulp of human spleen, as do PCs in rodent spleen.7,12
Expression and secretion of Ig by splenic CD38++CD20± B cells
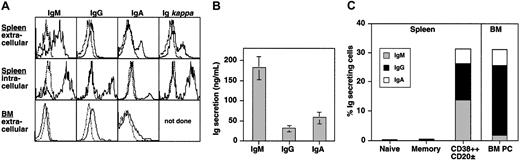
PCs have also been identified by the expression of cytoplasmic Ig molecules and the ability to spontaneously secrete Ig.1,17,25 Splenic CD38++CD20± B cells expressed low but detectable amounts of surface Ig (Figure 2A, upper panel), similar to PCs in human BM (Figure 2A, lower panel) and mouse spleen.6,11,30 However, the level of intracellular Ig in the cells was 10- to 100-fold greater than surface expression (Figure 2A, middle panel, for spleen; BM not shown), indicating their cytoplasms contained abundant amounts of Ig. In contrast, Ig was detected only on the surfaces of naive and memory B cells (data not shown). The presence of intracellular Ig suggested that splenic CD38++CD20± B cells might spontaneously secrete Ig. To test this, CD38++CD20± B cells were isolated and cultured without stimulation for 5 days. IgM, IgG, and IgA were secreted by CD38++CD20± B cells, with IgM comprising approximately 70% of the total secreted Ig (Figure 2B), consistent with a predominance of intracellular IgM+ CD38++CD20± cells (Figure 2A). In contrast, naive and memory B cells failed to secrete detectable amounts of Ig under the same in vitro culture conditions (data not shown).
CD38++CD20± B cells express and produce large amounts of Ig. (A) Spleen or BM MNCs were stained with anti-CD38 and CD20 mAb and then stained for surface or cytoplasmic immunoglobulin using heavy and light chain–specific antibodies. Results are representative of 3 different donor spleens or BM samples. (B) CD38++CD20± B cells were sorted and cultured (approximately 5 × 103 cells/200 μL) in medium only. After 5 days, the amount of secreted Ig was determined using ELISA. Values represent the mean ± SEM of 10 experiments using cells from 8 different donors. (C) Splenic naive, memory, and CD38++CD20± B cells, as well as BM PCs, were sorted directly into wells of ELISPOT plates precoated with anti-IgM, IgG, or IgA antisera. Percentages of IgM, IgG, and IgA ISCs within the different B-cell populations were then enumerated. Values represent the mean of 6 (spleen) or 3 (BM) experiments using cells from different donors.
CD38++CD20± B cells express and produce large amounts of Ig. (A) Spleen or BM MNCs were stained with anti-CD38 and CD20 mAb and then stained for surface or cytoplasmic immunoglobulin using heavy and light chain–specific antibodies. Results are representative of 3 different donor spleens or BM samples. (B) CD38++CD20± B cells were sorted and cultured (approximately 5 × 103 cells/200 μL) in medium only. After 5 days, the amount of secreted Ig was determined using ELISA. Values represent the mean ± SEM of 10 experiments using cells from 8 different donors. (C) Splenic naive, memory, and CD38++CD20± B cells, as well as BM PCs, were sorted directly into wells of ELISPOT plates precoated with anti-IgM, IgG, or IgA antisera. Percentages of IgM, IgG, and IgA ISCs within the different B-cell populations were then enumerated. Values represent the mean of 6 (spleen) or 3 (BM) experiments using cells from different donors.
To determine the frequency of ISCs within different B-cell populations, ELISPOT assays were performed using splenic naive, memory, and CD38++CD20± B cells and BM PCs. Consistent with their lack of Ig secretion during in vitro culture, less than 1% of naive and memory splenic B cells secreted Ig (Figure 2C). In contrast, approximately 30% of ex vivo isolated splenic CD38++CD20± B cells proved to be ISCs (range, 25%-45%; n = 6). This is comparable with the frequency of ISCs detected within the B220± CD138+ population of cells in mouse spleen after immunization with specific Ag (approximately 50%).31 Within the ISC population, 45%, 40%, and 15% (all approximate percentages) produced IgM, IgG, and IgA, respectively (Figure 2C). Strikingly, a similar proportion of BM PCs also produced Ig (approximately 30%; Figure 2C). However, in contrast to splenic CD38++CD20± B cells, more than 75% of ISC in BM produced IgG, whereas only approximately 15% produced IgA, and less than 2% produced IgM. Thus, the CD38++CD20± B-cell population spontaneously secreted Ig of all 3 major isotypes and contained most spleen cells capable of spontaneous Ig production.
IL-6 and IL-10 increase Ig secretion by splenic CD38++CD20± B cells
Cytokines such as IL-4, IL-6, and IL-10 have been shown to enhance B-cell differentiation by increasing Ig production by activated human B cells.32-34 Therefore, the effect of these cytokines on basal Ig production by purified splenic CD38++CD20± B cells during in vitro culture was determined. In the presence of IL-6 or IL-10, IgM and IgA secretion were increased 2-3-fold, and IgG secretion was increased approximately 1.5-fold compared with cells cultured in medium alone (Table 1). In contrast, IL-4 did not increase Ig secretion by these cells (total Ig in unstimulated culture, 334 ng/mL; plus IL-4, 280 ng/mL). Thus, the amount of Ig produced by splenic CD38++CD20± B cells can be heightened after culture in the presence of cytokines known to mediate the differentiation of B cells into ISCs.
Regulation of Ig production by ISC by cytokines
. | IgM . | . | IgG . | . | IgA . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Stimulus . | ng/mL . | Fold increase . | ng/mL . | Fold increase . | ng/mL . | Fold increase . | |||
| Nil | 301 ± 35 | 1.0 | 21 ± 6 | 1.0 | 87 ± 16 | 1.0 | |||
| IL-6 | 778 ± 72 | 1.9 ± 0.3 | 31 ± 3 | 1.3 ± 0.2 | 204 ± 33 | 1.9 ± 0.3 | |||
| IL-10 | 801 ± 132 | 2.5 ± 0.6 | 48 ± 19 | 1.5 ± 0.2 | 144 ± 31 | 2.6 ± 0.5 | |||
. | IgM . | . | IgG . | . | IgA . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Stimulus . | ng/mL . | Fold increase . | ng/mL . | Fold increase . | ng/mL . | Fold increase . | |||
| Nil | 301 ± 35 | 1.0 | 21 ± 6 | 1.0 | 87 ± 16 | 1.0 | |||
| IL-6 | 778 ± 72 | 1.9 ± 0.3 | 31 ± 3 | 1.3 ± 0.2 | 204 ± 33 | 1.9 ± 0.3 | |||
| IL-10 | 801 ± 132 | 2.5 ± 0.6 | 48 ± 19 | 1.5 ± 0.2 | 144 ± 31 | 2.6 ± 0.5 | |||
CD38++CD20± B cells were isolated by cell sorting and cultured in duplicate (approximately 5 × 103 cells/200 μL) in medium only (Nil) or in the presence of IL-6 (10 ng/mL) or IL-10 (100 U/mL). After 5 days, the level of Ig in culture supernatants was determined using ELISA. Values for Ig secretion are the means ± SD of 1 experiment (representative of 6) performed using cells from different donor spleens. Values for fold increase represent the mean increase ± SEM in Ig secretion induced by IL-6 or IL-10 (n = 6) relative to cultures performed in media alone.
CD38++CD20± B cells express genes characteristic of terminally differentiated plasma cells
Recent studies have defined a number of transcription factors that determine whether activated B cells differentiate into memory cells or PCs. Blimp-1 and XBP-1 are up-regulated in PCs and instigate molecular events that ultimately lead to Ig secretion and terminal differentiation. Conversely, BSAP and Bcl-6 must be down-regulated for PC differentiation to occur.35-39 Thus, differential expression of these transcription factors can indicate commitment to a particular lineage. Expression levels of Blimp-1, XBP-1, BSAP, and Bcl-6 were examined using sqPCR. Blimp-1 and XBP-1 mRNA were greatly increased in splenic CD38++CD20± B cells compared with naive and memory B cells (Figure 3A-B). In contrast, BSAP and, to a lesser extent, bcl-6, were detectable in naive B cells, down-regulated in memory B cells, and expressed at low levels in CD38++CD20± B cells (Figure 3C-D). Thus, the relative expression level of these transcription factors by splenic CD38++CD20± B cells is consistent with their commitment to the PC lineage.
CD38++CD20± B cells express genes indicative of commitment to the plasma cell lineage and derivation from germinal centers. (A-E) RNA was extracted from sort-purified naive (CD20+CD27–), memory (CD20+CD27+), and CD38++CD20± (ISC) B cells and transcribed into cDNA. Amounts of cDNA from the different populations were normalized for expression of GAPDH (E) and then used as a template to determine the relative expression levels of (A) Blimp-1, (B) XBP-1, (C) BSAP, and (D) Bcl-6 by sqPCR. Molecular grade dH2O was used as a negative control. (F) Immunoglobulin VH5 genes were amplified from CD38++CD20± B cell cDNA, cloned, and sequenced. Each line represents a single VH5 gene. Sequences with the no. 1 and no. 2 prefixes were derived from CD38++CD20± B cells sort-purified from 2 separate donor spleens. Vertical bars represent silent mutations; vertical bars with • represent replacement mutations. The total number of mutations detected in the different cloned genes is shown at the end of each sequence. The mutation frequency, percentage replacement mutations and R/S ratio within the entire immunoglobulin VH5 gene sequence and individual FR and CDR are indicated. These values were calculated for immunoglobulin VH5 sequences that contained somatic mutations. *Significant increase (P < .001) in the frequency of mutation in CDR1 compared with other regions and the total VH5 gene.
CD38++CD20± B cells express genes indicative of commitment to the plasma cell lineage and derivation from germinal centers. (A-E) RNA was extracted from sort-purified naive (CD20+CD27–), memory (CD20+CD27+), and CD38++CD20± (ISC) B cells and transcribed into cDNA. Amounts of cDNA from the different populations were normalized for expression of GAPDH (E) and then used as a template to determine the relative expression levels of (A) Blimp-1, (B) XBP-1, (C) BSAP, and (D) Bcl-6 by sqPCR. Molecular grade dH2O was used as a negative control. (F) Immunoglobulin VH5 genes were amplified from CD38++CD20± B cell cDNA, cloned, and sequenced. Each line represents a single VH5 gene. Sequences with the no. 1 and no. 2 prefixes were derived from CD38++CD20± B cells sort-purified from 2 separate donor spleens. Vertical bars represent silent mutations; vertical bars with • represent replacement mutations. The total number of mutations detected in the different cloned genes is shown at the end of each sequence. The mutation frequency, percentage replacement mutations and R/S ratio within the entire immunoglobulin VH5 gene sequence and individual FR and CDR are indicated. These values were calculated for immunoglobulin VH5 sequences that contained somatic mutations. *Significant increase (P < .001) in the frequency of mutation in CDR1 compared with other regions and the total VH5 gene.
CD38++CD20± B cells express somatically mutated Ig V region genes
Immune responses to T-cell–dependent Ag's yield 2 types of PCs—low-affinity, short-lived (extrafollicular) ISCs and high-affinity, GC-derived, long-lived PCs.6,8 Immunoglobulin affinity for Ag expressed by long-lived PCs increases as a result of somatic hypermutation occurring during the GC reaction.5,8,10 Thus, accumulations of somatic mutations in the Ig V region genes can be used to distinguish between Ag-selected, GC-derived, long-lived PCs and extrafollicular (ie, non-GC derived) short-lived PCs. The mutational status of Ig expressed by splenic CD38++CD20± B cells was investigated by cloning and sequencing the VH5 family of Ig V region genes from IgM-expressing cells. Twenty-three sequences were obtained from 2 separate donor spleens; 21 of these were unique. All but 1 of the sequences differed from the 2 characterized germline Ig VH5 genes (VH5-32 or VH5-251)40 and thus contained somatic mutations (range, 1-17; mean mutations/sequence, 7.35 ± 5.0; Figure 3F). The overall frequency of mutation was 2.5%. Mutations were present in the framework (FR) and complementarity-determining regions (CDRs); however, the mutational frequency was significantly increased in CDR1 (6.94%; Figure 3F) compared with the other regions and the complete Immunoglobulin V region gene. This is consistent with previous studies examining the mutational status of human memory B cells.26,41 More than two thirds (69.4%) of mutations yielded an amino acid replacement; the proportion of replacement mutations and the ratio of replacement to silent mutations (R/S) were appreciably higher in CDR1 and CDR2 than in the FR (Figure 3F), consistent with Ag selection.41 Thus, Ig molecules expressed by splenic CD38++CD20± B cells are highly mutated, suggesting they are derived from GC and, therefore, may be high-affinity, Agselected ISCs.
Phenotype of splenic CD38++CD20± B cells
Pan–B-cell molecules
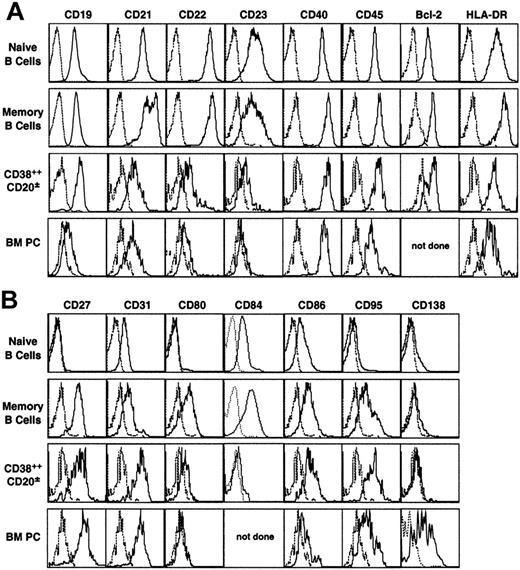
Expression of the pan–B-cell markers CD19, CD21, CD22, CD40, CD45, HLA-DR, and Bcl-2 was similar or slightly increased on memory B cells compared with naive B cells, whereas that of CD23 was reduced (Figure 4A). It varied, however, during the differentiation to PCs such that CD21, CD22, and CD23 expression was almost extinguished on splenic and BM CD38++CD20± cells, but that of HLA-DR and Bcl-2 was only slightly decreased (Figure 4A). In contrast, CD19, CD40, and CD45 expression was maintained on splenic CD38++CD20± B cells and BM PCs, though CD19 and CD45 expression was lower on BM than on splenic ISCs (Figure 4A). These data are consistent with our and other previous studies that examined naive and memory B cells in the human spleen26 and tonsils,42,43 defined by the expression of other cell surface molecules, and CD38++CD20± cells in human tonsil, BM, and gut.16,17,19
Expression of pan–B-cell markers and activation antigens by functionally distinct populations of human B cells. Spleen MNCs were stained with anti-CD27, CD38, and CD20 mAb. The fourth channel was then used to assess expression of (A) the pan–B-cell molecules CD19, CD21, CD22, CD23, CD40, CD45, Bcl-2, and HLA-DR and (B) the activation molecules CD27, CD31, CD80, CD86, CD95 and CD138. Electronic gates (shown in Figure 1A-B) were set on naive, memory, and CD38++CD20± B cells. BM MNCs from different donors were stained with mAbs specific for CD20, CD38, and the indicated molecule, and an electronic gate set to identify BM PCs (CD38++CD20±). Dotted line represents negative control; solid line represents marker of interest. Histograms represent cells from 1 donor but are representative of those from multiple donors (n > 3).
Expression of pan–B-cell markers and activation antigens by functionally distinct populations of human B cells. Spleen MNCs were stained with anti-CD27, CD38, and CD20 mAb. The fourth channel was then used to assess expression of (A) the pan–B-cell molecules CD19, CD21, CD22, CD23, CD40, CD45, Bcl-2, and HLA-DR and (B) the activation molecules CD27, CD31, CD80, CD86, CD95 and CD138. Electronic gates (shown in Figure 1A-B) were set on naive, memory, and CD38++CD20± B cells. BM MNCs from different donors were stained with mAbs specific for CD20, CD38, and the indicated molecule, and an electronic gate set to identify BM PCs (CD38++CD20±). Dotted line represents negative control; solid line represents marker of interest. Histograms represent cells from 1 donor but are representative of those from multiple donors (n > 3).
Activation molecules
Expression of CD27, CD31, CD86, and CD95 increased during differentiation of mature splenic B cells (ie, from naive to memory to CD38++CD20± B cells) (Figure 4). These molecules were low or absent on naive B cells, detectable on most, if not all, memory B cells, and further up-regulated on splenic CD38++CD20± B cells (Figure 4). GC B cells also express high levels of CD27, CD86, and CD95,42,44 consistent with an increase in expression of these molecules during B-cell differentiation. However, GC B cells do not express CD31,21 suggesting GC B cells down-regulate and then reacquire CD31 after differentiation to PCs. In contrast to these surface molecules, expression of CD80 and CD84 increased from naive to memory B cells, consistent with previous observations,26,27,42 whereas CD38++CD20± B cells express low levels of these molecules (Figure 4B). Although the expression of CD27 and CD80 was similar on CD38++CD20± B cells in BM and spleen, CD86 and CD95 were clearly down-regulated on BM PCs compared with splenic CD38++CD20± B cells (Figure 4B). On the other hand, BM PCs uniquely expressed CD138 (Figure 4B). Lastly, 2 molecules known to be up-regulated on PCs in multiple myeloma, CD28 and CD56,45,46 were absent from CD38++CD20±, as well as naive and memory, B cells, confirming that these markers are specific for malignant, but not normal, PCs (data not shown).47
Discussion
ISCs have been the focus of many studies designed to investigate mechanisms for maintaining humoral immunity. These studies, the majority performed in rodents, led to the identification of long-lived PCs that reside predominantly in the BM and to a lesser extent in the spleen.2,3,7,11,12 Identifying the counterpart in human spleen is important for 2 reasons. First, it is relevant to the investigation of disorders affecting ISCs in the spleen, PB, and BM, such as reactive plasmacytoses,25,48 systemic lupus erythematosus (SLE),49-51 and Waldenström macroglobulinemia.52 Second, asplenic individuals are highly susceptible to infection by encapsulated bacteria such as Streptococcus pneumoniae. The mechanism of this susceptibility may involve a deficiency in memory B cells that yield ISCs on exposure to Ag,53 a paucity of resident long-lived splenic ISCs, or both. In this paper we describe for the first time the identification and characterization of a population of human splenic ISCs that appears to correspond to long-lived PCs present in the spleens of mice.
Human splenic ISCs displayed a phenotype (CD38++CD20±) and a morphology reminiscent of human BM and tonsillar PCs15-17,25 (Figures 1, 2A). Importantly, increased expression of Blimp-1 and XBP-1, combined with decreased expression BSAP and Bcl-6 (Figure 3), provided further evidence of the commitment of these cells to the PC lineage.35-39 Cells expressing Blimp-1 and bcl-6 have been detected in tonsillar GCs and have been proposed to be progenitors of mature PCs, which, after leaving the GC, home to extrafollicular regions of lymphoid tissues.35,39 Our finding of decreased bcl-6 and BSAP in splenic ISCs, plus their positioning in the red pulp, suggests that they too have emigrated from the GC and undergone further differentiation along the PC pathway. Moreover, the presence of somatic mutations in Ig V genes and the high incidence of replacement mutations within the CDR (Figure 3) indicate that splenic ISCs were GC-derived and Ag-selected.8,10,41 Immunoglobulin V genes expressed by splenic IgM+ CD38++CD20± B cells had a mutation frequency similar to that reported for human splenic and tonsillar IgM+ memory B cells (2.5% vs 2.54%;26,27,41,54 ). Accordingly, IgM+ memory B cells may yield ISCs after re-exposure to specific Ag without undergoing further Ig isotype switching or somatic hypermutation. This interpretation is consistent not only with studies of murine immune responses in vivo13,55 but with the reported reduced humoral immune responses that result from a deficiency of IgM+ memory B cells in asplenic persons53 and, by inference, resident splenic IgM-secreting ISCs. Furthermore, this mutational frequency was similar to that found in some patients with IgM myeloma,56 Waldenström macroglobulinemia,57 and SLE,49,50 suggesting an involvement of splenic IgM+ ISCs in these diseases. Alternatively, splenic IgM+ memory B cells and ISCs may share similar mutational frequencies because they arise from the same GC B-cell clone.5 Irrespective of these possibilities, our data revealed that high-affinity ISCs localize to the red pulp of human spleen (Figure 1), as do the long-lived ISCs described in mice.7
Splenic ISCs spontaneously secreted a mixture of IgM, IgG, and IgA, though IgM was the predominant isotype produced (Figure 2B). However, when the proportion of cells secreting the various isotypes was enumerated, IgM and IgG ISCs occurred at similar frequencies (Figure 2C). Thus, the higher amount of IgM produced might have resulted from a higher rate of secretion rather than from the presence of a greater number of IgM ISCs. IgA ISCs also appeared to secrete Ig at a higher rate than did IgG ISCs, though the possibility that IgG ISCs undergo apoptosis more promptly in vitro compared with IgM and IgA ISCs cannot be excluded. The amount of Ig secreted could be increased in the presence of exogenous IL-6 or IL-10 (Table 1). Excessive production of IL-6 can cause polyclonal expansions of PC, resulting in plasmacytosis and hypergammaglobulinemia.25,48 Interestingly, affected cells in this condition have been found in all lymphoid tissues, including the spleen, of IL-6 transgenic mice.48 Thus, the ability of IL-6 alone to enhance the production of Ig by splenic (Table 1), but not tonsillar17 or BM,24 CD38++CD20± B cells suggests splenic ISCs could be a major source of Ig in these disorders. The responsiveness of splenic ISCs to IL-10 contrasted that of tonsillar ISCs.17 Serum levels of IL-10 are reported to be increased in patients with SLE (for a review, see Beebe et al58 ), and such patients have significant increases in the frequency and absolute number (3- to 10-fold) of CD38++CD20± B cells in their PB.49-51 IL-10 has also been found to be increased in the sera of patients with other B-cell dyscrasias, such as multiple myeloma and plasma cell leukemia,59,60 and it is a growth factor for myeloma cells.60 Thus, elevated levels of serum IL-10 may contribute to these diseases by enhancing survival, proliferation, and Ig production by splenic ISCs.
BM PCs are generally considered to be more differentiated than ISCs in secondary lymphoid tissues.16,20 However, the percentages of CD38++CD20± B cells in spleen and BM that secreted Ig, and the amounts secreted, were similar (Figure 2C).17,23,24,29 Our results, therefore, indicate that splenic ISCs are as functionally mature as BM PCs, a notion supported by a previous study of murine splenic and BM PCs.11 The major difference we observed was that human BM PCs predominantly expressed and produced IgG, whereas splenic ISCs produced all Ig isotypes, though IgM was predominant (Figure 2). Previous studies have reported that IgM ISCs represented less than 4% of the total population of ISCs in human tonsils, adenoids, and PB, with most (approximately 70%) secreting IgG and the remainder (25%) producing IgA.61 Similarly, PCs in the gut predominantly secrete IgA and only small amounts of IgG and IgM,19 whereas tonsillar ISCs were found to secrete comparable amounts of IgG and IgA but approximately 10-fold less IgM.17 Because the spleen is constantly exposed to a variety of blood-borne antigen, it is not surprising that a mixture of Ig isotypes would be required to combat such a diverse array of antigen. Ag-specific IgM ISCs have been detected in PB after systemic, but not intratonsillar or subcutaneous, immunization.61 However, the frequency of IgM ISCs in PB was still approximately 10-fold less than Ag-specific IgG and IgA ISCs.61 The paucity of IgM ISCs in PB and tonsils17,61 and their relatively high frequency in human spleens (Figure 2) suggests that these cells are generated in situ after Ag exposure, and most remain in the spleen thereafter. It is tempting to speculate that the few IgM ISCs detected in blood are derived from systemic exposure of an Ag that initiated an immune response in the spleen. The specific distribution of ISCs producing distinct Ig isotypes in spleen and BM, as well as tonsils and other mucosal sites, may reflect the unique responsiveness of IgM, IgG, and IgA ISCs to particular chemokines and expression of adhesion molecules.62,63 Detailed analysis of mechanisms underlying the positioning of ISCs in the red pulp of human spleen are under way.
Examining the ISC phenotype also yielded informative results. Compared with naive and memory B cells, CD21, CD22, and CD23 expression on spleen and BM ISCs was dramatically reduced, whereas CD40 and HLA-DR expression on all B-cell populations was similar but slightly higher on memory B cells (Figure 4). CD19 and CD45 expression of splenic ISCs was comparable to that of naive and memory B cells. These molecules were also expressed on BM PCs, albeit at reduced levels compared with splenic ISCs (Figure 4), consistent with studies comparing ISCs in human PB, tonsils, and BM.16 Splenic and BM CD38++CD20± B cells expressed elevated levels of CD27, CD31, and CD95 compared with naive and memory B cells (Figure 4), consistent with some recent observations on PCs in human tonsils, PB, and gut.16,18,19,44,64 Our findings of increased CD27 expression on splenic CD38++CD20± B cells were also consistent with our previous observation of intracellular IgMhiCD27hi cells in the red pulp of human spleen.26 Overall, based on the expression of the molecules examined in this study, the phenotype of splenic ISCs was more similar to that of tonsillar ISCs rather than ISCs detected in PB or BM.16,17 Ligation of CD27 or CD31 increases or antagonizes B-cell activation, respectively,65,66 whereas interaction between CD95 and its ligand induces apoptosis.67 Thus, the enhanced expression of CD27, CD31, and CD95 by ISCs may have an important function in regulating Ig production by these cells. A major difference between ISCs in spleen and BM was the reciprocal expression of CD86 and CD138 (Figure 4). CD86 was found on ISCs in spleen but not in BM. Interestingly, a subset of CD86+ myelomas has recently been reported,46 raising the possibility that this malignancy may arise from cells of the PC lineage present in the spleen rather than in BM. With respect to CD138, this marker is expressed on all murine ISCs,6,8,31 whereas in humans it is only up-regulated at the late stage of PC differentiation in the BM.16,25 Splenic CD38++CD20± B cells shared many molecular, cellular, and functional features in common with gut, tonsillar, and BM PCs. Thus, CD138 may not define all mature PCs but may facilitate migration to or retention of PCs, or possibly both, in the BM by interacting with ligands expressed on extracellular matrices at this site. Consistent with this proposition are the recent findings that most murine PCs in cervical lymph nodes lack CD138 expression68 and that some PB PCs express CD138.16,64 A significant proportion of PCs detected in the PB of SLE patients expressed CD95 and HLA-DR yet lacked expression of CD138.49-51 Curiously, CD86, but not CD80, has been reported to be expressed on 3- to 5-fold more PB CD19+ cells isolated from SLE patients compared with healthy controls,69,70 and the number of these CD19+CD86+ and CD38+CD20± B cells in SLE patients correlated with disease activity.51,70 Although the studies by Folzenlogen et al69 and Bijl et al70 did not resolve the B-cell compartment into naive, memory, or CD38++CD20± B cells, it is possible that the increased number of CD19+CD86+ cells detected in SLE correspond to PCs that are expanded to a similar extent in such patients.49-51 Thus, the PC population prominent in SLE has a phenotype reminiscent of that of splenic ISCs (ie, CD38++CD20± CD27++CD80–CD86+CD95+HLA-DR+CD138±), supporting our proposal that splenic ISCs may contribute to SLE.
In conclusion, identifying the human counterpart of murine splenic ISCs paves the way to investigate long-term serologic humoral immunity in healthy persons and in patients who have undergone splenectomy and to investigate human diseases, such as Waldenström macroglobulinemia,52 reactive plasmacytoses,25 SLE,49,50 and possibly CD86+46 or IgM-secreting56 myeloma, characterized by an accumulation of splenic ISCs.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-09-3109.
Supported by the National Health and Medical Research Council of Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the Australian Red Cross Blood Service for providing human spleens, Dr Joy Ho (Department of Haematology, Royal Prince Alfred Hospital, Sydney, Australia) for providing normal human bone marrow, Joseph Webster and Tara Macdonald for cell sorting, Michelle Amesbury for assisting with immunohistology, Dr Rene de Waal Malefyt for human IL-4 and IL-10, and Professor Tony Basten for critical review of this manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal