Abstract
The acute increase in vascular permeability produced by vascular endothelial growth factor (VEGF-A165) requires activation of endothelial Flk-1 receptors (VEGFR-2) and stimulation of platelet-activating factor (PAF) synthesis. Like PAF, VEGF-A165 promotes translocation of P-selectin to the endothelial cell (EC) surface. However, the mechanisms involved remain unknown. By treating human umbilical vein endothelial cells (HUVECs) with VEGF analogs, we show that activation of VEGFR-1 or VEGFR-2 or both induced a rapid and transient translocation of endothelial P-selectin and neutrophil adhesion to activated ECs. The effects mediated by VEGF-A165 and VEGF-A121 (VEGFR-1/VEGFR-2 agonists) were blocked by a selective VEGFR-2 inhibitor, SU1498. VEGF-A165 was twice as potent as VEGF-A121, which can be explained by the binding capacity of VEGF-A165 to its coreceptor neuropilin-1 (NRP-1). Indeed, treatment with NRP-1 antagonist (GST-Ex7) reduced the effect of VEGF-A165 to the levels observed upon stimulation with VEGF-A121. Finally, the use of selective PAF receptor antagonists reduced VEGF-A165–mediated P-selectin translocation. Together, these data show that maximal P-selectin translocation and subsequent neutrophil adhesion was mediated by VEGF-A165 on the activation of VEGFR-2/NRP-1 complex and required PAF synthesis.
Introduction
Although multiple growth factors induce the proliferation and migration of endothelial cells (ECs), vascular endothelial growth factor (VEGF) is the only growth factor capable of promoting protein extravasation,1-3 which is linked to its angiogenic properties.1-4 There are 5 different VEGF isoforms, of 206, 189, 165, 145 and 121 amino acids, termed as VEGF-A206, 189, 165, 145, and 121, and which are produced from a single gene by alternative splicing. The VEGF family also includes 5 different analogs: placental growth factor (PlGF-1 and -2), VEGF-B, VEGF-C, VEGF-D, and a viral homolog, VEGF-E. The actions of VEGF family members are mediated by the activation of selective tyrosine kinase receptors including VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1/KDR), which are almost exclusively expressed on ECs, and VEGFR-3 (Flt-4), which is mainly limited to lymphatic endothelium. VEGF-A binds to VEGFR-1 and VEGFR-2; PlGF-1, PlGF-2, and VEGF-B bind to VEGFR-1; VEGF-C and -D bind to VEGFR-2 and VEGFR-3; and VEGF-E interacts only with VEGFR-2 (for reviews, see Petrova et al5 and Ferrara6 ). Recent studies also reported that neuropilin 1 (NRP-1), a transmembrane receptor, acts as a coreceptor, complexing with VEGFR-1 and VEGFR-2.7-10 NRP-1 specifically enhances the binding of VEGF-A165 to VEGFR-2 and potentiates various VEGF-A165 biologic activities.8 Such selectivity is attributable to the presence of VEGF-A exon 7 in VEGF-A165, a domain that is lacking in VEGF-A121, VEGF-C, VEGF-D, and PlGF-1.8,11 On the other hand, although NRP-1 can interact with PlGF-2 and VEGF-B isoforms, it does not potentiate their biologic activities on VEGFR-1 stimulation.9,10
We have recently shown that VEGF-A165 permeability effect involves endothelial platelet-activating factor (PAF) synthesis.12 We subsequently investigated the role of VEGF-A receptors and showed that VEGF-A165 induces EC migration and proliferation and PAF synthesis through the activation of VEGFR-2, and that such effects can be potentiated by the expression of NRP-1,13,14 whereas the activation of VEGFR-1 in the presence or absence of NRP-1 had no or marginal contribution on these activities.13,14
The acute induction of PAF synthesis induced by VEGF-A165 may also play a pivotal role in the interaction between inflammatory cells and ECs.15-18 Indeed, VEGF-A165, like other inflammatory mediators including histamine and thrombin, promotes the fusion of cytosolic Weibel-Palade bodies (WPBs) with the plasma membrane, leading to the release of von Willebrand factor (VWF) and to a rapid and transient translocation of P-selectin to the endothelial apical surface.19-24 At the cell surface, P-selectin is able to interact with its high-affinity counterreceptor, P-selectin–glycoprotein ligand 1 (PSGL-1), on neutrophils to promote the rolling and transient adhesion of neutrophils.25,26 In addition, newly synthesized PAF, which remains associated with ECs,27-29 may interact with PAF receptors (PAFRs) on neutrophils.30 Together, these interactions might enhance the adhesion of neutrophils to ECs, which is critical for the early/acute recruitment of leukocytes, and thus contribute to the induction of pathologic angiogenesis.15-18
In the present study, using VEGF analogs, in conjunction with selective inhibitors and antagonists, we studied the mechanisms whereby VEGF-A165 promotes P-selectin translocation in human umbilical vein endothelial cells (HUVECs) and the role of P-selectin and PAF in VEGF-A165–mediated neutrophil adhesion to ECs.
Materials and methods
Chemicals
Histamine was purchased from Sigma (St Louis, MO), human recombinant VEGF165 from PeproTech (Rocky Hill, NJ), human recombinant VEGF121, VEGF-B, VEGF-C, and VEGF-D and PlGF152 (PlGF-1) from R&D Systems (Minneapolis, MN.). SU1498 was from Calbiochem (San Diego, CA), BN 52021 and CV-3988 were from Biomol Research Laboratories (Plymouth Meeting, PA), and LAU 8080 (formerly known as BN 50730) was synthesized and obtained from LSU Health Sciences Center (New Orleans, LA). Dulbecco modified Eagle medium (DMEM), F-12 medium, and Dulbecco phosphate-buffered saline (DPBS) were obtained from Life Technologies (Burlington, ON, Canada).
Cell culture
HUVECs were isolated from fresh umbilical cords and cultured as described previously.31 Briefly, HUVECs were seeded on gelatin-coated (0.25%) plates and cultured in DMEM/F-12 (3:2) medium containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT), EGM-2 singlequot without VEGF (Clonetics, Walkersville, MD), and 2% antibiotics (penicillin and streptomycin; Sigma). HUVECs were characterized by cobblestone appearance and used at passage 1 or 2.
Confocal microscopy
HUVECs were grown on glass coverslips, rinsed with DPBS (37° C), stimulated with histamine or VEGF-A165, and fixed with 1% paraformaldehyde-DPBS solution. In another series of experiments, upon paraformaldehyde fixation, HUVECs were permeabilized with DPBS-Triton X-100 (0.5%). Nonspecific binding of primary antibodies was prevented by preincubating fixed HUVECs with 10% serum from the species used to raise the secondary antibodies. Cells were incubated with rabbit polyclonal antihuman P-selectin antibodies (1:100 dilution), or with goat polyclonal antihuman VWF antibodies (1:200 dilution; Research Diagnostics, Flanders, NJ) for 90 minutes. Cells were rinsed with DPBS and incubated with swine antirabbit tetramethylrhodamine isomer-R (TRITC)–conjugated IgG (1:100 dilution) or donkey antigoat fluorescein isothiocyanate (FITC)–conjugated IgG (1:100 dilution; Dako Diagnostics, Mississauga, ON, Canada) for 60 minutes. Glass coverslips were mounted using 1,4-diazabicyclo-2-2-2-octane (DABCO)/glycerol (1:1) solution. HUVECs were observed on a Zeiss Axiovert 100 M microscope adapted with an LSM 510 confocal system. Images were recorded with the LSM 510 software.
Cell surface ELISA
For enzyme-linked immunosorbent assay (ELISA), flat-bottom 96-well plates were coated with 0.25% gelatin; HUVECs (20 000 cells/well) were seeded and grown up to 3 days after confluence. HUVECs were rinsed with DPBS (37° C), pretreated with a DPBS-CaCl2 (1 mM) solution with or without selective antagonists 15 minutes prior to stimulation with VEGF analogs, PAF, or histamine. Reactions were stopped by removing stimulation medium and adding 1% paraformaldehyde for 20 minutes. Following a rinse with DPBS, cells were incubated with blocking solution (5% BSA in DPBS) for 15 minutes. Cells were then incubated with antihuman P-selectin antibodies (1:100 dilution, 90 minutes), rinsed with DPBS, and then incubated with horseradish peroxidase (HRP)–conjugated goat antirabbit IgG (1:5 000 dilution, 45 minutes). Peroxidase activity was quantified at 450 nm using a plate reader. Nonspecific binding was assessed by substituting primary antibodies with normal rabbit serum (Santa Cruz Biotechnology, Santa Cruz, CA).
Western blot analyses of VEGFRs
Confluent HUVECs (100-mm tissue culture plate) were rinsed with fresh Hanks balanced salt solution (HBSS)–HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]; 10 mM, pH 7.4) and stimulated either with DPBS, VEGF-A165, VEGF-A121, VEGF-C, or VEGF-D. In a series of experiments, HUVECs were pretreated for 15 minutes with VEGFR-2 inhibitor (SU1498) or PAFR antagonists prior to stimulation. Following stimulation, the medium was removed, cells were washed, and lysates were prepared. Immunoprecipitation was from 1 mg lysate. Western blot analyses were performed as described previously.13,14 Primary antibodies used were rabbit antihuman VEGFR-1, rabbit antimouse VEGFR-2, rabbit antihuman VEGFR-3, or goat antihuman NRP-1 IgG polyclonal antibodies (Santa Cruz Biotechnology). Membranes were stripped and reprobed to determine receptor phosphorylation (with the exception of NRP-1 because VEGF-A165, and its analogs, do not induce NRP-1 phosphorylation).14 Briefly, membranes were stripped using Re-Blot Plus Strong stripping solution (Chemicon International, Temecula, CA.) for 20 minutes, followed by a brief rinse with 0.1% Tween–Tris-buffered saline (tris(hydroxymethyl)aminomethane; TTBS). Following reblocking, receptor tyrosine phosphorylation was determined as described; the primary antiserum was mouse antiphosphotyrosine clone 4G10 (1:4000 dilution; Upstate Biotechnology, Lake Placid, NY). Kaleidoscope molecular weight markers (Bio-Rad, Hercules, CA) were used as molecular mass standards for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) immunoblotting experiments.
Neutrophil purification
Venous blood was obtained from healthy donors, free from medication for at least 10 days before the experiments, as described previously.31 This procedure yielded a neutrophil population that was 95% pure, as determined by a Coulter counter and Wright-Giemsa staining, and over 98% viable when assessed by trypan blue dye exclusion assay.
Neutrophil adhesion assay
HUVECs were seeded and cultured (40 000 cells/well; 24-well plates) as described (“Cell surface ELISA”). Confluent HUVECs were rinsed with DPBS (37° C) and pretreated with DPBS containing 1 mM CaCl2 (DPBS-CaCl2) alone or containing PAFR antagonists, which bind selectively to intracellular PAFR (LAU 8080; 10–10-10–7 M), extracellular PAFR (BN 52021; 10–8-10–5 M), or to intracellular and extracellular PAFR (CV-3988; 10–8-10–5 M), or a P-selectin antagonist, recombinant soluble P-selectin glycoprotein ligand immunoglobulin molecule; (rPSGL-Ig; 1 ng to 50 μg/mL; kindly provided by Dr A. Kumar, Genetics Institute, Andover, MA) for 15 minutes at 37° C. Neutrophils (1 × 105 in 500 μL DPBS-CaCl2) were added to each well and HUVECs were stimulated with PAF (10–9 M) or VEGF analogs (10–9 M) for 7.5 minutes. The wells were rinsed with DPBS, to remove nonadherent neutrophils, and fixed with a 1% paraformaldehyde/1% glutaraldehyde-DPBS solution. Adhesion of neutrophils to HUVEC monolayers was assessed with a color video digital camera adapted to a binocular microscope. For each well, 3 fields of view were randomly selected and the neutrophils in each field of view were counted and recorded as the number of adhered neutrophils/mm2; because there were slight variations of basal neutrophil adhesion between experiments, we then reported our data as relative neutrophil adhesion (%).
Preparation of GST-VEGF-A165 exon 7 fusion protein
To assess the potential contribution of NRP-1 in potentiating the effects of VEGF-A165 over VEGF-A121 on P-selectin translocation and neutrophil adhesion to HUVECs, we produced a glutathione-S-transferase (GST) fusion protein encoding exon 7 of human VEGF-A165 (GST-Ex7). This sequence is not present in VEGF-A121 and is responsible for the binding of VEGF-A165 to NRP-1.8 The sequence encoding exon 7 of human VEGF-A165 was cloned into the vector pGEX-2TK (Amersham Biosciences, Baie d'urfé, QC, Canada). Escherichia coli (DH5α) was transformed with pGEX-2TK or p2TK-exon 7 vectors to produce GST and GST-exon 7 proteins. The recombinant proteins were purified from bacterial lysates using glutathione and heparin affinity chromatographies, as described previously.32
Measurement of PAF synthesis
PAF production by HUVECs was measured by determining the incorporation of 3H-acetate into lyso-PAF. Briefly, HUVECs were cultured to confluence in 6-well plates as detailed and stimulated with VEGF analogs (10–9 M) for 7.5 minutes. Reactions were stopped and synthesized 3H-PAF was extracted, purified by high-performance liquid chromatography (HPLC), and quantified by β-counting.33
Statistical analysis
Data are presented as mean ± SEM. Statistical comparisons were made by analysis of variance, followed by a Bonferroni test for multiple comparisons. Differences were considered significant at P values less than .05.
Results
Localization of P-selectin and VWF in HUVECs
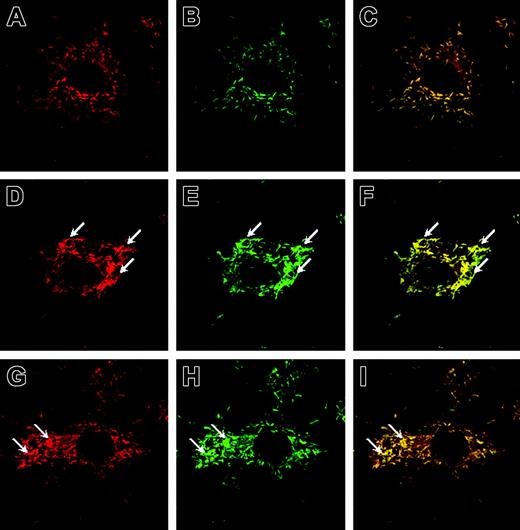
The subcellular localization of P-selectin and VWF was assessed by dual labeling, using selective antibodies against P-selectin or VWF or both, in conjunction with confocal microscopy. In control (DPBS)–treated HUVECs, we observed a homogenous cytosolic distribution of P-selectin and VWF (Figure 1A-C). Treatment with VEGF-A165 (10–9 M) for 7.5 minutes induced a clustering of WPBs along the cytoplasmic membrane (Figure 1D-F). As positive control, HUVECs were treated with histamine (10–4 M) for 7.5 minutes, which promoted a clustering pattern of WPBs similar to that observed in VEGF-A165–treated cells (Figure 1G-I).19,24 Negative control experiments performed with purified preimmune rabbit and goat IgG as primary antibodies did not produce detectable staining (data not shown).
P-selectin and VWF colocalize in rod-shaped WPBs of ECs and cluster to cell membrane on stimulation. HUVECs were stimulated with solution buffer (DPBS), histamine, or VEGF-A165 for 7.5 minutes, fixed, labeled with antisera, and visualized with a Zeiss LSM 510 confocal fluorescent microscope. Shown is the subcellular localization of P-selectin (A,D,G; TRITC staining, colored red), VWF (B,E,H; FITC staining, colored green), and their colocalization in WPBs (C,F,I; colocalized pixels are colored yellow). In DPBS-treated cells, WPBs were distributed homogeneously (A-C), whereas in cells treated with VEGF-A165 (D-F) and histamine (G-I), WPBs clustered along the cell membrane (arrows). For control experiments, HUVECs were prepared according to the labeling procedure except that primary antibodies were omitted (not shown). Original magnification: ×630.
P-selectin and VWF colocalize in rod-shaped WPBs of ECs and cluster to cell membrane on stimulation. HUVECs were stimulated with solution buffer (DPBS), histamine, or VEGF-A165 for 7.5 minutes, fixed, labeled with antisera, and visualized with a Zeiss LSM 510 confocal fluorescent microscope. Shown is the subcellular localization of P-selectin (A,D,G; TRITC staining, colored red), VWF (B,E,H; FITC staining, colored green), and their colocalization in WPBs (C,F,I; colocalized pixels are colored yellow). In DPBS-treated cells, WPBs were distributed homogeneously (A-C), whereas in cells treated with VEGF-A165 (D-F) and histamine (G-I), WPBs clustered along the cell membrane (arrows). For control experiments, HUVECs were prepared according to the labeling procedure except that primary antibodies were omitted (not shown). Original magnification: ×630.
Effect of VEGF analogs and corresponding receptors on P-selectin translocation
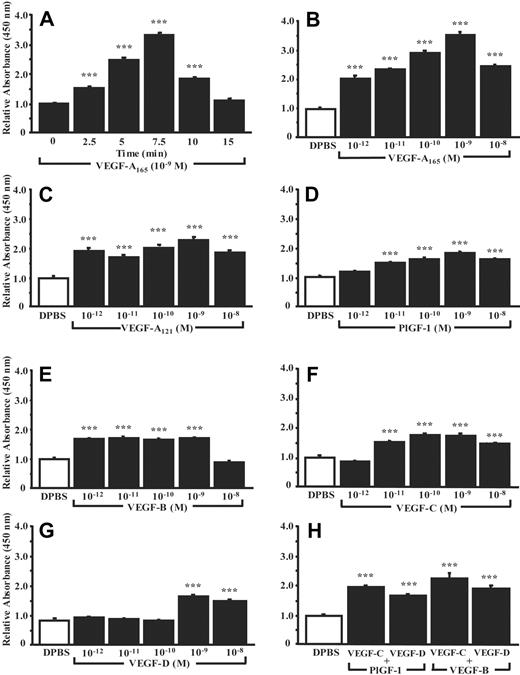
The effect of VEGF analogs, plus the contribution of their receptors and the coreceptor NRP-1, on P-selectin translocation in HUVECs was assessed by cell surface ELISA. First, HUVECs were treated with VEGF-A165, and the time dependence (0-15 minutes, 10–9 M) and dose dependence (10–12-10–8 M, 7.5 minutes) of P-selectin translocation were determined. The accumulation of P-selectin at the cell surface reached a maximum following 7.5 minutes of stimulation and then declined (Figure 2A). The optimum concentration of VEGF-A165 was 10–9 M, which increased cell surface P-selectin by up to 270% within 7.5 minutes (Figure 2B). Hence, these conditions were used for the characterization of other VEGF analogs. VEGF-A121 increased cell surface P-selectin by 135% (Figure 2C), whereas PlGF-1 (VEGFR-1 ligand) or VEGF-B (VEGFR-1 and NRP-1 ligand) produced increases of 79% and 72%, respectively (Figure 2D-E). VEGF-C and VEGF-D (VEGFR-2 and VEGFR-3 analogs) increased cell surface P-selectin by 77% and 69%, respectively (Figure 2F-G). To determine if the activation of VEGFRs by various analogs was additive, combinations of VEGF analogs (10–9 M) were used; the combination of VEGF-B with VEGF-C or VEGF-D increased P-selectin translocation by 130% and 94%, respectively, whereas the combination of PlGF-1 with VEGF-C or VEGF-D increased it by 99% and 70%, respectively (Figure 2H).
VEGF analogs induce P-selectin cell surface expression in HUVECs. P-selectin translocation was quantified by cell surface ELISA. DPBS was used as negative control and the basal levels of cell surface P-selectin were normalized to 1. The translocation of P-selectin induced by VEGF-A165 (10–9 M) was determined as a function of the time (0-15 minutes; A) and at various concentrations (10–12-10–8 M) in cells treated for 7.5 minutes (B). Translocation of P-selectin induced by VEGF analogs was assessed at various concentrations (10–12-10–8 M) in cells treated for 7.5 minutes (C-G). The combination of VEGFR-1 and VEGFR-2 agonists (10–9 M; 7.5 minutes) on P-selectin translocation was assessed (H). In each experiment, a group of cells was treated with normal rabbit IgG to evaluate the nonspecific binding, which was subtracted from the values obtained on stimulation with agonists. Data are means ± SEM of at least 12 experiments; ***P ≤ .001 as compared to DPBS.
VEGF analogs induce P-selectin cell surface expression in HUVECs. P-selectin translocation was quantified by cell surface ELISA. DPBS was used as negative control and the basal levels of cell surface P-selectin were normalized to 1. The translocation of P-selectin induced by VEGF-A165 (10–9 M) was determined as a function of the time (0-15 minutes; A) and at various concentrations (10–12-10–8 M) in cells treated for 7.5 minutes (B). Translocation of P-selectin induced by VEGF analogs was assessed at various concentrations (10–12-10–8 M) in cells treated for 7.5 minutes (C-G). The combination of VEGFR-1 and VEGFR-2 agonists (10–9 M; 7.5 minutes) on P-selectin translocation was assessed (H). In each experiment, a group of cells was treated with normal rabbit IgG to evaluate the nonspecific binding, which was subtracted from the values obtained on stimulation with agonists. Data are means ± SEM of at least 12 experiments; ***P ≤ .001 as compared to DPBS.
Activation and regulation of VEGFRs
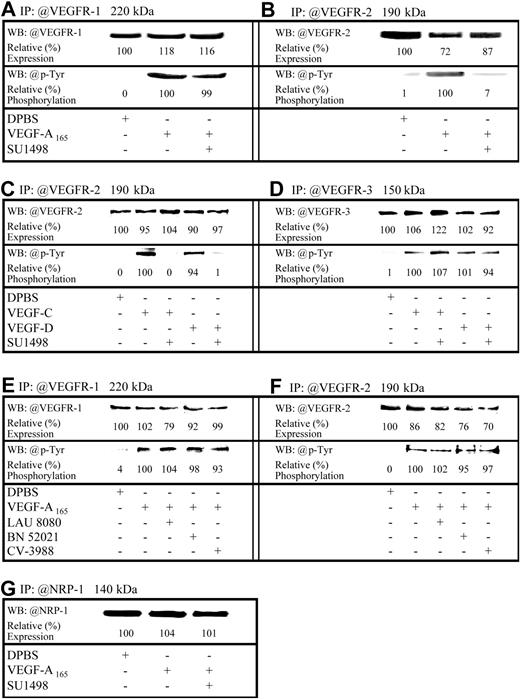
Because VEGF analogs induced P-selectin translocation, Western blot analyses were used to both confirm the expression of VEGFRs and NRP-1 in HUVECs and assess the capacity of VEGF-A165 to promote VEGFR-1/VEGFR-2 phosphorylation and VEGF-C and VEGF-D to induce VEGFR-2/VEGFR-3 phosphorylation. In addition, using a selective VEGFR-2 inhibitor (SU1498; 5 × 10–6 M, inhibitory concentration of 50% [IC50] = 700 nM]), we determined whether the translocation of P-selectin induced by VEGF-A165 and VEGF-A121 results from the activation of VEGFR-1 or VEGFR-2 or both, and if VEGFR-3 contributes to VEGF-C and VEGF-D effect. VEGFR-1, VEGFR-2, VEGFR-3, and NRP-1 were detected by immunoblotting, which is in agreement with previous reports14,34 (Figure 3A-G upper bands). VEGF-A165 increased tyrosine phosphorylation in both VEGFR-1 and VEGFR-2 (Figure 3A-B lower bands). Similarly, VEGF-C and VEGF-D induced phosphorylation of VEGFR-2 and VEGFR-3 (Figure 3C-D lower bands). Pretreatment with SU1498 abrogated the phosphorylation of VEGFR-2 mediated by VEGF-A165, VEGF-C, or VEGF-D (Figure 3B-C lower bands) without affecting the phosphorylation of VEGFR-1 and VEGFR-3 (Figure 3A,D lower bands). For each receptor subtype investigated, in the absence of primary antibody, no bands were detected (data not shown).
Expression and regulation of VEGFRs in HUVECs. HUVECs were treated either with DPBS or VEGF analogs (10–9 M; 7.5 minutes). Where indicated, HUVECs were pretreated for 15 minutes either with a VEGFR-2 inhibitor or PAFR antagonists prior to stimulation with VEGF analogs. Lysates were prepared and VEGFR-1 (A,E), VEGFR-2 (B,C,F), VEGFR-3 (D), or NRP-1 (G) were immunoprecipitated (IP) from 1 mg lysate. Following resolution on SDS-PAGE and transfer onto polyvinylidene difluoride (PVDF) membranes, immunoreactive bands were visualized by enhanced chemiluminescence (ECL), digitized using a 2-dimensional gel scanner, and quantified using Quantity One software (Bio-Rad). Results were normalized to those from DPBS-treated cells (upper bands). Membranes were subsequently stripped using Re-Blot Plus Strong stripping solution and the detection of VEGFR-1, VEGFR-2, or VEGFR-3 phosphorylation was performed with antiphosphotyrosine antibodies. Phosphorylation results were normalized to those from cells treated with VEGF analogs (lower bands).
Expression and regulation of VEGFRs in HUVECs. HUVECs were treated either with DPBS or VEGF analogs (10–9 M; 7.5 minutes). Where indicated, HUVECs were pretreated for 15 minutes either with a VEGFR-2 inhibitor or PAFR antagonists prior to stimulation with VEGF analogs. Lysates were prepared and VEGFR-1 (A,E), VEGFR-2 (B,C,F), VEGFR-3 (D), or NRP-1 (G) were immunoprecipitated (IP) from 1 mg lysate. Following resolution on SDS-PAGE and transfer onto polyvinylidene difluoride (PVDF) membranes, immunoreactive bands were visualized by enhanced chemiluminescence (ECL), digitized using a 2-dimensional gel scanner, and quantified using Quantity One software (Bio-Rad). Results were normalized to those from DPBS-treated cells (upper bands). Membranes were subsequently stripped using Re-Blot Plus Strong stripping solution and the detection of VEGFR-1, VEGFR-2, or VEGFR-3 phosphorylation was performed with antiphosphotyrosine antibodies. Phosphorylation results were normalized to those from cells treated with VEGF analogs (lower bands).
P-selectin translocation mediated by VEGF analogs: role of VEGFR-1, VEGFR-2, and NRP-1
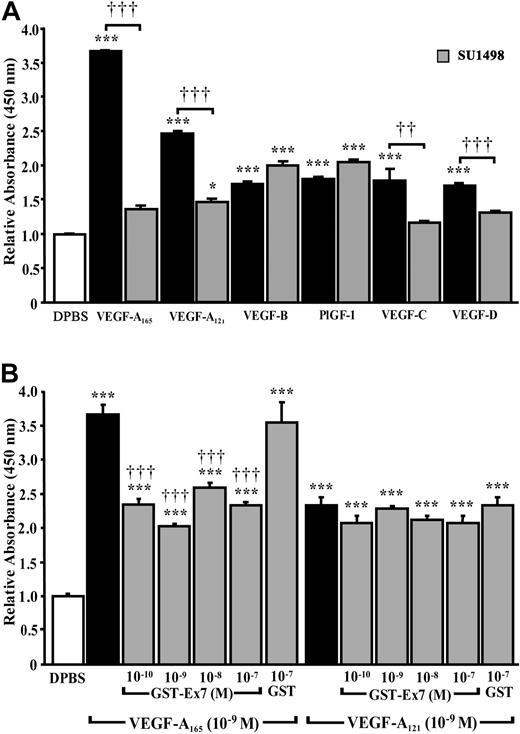
To assess the relative contributions of VEGFR-1 and VEGFR-2 in mediating P-selectin translocation, HUVECs were pretreated with a VEGFR-2 inhibitor, SU1498. Blockade of VEGFR-2 activation reduced VEGF-A165– and VEGF-A121–induced P-selectin translocation by 91% and 85%, respectively (Figure 4A). SU1498 was also efficient at preventing the effect of VEGF-A165 and VEGF-A121 at lower concentrations (10–11 and 10–10 M; data not shown). SU1498 also reduced by 80% and 56% the translocation of P-selectin produced by VEGF-C and VEGF-D. P-selectin translocation produced by VEGFR-1 analogs (PlGF-1, VEGF-B) was unaffected by VEGFR-2 inhibition (Figure 4A).
Contribution of VEGFRs to P-selectin translocation. Prior to stimulation with the indicated VEGF analogs (10–9 M; 7.5 minutes), HUVECs were pretreated for 15 minutes with (A) a selective VEGFR-2 inhibitor (SU1498; 5 × 10–6 M) or (B) either a GST fusion protein containing either exon 7 of human VEGF-A165 (GST-Ex7) or GST. Data are means ± SEM of at least 6 experiments, *P ≤ .05 and ***P ≤ .001 as compared to DPBS, ††P ≤ .01 and †††P ≤ .001 as compared to corresponding VEGF analogs.
Contribution of VEGFRs to P-selectin translocation. Prior to stimulation with the indicated VEGF analogs (10–9 M; 7.5 minutes), HUVECs were pretreated for 15 minutes with (A) a selective VEGFR-2 inhibitor (SU1498; 5 × 10–6 M) or (B) either a GST fusion protein containing either exon 7 of human VEGF-A165 (GST-Ex7) or GST. Data are means ± SEM of at least 6 experiments, *P ≤ .05 and ***P ≤ .001 as compared to DPBS, ††P ≤ .01 and †††P ≤ .001 as compared to corresponding VEGF analogs.
Because VEGF-A165 and VEGF-A121 induced P-selectin translocation through activation of VEGFR-2, and VEGF-A165 was more potent than VEGF-A121, the effects of VEGF-A165 may be potentiated by the coreceptor NRP-1. Thus HUVECs were pretreated with a GST fusion protein containing exon 7 of human VEGF-A165 (GST-Ex7) to block the interaction of VEGF-A165 with NRP-1. Exon 7 encodes a domain not present in VEGF-A121 that is responsible for the binding of VEGF-A165 to NRP-1.8 Pretreatment of HUVECs with GST-Ex7 (10–10-10–7 M) 15 minutes prior to stimulation with VEGF-A165 reduced P-selectin translocation to the level induced by VEGF-A121 (Figure 4B). GST (up to 10–7 M), without the exon 7 insert, did not alter VEGF-A165–induced P-selectin translocation. Neither GST-Ex7 nor GST (up to 10–7 M) altered the basal level of P-selectin translocation (data not shown) or VEGF-A121–induced P-selectin translocation (Figure 4B).
Contribution of PAF to VEGF-A165–induced P-selectin translocation
We have previously shown that VEGF-A165 induces PAF synthesis.12 Furthermore, exposure of ECs to PAF promotes P-selectin translocation.31 To determine whether PAF and its receptors contribute to VEGF-A165–mediated P-selectin translocation, the presence of cell membrane and intracellular PAFRs was confirmed in HUVECs by confocal microscopy35,36 (data not shown). The capacity of each VEGF analog to mediate PAF synthesis was then assessed. VEGF analogs capable of activating VEGFR-2 (VEGF-A165, VEGF-A121, VEGF-C, and VEGF-D) increased PAF synthesis by 75% to 95% (Table 1). VEGFR-1 agonists (PlGF-1, VEGF-B) had no effect on PAF synthesis (Table 1).
PAF synthesis mediated by VEGF analogs
Treatment . | 3H-PAF synthesis (dpm) . |
|---|---|
| DPBS | 299 ± 34 |
| VEGF-A165 | 537 ± 85* |
| VEGF-A121 | 580 ± 51* |
| PIGF-1 | 242 ± 44 |
| VEGF-B | 276 ± 36 |
| VEGF-C | 525 ± 69* |
| VEGF-D | 587 ± 42† |
Treatment . | 3H-PAF synthesis (dpm) . |
|---|---|
| DPBS | 299 ± 34 |
| VEGF-A165 | 537 ± 85* |
| VEGF-A121 | 580 ± 51* |
| PIGF-1 | 242 ± 44 |
| VEGF-B | 276 ± 36 |
| VEGF-C | 525 ± 69* |
| VEGF-D | 587 ± 42† |
HUVECs were stimulated with DPBS or VEGF analogs (10-9 M) for 7.5 minutes. Data are means ± SEM of at least 6 experiments.
dpm indicates disintegrations per minute.
P ≤ .05 as compared to DPBS.
P ≤ .01 as compared to DPBS.
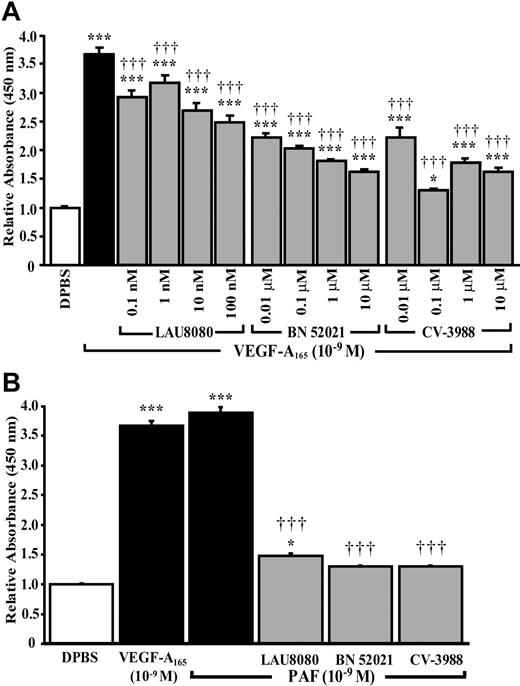
We then examined if newly synthesized PAF, and its receptors, were involved in P-selectin translocation induced by VEGF-A165. Selective intracellular, extracellular, and both intracellular and extracellular PAFR antagonists (LAU 8080, BN 52021, and CV-3988) produced dose-dependent reductions in VEGF-A165–induced P-selectin translocation of, respectively, 39%, 65%, and 88% (Figure 5A). PAF induced P-selectin translocation and pretreatment with PAFR antagonists prior to a stimulation with PAF (10–9 M) prevented P-selectin translocation by 88% to 95% (Figure 5B). Treatment with PAF antagonists alone had no effect on basal P-selectin translocation (data not shown). Similarly, PAFR antagonists (LAU 8080, 100 nM [IC50 = 590 pM]; BN 52021, 10 μM [IC50 = 164 nM]; and CV-3988, 10 μM [IC50 = 254 nM])37-39 did not affect the capacity of VEGF-A165 to induce the phosphorylation, and therefore activation, of VEGFR-1 and VEGFR-2 (Figure 3E-F lower bands).
Role of PAF and its receptors on VEGFA165–induced P-selectin translocation. HUVECs were pretreated with selective PAFR antagonists, at the indicated concentrations, for 15 minutes prior to stimulation with VEGF-A165 (10–9 M, 7.5 minutes; A) or PAF (10–9 M, 7.5 minutes; B). Pretreatment with intracellular (LAU 8080), extracellular (BN 52021) and nonspecific PAFR (CV-3988) antagonists reduced significantly the translocation of P-selectin induced by VEGF-A165 and PAF. Data are means ± SEM of at least 6 experiments, *P ≤ .05 and ***P ≤ .001 as compared to DPBS, †††P ≤ .001 compared to VEGF-A165 (A) or PAF (B).
Role of PAF and its receptors on VEGFA165–induced P-selectin translocation. HUVECs were pretreated with selective PAFR antagonists, at the indicated concentrations, for 15 minutes prior to stimulation with VEGF-A165 (10–9 M, 7.5 minutes; A) or PAF (10–9 M, 7.5 minutes; B). Pretreatment with intracellular (LAU 8080), extracellular (BN 52021) and nonspecific PAFR (CV-3988) antagonists reduced significantly the translocation of P-selectin induced by VEGF-A165 and PAF. Data are means ± SEM of at least 6 experiments, *P ≤ .05 and ***P ≤ .001 as compared to DPBS, †††P ≤ .001 compared to VEGF-A165 (A) or PAF (B).
Effect of VEGF analogs and corresponding receptors on the adhesion of neutrophils to HUVECs: role of P-selectin translocation
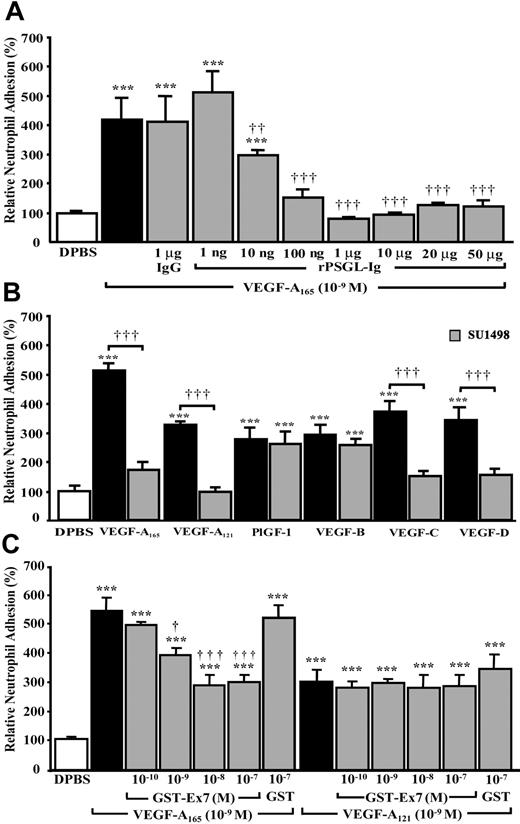
Because VEGF analogs induced P-selectin translocation, we wanted to assess their capacity to enhance the adhesion of neutrophils to HUVECs. Treatment of confluent HUVECs (1485 ± 72 cells/mm2) with VEGF-A165 (10–9 M) for 7.5 minutes increased by 318% the adhesion of neutrophils to confluent HUVECs from a basal value of 65 ± 5 to 274 ± 50/mm2 (Figure 6A). Pretreatment of HUVECs with the P-selectin antagonist, rPSGL-Ig (10 ng to 50 μg/mL),32 15 minutes prior to stimulation with VEGF-A165 (10–9 M, 7.5 minutes) resulted in a concentration-dependent inhibition of neutrophil adhesion to HUVECs (Figure 6A). At a concentration of 1 μg/mL, and in the presence of VEGF-A165 (10–9 M), rPSGL-Ig reduced neutrophil adhesion to basal levels (Figure 6A). Treatment with normal mouse IgG (1 μg/mL) did not affect VEGF-A165–mediated neutrophil adhesion to HUVECs (Figure 6A).
Effect of VEGF analogs and their corresponding receptors on neutrophil adhesion to HUVECs and role of P-selectin translocation. HUVECs were pretreated with rPSGL-Ig (A), SU1498 (B), or either with a GST-Ex7 or GST (C) for 15 minutes prior to the addition of neutrophils (1 × 105) and to the stimulation of confluent HUVECs with VEGF analogs (10–9 M, 7.5 minutes). Data are means ± SEM of 6 to 12 experiments; ***P ≤ .001 as compared to DPBS and ††P ≤ .01, †††P ≤ .001 as compared to corresponding VEGF analogs.
Effect of VEGF analogs and their corresponding receptors on neutrophil adhesion to HUVECs and role of P-selectin translocation. HUVECs were pretreated with rPSGL-Ig (A), SU1498 (B), or either with a GST-Ex7 or GST (C) for 15 minutes prior to the addition of neutrophils (1 × 105) and to the stimulation of confluent HUVECs with VEGF analogs (10–9 M, 7.5 minutes). Data are means ± SEM of 6 to 12 experiments; ***P ≤ .001 as compared to DPBS and ††P ≤ .01, †††P ≤ .001 as compared to corresponding VEGF analogs.
To examine the effectiveness of VEGF analogs in promoting neutrophil adhesion, HUVECs were treated as described (“Neutrophil adhesion assay”). VEGF-A165, VEGF-A121, PlGF-1, VEGF-B, VEGF-C, and VEGF-D (10–9 M, 7.5 minutes) increased the adhesion of neutrophils to HUVECs by 415%, 230%, 176%, 192%, 272%, and 242%, respectively (Figure 6B). To assess if the effects of VEGF-A165 and VEGF-A121 were mediated by the activation of VEGFR-1 or VEGFR-2 or both, and if the effects of VEGF-C and VEGF-D were mediated by the activation of VEGFR-2 or VEGFR-3 or both, HUVECs were pretreated with VEGFR-2 inhibitor (SU1498; 5 × 10–6 M) 15 minutes prior to stimulation with VEGF analogs. SU1498 reduced VEGF-A165– and VEGF-A121–induced adhesion by 83% and 100% and VEGF-C– and VEGF-D–induced adhesion by 81% and 77%. SU1498 did not alter the capacity of VEGFR-1 agonists PlGF-1 and VEGF-B to induce neutrophil adhesion to HUVECs (Figure 6B).
VEGF-A165 was twice as potent as VEGF-A121 at inducing the adhesion of neutrophil to HUVECs, despite the fact that both ligands acted through VEGFR-2. Such differences might result from the ability of VEGF-A165, but not VEGF-A121, to bind NRP-1. Pretreatment of HUVECs with GST-Ex7 (10–10-10–7 M) 15 minutes prior to stimulation with VEGF-A165 (10–9 M) reduced neutrophil adhesion to the level observed with VEGF-A121 (Figure 6C). Such pretreatment did not alter VEGF-A121–induced neutrophil adhesion (Figure 6C). Pretreatment of HUVECs with GST (10–7 M) did not alter VEGF-A165– or VEGF-A121–induced neutrophil adhesion (Figure 6C). GST-Ex7 or GST (10–7 M) alone had no effect on basal neutrophil adhesion (data not shown).
Contribution of PAF to VEGF-A165–mediated neutrophil adhesion to HUVECs
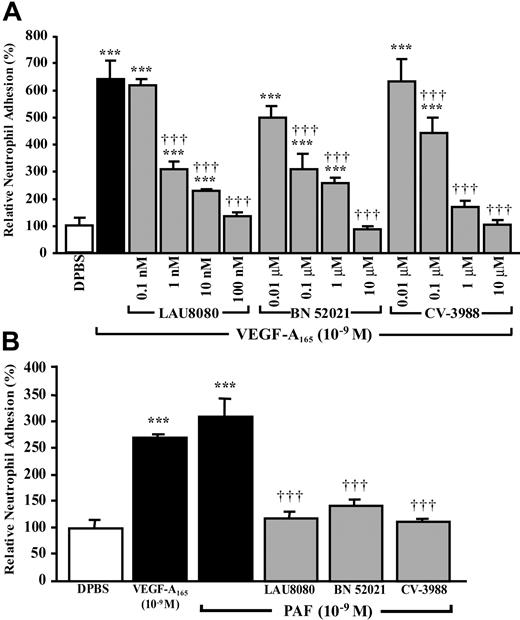
Because the activation of VEGFR-2 with selective VEGF analogs led to PAF synthesis and the blockade of intracellular and extracellular PAFRs reduced VEGF-A165–mediated P-selectin translocation, the effect of PAFR antagonists on neutrophil adhesion was examined. Pretreatment with the intracellular PAFR antagonist, LAU 8080 (up to 100 nM), reduced VEGF-A165–induced neutrophil adhesion by 94%, whereas an extracellular PAFR antagonist, BN 52021, or an antagonist of both intracellular and extracellular PAFRs, CV-3988, completely prevented the adhesion of neutrophils to HUVECs (Figure 7A). When HUVECs were pretreated with the highest effective concentrations of PAF antagonists prior to a stimulation with PAF (10–9 M), the adhesion of neutrophils to HUVECs was reduced by 81% to 96% (Figure 7B). Treatment with PAF antagonists alone had no effect on basal neutrophil adhesion to HUVECs (data not shown).
Role of PAF and its receptors on VEGF-A165–induced neutrophil adhesion to HUVECs. HUVECs were pretreated with the indicated PAFR antagonists for 15 minutes prior to the addition of neutrophils (1 × 105) and to the stimulation of confluent HUVEC with VEGF-A165 (10–9 M, 7.5 minutes; A) or PAF (10–9 M, 7.5 minutes; B). Pretreatment with an intracellular (LAU 8080), an extracellular (BN 52021), and nonspecific PAFR antagonist (CV-3988) reduced significantly the adhesion of neutrophils to HUVECs mediated by VEGF-A165 and PAF. Data are means ± SEM of at least 6 experiments; ***P ≤ .001 as compared to DPBS, †††P ≤ .001 compared to VEGF-A165 (A) or PAF (B).
Role of PAF and its receptors on VEGF-A165–induced neutrophil adhesion to HUVECs. HUVECs were pretreated with the indicated PAFR antagonists for 15 minutes prior to the addition of neutrophils (1 × 105) and to the stimulation of confluent HUVEC with VEGF-A165 (10–9 M, 7.5 minutes; A) or PAF (10–9 M, 7.5 minutes; B). Pretreatment with an intracellular (LAU 8080), an extracellular (BN 52021), and nonspecific PAFR antagonist (CV-3988) reduced significantly the adhesion of neutrophils to HUVECs mediated by VEGF-A165 and PAF. Data are means ± SEM of at least 6 experiments; ***P ≤ .001 as compared to DPBS, †††P ≤ .001 compared to VEGF-A165 (A) or PAF (B).
Viability of HUVECs and neutrophils
Finally, to ensure that the blockade of P-selectin translocation and neutrophil adhesion to HUVECs produced by the agonists and antagonists used in these studies were not due to a cytotoxic effect, the effect of all antagonists and inhibitors was assessed at the highest concentration used, either alone or in the presence of VEGF analogs on viability of HUVECs and neutrophils by trypan blue exclusion (data not shown). In all cases, the reagents and conditions used herein did not increase cell mortality. Furthermore, no increase in detachment of HUVECs or the formation of membrane ghosts in HUVECs and neutrophils were detected up to 120 minutes after treatment.
Discussion
Using receptor subtype-selective VEGF analogs, we have shown that VEGFR-1 and VEGFR-2 activation induced a rapid and transient translocation of endothelial P-selectin. VEGF-A165 and VEGF-A121 induced P-selectin translocation primarily through activation of VEGFR-2 and the binding of VEGF-A165 to the coreceptor NRP-1 potentiated its effect as compared to VEGF-A121. Finally, by pretreating HUVECs with selective PAFR and P-selectin antagonists, we were able to demonstrate that PAF synthesis is essential for VEGF-A165–induced P-selectin translocation, which, in turn, is essential for the adhesion of neutrophils onto activated HUVECs.
Involvement of VEGFRs in P-selectin translocation
VEGF-C and VEGF-D, which activate VEGFR-2 and VEGFR-3, induced P-selectin translocation in HUVECs to about 30% of the maximal effect obtained with VEGF-A165 (Figure 2). Pretreatment with SU1498, an inhibitor of VEGFR-2 kinase activity, prior to stimulation with VEGF-C or VEGF-D inhibited VEGFR-2 phosphorylation and reduced P-selectin translocation by 80% without affecting the phosphorylation of VEGFR-3 (Figures 3-4). Because VEGF-C and VEGF-D bind to VEGFR-2, without interacting with NRP-1,40 we can therefore state that VEGFR-2 stimulation is sufficient to promote partial P-selectin translocation. Treatment of HUVECs with VEGFR-1 agonists PlGF-1 and VEGF-B also induced a partial P-selectin translocation as compared to VEGF-A165. Our data demonstrate that VEGFR-1 stimulation can promote selective biologic activities. Treatment with VEGF-A121, which binds to VEGFR-1 and VEGFR-2 but not to NRP-1, increased P-selectin translocation by 135%, which represents 50% of the effect of VEGF-A165, suggesting that the ability of VEGF-A165 to bind to NRP-1 potentiates the effect of this agonist on endothelial P-selectin translocation. This latter hypothesis is supported by experiments where pretreatment of HUVECs with a GST-VEGF-A165 exon 7 fusion protein (GST-Ex7) reduced the translocation of P-selectin mediated by VEGF-A165 to the level mediated by VEGF-A121. Finally, pretreatment of HUVECs with SU1498 prior to stimulation with VEGF-A121 or VEGF-A165 reduced P-selectin translocation by 80% to 90% (Figure 4A). Therefore, in HUVECs, VEGFR-2 is favored over VEGFR-1 in mediating P-selectin translocation in response to VEGF-A isoforms, and the capacity to bind NRP-1 enhances effectiveness of VEGF-A165, as compared to VEGF-A121, to induce of P-selectin translocation.
Despite the fact that VEGF-A165 and VEGF-A121 activate both VEGFR-1 and VEGFR-2, in HUVECs, P-selectin translocation appears to be mediated predominantly by VEGFR-2. This is in agreement with previous reports suggesting that VEGFR-1 might act as a decoy receptor for VEGF-A165 and VEGF-A121.13,41 Alternatively, VEGF-A isoforms may activate VEGFR-1 differently, compared to other VEGFR-1 agonists, resulting in a different profile of effector activation.9,41-43 This hypothesis is in agreement with our results, because VEGFR-1 agonists VEGF-B and PlGF-1 increased cell surface P-selectin to about 30% that produced by VEGF-A165. Furthermore, Auterio and colleagues showed recently that VEGF-A165 induced a strong phosphorylation of VEGFR-1 tyrosine residue Tyr1213 and to a lesser extent Tyr1242 and Tyr1333, whereas PlGF induced the phosphorylation of Tyr1309 but not Tyr1213.44 Such differences in the activation of VEGFR-1 by various agonists, termed “agonist trafficking,” might explain the distinct biologic activities of VEGF-A165 and its analogs.
Contribution of endogenous PAF synthesis on P-selectin translocation
Stimulation of ECs with VEGF-A165, or other inflammatory mediators, leads to rapid and transient PAF synthesis. This newly formed PAF remains associated with ECs, 20% or less being exposed on the cell surface, whereas the major portion remains inside the cell.12-14,27-29 PAFR antagonists have revealed that endogenous PAF acts as a second messenger to promote a rapid and transient synthesis of prostacyclin (PGI2) and inducing c-fos and c-jun transcription.37-39,45 Consequently, we hypothesized that PAF, acting via cell membrane or intracellular PAFRs, might be involved in regulating P-selectin translocation.
VEGF analogs capable of activating VEGFR-2 doubled the cellular PAF content within 7.5 minutes (Table 1), whereas VEGFR-1–selective agonists (eg, PlGF-1, VEGF-B) had no effect on PAF synthesis. Pretreatment with selective intracellular, extracellular, and both intracellular and extracellular PAFR antagonists prior to stimulation with VEGF-A165 reduced P-selectin translocation by 39%, 65%, and 88%, respectively (Figure 5A). Together, our data demonstrate that VEGF-A165–induced P-selectin translocation is mediated by VEGFR-2 and may require the activation of both cell membrane and intracellular PAFRs. It is unlikely that the blockade of VEGF-A165–induced P-selectin translocation by PAFR antagonists was due to cytotoxic or nonspecific effects because HUVEC viability and VEGFR-1/VEGFR-2 phosphorylation were unaffected. Furthermore, these antagonists have no effect on VEGF-A165–induced cyclic guanosine monophosphate (cGMP) production.46 Interestingly, VEGFR-1–selective agonists PlGF-1 and VEGF-B induced P-selectin translocation independent from PAF synthesis.
VEGF-mediated neutrophil adhesion to HUVECs is P-selectin dependent
Previous reports have shown that endothelial P-selectin and PAF contribute to neutrophil adhesion in response to activation by inflammatory mediators.24-30 Although the pivotal role played by P-selectin has been established, it is less clear whether PAF is essential. Some reports suggest that PAF might act in concert with P-selectin, whereas others show that the blockade of P-selectin is sufficient to prevent neutrophil adhesion to ECs.47-50 Such discrepancies might be explained by the fact that newly synthesized PAF, as shown in our study, appears to direct the translocation of P-selectin. Consequently, PAF synthesis is essential for the induction of P-selectin translocation but not for neutrophil adhesion.
This study demonstrates that treatment of HUVECs with VEGF-A165 (10–9 M), which induced maximal P-selectin translocation and PAF synthesis, increased the adhesion of neutrophils to HUVECs by over 300%. A P-selectin antagonist, rPSGL-Ig, resulted in complete inhibition of VEGF-A165–induced neutrophil adhesion (Figure 6A). Hence, P-selectin translocation is essential for the adhesion of neutrophils to activated ECs, whereas the interaction between endothelial PAF and PAFRs expressed on neutrophils is not itself sufficient to support the adhesion of neutrophils to ECs. These data strengthen the hypothesis, at least under static conditions, that P-selectin itself is sufficient to promote neutrophil adhesion to activated ECs. However, we cannot rule out a role for PAF under conditions of shear stress in supporting the adhesion of neutrophils to activated ECs.
In conclusion, stimulation of VEGFRs led to endothelial P-selectin translocation. The major pathway involved the VEGFR-2/NRP-1 complex by VEGF-A165 and required PAF synthesis. Furthermore, PAF regulated P-selectin translocation via both autocrine and intracrine signaling. Interestingly, stimulation of VEGFR-1 either by PlGF-1 or VEGF-B led to P-selectin translocation that was PAF independent and of lesser magnitude than that included by VEGF-A165. Finally, the translocation of P-selectin to the surface of activated ECs was sufficient to support the adhesion of neutrophils, whereas PAF synthesis was essential for P-selectin translocation but not for neutrophil adhesion. Because the angiogenic properties of VEGF appear to be linked to increased vascular permeability, the increase in PAF synthesis or P-selectin translocation induced by VEGF may represent useful therapeutic targets in preventing or slowing the development of diseases involving pathologic angiogenesis.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-07-2272.
Supported by the Canadian Institutes of Health Research (CIHR) (scholarship to M.G.S.) grants from CIHR and Heart and Stroke Foundation of Québec.
S.R. and C.L. contributed equally.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Danielle Libersan for her technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal