Abstract
Human α2-antiplasmin (α2AP), also known as α2-plasmin inhibitor, is the major inhibitor of the proteolytic enzyme plasmin that digests fibrin. There are 2 N-terminal forms of α2AP that circulate in human plasma: a 464-residue protein with Met as the N-terminus, Met-α2AP, and a 452-residue version with Asn as the N-terminus, Asn-α2AP. We have discovered and purified a proteinase from human plasma that cleaves the Pro12-Asn13 bond of Met-α2AP to yield Asn-α2AP and have named it antiplasmin-cleaving enzyme (APCE). APCE is similar in primary structure and catalytic properties to membrane-bound fibroblast activation protein/seprase for which a physiologic substrate has not been clearly defined. We found that Asn-α2AP becomes cross-linked to fibrin by activated factor XIII approximately 13 times faster than native Met-α2AP during clot formation and that clot lysis rates are slowed in direct proportion to the ratio of Asn-α2AP to Met-α2AP in human plasma. We conclude that APCE cleaves Met-α2AP to the derivative Asn-α2AP, which is more efficiently incorporated into fibrin and consequently makes it strikingly resistant to plasmin digestion. APCE may represent a new target for pharmacologic inhibition, since less generation and incorporation of Asn-α2AP could result in a more rapid removal of fibrin by plasmin during atherogenesis, thrombosis, and inflammatory states.
Introduction
Human α2-antiplasmin (α2AP), also known as α2-plasmin inhibitor, is the main inhibitor of plasmin.1 Plasmin plays a critical role in fibrin proteolysis and tissue remodeling. The physiologic relevance of plasmin inhibition by α2AP to blood clotting and fibrinolytic homeostasis is supported by the following observations: (1) the rate of free plasmin inactivation by circulating α2AP is much faster than fibrin(ogen) digestion by plasmin,2 thereby eliminating the possibility of a systemic lytic state and consequent bleeding; (2) α2AP is cross-linked to forming fibrin by activated blood clotting factor XIII (FXIIIa) and inhibits plasmin-mediated lysis in direct proportion to the amount incorporated3-5 ; and (3) patients with homozygous α2AP deficiency manifest serious hemorrhagic tendencies, while heterozygotes tend to bleed only after major trauma or surgery.6 Human α2AP is synthesized primarily in the liver, and during circulation in plasma, the secreted precursive Met-α2AP form, a 464-residue protein having Met as the N-terminus, undergoes proteolytic cleavage between Pro12 and Asn13 to yield Asn-α2AP, a 452-residue version with Asn as the N-terminus.7 Met-α2AP accounts for approximately 30% of circulating α2AP, and Asn-α2AP, approximately 70%.7,8 While 3-fold more Asn-α2AP than recombinant Met-α2AP was shown to cross-link to fibrin,9 no data have been reported for native circulating Met-α2AP. Moreover, the effect of different ratios of the 2 α2AP forms on clot lysis has not been reported. Finally, the enzyme responsible for converting Met-α2AP to Asn-α2AP has not been identified, although it is presumed to be in plasma.7
We report here the purification and partial characterization of antiplasmin-cleaving enzyme (APCE), a serine proteinase from human plasma that cleaves the Pro12-Asn13 bond of Met-α2AP to yield Asn-α2AP, and the comparison of these 2 circulating forms of α2AP for the ability to cross-link with fibrin and to inhibit fibrinolysis.
Materials and methods
Isolation of α2AP
A mixture of Met-α2AP and Asn-α2AP was isolated using a modification of a published procedure.10 Briefly, human plasma was precipitated using polyethylene glycol 6000 (final concentration 5%), and the supernatant was applied to a lysine–Sepharose 4B (Amersham Biosciences, Piscataway, NJ) affinity column to remove plasminogen. The flow-through volume was applied to an affinity column consisting of plasminogen kringles 1 to 3 bound to Sepharose 4B. After washing with 40 mM sodium phosphate–0.5 M NaCl, pH 7.0, α2AP was eluted from this column using a 0- to 50-mM linear gradient of ϵ-aminocaproic acid in the same buffer. To separate Met-α2AP and Asn-α2AP, a goat antibody to the N-terminal 12–amino acid sequence unique to Met-α2AP was prepared, using as the immunogen a multiple antigenic peptide (MAP) that contained 8 copies of the N-terminal peptide linked via their C-termini to a core peptide of 7 lysines.11 The α2AP mixture was applied to an immunoaffinity column made by conjugating the purified goat antibody to Affigel-10 (Bio-Rad, Richmond, CA). After washing with 40 mM Tris (tris(hydroxymethyl)aminomethane) · HCl–0.5 M NaCl, pH 7.5, Met-α2AP was eluted with 0.1 M glycine, pH 2.5. Fractions were immediately neutralized by collection into tubes containing 1.0 M Tris · HCl, pH 9.0. Asn-α2AP eluted in the flow-through fractions from the immunoaffinity column. N-terminal amino acid sequence analyses confirmed that unbound and bound fractions were Asn-α2AP and Met-α2AP, respectively. FXIII was purified from fresh human plasma according to published methods.12
Analysis of α2AP cross-linking to fibrin
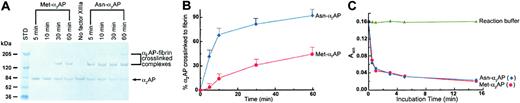
Factor XIIIa–catalyzed cross-linking reactions were performed, and free α2AP and α2AP-fibrin cross-linked complexes were detected by immunostaining with an antibody to α2AP as previously described.13 The rate of formation of α2AP-fibrin cross-linked complexes (Figure 1A-B) was determined by measuring the appearance of bands of α2AP-fibrin complexes at approximately 140 kDa and approximately 210 kDa and the concomitant disappearance of the 70-kDa α2AP band from gel scans using a Personal Densitometer SI (Molecular Dynamics, Piscataway, NJ) and image analysis software (ImageQuant, version 5.2; Molecular Dynamics, Piscataway, NJ). The percentage of α2AP-fibrin cross-linked complexes was expressed as [(densitometric values for the ∼ 140 kDa and ∼ 210 kDa bands)/(densitometric values for the ∼ 70 kDa, ∼ 140 kDa, and ∼ 210 kDa bands)] × 100 (Figure 1B).
Met-α2AP and Asn-α2AP cross-linking to fibrin and their plasmin-inhibitory activity. (A) Time-dependent cross-linking of Met-α2AP and Asn-α2AP to fibrin by FXIIIa catalysis. Solutions of purified human α2AP, fibrinogen, and FXIII were clotted with thrombin and CaCl2, and at the indicated times above each gel lane, clotting was terminated by solubilizing in urea-SDS-DTT. Non–cross-linked α2AP and α2AP-fibrin cross-linked complexes were detected by immunoblot analysis. (B) Densitometric analysis of the percent incorporation of α2AP into fibrin. Each data point is the mean ± SD of 3 experiments. (C) Plasmin-inhibitory activity of Met-α2AP and Asn-α2AP. In control sample, α2AP was replaced with reaction buffer. Each α2AP was incubated with an equimolar amount of plasmin for selected incubation periods, and then using a plasmin chromogenic substrate (S-2251) residual plasmin activity was assayed by a published method.10 Each data point is the average of 2 experiments.
Met-α2AP and Asn-α2AP cross-linking to fibrin and their plasmin-inhibitory activity. (A) Time-dependent cross-linking of Met-α2AP and Asn-α2AP to fibrin by FXIIIa catalysis. Solutions of purified human α2AP, fibrinogen, and FXIII were clotted with thrombin and CaCl2, and at the indicated times above each gel lane, clotting was terminated by solubilizing in urea-SDS-DTT. Non–cross-linked α2AP and α2AP-fibrin cross-linked complexes were detected by immunoblot analysis. (B) Densitometric analysis of the percent incorporation of α2AP into fibrin. Each data point is the mean ± SD of 3 experiments. (C) Plasmin-inhibitory activity of Met-α2AP and Asn-α2AP. In control sample, α2AP was replaced with reaction buffer. Each α2AP was incubated with an equimolar amount of plasmin for selected incubation periods, and then using a plasmin chromogenic substrate (S-2251) residual plasmin activity was assayed by a published method.10 Each data point is the average of 2 experiments.
Measurement of plasma clot lysis
After adding Met-α2AP, Asn-α2AP, or selected mixtures of the 2 forms (0.5 μM) to α2AP-depleted plasma (Enzyme Research, South Bend, IN), a mixture of 1 U/mL thrombin, 16 mM CaCl2, and 45 IU/mL urokinase (uPA) (Abbott, Chicago, IL) was added to catalyze almost instant fibrin clot formation and to initiate fibrinolysis. The rate of plasma clot lysis was determined by a turbidimetric microtiter plate method.14-16
Purification of antiplasmin-cleaving enzyme (APCE)
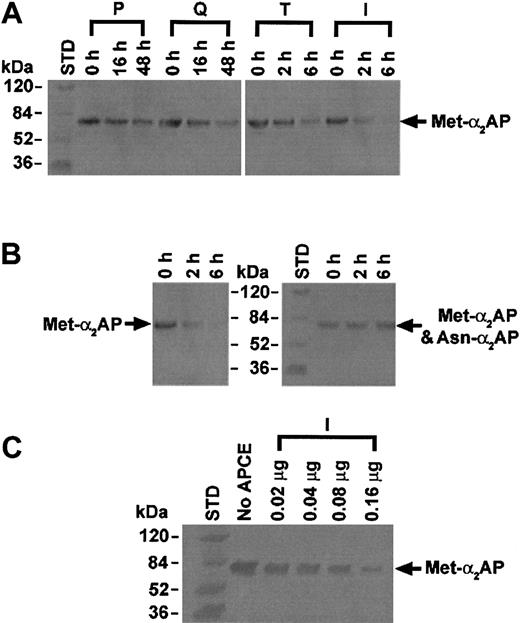
APCE was isolated from 2.0 L of human citrated plasma (purchased from Sylvan Goldman Blood Institute, Oklahoma City, OK) by sequential chromatographic steps that followed ammonium sulfate fractionation (15%-40%). The pellet was dissolved in 25 mM sodium phosphate–1 mM EDTA (ethylenediaminetetraacetic acid) buffer, pH 7.5, containing 10% ammonium sulfate and applied to a phenyl-Toyopearl hydrophobic interaction chromatography column (Tosohaas, Montgomeryville, PA). After washing with 10% ammonium sulfate–25 mM sodium phosphate–1 mM EDTA, pH 7.5, bound proteins were eluted by a decreasing ammonium sulfate gradient (linear, 5%-0%) in the same buffer. To monitor APCE activity in chromatographic fractions, hydrolysis of the fluorogenic substrate Z-Gly-Pro-AMC was measured at excitation/emission wavelengths of 360/460 nm. Active fractions were pooled and further purified by elution from a Q-Sepharose anion exchange column (Amersham Biosciences) using a 0- to 0.5-M NaCl linear gradient in the buffer described above. The activity peak was applied to a T-gel thiophilic column (Pierce, Rockford, IL) equilibrated in 0.5 M ammonium sulfate in the same phosphate buffer, and APCE activity was eluted by a decreasing ammonium sulfate gradient (linear, 0.5-0 M). N-terminal sequence of the dominant protein in the active fractions, as judged by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), was determined by Edman sequence analysis. Based upon the N-terminal sequence (IVLRPSRVHNSEENT) obtained, a multiple antigenic peptide (MAP) was prepared for immunizing a goat. The linear form of the peptide was used to make an affinity column from which the antibody was purified from goat serum. An immunoaffinity column made with this antibody was used to purify the cleaving activity of Z-Gly-Pro-AMC from the T-gel column. Following application and washing, the immunoaffinity column was eluted with 3 M sodium thiocyanate, pH 7.5. The active fractions were pooled, dialyzed against 25 mM sodium phosphate–1 mM EDTA–20% glycerol, pH 7.5, and stored at –80° C. On the basis of the Z-Gly-Pro-AMC assay, selected fractions were pooled from each chromatographic step, and each pooled sample was also assayed for Met-α2AP cleaving activity (Figure 4A). Met-α2AP (5 μg) and each chromatographic sample (54 μg phenyl-Toyopearl chromatography fraction, 9 μg Q-Sepharose chromatography fraction, 0.6 μg T-gel thiophilic chromatography fraction, or 0.2 μg immunoaffinity chromatography fraction) was incubated at 22° C. At selected times, an aliquot of each reaction mixture was subjected to SDS-PAGE and transferred to a nitrocellulose membrane. Met-α2AP was treated with antibody specific for its N-terminal sequence and then detected by the horseradish-peroxidase system. Met-α2AP and Asn-α2AP were detected by a monoclonal antibody (American Diagnostica, Greenwich, CT), which was reactive to the C-terminal region of either form of α2AP (Figure 4B, right panel). Also, Met-α2AP (5 μg) was incubated with selected amounts of APCE (0.02-0.16 μg) for 6 hours, and Western blot analysis was performed using Met-α2AP–specific antibody (Figure 4C).
Immunoblot analysis of Met-α2AP cleaving activity of APCE in pooled fractions from each chromatographic step. (A) APCE activity increased at each successive purification step. P, Q, T, or I represents APCE activity of each chromatographic pool of samples with Z-Gly-Pro-AMC cleaving activity. The total protein used from each chromatographic step was as follows: P, 54 μg; Q, 9 μg; T, 0.6 μg; or I, 0.2 μg. Met-α2AP cleavage was demonstrated by immunoblot analysis using an antibody specific for its N-terminal peptide. (B) Incubation of Met-α2AP with the APCE activity pool from immunoaffinity chromatography (0.2 μg) diminished in a time-dependent manner when assessed by an antibody specific for the N-terminal peptide of Met-α2AP (left panel), but did not diminish α2AP band intensity or generate bands less than 70-kDa when assessed by a monoclonal antibody to the C-terminal region of Met-α2AP or Asn-α2AP (right panel). (C) APCE (amount shown above each gel lane) concentration-dependent cleavage of Met-α2AP (5 μg) detected by an antibody specific for Met-α2AP.
Immunoblot analysis of Met-α2AP cleaving activity of APCE in pooled fractions from each chromatographic step. (A) APCE activity increased at each successive purification step. P, Q, T, or I represents APCE activity of each chromatographic pool of samples with Z-Gly-Pro-AMC cleaving activity. The total protein used from each chromatographic step was as follows: P, 54 μg; Q, 9 μg; T, 0.6 μg; or I, 0.2 μg. Met-α2AP cleavage was demonstrated by immunoblot analysis using an antibody specific for its N-terminal peptide. (B) Incubation of Met-α2AP with the APCE activity pool from immunoaffinity chromatography (0.2 μg) diminished in a time-dependent manner when assessed by an antibody specific for the N-terminal peptide of Met-α2AP (left panel), but did not diminish α2AP band intensity or generate bands less than 70-kDa when assessed by a monoclonal antibody to the C-terminal region of Met-α2AP or Asn-α2AP (right panel). (C) APCE (amount shown above each gel lane) concentration-dependent cleavage of Met-α2AP (5 μg) detected by an antibody specific for Met-α2AP.
Specificity of APCE activity in plasma
To assess APCE activity in human plasma and its recovery in each purification step, we designed and synthesized a fluorescence resonance energy transfer (FRET) peptide substrate that contained the APCE-sensitive Pro12-Asn13 bond within the Thr9-Gln16 sequence of Met-α2AP as follows: Arg-Lys(DABCYL)-Thr-Ser-Gly-Pro-Asn-Gln-Glu-Gln-Glu(EDANS)-Arg. Hydrolysis of the Pro-Asn bond separates the fluorophore, EDANS (5-[(2-aminoethyl)amino]-naphthalene-1-sulfonic acid), from the quenching group, DABCYL (4-(4-dimethylaminophenylazo)benzoyl), to give an increase in fluorescence. APCE cleavage of the Pro-Asn bond in the FRET peptide substrate was confirmed by analysis of products by liquid chromatography–mass spectrometry (LC/MS; data not shown). APCE was selectively removed from normal human plasma by an antibody specific for the N-terminal region of APCE (see above for purified goat antibody as made for APCE purification). A nonspecific goat antibody was used as a control. Each antibody was coupled to POROS EP20 beads (Applied Biosystems, Framingham, MA), and the coupled beads were incubated separately with plasma diluted 1:5 with 25 mM sodium phosphate–1 mM EDTA, pH 7.5, on a rocker for 15 hours at 4° C. Any APCE activity remaining in the supernatant, and therefore not bound to the antibody-POROS beads, was measured as a function of its ability to cleave the APCE-specific FRET peptide (0.1 mM, 10 μL), which was added to 200 mL of the supernatant and incubated at 22° C. Fluorescence was monitored with time at excitation and emission wavelengths of 360 and 460 nm, using a BIO-TEK FL600 fluorescence plate reader (Winooski, VT).
Results and discussion
FXIIIa-catalyzed cross-linking of Met-α2AP or Asn-α2AP to fibrin
We determined the N-terminal sequence of purified α2AP from 52 normal plasma samples and found that Met-α2AP is present as 33% ± 10% (mean ± SD) of the total α2AP, which is in accord with previous values from 4 pools of human plasma.8 We isolated native forms of both Met-α2AP and Asn-α2AP from human plasma and studied these instead of recombinant forms.9 To compare cross-linking rates of Met-α2AP versus Asn-α2AP to fibrin, mixtures of fibrinogen, Ca2+, FXIII, and Met-α2AP or Asn-α2AP were clotted by thrombin and allowed to cross-link for selected times; then solubilized in urea–sodium dodecyl sulfate (SDS)–dithiothreitol (DTT); subjected to polyacrylamide gel electrophoresis (PAGE); analyzed by Western blots; and quantified by densitometry. The content of α2AP immunoreactivity in high-molecular-weight cross-linked complexes of α2AP-fibrin increased with time, and simultaneously immunoreactivity decreased in the lower molecular-weight α2AP band (Figure 1A). Using essentially physiologic concentrations, we found that the extent of purified native Asn-α2AP cross-linking to fibrin was approximately 13 times greater than purified native Met-α2AP during the initial 10 minutes as fibrin gel formation achieved completion (Figure 1B). Our finding that native Asn-α2AP is far more rapidly incorporated into fibrin than its native precursive form, Met-α2AP, clarifies and extends a prior observation that recombinant Met-α2AP cross-linked to fibrin at one-third of the amount of purified plasma Asn-α2AP.9 Holmes et al17 reported a similar finding in that recombinant Asn-α2AP having an extension of 3 additional N-terminal amino acids could no longer be cross-linked to fibrin. Our results can be explained by comparing the 2 purified plasma forms, rather than plasma α2AP with recombinant Met-α2AP as in the previous report. Recombinant Met-α2AP has been shown to have a bit slower initial rate of free plasmin inhibition than plasma α2AP,9 raising the question of whether slight structural differences might exist between circulating α2AP and recombinant Met-α2AP that could also impact cross-linking. We could detect no differences between the 2 purified circulating forms in this regard (Figure 1C). Important to note is that the purified plasma α2AP used by Sumi et al9 was not characterized for content of the 2 α2AP forms, and no separation method was described. Hence, if their plasma α2AP preparation were in fact a mixture of Met-α2AP and Asn-α2AP, this could account for the much narrower difference between the 2 forms for becoming cross-linked to fibrin than we report here.9 Finally, the molar ratio of fibrinogen to α2AP used by Sumi et al9 was around 70:1, which is significantly greater than the physiologic ratio of 7:1 used in our work, and for that matter, even higher than ratios in pathologic settings where fibrinogen concentration is markedly elevated.
Effect of Met-α2AP or Asn-α2AP on plasma clot lysis
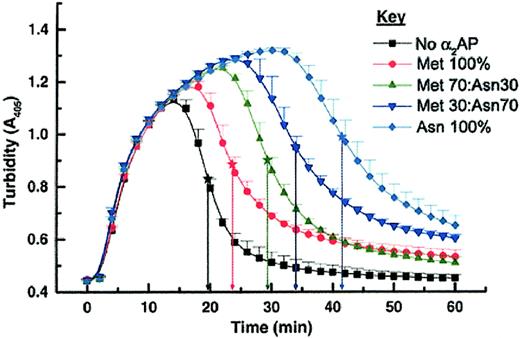
It has not been established whether Asn-α2AP protects fibrin from fibrinolysis more effectively than Met-α2AP. Therefore we compared the 2 forms by urokinase (uPA)–induced plasma clot lysis. After addition of selected ratios of purified Met-α2AP and/or Asn-α2AP to give a total α2AP concentration of 0.50 μM in α2AP-depleted plasma, a mixture of thrombin, Ca2+, and uPA was added to catalyze cross-linked fibrin formation and to initiate fibrinolysis. As shown in Figure 2, turbidity was monitored at 405-nm wavelength, and plasma clot lysis times (PCLTs) were defined as the midpoint between the highest and lowest absorbances. A control clot prepared from α2AP-depleted plasma was rapidly digested by plasmin (PCLT = 20 minutes). When only Met-α2AP was added, clot lysis was slightly prolonged (PCLT = 23 minutes). As the proportion of Asn-α2AP increased, clot lysis was progressively delayed. When 100% Asn-α2AP was added, clot lysis was maximally delayed (PCLT = 42 minutes), becoming about 2-fold longer than when only Met-α2AP was present. These results indicated a dose-response effect for ratios of the 2 forms of circulating α2AP on fibrinolysis. It should be noted that normal human plasma that had been depleted of α2AP using immobilized polyclonal antibodies to α2AP was used in these studies, thereby negating any competitive impact that C-terminal shortened, non–plasminogen-binding forms of α2AP may exhibit for becoming cross-linked to fibrin.18,19 The Met-α2AP and Asn-α2AP added to α2AP-depleted plasma were purified using their C-terminal affinities for immobilized plasminogen kringles 1-3; hence, C-terminal degraded forms of α2AP would not bind. These studies demonstrate clearly that Asn-α2AP inhibits plasmin-mediated fibrin digestion more effectively than Met-α2AP.
Plasma clot lysis times (PCLTs) determined with different Met-α2AP/Asn-α2AP ratios. Each data point was determined in quadruplicate (mean ± SD). PCLTs were defined as midpoint times (★) between the peak of clot formation (highest absorbance) and the maximal fibrinolysis (lowest absorbance).
Plasma clot lysis times (PCLTs) determined with different Met-α2AP/Asn-α2AP ratios. Each data point was determined in quadruplicate (mean ± SD). PCLTs were defined as midpoint times (★) between the peak of clot formation (highest absorbance) and the maximal fibrinolysis (lowest absorbance).
Purification and identification of proteinase that converts Met-α2AP to Asn-α2AP
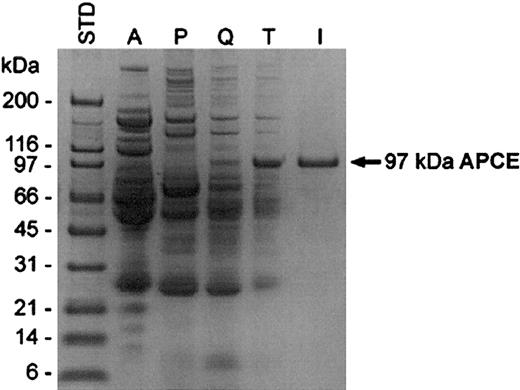
Given the greater efficiency of Asn-α2AP incorporation into fibrin, we reasoned that the unidentified plasma proteinase responsible for converting Met-α2AP to Asn-α2AP might be important in modulating the susceptibility of fibrin to plasmin; we therefore attempted to isolate the proteinase. The proteinase was purified from human plasma using an initial ammonium sulfate fractionation (A), followed by phenyl-Toyopearl hydrophobic interaction (P), Q-Sepharose anion exchange (Q), T-gel thiophilic (T), and immunoaffinity (I) chromatographies. Through the successive purification steps prior to the immunoaffinity chromatography, the enzyme activity increased concomitantly with the increase of the dominant 97-kDa protein band as detected by SDS-PAGE (Figure 3). This band was subjected to protein sequencing following transfer to a polyvinylidene difluoride membrane, and the identification of the first 15 amino acids (IVLRPSRVHNSEENT) allowed the synthesis of an identical peptide, which was used as an immunogen to prepare a goat antibody. After purification, the antibody was linked to a gel support for immunoaffinity chromatography (I). Throughout the purification processes, proteolytic activity was monitored by hydrolysis of either Z-Gly-Pro-AMC20 or the FRET peptide. Active fractions in each purification step were pooled and analyzed by SDS-PAGE (Figure 3). Cleavage of Met-α2AP to Asn-α2AP was assessed by immunoblot analyses; the rate of Met-α2AP conversion by the proteinase clearly increased as its purification progressed (Figure 4A). We termed this proteinase “antiplasmin-cleaving enzyme” (APCE). Table 1 summarizes a typical purification of APCE from human plasma.
Purification of APCE. Coomassie blue–stained gel analysis of the activity pool from each purification step. Proteins were analyzed on a 10% to 20% gradient SDS-PAGE under reducing conditions. STD indicates molecular-weight standards; A, ammonium sulfate fraction (15%-40%); P, phenyl-Toyopearl chromatography fraction; Q, Q-Sepharose chromatography fraction; T, T-gel thiophilic chromatography fraction; and I, immunoaffinity chromatography fraction.
Purification of APCE. Coomassie blue–stained gel analysis of the activity pool from each purification step. Proteins were analyzed on a 10% to 20% gradient SDS-PAGE under reducing conditions. STD indicates molecular-weight standards; A, ammonium sulfate fraction (15%-40%); P, phenyl-Toyopearl chromatography fraction; Q, Q-Sepharose chromatography fraction; T, T-gel thiophilic chromatography fraction; and I, immunoaffinity chromatography fraction.
Purification of APCE from human plasma
Purification step . | Total activity,*FI × 10-3 . | Total protein, mg . | Specific activity, FI × 10-3/mg . | Purification, fold . | Yield, % . |
|---|---|---|---|---|---|
| Plasma, 2 L | 58 950 | 185 650 | 0.3 | 1 | 100.0 |
| Ammonium sulfate | 41 520 | 48 840 | 0.9 | 3 | 70.4 |
| Phenyl-Toyopearl | 27 964 | 320 | 87.4 | 291 | 47.4 |
| Q-Sepharose anion | 17 687 | 32 | 552.7 | 1 842 | 30.0 |
| T-gel thiophilic | 8 576 | 0.45 | 19 057.8 | 63 526 | 14.5 |
| Immunoaffinity | 4 738 | 0.09 | 52 644.4 | 175 481 | 8.0 |
Purification step . | Total activity,*FI × 10-3 . | Total protein, mg . | Specific activity, FI × 10-3/mg . | Purification, fold . | Yield, % . |
|---|---|---|---|---|---|
| Plasma, 2 L | 58 950 | 185 650 | 0.3 | 1 | 100.0 |
| Ammonium sulfate | 41 520 | 48 840 | 0.9 | 3 | 70.4 |
| Phenyl-Toyopearl | 27 964 | 320 | 87.4 | 291 | 47.4 |
| Q-Sepharose anion | 17 687 | 32 | 552.7 | 1 842 | 30.0 |
| T-gel thiophilic | 8 576 | 0.45 | 19 057.8 | 63 526 | 14.5 |
| Immunoaffinity | 4 738 | 0.09 | 52 644.4 | 175 481 | 8.0 |
Total activity is defined as total fluorescence intensity (FI) produced by APCE-catalyzed FRET peptide cleavage during 2-hour incubation at 22°C.
Recently it was reported that phenyl hydrophobic interaction chromatography could be used to separate 2 distinct Z-Gly-Pro-AMC–hydrolyzing activities from bovine serum: one bound to the phenyl column, while the other did not.21 We obtained a similar result when ammonium sulfate–fractionated human plasma was subjected to phenyl-column chromatography, with each fraction being assayed using Z-Gly-Pro-AMC. Importantly, however, only the phenyl-bound fraction possessed proteinase activity that cleaved the FRET peptide substrate and Met-α2AP (Figure 4A, P). Table 1 shows a dramatic increase in purification with a high yield of activity for this phenyl-Toyopearl step. To determine APCE activity in human plasma, commercially available Z-Gly-Pro-AMC cannot be used, since more than 1 Pro-Xaa cleaving enzyme is present in human plasma. Therefore, taking advantage of the FRET peptide as a specific substrate, total APCE activity was assessed in 3 different pooled normal plasma samples, using the total activity in each plasma sample and specific activity of purified APCE from each to estimate the concentration of APCE in each plasma sample as 560, 561, and 493 μg/L.
When Met-α2AP was incubated with APCE activity from the final immunoaffinity step (Figure 4B), it was completely and specifically cleaved at the Pro12-Asn13 bond as shown by the following: (1) The 70-kDa Met-α2AP band, which was easily demonstrable at 0 time by antibody specific for Met-α2AP, had completely disappeared by 6 hours (Figure 4B, left panel). When immunostained with an antibody to both α2AP forms, however, the intensities of the 70-kDa band at 0 hours and 6 hours did not change (Figure 4B, right panel), but the Met-α2AP band disappeared in direct proportion to the concentration of APCE when analyzed with an antibody that detected only Met-α2AP (Figure 4C). (2) N-terminal analyses of the 0-hour sample (Figure 4B) showed only the Met-α2AP sequence, MEPLGRQLTSGP, while the 6-hour sample had only the Asn-α2AP N-terminal sequence, NQEQVSPLTLLK. The proteinase isolated by the last purification step showed a single 97-kDa band (Figure 3, I), with an N-terminal sequence identical to that of the major protein band in sample T. Interestingly, the sequence matched that deduced from the cDNA sequence for Ile24-Thr38 of fibroblast activation protein (FAP),22 a proline-specific serine proteinase also termed seprase by another group.23
To determine additional sequence information from the protein, the 97-kDa protein band from the SDS-PAGE gel was reduced, alkylated, and digested with trypsin.24 The tryptic digest was subjected to liquid chromatography–mass spectrometry–mass spectrometry (LC/MS/MS) to obtain molecular weights and MS/MS fragment ion spectra and to use the MASCOT MS/MS ion search engine to query the OWL nonidentical protein database (http://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=MIS). From our search results, 5 peptides had sequences identical with FAP/seprase22,23 : residues 210-219 (YALWWSPNGK); residues 247-254 (TINIPYPK); residues 487-499 (ILEENKELENALK); residues 500-509 (NIQLPKEEIK); and residues 522-530 (MILPPQFDR). This degree of homology suggested that the 97-kDa APCE proteinase had similarity to FAP/seprase; however, significant differences remained. FAP/seprase is reported to be expressed on the cell surface of reactive stromal fibroblasts of healing wounds and epithelial cancers, but not on normal tissue or benign epithelial tumors.25,26 It is believed to be particularly associated with an invasive phenotype of human melanoma and carcinoma cells.23 Despite the plausibility that FAP/seprase may have a role in tissue remodeling, a physiologic substrate has not been demonstrated for the native enzyme, and hence a clearly defined biologic function remains obscure. Although gelatin and collagen I appeared to serve as substrates for recombinant FAP expressed on a human embryonic kidney epithelial cell line (293 cells), products of fragmentation or actual cleavage sites have not been identified.27 Importantly, a previous report indicated that neither soluble FAP nor its cleavage products exist in the circulation.28 The APCE we describe here was isolated from normal human plasma.
The possibility that human plasma APCE could be proteolytically derived from cell membrane–bound FAP/seprase is raised by the following observations: (1) The N-terminal 15 amino acids of APCE are identical to residues 24 to 38 of FAP/seprase, the latter estimated to have its first 6 N-terminal amino acids in the fibroblast cytoplasm, with another predicted 20-residue transmembrane domain, and then a 734-residue extracellular C-terminal catalytic domain.22,23 (2) Internal sequences of APCE obtained from 5 tryptic peptides are identical to those deduced from the cDNA sequence of a fairly long segment of FAP/seprase (amino acids 210 through 530). (3) Nondenaturing gel filtration chromatography of APCE indicates a native mass of approximately 180 kDa, and, whether or not reduced, its denatured molecular weight by SDS-PAGE is 97 kDa (Figure 3, I). Both values are consistent with the reported dimeric structure of 190 kDa for FAP/seprase.27 (4) APCE is inhibited by diisopropyl fluorophosphate (DFP), phenylmethyl-sulfonyl fluoride (PMSF), and AEBSF (4-(2-aminoethyl)-benzene-sulfonyl fluoride), but not by iodoacetate, E-64, or EDTA, just as reported for FAP/seprase.27 (5) Analogously, a closely related type II transmembrane serine proteinase with prolyl specificity apparently undergoes proteolytic cleavage of its N-terminal 38-residue cytoplasmic/transmembrane domain to give rise to the circulating human dipeptidyl peptidase IV (DPP IV, aka T-cell activation CD26, with 50% homology to FAP/seprase)29 ; however, DPP IV is strictly a dipeptidyl aminopeptidase,20 and we found that it does not cleave Pro12-Asn13 of Met-α2AP as does APCE. If APCE indeed results from proteolysis of the transmembrane domain of FAP/seprase, a proteinase that might cleave the Cys23-Ile24 bond has yet to be identified. The hydrophobic residue Ile24 as the P1′ position of the bond could make this a preferred cleavage site for an extracellular enzyme such as a matrix metalloproteinase.30
Alternatively, APCE may be a product of intracellular processing. Conceivably, 2 pathways could exist in the same cell: one that normally synthesizes, processes, and secretes APCE without a transmembrane segment; and another that produces a membrane-bound enzyme (FAP/seprase) when fibroblasts become activated and proliferate in inflammatory states, embryogenesis, or metastases of certain epithelial-derived malignancies.25,26 Finally, the possibility remains that APCE is a product of a different gene or that it results from alternative splicing. Our data indicate that APCE is produced under normal physiologic states, and unlike the case of FAP/seprase, pathologic disorders are not required to stimulate gene expression. The fact that APCE is found in normal plasma and cleaves Met-α2AP suggests that one of its physiologic functions is the regulation of Asn-α2AP availability for plasmin inhibition within cross-linked fibrin.
To determine if whole-plasma APCE is specifically responsible for cleaving the Met-α2AP sequence that generates Asn-α2AP, antibody to the N-terminal 15 residues of APCE was coupled to beads and compared with a control antibody for the ability to remove significant amounts of APCE activity from human plasma as described in “Materials and methods.” Control antibody did not remove FRET peptide cleaving activity, whereas antibody specific for APCE removed approximately 50%. Since the FRET peptide substrate is specific for APCE activity, the remaining FRET peptide cleaving activity in this experiment may be due to N-terminally degraded forms of APCE that react poorly or not at all with the antibody, particularly given that only the linear epitopes among the first 15 residues of APCE would be recognized. Although it must still be considered that human plasma may contain other proteinases that cleave the N-terminal 12-residue peptide of Met-α2AP, our data indicate that this is unlikely because (1) only the 15% to 40% ammonium sulfate fraction was found to contain Met-α2AP cleaving activity; (2) when ammonium sulfate–fractionated human plasma was subjected to phenyl-column chromatography, phenyl-bound and unbound cleaving activities toward Z-Gly-Pro-AMC were indeed observed, but only the bound activity cleaved the FRET peptide and Met-α2AP; (3) only one peak of proteolytic activity for the FRET peptide and Met-α2AP was observed in elution profiles from Q-Sepharose and T-gel chromatographies (Figure 4A); and (4) on reduced SDS-PAGE, the peak from the immunoaffinity chromatography contained only one band and it had a single N-terminal sequence (Figure 3, I).
We conclude that human Met-α2AP is a physiologically important substrate of APCE, since proteolytic cleavage between Pro12-Asn13 yields the derivative Asn-α2AP, which becomes cross-linked significantly faster to fibrin by FXIIIa, and, as a consequence, enhances the resistance of fibrin to digestion by plasmin. Since human APCE augments the inhibition of fibrinolysis by increasing the availability of the faster cross-linking form (ie, Asn-α2AP), potent and selective inhibitors of APCE might allow titration of Asn-α2AP production to lower in vivo levels, and thereby enhance both endogenous and exogenous fibrin removal. Bleeding complications would be unlikely, based on the observation that persons heterozygous for α2AP deficiency have minimal hemorrhagic risk.6 Hence, a window of safety may exist for lowering α2AP function in healthy persons. Particularly in clinical situations where fibrin formation is likely, the development of an agent that inhibits APCE might result in decreased amount of Asn-α2AP available for cross-linking to fibrin as thrombi develop or inflammation progresses. Then endogenous levels of generated plasmin, or plasmin produced by administering small amounts of a plasminogen activator, might be sufficient to effect fibrin removal so that vessel patency and organ function are maintained and bleeding risk is minimized. We believe that this hitherto unknown circulating enzyme, APCE, may provide a valuable target for pharmacologic modulation of the fibrinolytic system.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-12-4240.
Supported by the William K. Warren Medical Research Center, the Oklahoma Center for the Advancement of Science and Technology, and National Institutes of Health (NIH) grant HL072995.
Presented in part as an abstract to the 19th annual meeting of the International Society on Thrombosis and Haemostasis, Birmingham, United Kingdom, July 12-18, 2003.31
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. S. Lee and J. G. Chun for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal