Abstract
Patients with relapsed or primary refractory diffuse large B-cell lymphoma (DLBCL) who achieve complete response (CR) before autologous stem cell transplantation (ASCT) generally have better outcomes than those who achieve only partial response (PR). We investigated whether adding rituximab to the ifosfamide-carboplatin-etoposide (ICE) chemotherapy regimen (RICE) could increase the CR rate of patients with DLBCL under consideration for ASCT. Thirty-six eligible patients were treated with RICE, and 34 received all 3 planned cycles. The CR rate was 53%, significantly better than the 27% CR rate (P = .01) achieved among 147 similar consecutive historical control patients with DLBCL treated with ICE; the PR rate was 25%. Febrile neutropenia was the most frequent grade 3 or 4 nonhematologic toxicity; it occurred in 7.5% of delivered cycles. No patient had RICE-related toxicity that precluded ASCT. The median number of CD34+ cells per kilogram mobilized was 6.3 × 106. Progression-free survival rates of patients who underwent transplantation after RICE were marginally better than those of 95 consecutive historical control patients who underwent transplantation after ICE (54% vs 43% at 2 years; P = .25). RICE appears to induce very high CR rates in patients with relapsed and refractory DLBCL; however, further studies are necessary to determine whether this treatment regimen will improve outcomes after ASCT.
Introduction
Approximately 40% to 60% of patients with aggressive non-Hodgkin lymphoma (NHL) treated with standard anthracycline-based regimens either fail to achieve complete response (CR) or have relapses after attaining CR.1,2 High-dose chemotherapy with autologous stem cell transplantation (ASCT) can be curative in a proportion of patients with relapsed or primary refractory disease provided that CR or partial response (PR) can be induced with second-line chemotherapy.3-6
Among patients with chemosensitive disease, the remission status at transplantation appears to have a significant impact on outcome, because patients who undergo transplantation in CR have better long-term progression-free survival (PFS) than patients who undergo transplantation in PR.7,8 This observation may suggest that the response to second-line chemotherapy is a reflection of the underlying chemosensitivity of a lymphoma, and that a lymphoma induced into CR by second-line chemotherapy inherently is more likely to be eradicated by high-dose therapy than is a lymphoma that is induced into PR by second-line chemotherapy. Alternatively, among chemosensitive patients, the efficacy of high-dose therapy may be dependent on tumor burden, such that potentiating the response to existing second-line regimens may improve the outcome of high-dose therapy.
The ifosfamide-carboplatin-etoposide (ICE) regimen is an effective, dose-intense, short-course cytoreductive regimen capable of mobilizing peripheral blood progenitor cells (PBPCs) with minimal extramedullary toxicity.7 The overall response rate to ICE in patients with relapsed or primary refractory diffuse large B-cell lymphoma (DLBCL) is approximately 70%, with a CR rate of 25% to 30%.7,9 Disease status—that is, whether the patient has relapsed or primary refractory disease—and the second-line age-adjusted international prognostic index (sAAIPI)9,10 are the primary determinants of the response to ICE.
Rituximab is a chimeric anti-CD20 immunoglobulin G1 κ (IgG1κ) monoclonal antibody that contains murine variable regions and human constant regions.11 Although it has received United States Food and Drug Administration (FDA) approval for the treatment of low-grade lymphoma, rituximab has single-agent activity in DLBCL, with an overall response rate of approximately 30% to 35% in pretreated patients.12,13 Recent studies suggest that adding rituximab to cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) significantly improves the CR rate and survival in patients with untreated DLBCL.14,15
We hypothesized that minimizing a patient's disease burden before transplantation by enhancing sensitivity to ICE would improve the outcome ofASCT. Therefore, we investigated whether adding rituximab to ICE (RICE) could increase the CR rate of transplant-eligible patients with relapsed or primary refractory DLBCL.
Patients and methods
Patients
Patients aged 18 to 72 years who had DLBCL (according to the World Health Organization classification16 ) that relapsed after, or was refractory to, a single standard anthracycline-based regimen were eligible. Before enrollment, all patients were required to have confirmation of active CD20+ DLBCL by biopsy or fine-needle aspiration of an involved site. All biopsy specimens were reviewed by 1 of 2 hematopathologists (D.F., J.T.F.). All patients underwent pretreatment staging studies that included computed tomography (CT) of the chest, abdomen, and pelvis; nuclear imaging with gallium scans, fluorine-18-fluorodeoxyglucose positron emission tomography (PET), or both; and unilateral bone marrow biopsies. Patients were required to have normal cardiac, renal, and hepatic functions. Patients were ineligible if they had lymphoma of any histology other than DLBCL (although a history of indolent lymphoma or Hodgkin disease was permitted); central nervous system involvement; previous treatment with carboplatin, cisplatin, or etoposide; positive serologic test findings for HIV; active hepatitis B; previous cancer for which the disease-free duration was less than 5 years, excluding basal cell carcinoma, cutaneous squamous cell carcinoma, or carcinoma in situ of the cervix, for which they received definitive treatment; or any other illness that, in the opinion of the treating investigator, would preclude the safe administration of rituximab or ICE. Our institutional review board approved this study, and all patients gave written, informed consent before enrollment.
The sAAIPI
The sAAIPI is determined before the initiation of second-line therapy and is composed of 3 risk factors: Karnofsky performance status less than 80%, lactate dehydrogenase (LDH) level greater than normal, and stage III or IV disease.9,10 Patients with 0, 1, 2, or 3 risk factors are considered to have low, low-intermediate, high-intermediate, or high-risk disease, respectively.
Treatment
The planned treatment consisted of 3 cycles of RICE. Rituximab (375 mg/m2) was administered on an outpatient basis on day 1 of each cycle and 48 hours before the initiation of the first cycle. Patients completing all 3 cycles received 4 doses of rituximab. After the administration of oral acetaminophen (650 mg) and intravenous diphenhydramine (50 mg), rituximab was infused according to standard prescribing guidelines. ICE chemotherapy was administered on an inpatient basis beginning on day 3 of each cycle, as previously described.7 Briefly, a 12-hour urine sample was obtained on admission for measurement of the creatinine clearance (Clcr). Etoposide (100 mg/m2) was administered as an intravenous bolus daily for 3 days, from days 3 to 5. Carboplatin (area under the curve [AUC], 5; dose = 5 × [25 + Clcr]), capped at 800 mg, was administered as a bolus infusion on day 4. Ifosfamide (5000 mg/m2), mixed with an equal amount of mesna, was administered as a continuous intravenous infusion over 24 hours beginning on day 4. Beginning on day 7, granulocyte–colonystimulating factor (G-CSF) was administered subcutaneously at 5 μg/kg each day for 8 days (days 7-14), after the first 2 cycles of RICE, and at 10 μg/kg per day after the third cycle, until the end of leukapheresis. It was intended that the cycles would be administered at 2-week intervals such that the second and third cycles of RICE would begin on day 15 of the previous cycle. Cycles were delayed if the absolute neutrophil count was less than 1 × 109/L (1000 neutrophils/μL) or if the platelet count was less than 50 × 109/L (50 000 platelets/μL). Patients were not treated with prophylactic antimicrobials.
PBPC collection
After the third cycle of RICE, leukapheresis was initiated once the white blood cell count recovered from the nadir to more than 5 × 109/L (5000 cells/μL). Leukapheresis was performed daily until either more than 6 × 106 CD34+ cells/kg had been collected or 5 apheresis procedures were performed, whichever occurred first. Patients in whom fewer than 2 × 106 CD34+ cells/kg were collected were considered to have experienced mobilization failure.
Assessment of response and toxicity
Response to RICE was assessed by CT of the chest, abdomen, and pelvis and gallium imaging or PET, or both, approximately 10 to 14 days after the third cycle of RICE. Bone marrow biopsies were repeated only if samples were abnormal before treatment. Response to RICE was assessed using the International Working Group criteria,17 taking into consideration the results of nuclear imaging studies: CR required that there be no evidence of disease by nuclear imaging. Toxicity was graded according to the National Cancer Institute common toxicity criteria, version 2.0.
ASCT
Only those patients who achieved CR or PR after 3 cycles of RICE were considered candidates for ASCT, though ASCT was not a component of the study evaluating the RICE regimen. Patients undergoing ASCT were required to have a cardiac ejection fraction greater than 50% (assessed before treatment with RICE), pulmonary diffusing capacity greater than 50% of predicted, and normal renal function. The choice of conditioning regimen depended on the patient's age, the extent of previous therapy, and the clinical trials active at the time of transplantation. All patients received G-CSF (5 μg/kg subcutaneously) twice daily beginning the day after stem cell infusion until the absolute neutrophil count (ANC) exceed 0.5 × 109/L (500 neutrophils/μL) for 3 consecutive days or 5 × 109/L (5000 neutrophils/μL) on a single day, whichever occurred earlier. No patient received post-ASCT “adjuvant” therapy.
Statistics
The goal of this study was to test the hypothesis that adding rituximab to ICE would improve the CR rate from 25% to 45%. To detect this difference with 80% power at the .05 level of significance required that 36 patients be treated. Categorical variables were compared using the 2-sided Fisher exact test.18 PFS and overall survival (OS) for patients who underwent transplantation were assessed from the day of stem cell infusion. Survival curves were generated using the method of Kaplan and Meier19 and were compared using the log-rank test.20 In assessing PFS, patients who died without evidence of disease were censored at the time of death.
Results
Thirty-seven patients were enrolled in this study, but one was removed after receiving 1 cycle of RICE when a review of her biopsy demonstrated indolent lymphoma; this patient was considered evaluable for toxicity but not for response. Patients' characteristics are outlined in Table 1. Sixteen patients had been treated with NHL-15, a dose-intense regimen consisting of biweekly administration of doxorubicin and vincristine for 4 cycles followed by high-dose cyclophosphamide for 3 cycles.21 Two patients initially presented with Hodgkin disease and were treated with ABVD; both had relapses with biopsy-proven DLBCL. Although previous treatment with rituximab was not an exclusion criterion, no patient had received rituximab before enrollment. Approximately half the patients had high-intermediate or high-risk disease as assessed by the sAAIPI. Patients treated with RICE were similar to a group of 147 consecutive historical patients with DLBCL treated with ICE alone, except that a greater proportion of patients treated with RICE were older than 60 years (29% vs 14%).
Patient characteristics
. | No. patients . | . | . | |
|---|---|---|---|---|
| Parameter . | RICE (n = 36) . | ICE historical controls (n = 147) . | P . | |
| Age, y | .10 | |||
| 60 and younger | 28 | 129 | ||
| Older than 60 | 8 | 18 | ||
| Median (range) | 45 (23-72) | 48 (18-68) | ||
| Previous chemotherapy | ||||
| CHOP | 16 | 65 | ||
| CHOP-like | 2 | 31 | ||
| NHL-1521 | 16 | 50 | ||
| ABVD | 2 | 0 | ||
| Stanford-V | 0 | 1 | ||
| Disease status | .35 | |||
| Relapsed | 23 | 80 | ||
| Primary refractory | 13 | 67 | ||
| Karnofsky performance status | .23 | |||
| 80 or greater | 28 | 97 | ||
| Less than 80 | 8 | 50 | ||
| LDH level | .45 | |||
| Elevated | 18 | 61 | ||
| Normal | 18 | 86 | ||
| Stage at protocol entry | .38 | |||
| I or II | 10 | 31 | ||
| III or IV | 26 | 116 | ||
| Second-line age-adjusted IPI | .19 | |||
| Low/low-intermediate | 19 | 59 | ||
| High-intermediate/high | 17 | 88 | ||
. | No. patients . | . | . | |
|---|---|---|---|---|
| Parameter . | RICE (n = 36) . | ICE historical controls (n = 147) . | P . | |
| Age, y | .10 | |||
| 60 and younger | 28 | 129 | ||
| Older than 60 | 8 | 18 | ||
| Median (range) | 45 (23-72) | 48 (18-68) | ||
| Previous chemotherapy | ||||
| CHOP | 16 | 65 | ||
| CHOP-like | 2 | 31 | ||
| NHL-1521 | 16 | 50 | ||
| ABVD | 2 | 0 | ||
| Stanford-V | 0 | 1 | ||
| Disease status | .35 | |||
| Relapsed | 23 | 80 | ||
| Primary refractory | 13 | 67 | ||
| Karnofsky performance status | .23 | |||
| 80 or greater | 28 | 97 | ||
| Less than 80 | 8 | 50 | ||
| LDH level | .45 | |||
| Elevated | 18 | 61 | ||
| Normal | 18 | 86 | ||
| Stage at protocol entry | .38 | |||
| I or II | 10 | 31 | ||
| III or IV | 26 | 116 | ||
| Second-line age-adjusted IPI | .19 | |||
| Low/low-intermediate | 19 | 59 | ||
| High-intermediate/high | 17 | 88 | ||
Thirty-four patients completed all 3 cycles of RICE; 2 patients did not complete treatment because of progressive disease. Transient grade 3 or 4 infusion-related toxicity was noted only during the first rituximab infusion and occurred in only 4 patients; all 4 patients received additional rituximab with little or no infusion-related toxicity. The median time to complete 3 cycles of RICE, from the first to the last day of treatment, was 45 days (range, 35-59 days). The primary reason for delay was grade 3 or 4 hematologic toxicity (Table 2), and only 29% of patients received all treatments on time. Nonhematologic toxicity was minimal and is outlined in Table 3. Cardiovascular disease was diagnosed in 2 patients who reported angina-like symptoms; their symptoms were thought to be unrelated to the treatment. Febrile neutropenia, the most common grade 3 or 4 nonhematologic toxicity, developed during 8 (7.5%) of 106 delivered cycles. In no patient did RICE-related toxicity preclude transplantation.
Grade 3 or 4 hematologic toxicity resulting in treatment delays
. | No. patients . | . | |
|---|---|---|---|
| Toxicity . | Cycle 1 . | Cycle 2 . | |
| Neutropenia | 6 | 5 | |
| Thrombocytopenia | 6 | 8 | |
| Neutropenia and thrombocytopenia | 6 | 5 | |
. | No. patients . | . | |
|---|---|---|---|
| Toxicity . | Cycle 1 . | Cycle 2 . | |
| Neutropenia | 6 | 5 | |
| Thrombocytopenia | 6 | 8 | |
| Neutropenia and thrombocytopenia | 6 | 5 | |
Grade 3 or 4 nonhematologic toxicity
Toxicity . | No. incidents . |
|---|---|
| Grade 3 | |
| Neutropenic fever | 8 |
| Infection | 4 |
| Cardiac ischemia | 2 |
| Deep venous thrombosis/pulmonary embolus | 2 |
| Hemorrhagic cystitis | 2 |
| Nausea/vomiting | 2 |
| Syncope | 1 |
| Grade 4 | |
| Fever of unknown origin | 1 |
Toxicity . | No. incidents . |
|---|---|
| Grade 3 | |
| Neutropenic fever | 8 |
| Infection | 4 |
| Cardiac ischemia | 2 |
| Deep venous thrombosis/pulmonary embolus | 2 |
| Hemorrhagic cystitis | 2 |
| Nausea/vomiting | 2 |
| Syncope | 1 |
| Grade 4 | |
| Fever of unknown origin | 1 |
All 34 patients who completed 3 cycles of RICE underwent leukapheresis. The median number of CD34+ cells per kilogram collected was 6.3 × 106 (range, 0-15.6 × 106), in a median of 3 apheresis sessions (range, 1-5 sessions). PBPC yields were inadequate (less than 2 × 106 CD34+ cells/kg) in 6 (18%) patients.
Nineteen (53%) patients attained CR, with gallium or PET, or both, scans that showed no abnormal sites of uptake, and 9 patients (25%) achieved PR, for an overall response rate of 78% (Table 4); 104 of the 147 historical control patients responded to ICE, for an overall response rate of 71%. Patients treated with RICE had a significantly better CR rate than patients treated with ICE (53% [95% confidence interval (CI), 36%-69%] vs 27% [95% CI, 20%-34%]; P = .01), particularly if they had relapsed disease (65% [95% CI, 43%-83%] vs 34% [95% CI, 24%-44%]; P = .01) or sAAIPI high-intermediate or high-risk disease (53% [95% CI, 29%-76%] vs 19% [95% CI, 11%-28%]; P = .01) (Table 4). Similar to our results with ICE,7 disease status (relapsed vs primary refractory disease) predicted response to RICE (96% vs 46%; P < .01). However, the sAAIPI, predictive of overall response (P < .01) and CR (P = .01) to ICE, was not predictive for the RICE-treated patients. In fact, RICE-treated patients with high-intermediate or high-risk disease did as well as those with low or low-intermediate risk disease with respect to overall response and CR rates (P = 1.00 for each).
Response rates to RICE compared with ICE historical controls
. | Overall response rate, % . | . | . | Complete response rate, % . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient subgroup . | RICE . | ICE historical controls . | P . | RICE . | ICE historical controls . | P . | ||||
| All patients | 78 | 71 | .53 | 53 | 27 | .01 | ||||
| Relapsed | 96 | 79 | .07 | 65 | 34 | .01 | ||||
| Refractory | 46 | 63 | .36 | 31 | 19 | .46 | ||||
| sAAIPI L/LI | 79 | 86 | .47 | 53 | 39 | .42 | ||||
| sAAIPI HI/H | 76 | 61 | .28 | 53 | 19 | .01 | ||||
. | Overall response rate, % . | . | . | Complete response rate, % . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient subgroup . | RICE . | ICE historical controls . | P . | RICE . | ICE historical controls . | P . | ||||
| All patients | 78 | 71 | .53 | 53 | 27 | .01 | ||||
| Relapsed | 96 | 79 | .07 | 65 | 34 | .01 | ||||
| Refractory | 46 | 63 | .36 | 31 | 19 | .46 | ||||
| sAAIPI L/LI | 79 | 86 | .47 | 53 | 39 | .42 | ||||
| sAAIPI HI/H | 76 | 61 | .28 | 53 | 19 | .01 | ||||
L indicates low risk; LI, low-intermediate risk; HI, high-intermediate risk; H, high risk.
Three of the 28 responding patients did not undergo ASCT: 1 patient refused, one patient did not have an adequate number of mobilized stem cells and underwent allogeneic transplantation from his HLA-matched sibling, and one patient, who did not have any mobilized PBPCs even after a repeated attempt with G-CSF alone, was found to have adenocarcinoma of the lung after completing RICE. Among the 25 responding patients who underwent ASCT, 4 did not have more than 2 × 106 CD34+ cells/kg mobilized after RICE. Two of these patients underwent transplantation after an adequate number of PBPCs were mobilized with G-CSF alone, and 1 patient underwent transplantation with autologous bone marrow. One patient underwent transplantation with 1.6 × 106 CD34+ cells/kg. He had persistent pancytopenia after ASCT and was found to have hypocellular myelodysplasia with a 7q–abnormality. He underwent allogeneic transplantation from his HLA-matched sibling and is alive without recurrent lymphoma 2.5 years after transplantation. He is excluded from the transplantation analyses.
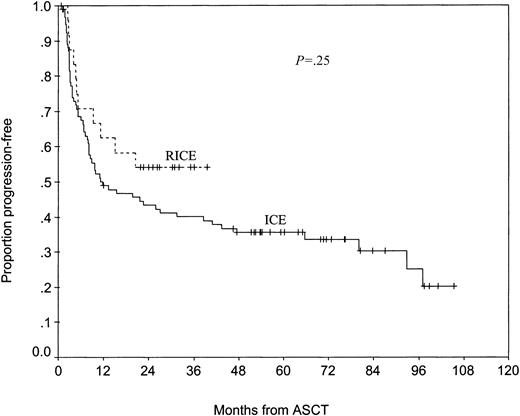
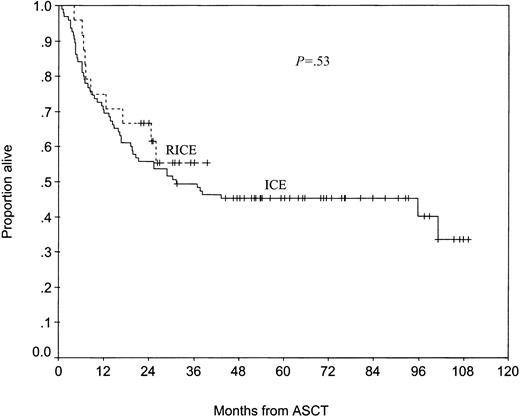
High-dose therapy consisted of carmustine, etoposide, cytarabine, and melphalan (BEAM) in 12 patients; total body irradiation (TBI), ifosfamide, and etoposide in 10 patients; TBI, cyclophosphamide, and etoposide in 1 patient; and cyclophosphamide, carmustine, and etoposide (CBV) in 1 patient. The median time to neutrophil engraftment (ANC 500 neutrophils/μL or higher) was 9 days (range, 8-13 days). With a median posttransplantation follow-up of 29 months for surviving patients, the median 2-year PFS and OS have not been reached. Ninety-five of the 104 control patients who responded to ICE underwent ASCT; they were not different from the RICE-treated patients who underwent ASCT with respect to the sAAIPI (P = .65). After ASCT, the RICE-treated patients had a better PFS than the ICE-treated patients, although the difference was not statistically significant (54% [95% CI, 38%-78%] vs 43% [95% CI, 34%-55%] at 2 years) (Figure 1). The OS rates of RICE- and ICE-treated patients who underwent ASCT were similar (67% [95% CI, 50%-89%] vs 56% [95% CI, 47%-67%] at 2 years) (Figure 2).
Progression-free survival of RICE- and ICE-treated patients who underwent autologous transplantation.
Progression-free survival of RICE- and ICE-treated patients who underwent autologous transplantation.
Overall survival of RICE- and ICE-treated patients who underwent autologous transplantation.
Overall survival of RICE- and ICE-treated patients who underwent autologous transplantation.
Discussion
The benefit of ASCT for relapsed or primary refractory aggressive NHL is largely restricted to patients with chemosensitive disease3,6,22-25 because response to second-line chemotherapy likely predicts sensitivity to high-dose chemotherapy. The observation that patients who undergo transplantation in CR have better outcomes than patients who undergo transplantation in PR7,8 suggests that the efficacy of high-dose therapy is influenced by the inherent sensitivity of the lymphoma or that it is dependent on tumor burden or both. One way to examine the relative contributions of inherent chemosensitivity and tumor burden to transplantation outcome is to potentiate the effects of a standard second-line chemotherapy regimen and increase the CR rate using nonchemotherapeutic approaches.
The CR rates of the commonly used second-line regimens dexamethasone-cytarabine-cisplatin (DHAP),26 etoposide-methylprednisolone-cytarabine-cisplatin (ESHAP),27 and mini-BEAM28 are approximately 25% to 35%, and no single regimen appears superior, although studies comparing these regimens directly have not been performed. Similarly, the CR rate among patients with relapsed or primary refractory DLBCL treated with ICE is approximately 25% to 30%.7,9 Rituximab induces responses in approximately 30% to 35% of patients with relapsed or primary refractory DLBCL12,13 and sensitizes tumor cells to the effects of chemotherapy.29-31 When added to CHOP, rituximab significantly increases the CR rate among patients with previously untreated DLBCL.14 We show that adding rituximab to ICE appears to double the CR rate of ICE alone (53% vs 27%) in patients with relapsed or primary refractory DLBCL. Given that RICE- and ICE-treated patients were similar with respect to disease status and sAAIPI, known prognostic factors for response to ICE, it is unlikely that these results were caused by selection bias. Rather, the results likely reflect an effect of rituximab on potentiating chemotherapy sensitivity, thereby improving the quality of response and further reducing tumor burden before ASCT.
RICE appears particularly beneficial in patients with relapsed disease; the CR rate in patients treated with RICE was 65% compared with 34% in patients treated with ICE (P = .01). The CR rate (53%) in patients with high-intermediate or high-risk disease was the same as in patients with low or low-intermediate risk disease, suggesting that adding rituximab to ICE may overcome the adverse effects of the sAAIPI. The basis for this observation is unclear, and these results should be confirmed in a larger cohort of patients. Vose and Sneller32 recently reported that the CR rate in 28 patients with relapsed or refractory B-cell lymphoma who were treated with rituximab and an outpatient ICE regimen was 25%; however, only 12 of those 28 patients had DLBCL. However, these patients received only 2 cycles of treatment, and the CR rate in the patients with DLBCL was not reported.
The overall response rate of RICE-treated patients was not different from that of ICE-treated patients. Given the independent activity of rituximab in relapsed or primary refractory DLBCL, it is likely that a proportion of patients who did not respond to ICE responded to rituximab, resulting in the slightly higher response rate to RICE compared with ICE. However, to detect a modest improvement in the overall response rate with sufficient statistical confidence would require more patients than were included in this cohort; thus, the overall response rates to RICE and ICE appear similar.
A critical question is whether improving the CR rate will translate into improved outcomes after ASCT. The PFS of patients who underwent transplantation after RICE appears slightly better than the PFS of patients who underwent transplantation after ICE (54% vs 43% at 2 years). This difference was not statistically significant, but this study was not powered to detect modest improvements in survival rates. Nevertheless, these results are encouraging and warrant evaluation in a larger study designed to detect such a difference. Confirming a clinically meaningful difference in PFS would lend further support to the hypothesis that the response to high-dose therapy in chemosensitive patients depends on tumor burden and that the induction of CR should be a primary goal of second-line therapy.
RICE was well tolerated, and, despite its hematologic toxicity, febrile neutropenia occurred in only 7.5% of delivered cycles, much lower than the incidence with several of the other commonly used second-line regimens associated with febrile neutropenia rates of 30% to 65%.26-28,33 Similar to our experience, Vose and Sneller32 noted febrile neutropenia in only 4 of 28 patients treated with rituximab and outpatient ICE. In addition, RICE-related toxicity did not preclude transplantation in any patients. In contrast, the cisplatin-containing second-line regimens DHAP and ESHAP are associated with renal insufficiency in significant proportions of patients, especially when 2 or more cycles are administered,26,27,33 which could render chemosensitive patients ineligible for ASCT.
Adding rituximab to ICE appears to significantly improve the CR rate of patients with relapsed or primary refractory DLBCL, with more than 50% of patients achieving CR. Our data suggest that RICE may be particularly beneficial to patients with relapsed disease and may overcome the adverse features of the sAAIPI; however, such conclusions must be interpreted cautiously given the small number of patients studied. An international consortium led by the Groupe d'Etudes des Lymphomes Adultes (GELA) has initiated a randomized trial of rituximab and ICE compared with rituximab and DHAP for patients with relapsed or primary refractory DLBCL. This large study will address whether disease status and sAAIPI affect response rates to rituximab-containing regimens. It will also help address whether improving remission quality before transplantation will improve PFS after ASCT.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-11-3911.
Supported in part by Genentech, Inc, the National Institutes of Health (grant 5P01CA5826-34), the Lymphoma Research Fund, the Lymphoma Foundation, the Byrne Fund, and the Mortimer J. Lacher Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal