Abstract
Women with systemic lupus erythematosus (SLE) are at risk for premature atherothrombosis independent of Framingham risk factors. We investigated whether endothelial cell (EC) apoptosis predicts abnormal vasomotor tone and contributes to circulating tissue factor (TF) levels in this disease. Brachial artery flow-mediated dilation (FMD) and nitroglycerin-mediated dilation were determined in women with SLE, healthy control subjects, and subjects with coronary artery disease (CAD) (n = 43/group). Quantification of circulating apoptotic ECs was performed by flow cytometry (CD146+ cells that stained for Annexin V [CD146AnnV+]) and immunofluorescent microscopy. Plasma TF was measured by enzyme-linked immunosorbent assay (ELISA). Compared with healthy control and CAD subjects, patients with SLE had higher numbers of circulating CD146AnnV+ cells (10 ± 3, 18 ± 5, and 89 ± 32 cells/mL, respectively, mean ± SEM; P < .01). Increased CD146AnnV+ cells correlated strongly with abnormal vascular function (P = .037). After adjusting for known predictors of endothelial function, CD146AnnV+ was the only variable that predicted FMD (β = –4.5, P < .001). Increased CD146AnnV+ was strongly associated with elevated levels of circulating TF (r = .46, P = .002). Circulating apoptotic ECs are elevated in young women with SLE and strongly correlate with markedly abnormal vascular function and elevated TF levels. Heightened endothelial apoptosis may represent an important mechanism for development of atherothrombosis in SLE.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that primarily affects young women.1 Women with SLE have strikingly higher rates of premature cardiovascular disease, with up to a 50-fold increase in the incidence of cardiovascular complications, over age- and sex-matched control subjects.2 Standard Framingham risk factors are not predictive for this heightened risk.3 Although several groups have proposed that immune dysregulation characteristic of SLE plays the dominant role in atherogenesis, the exact mechanisms involved in this process remain unclear.4,5
Extensive evidence suggests that enhanced apoptosis may play a major role in the pathogenesis of SLE.6-8 Increased detection of apoptotic cells, including monocytes, macrophages, neutrophils, and lymphocytes, has been reported both in vitro and ex vivo in patients with lupus, as well as in vivo in murine SLE models.8-13 However, whether endothelial cells (ECs) are also an apoptotic target in this disease has not been systematically investigated.
Evidence suggests that in vitro apoptosis of ECs results in generation of phospholipid-rich microparticles and enhanced procoagulant potential through tissue factor (TF)–dependent pathways.14 The origin of elevated TF activity is believed to be secondary to externalization of phospholipids, such as phosphatidylserine, during apoptosis which then confers potent procoagulant activity to the apoptotic cell.15,16 TF levels and activity are elevated in a variety of conditions associated with thrombosis propensity, such as unstable coronary syndromes and autoimmune conditions, including SLE.17-19 The mechanisms by which elevated levels of TF are generated in SLE remain unclear.20,21 Enhanced EC apoptosis may also result in loss of nitric oxide (NO) release, further amplification of platelet thrombosis, and potentiation of atherogenesis.22,23 Loss of endothelial-derived NO manifests as abnormal vascular function and precedes clinical atherosclerosis.24 A number of studies have confirmed the prognostic significance of abnormal endothelial function for future risk of atherosclerosis-related complications.25-27
Although circulating ECs have been documented in autoimmune conditions, including lupus and vasculitis,28,29 the relationship of EC injury/apoptosis with development of vascular dysfunction in SLE has not been prospectively inv estigated. Furthermore, the correlation of increased TF levels with the presence of EC apoptosis in SLE has not been determined. We hypothesized that enhanced circulating EC apoptosis in SLE correlates with reduced endothelial function and elevated TF activity.
Patients, materials, and methods
Study population
The University of Michigan Institutional Review Board (IRB) approved this study. Informed consent was provided according to the Declaration of Helsinki. Procedures followed were in accordance with institutional guidelines. Blood was collected from 43 women with SLE who fulfilled the American College of Rheumatology criteria for lupus.30 Lupus disease activity was assessed with the SLE-Disease Activity Index (SLEDAI).31 Anti–β-2 glycoprotein (immunoglobulin G [IgG], IgM, and IgA) and antiphospholipid antibodies (IgG and IgM) were determined by enzyme-linked immunosorbent assay (ELISA); lupus anticoagulant was determined by using dilute Russell viper venom test (DRVVT). Following international guidelines, all patients underwent determinations of antiphospholipid antibodies and/or lupus anticoagulant at least twice, and 6 weeks or more apart. For the purposes of this study, we used the titers determined on the day when the vascular studies and endothelial cell apoptosis determinations were done. Of the subjects with SLE, 33% tested positive for anticardiolipin antibody, whereas 30% had a positive lupus anticoagulant and 48% had an anti-β2 glycoprotein antibody, reflective of a prothrombotic state.
We excluded pregnant or postmenopausal women, smokers, persons with diabetes, patients with significant hyperlipidemia, and those receiving cholesterol-lowering medications, including statins. In addition, we excluded patients with a history of venous or arterial thrombosis. We included 43 age-matched healthy subjects that met the same exclusion criteria as those of the SLE cohort. We also included 43 individuals with known coronary artery disease (CAD), confirmed by coronary angiography (> 50% stenosis in at least one vessel). Subjects were recruited through advertisements from within the University of Michigan, the Michigan Lupus Cohort, and cardiology clinics. Subjects with CAD had endothelial function studies performed a minimum of 8 weeks following cardiac catheterization.
Concomitant therapy
A total of 55% of subjects with CAD were on lipid-lowering therapy, including statins, 76% were receiving β-blockers and acetylsalicylic acid (ASA), and 32% were on angiotensin-converting enzyme (ACE) inhibitor therapy. Of the subjects with SLE, 60% were receiving steroids with a mean dosage of 7 ± 4 mg prednisone, 45% received hydroxychloroquine, and 40% received other immunosuppressant therapy.
Study protocol
Determination of vascular function. Flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD) were performed in fasting state during morning hours and prior to procurement of blood samples, with the subject resting quietly in a light- and temperature-controlled room. Images were obtained with a 10-mHz linear array transducer and an HP Image Point ultrasound system (Hewlett Packard, Andover, MA). After baseline measurements of brachial artery diameter, a blood pressure cuff was inflated to 50 mm Hg above the subject's systolic blood pressure over the proximal portion of the right forearm for 4 minutes. FMD was determined 1 minute after release of the cuff. Brachial artery diameter was then allowed to return to baseline over a period of 15 minutes. Endothelial-independent responsiveness was then evaluated with 0.4 mg nitroglycerin administered sublingually with brachial artery images being obtained 3 minutes later. Four R wave–triggered events from the electrocardiogram (EKG; 24 sequential frames) for each intervention were procured digitally through a frame grabber attached to a computer. The end point of measurement was the percentage of change in diameter in response to reactive hyperemia (FMD) or to nitroglycerin (NMD). An independent reviewer not familiar with the status of the patient reviewed the images. The reproducibility of our technique has been reported previously.32,33
Detection of apoptotic circulating ECs. These studies were performed at the time of the vascular measurements. Twenty milliliters of blood were obtained by venipuncture, with the first 2 mL discarded to avoid contamination from the punctured vessel wall. Peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Hypaque (Amersham, Uppsala, Sweden) and after suspension of 106 cells/mL in phosphate-buffered saline (PBS), ethylenediaminetetraacetic acid (EDTA), and 2% horse serum incubated with mouse antihuman CD146 (Chemicon, Temecula, CA), conjugated to phycoerythrin (PE) or isotype control. With the exception of certain tumor lines, CD146 (also known as MCAM, MUC18, P1H12, S-Endo-1, and Mel-CAM) is exclusively expressed on mature ECs.34 To confirm the endothelial lineage of these cells, we performed additional studies with monoclonal antibodies to alternate EC antigens such as flk-1/KDR (kinase domain receptor) (vascular endothelial growth factor receptor-2 [VEGFR-2]; Research Diagnostics, Flanders, NJ) and platelet endothelial cell adhesion molecule 1 (PECAM-1; CD31; BD Biosciences, San Diego, CA). This was followed by 2 washes in PBS/EDTA/2% fetal bovine serum (FBS). When VEGFR-2 staining was performed, a second step that involved adding a goat-antimouse–PE antibody and costaining with anti–CD45-CYC (BD Biosciences) was also performed. To further confirm the specificity of CD146 expression as a marker of EC lineage, we performed 2-color flow cytometry using anti-CD3, anti-CD21, and anti-CD14 monoclonal antibodies (BD Biosciences), as markers of T-cell, B-cell, and monocyte lineage, respectively. CD146+ cells did not coexpress the latter markers. Apoptosis was then evaluated by resuspending these cells in 100 μL Annexin V binding buffer and incubating them with 5 μL Annexin V–fluorescein isothiocyanate (FITC; BD Biosciences) for 15 minutes. Annexin V detects the externalization of phosphatidylserine during apoptosis and represents an early and sensitive marker for this type of cell death.35 Cells were then analyzed by using a FACScan flow cytometer (BD Biosciences) to assess the cells that were positive for CD146 and Annexin V (CD146AnnV+). A total of 10 000 events were measured per sample. On the basis of the PBMC counts, we calculated the absolute number of circulating CD146AnnV+/mL. Samples derived from cohorts were paired and studied on the same day and under the same conditions to ensure that daily variations would not introduce errors in the data.
In additional experiments, we evaluated apoptotic ECs with an alternate approach by using fluorescent microscopy and analyzing nuclear morphology.36,37 Cells were incubated with anti-CD146 as earlier, washed with Hanks balanced salt solution (HBSS), and fixed with ice-cold 4% paraformaldehyde for 10 minutes. Cells were then washed, incubated with Annexin V–FITC for 15 minutes on ice, washed again, and stained with the nuclear dye Hoechst 33342 (Molecular Probes Eugene, OR). After 15-minute incubation on ice, cells were washed, mounted onto poly-l-lysine–coated glass slides, and viewed with an LSM 510 laser-scanning microscope (Zeiss, Thorwood, NY).
Determination of circulating TF levels in subjects with SLE. Plasma TF levels were detected by an IMUBIND TF ELISA test kit obtained from American Diagnostica (Stamford, CT), following manufacturer's instructions. The absorbance was read on a microplate reader, and a standard curve was generated with standards. The concentration of TF in each sample was calculated from the standard curve equation in picogram per milliliter concentrations.
Statistical analysis
Baseline continuous data, stratified by group, are summarized as means ± SD unless otherwise noted. Analysis of variance (ANOVA) was used to compare vascular reactivity data and CD146/Ann V+ cells in the 3 groups of subjects, with P values less than .05 considered statistically significant. A nonparametric test for correlation (Spearman) between FMD, CD146/Ann V+, TF, and SLEDAI was performed. Log transformation was performed on variables that were not normally distributed. To estimate the effect of CD146AnnV+ on FMD, univariate linear regression was performed; multi-variable regression was performed to adjust for confounders. Statistical analysis was performed using Stata version 8 (StataCorp, College Station, TX).
Results
Patient population
Table 1 depicts baseline demographic and clinical data. The patients with CAD, as expected, were considerably older than the SLE and control cohorts (65 ± 10 versus 37 ± 9, and 35 ± 9 years, respectively). Lipid and adiposity parameters did not significantly differ across groups. Baseline diameter of the brachial artery was increased in the CAD cohort, likely reflecting the higher age of the cohort as has been demonstrated previously.38 C-reactive protein (CRP) and homocysteine values were normal in the SLE group (0.8 ± 0.4 mg/L and 8.4 ± 4 μM, respectively). In the CAD group, 20% of subjects were diabetic, whereas 21% had a history of smoking, representing confluence of multiple risk factors as is typical for this population. SLEDAI, a commonly used and validated index of activity status that combines clinical and serologic markers of disease severity, was 4 ± 3.7 (mean ± SD) on a scale of 0 to 105. Most (39 of 43) subjects with SLE had no proteinuria, and all had normal creatinine clearance.
Baseline demographic and clinical data
Group characteristics . | Control . | SLE . | CAD . |
|---|---|---|---|
| Age, y | 35 ± 9 | 37 ± 9 | 65 ± 10 |
| Women, % | 50 | 100 | 29 |
| BMI | 25 ± 7 | 26 ± 6 | 28 ± 5 |
| Total cholesterol, mg/dL | 175.9 ± 35 | 196 ± 33 | 177 ± 38 |
| HDL cholesterol, mg/dL | 49 ± 15 | 54 ± 18 | 47 ± 12 |
| LDL cholesterol, mg/dL | 98 ± 26 | 106 ± 42 | 101 ± 35 |
| Systolic blood pressure, mm Hg | 137 ± 12 | 136 ± 24 | 134 ± 17 |
| Diastolic blood pressure, mm Hg | 73 ± 13 | 70 ± 11 | 72 ± 10 |
| Baseline diameter of brachial artery, mm | 3.3 ± 0.4 | 3.5 ± 0.3 | 4.3 ± 0.2 |
Group characteristics . | Control . | SLE . | CAD . |
|---|---|---|---|
| Age, y | 35 ± 9 | 37 ± 9 | 65 ± 10 |
| Women, % | 50 | 100 | 29 |
| BMI | 25 ± 7 | 26 ± 6 | 28 ± 5 |
| Total cholesterol, mg/dL | 175.9 ± 35 | 196 ± 33 | 177 ± 38 |
| HDL cholesterol, mg/dL | 49 ± 15 | 54 ± 18 | 47 ± 12 |
| LDL cholesterol, mg/dL | 98 ± 26 | 106 ± 42 | 101 ± 35 |
| Systolic blood pressure, mm Hg | 137 ± 12 | 136 ± 24 | 134 ± 17 |
| Diastolic blood pressure, mm Hg | 73 ± 13 | 70 ± 11 | 72 ± 10 |
| Baseline diameter of brachial artery, mm | 3.3 ± 0.4 | 3.5 ± 0.3 | 4.3 ± 0.2 |
All values are expressed as means ± SD except for percentage of females. The baseline diameter in the CAD group was different compared with the lupus and control cohort (P < .05). BMI indicates body mass index.
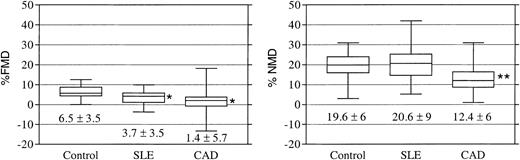
Female patients with lupus have significant impairment in endothelium-dependent vascular responses
Figure 1 represents box-whisker plots for the FMD responses in the various cohorts. Mean FMD responses in the SLE cohort were significantly impaired compared with healthy control subjects, whereas they did not differ from the CAD cohort (6.5% ± 3.5% in control subjects, 3.7% ± 3.5% in SLE, and 1.4% ± 5.7% in CAD, respectively, P < .05 for controls versus SLE or CAD), indicating abnormal endothelial function. NMD differed significantly between the CAD cohort and the subjects in the SLE and normal cohorts (12.4 ± 6.2, 19.6 ± 5.9, and 20.6 ± 8.5, respectively, P < .001 for controls versus CAD and for CAD versus SLE), indicating impairment in smooth muscle responses to NO donors in the CAD cohort.
Patients with SLE have impaired endothelial function. Box-whisker plots representing flow-mediated vasodilation (FMD) and nitroglycerin-mediated vasodilation (NMD) in control subjects and SLE and CAD cohorts. Results below the box plots are means ± SEM. *P < .01 versus controls and **P < .001 versus controls and SLE.
Patients with SLE have impaired endothelial function. Box-whisker plots representing flow-mediated vasodilation (FMD) and nitroglycerin-mediated vasodilation (NMD) in control subjects and SLE and CAD cohorts. Results below the box plots are means ± SEM. *P < .01 versus controls and **P < .001 versus controls and SLE.
Increased circulating apoptotic ECs are present in women with lupus and correlate with abnormal vascular responses and disease activity
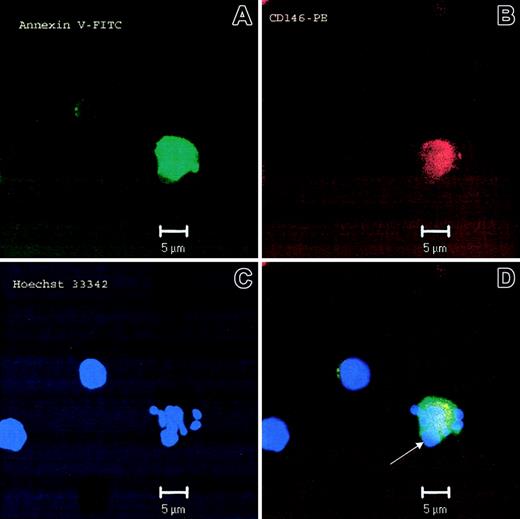
When compared with healthy control subjects and individuals with CAD, patients with SLE had higher numbers of circulating CD146AnnV+ cells (10 ± 3, 18 ± 5, and 89 ± 32 cells/mL, respectively, mean ± SEM, P < .01; Figure 2A). Although patients with a history of CAD had higher levels of circulating CD146AnnV+ cells than healthy control subjects, the differences were not statistically significant (P > .05). Figure 2B shows dot-plots of CD146AnnV+ cells from 1 healthy control subject and 3 patients with SLE with different degrees of disease activity, as measured by SLEDAI. Staining specificity was additionally confirmed by using other EC-specific antibodies (anti-flk-1/KDR, CD31, CD62P, and CD106) and determining nonexpression of leukocyte lineage markers (CD3, CD21, CD14). As an alternate method to confirm that lupus ECs display an apoptotic phenotype, we performed immunofluorescent microscopy by using Hoechst 33342 to assess nuclear morphology and distribution. As shown in Figure 3, lupus ECs demonstrate nuclear fragmentation and redistribution of nuclear material to the cell membrane characteristic of apoptosis.
Patients with SLE have increased numbers of circulating apoptotic endothelial cells that correlate with disease activity. (A) Number of circulating CD146+ cells expressing annexin V (CD146AnnV) per volume of blood (milliliter) in healthy control subjects and in patients with SLE and CAD. (*P < .01, results represent mean ± SD). (B) Dot-plots displaying circulating apoptotic ECs in a healthy control subject, and 3 patients with SLE with different degrees of disease activity, as measured by SLEDAI. On the basis of the PBMC counts, we calculated the absolute number of circulating CD146AnnV+ per milliliter, represented in the right upper quadrant.
Patients with SLE have increased numbers of circulating apoptotic endothelial cells that correlate with disease activity. (A) Number of circulating CD146+ cells expressing annexin V (CD146AnnV) per volume of blood (milliliter) in healthy control subjects and in patients with SLE and CAD. (*P < .01, results represent mean ± SD). (B) Dot-plots displaying circulating apoptotic ECs in a healthy control subject, and 3 patients with SLE with different degrees of disease activity, as measured by SLEDAI. On the basis of the PBMC counts, we calculated the absolute number of circulating CD146AnnV+ per milliliter, represented in the right upper quadrant.
Circulating ECs display an apoptotic phenotype. Microphotograph of an apoptotic EC from a patient with SLE. Annexin V was visualized with FITC-conjugated Annexin V, CD146 was visualized by anti-CD146 conjugated to phycoerythrin, and nuclear material was visualized with Hoechst 33342. (A-C) Micrographs show the staining with each individual fluorochrome and the composite image (D). Panel C displays evidence of nuclear fragmentation, characteristic of apoptosis. Arrowhead denotes a prominent surface bleb containing nuclear material, characteristic of apoptosis. The other 2 cells in the figure represent cells that are CD146– and, therefore, not of endothelial origin.
Circulating ECs display an apoptotic phenotype. Microphotograph of an apoptotic EC from a patient with SLE. Annexin V was visualized with FITC-conjugated Annexin V, CD146 was visualized by anti-CD146 conjugated to phycoerythrin, and nuclear material was visualized with Hoechst 33342. (A-C) Micrographs show the staining with each individual fluorochrome and the composite image (D). Panel C displays evidence of nuclear fragmentation, characteristic of apoptosis. Arrowhead denotes a prominent surface bleb containing nuclear material, characteristic of apoptosis. The other 2 cells in the figure represent cells that are CD146– and, therefore, not of endothelial origin.
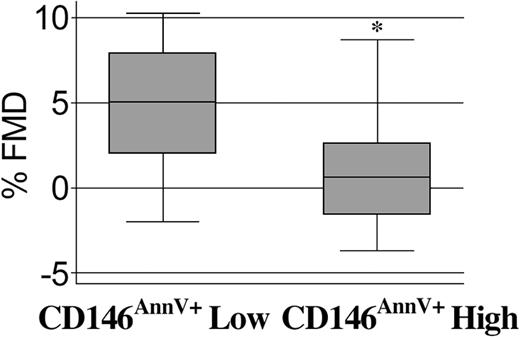
In the SLE group, there was a significant correlation between the presence of circulating apoptotic ECs and abnormal FMD by using a nonparametric measure (Spearman r =–0.32, P = .037). In addition, the correlation between circulating apoptotic ECs and lupus disease activity (by SLEDAI) approached statistical significance (r = 0.27, P = .08). We then divided subjects into 2 subgroups on the basis of their levels of circulating apoptotic ECs, using a cutoff of 85 cells/mL which corresponded to the 75th percentile among the patients with lupus. In a multiple regression analysis that adjusted for known predictors of endothelial function (age, systolic blood pressure, and low-density lipoprotein [LDL] cholesterol), the level of apoptotic endothelial cells was the only variable that predicted FMD (β=–4.5, P < .001). Prednisone use, SLEDAI, SLE disease duration, and presence of lupus anticoagulant, anti–β2-glycoprotein, or antiphospholipid antibody did not predict endothelial function. When we investigated patients with the highest levels of these autoantibodies, we confirmed no correlation with SLEDAI, CD146AnnV+, and/or abnormal FMD. As shown in Figure 4, attenuation of FMD was significantly pronounced in the group of subjects with the highest numbers of increased circulating apoptotic ECs (P = .0026 by t test).
Increased circulating apoptotic endothelial cells (CD146AnnV+) predict vascular impairment. Patients with SLE were subdivided into 2 subgroups on the basis of their levels of circulating apoptotic ECs per milliliter, using a cutoff of 85 cells/mL which corresponded to the 75th percentile among the patients with lupus. Therefore, 85 cells/mL or more is designated as a high number of circulating CD146AnnV+ cells, and less than 85 cells/mL is designated as low CD146AnnV+; *P = .0026 by t test. FMD indicates flow-mediated vasodilation.
Increased circulating apoptotic endothelial cells (CD146AnnV+) predict vascular impairment. Patients with SLE were subdivided into 2 subgroups on the basis of their levels of circulating apoptotic ECs per milliliter, using a cutoff of 85 cells/mL which corresponded to the 75th percentile among the patients with lupus. Therefore, 85 cells/mL or more is designated as a high number of circulating CD146AnnV+ cells, and less than 85 cells/mL is designated as low CD146AnnV+; *P = .0026 by t test. FMD indicates flow-mediated vasodilation.
Circulating apoptotic endothelial cells correlate with plasma TF levels in SLE
CD146AnnV+ levels correlated strongly with circulating TF levels (Spearman r = 0.46, P = .002) in SLE. As shown in Figure 5, levels of apoptotic ECs increase according to quartile of TF (P < .001 by nonparametric test for trend). TF levels did not correlate with presence of antiphospholipid antibodies, lupus anticoagulant, or anti–β2-glycoprotein, but they correlated with abnormal FMD (Spearman r =–0.47, P = .007).
Increased circulating apoptotic ECs (CD146AnnV+) correlates with TF levels in SLE. TF levels are expressed in quartiles in the x-axis. The y-axis represent Log (CD146AnnV+ per milliliter); P < .001 by nonparametric test for trend across TF quartiles.
Increased circulating apoptotic ECs (CD146AnnV+) correlates with TF levels in SLE. TF levels are expressed in quartiles in the x-axis. The y-axis represent Log (CD146AnnV+ per milliliter); P < .001 by nonparametric test for trend across TF quartiles.
Discussion
The major findings of this investigation included the following: (1) Young female subjects with SLE have high levels of circulating apoptotic ECs; (2) Levels of apoptotic ECs are highly correlated with abnormal endothelial-dependent tone, an important predictor of future atherosclerosis-related events; and (3) EC apoptosis in SLE correlates strongly with levels of circulating TF, the major procoagulant in vivo. Taken together these findings provide a potential link between endothelial apoptosis, thrombosis, and abnormal endothelial tone with its implications for atherothrombotic events in SLE.
Although circulating ECs have been reported in diverse conditions associated with endothelial injury, including acute coronary syndromes,39 SLE,28 and antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis,29 an apoptotic phenotype of circulating ECs in SLE has not been investigated. To the best of our knowledge, this is the first study to demonstrate evidence of circulating apoptotic ECs (CD146AnnV+) in the peripheral circulation in SLE. These markers specifically correlated with vascular dysfunction and TF levels in a cohort of young subjects with SLE, with low Framingham risk scores (score < 1%), and independent of other traditional predictors of atherosclerosis.
These findings may potentially indicate an additional mechanism for the predisposition of these individuals to premature vascular disease, heightened thrombogenicity, and the development of cell-mediated and humoral-immune responses.7 The increased propensity for vascular disease in SLE, despite adjustment for risk factors, is at least 10-fold higher for myocardial infarction and 6.3-fold higher for stroke.40 Individuals with SLE with no prior vascular event have evidence of incipient vascular dysfunction as evidenced by subtle alterations in vascular function and asymptomatic perfusion abnormalities, suggestive of altered coronary flow reserve.41,42 A number of lupus-specific mechanisms have been hypothesized to play a role in the pathogenesis of this vasculopathy. These mechanisms include, among others, antiendothelial cell and anticardiolipin antibodies as well as direct cell-mediated cytotoxicity, all of which have been described to cause EC apoptosis.43-46 The subjects with SLE in this study were young and exclusively women, and yet they had FMD measurements that were comparable to those of an older CAD cohort. Per our protocol design, subjects with SLE were required not to have other known risk factors for vascular disease, including a history of smoking, significant elevations of LDL and non–high-density lipoprotein (HDL) cholesterol, obesity, hypertension, and had normal plasma homocysteine and C-reactive protein levels. In addition, nearly half of our SLE cohort was on antimalarial treatment, a modality that is known to increase HDL levels.47 This renders our findings all the more significant, because it is well known that women in the reproductive age group have significantly better FMD compared with men.38 Furthermore, the cohort studied was clinically stable, with an average SLEDAI score of 4. This finding suggests that even those patients with fairly controlled SLE have significant abnormalities in endothelial function.
Antiphospholipid antibody status could have additionally conferred an adverse vascular phenotype and increased propensity for apoptosis. We, therefore, evaluated the influence of this parameter as well. Although previous studies have reported that plasma with antiphospholipid antibody, including lupus anticoagulant activity, induces EC apoptosis and binds annexin,44 we did not find an association between the presence and/or titers of antiphospholipid antibodies, β-2-glycoprotein antibodies, or lupus anticoagulant and CD146AnnV+, TF levels or abnormal FMD. We must note that we only used one clot-based assay (DRVVT) to detect lupus anticoagulant, and in future studies we plan to confirm our findings by using a second clot-based assay following current international guidelines.
Our control CAD cohort represented a positive control group for the presence of endothelial dysfunction and included a diverse group of individuals with atherosclerosis and additional classic risk factors for CAD. Interestingly, these individuals did not demonstrate significant elevations in circulating ECs, confirming a previous observation that demonstrated these cells were found only in subjects with unstable coronary syndromes but not in those with stable CAD.17 Thus, on the basis of our observations at least in stable CAD, release of apoptotic cells into the circulation does not seem to occur to the degree observed in SLE. The presence of circulating apoptotic ECs in SLE, but not in stable CAD, argues for additional defects in clearance of these cells in SLE and/or inappropriately high rates of apoptosis in combination with additional defects in marrow reserve, as potential mechanisms.48,49
Statins have been reported to improve arterial function in patients with CAD in the presence of risk factors such as hyperlipidemia, diabetes, and smoking.50 The CAD group included in this study represented patients who were undergoing treatment with standard-of-care approaches that included statins that may potentially influence endothelial function. Despite usage of statins in this study in the CAD cohort, endothelial function was still markedly depressed. More important though was the finding that function in the lupus cohort was also markedly depressed and was identical to the significantly older CAD cohort. Thus, the purpose of the CAD group was to provide a control patient population with manifest endothelial dysfunction with whom vascular function in SLE could be contrasted. It is certainly possible that treatment with statins may have modified circulating apoptotic cell burden, and future studies will address this possibility. We must also note that the healthy control subjects as well as the CAD control subjects were not matched precisely with regard to age or sex ratio. However, we believe that this lack of homogeneity strengthens rather than weakens our arguments. Despite the presence of men in the healthy control group, who had similar lipid profile, blood pressure, and were of the same age when compared with patients with lupus, endothelial function was nearly 2 times better in this patient population. The exclusion of men in this healthy cohort would have, if anything, markedly increased the FMD in the control population because it is well known that women in the reproductive age have far better FMD responses.51 Thus, despite the inclusion of men, endothelial function seemed to be far better in the control patient population compared with the exclusively female lupus cohort. The inclusion of men in the population of patients with CAD was a reflection of clinical practice. CAD is more common in men compared with women in premenopausal age groups, and this predilection evens up in the postmenopausal age group. It is possible that inclusion of more women in the CAD group might have improved endothelial function in this group.
EC apoptosis is a prominent feature of atherosclerosis and colocalizes with TF activity in the plaque.52,53 Practically all risk factors for atherosclerosis may cause in situ EC apoptosis, perhaps through activation of redox-sensitive pathways.54-56 Apoptotic ECs in vitro have been found to have marked thrombotic potential, owing to redistribution of phosphatidylserine residues, activation of the TF cascade,57 and potentiation of atherothrombosis.52,53,58,59 Furthermore, the loss of ECs in the vessel wall reduces the generation of NO, with its proatherogenic consequences. Our findings of elevated apoptotic ECs in conjunction with elevated TF levels and attenuation of vascular reactivity, seems to fortify the central role of endothelial injury in the pathogenesis of vascular abnormalities in this disorder.
One possible limitation of the study is the use of immunomodulatory agents in the SLE cohort. It is difficult to perform studies in patients with SLE on no treatment, as most patients are on active therapy for the condition. Although difficult to exclude, we believe that treatment with these agents is an unlikely explanation for our findings. The effects of glucocorticoids on endothelial function and NO synthase activity are complex, and studies in experimental model systems as well as short-term studies in humans have yielded conflicting data.60-63 Similarly, the effects (proapoptotic versus antiapoptotic) of corticosteroids and other immunomodulatory agents, including antimalarials, appear to be predicated by a number of factors, including the type of corticosteroid (dexamethasone versus prednisone) and the specific cell examined.64-66 In a recent study of women with SLE without manifest vascular disease (mean age, 29 ± 6 years), although there were marked abnormalities in brachial artery endothelial function, these findings did not correlate with steroid use,41 in agreement with our findings. This result is compatible with the observation that corticosteroids and antimalarials in individuals with rheumatologic diseases are extremely effective in reducing the effects of inflammatory cytokines, which may play a major role in worsening endothelial function. Indeed, the administration of these agents to such patients is associated with improvement in endothelial function.67 However, it is still possible that the duration of steroid use may potentially accentuate vascular dysfunction and heighten propensity to vascular disease.
In conclusion, young women with SLE with a low Framingham risk score have increased circulating apoptotic ECs that correlate with pronounced abnormalities in endothelial function and elevated TF levels. Future studies should address the role of therapies specifically targeting endothelial apoptosis and evaluate the effects of these strategies on vascular function, TF levels, and atherothrombotic events.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-09-3198.
Supported by the Lupus Research Institute, University of Michigan Cardiovascular Center, Arthritis Foundation, Herbert and Carol Amster Lupus Fund and supported in part by the Public Health Service (PHS) (grants AR48234, AG014783, AR42525, ES011196), General Clinical Research Center (GCRC) (grant M01-RR00042), the University of Michigan and Cancer Center Support (grant 5 P30 CA46592).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal