Abstract
From 1990 to 1996, a total of 386 adult patients with early/intermediate Hodgkin disease (HD) were randomly assigned to receive 3 cycles of adriamycin, bleomycin, vinblastine, dacarbazine (an alkylating agent), and methylprednisolone (ABVDm, arm A) or epirubicin, bleomycin, vinblastine, methotrexate, and methylprednisolone (EBVMm, arm E), a combination without alkylating agent. Responding patients received extended field radiation therapy (RT). Postchemotherapy complete remission and 10-year freedom from progression rates were higher in arm A (79.5% and 91.4%) than in arm E (70.4%, P = .04, and 80%, P < .002). HD mortality (HDM), treatment-related mortality (TRM), and overall survival (OS) were similar in both arms (A, 2.1%, 7.5%, and 90.4%; B, 3.9%, 5.5%, and 90.3%). However TRM and OS rates were lower in patients aged 40 years or older (P < .005), reflecting the increasing incidence of background fatal events with increasing age. Finally, event-free survival (EFS) was higher in arm A (84.6%) than in arm E (74.9%, P < .02). In patients aged younger than 40 years in arm A (74%), 10-year EFS and OS rates were 88.9% and 95.4% with HDM and TRM rates as low as 0.7% and 3%. Three courses of ABVDm plus RT are the best available option for treating early or intermediate HD.
Introduction
Over the past 30 years, Hodgkin disease (HD) has been shown to be extremely sensitive to both radiation therapy (RT) and multiagent chemotherapy (CT). As a result, a large array of treatments, ranging from RT alone to various CT and to several combinations of CT and RT, has been offered to patients suffering from HD with increasing successes.1-3 However, these treatments have been shown to have long-term morbidity and mortality.4-12
The objective of this study was to evaluate in the setting of a randomized trial comparing 2 anthracycline-based CTs (one of which with a low-toxicity anthracycline and without alkylating agents) combined with the same RT, the 2 components of the 10-year mortality rate: that resulting from HD itself and that occurring in complete remission (CR) from fatal events attributable to the treatment.
In 1989, on the basis of the 5-year results of our previous trial (H81, 1981-1988, 407 patients with clinical stages [CSs] IA to IVB) combining 3 courses of ABVDm (Adriamycin, bleomycin, vinblastine, dacarbazine, and methylprednisolone) and high-dose extended RT,13-15 we identified a group of 264 patients with early/intermediate HD (ie, with CS IA to IIIB with a mediastinal mass ratio < 0.45 and without involvement of both pelvic and lumboaortic areas) who had 5-year freedom from progression and survival rates peaking at 95.1% and 95.9%, respectively; these figures were very significantly different (P < .0001) from those observed in the group of the 143 remaining patients with advanced HD (65.7% and 75.1%, respectively).
When we started preparing our H90-NM trial for early/intermediate HD, we were faced with the fact that it would be difficult to further improve the remarkable above-mentioned results. We, thus, decided to test in a randomized trial whether it would be possible to maintain these excellent figures with an initial CT made of a combination of drugs potentially less toxic than those of the ABVD association.
In ABVD, Adriamycin is known to cause severe or even fatal cardiac complications in some patients, particularly when the drug is combined with mediastinal RT10,16,17 ; moreover, dacarbazine, which is an alkylating agent,18 thus, could be potentially responsible for secondary tumors. On the basis of previous reports in which epirubicin or methotrexate were given in association with bleomycin and vinblastine (Velban) in HD treatment,19,20 we designed the EBVMm regimen, an association without any alkylating agent comprising epirubicin, bleomycin, vinblastine, methotrexate, and methylprednisolone. In 1990, we, thus, initiated the H90-NM randomized program (1990-1996), in which patients with early/intermediate HD were randomly assigned to 3 monthly courses of EBVMm (experimental arm) or ABVDm (reference arm). Patients of both arms enjoying complete or partial remission after CT received the same tailored high-dose extended RT as that given in our previous H81 trial. Besides recording usual end points such as response to CT and to RT, relapses, and deaths, we also prospectively recorded all severe complications and their outcome.
Here, we report the 10-year results of this randomized study that included 386 patients. The objective of the trial was to compare the freedom from progression and the HD mortality rates as well as the incidence of life-threatening events occurring in CR and their resulting mortality in both arms of the trial.
Patients and methods
Description of the H90-NM protocol, inclusion criteria
From January 1990 to December 1996, all consecutive patients aged 18 to 65 years with untreated early/intermediate HD recruited in the 14 French centers affiliated to the “Groupe Ouest-Est d'Etude des Leucémies et Autres Maladies du Sang” (GOELAMS) were enrolled in the H90-NM trial. Patients with early/intermediate HD were defined as those having clinical stage IA to IIIB with a mediastinal mass ratio less than 0.4514,21-23 (or no mediastinal involvement) and without involvement of both (unilateral or bilateral) pelvic and lumboaortic nodes.
Initial investigations included lymph node biopsy for diagnosis and histologic classification, physical examination, chest x-ray, bipedal lymphangiography (or abdominopelvic computerized tomography scan), bone marrow biopsy, and biologic evaluation, including blood count, sedimentation rate, and kidney and liver chemistries. A liver biopsy was performed in case of liver enlargement and/or a greater than 2-fold increase in serum alkaline phosphatase level. Clinical and radiologic examination of Waldeyer ring, with a biopsy when necessary, was performed in patients with upper cervical nodes. Patients were classified in CS IA to IIIB according to the Ann Arbor criteria.24 The mediastinal mass ratio (MMR; ie, the maximum mediastinal tumor width divided by the thoracic width at vertebra T6) was measured in all patients with mediastinal disease. The trial was conducted according to the guidelines of the Helsinki Declaration.
After verification of inclusion criteria (diagnosis of Hodgkin disease, age ≥ 18 and ≤ 65 years, CS IA-IIIB with a mediastinal mass ratio < 0.45 or no mediastinal mass, and without involvement of both pelvic and lumboaortic nodes, no history of malignancy except nonmelanoma skin tumors and carcinoma in situ of the cervix, no serious illness that would limit survival or preclude CT or RT, absence of pregnancy, and informed signed consent), patients were randomly assigned (center by center) by a phone call to the central secretary of GOELAMS into one of the following arms: arm A, 3 cycles of ABVDm; arm E, 3 cycles of EBVMm. Only one course of CT (ABVDm or EBVMm) was administered to patients with cervical, axillary, or inguinal CS IA with one pathologic node not exceeding 2 cm in diameter. Schedules and dosages of the 2 CT regimens are listed in Table 1. The dosage of each drug was reduced to two thirds when the leukocyte count was between 2 and 2.5 g/L and/or the platelet count was between 60 and 80 g/L; chemotherapy infusion was postponed for 1 week when leukocyte and/or platelet counts were less than 2 and/or 20 g/L, respectively.
Chemotherapies of the randomized H90-NM trial
Intravenous drugs . | Dose, mg/m2, d 1 and 14 . |
|---|---|
| Arm A (ABVDm), 1 cycle every 4 wk | |
| Adriamycin | 25 |
| Bleomycin | 10 |
| Vinblastine | 6 |
| Dacarbazine | 375 |
| Methylprednisolone | 120 |
| Arm E (EBVMm), 1 cycle every 4 wk | |
| Epirubicin | 30 |
| Bleomycin | 10 |
| Vinblastine | 6 |
| Methotrexate | 30 |
| Methylprednisolone | 120 |
Intravenous drugs . | Dose, mg/m2, d 1 and 14 . |
|---|---|
| Arm A (ABVDm), 1 cycle every 4 wk | |
| Adriamycin | 25 |
| Bleomycin | 10 |
| Vinblastine | 6 |
| Dacarbazine | 375 |
| Methylprednisolone | 120 |
| Arm E (EBVMm), 1 cycle every 4 wk | |
| Epirubicin | 30 |
| Bleomycin | 10 |
| Vinblastine | 6 |
| Methotrexate | 30 |
| Methylprednisolone | 120 |
Two weeks after the last infusion of CT, patients' status was assessed. Complete remission (CR) was defined as the complete disappearance of clinical or radiologic disease, partial remission (PR) as a 50% or more decrease of tumor burden, and failure as the persistence (< 50% decrease), progression, or reappearance of the disease. In case of failure to CT, patients received salvage CT combinations, followed by RT whenever possible.
Patients in CR or PR after CT were irradiated. RT started 4 to 5 weeks after the last infusion of CT. All patients were treated with megavoltage beam energy of 15 MV to 25 MV. RT of initially involved nodes was given at a daily dose of 1.8 Gy per day (by equally weighted parallel opposed anteroposterior-posteroanterior [AP-PA] fields), 9 Gy per week up to 40 Gy. Noninvolved sites received prophylactic RT (30 Gy).
In supradiaphragmatic CS I and II, the width of mediastinal radiation field was planned according to the situation observed after CT. Moreover, when the upper cervical areas were not initially involved, the upper limit of the RT field was the vertebra C3. When supraclavicular nodes were involved, homolateral axillary area(s) received prophylactic RT (30 Gy); however, the mediastinum was not irradiated when it was initially free of disease. However, all patients with supradiaphragmatic CS I and II received prophylactic RT (30 Gy) to the spleen and lumboaortic nodes (from vertebra T11/T12 to vertebra L3/L4).
In infradiaphragmatic CS I and II, unilateral or bilateral inguinal and pelvic areas (according to initial involvement) were irradiated (40 Gy), whereas the noninvolved lumboaortic area (up to T11/T12) and the spleen received prophylactic RT (30 Gy).
In CS III, supradiaphragmatic RT was the same as that given in CSI/II, except that the mediastinum was prophylactically irradiated (30 Gy) when not initially involved. However, infradiaphragmatic RT (40 Gy) encompassed the spleen and the lumboaortic area until vertebra L5/S1.
Patient status was reassessed 1 month after completing RT. Patients without clinical and radiologic disease were considered in CR. Patients with a residual mediastinal mass after RT were also considered in posttreatment CR, provided their residual mass had not re-increased within the 6 months after completion of RT. Other patients were considered as failures to RT.
Remission status was checked every 3 months during the first year, then twice a year until the fifth year, and once a year thereafter. At each follow-up consultation, the occurrence (or absence of occurrence) of the following events was carefully recorded and dated: type and site of second primary tumors (excluding nonmelanoma skin cancers and carcinoma in situ of the cervix), constrictive pericarditis, angina pectoris, myocardial infarction, cardiac dysrhythmia, chronic dyspnea, or severe lung or systemic infections. All other life-threatening or fatal events were also recorded. Relapse was defined as the reappearance of the signs of the disease; pathologic material obtained at the time of relapse (biopsy or fine needle aspiration) was examined in parallel with the initial material. Relapses were treated by salvage CT and RT whenever possible. The pathologic material of all secondary tumors was reviewed by a panel of pathologists coordinated by one of us (J.B.).
Statistics
Probabilities of freedom from progression (FFP), event-free survival (EFS), and overall survival (OS), as well as probabilities of HD mortality (HDM) and treatment-related mortality (TRM), were calculated from the starting date of CT. Patients who were lost to follow-up were censored at the date of their last examination. To calculate the probability of OS, all deaths were taken into account; for FFP, events were failures (to CT or RT) and relapses. For EFS, events were failures, relapses, and deaths in first CR whatever their cause. For HDM, only deaths from HD were considered as events, whereas patients who died of other causes were censored at the time of death; for TRM, events were restricted to deaths directly resulting from the treatment or from all fatal events potentially attributable to the treatment (whereas patients who died either of HD or of a cause clearly without any potential relation with HD treatment were censored). Probabilities of development of second tumors and cardiac events were calculated from the date of treatment completion. All the above-mentioned probabilities were calculated by the Kaplan-Meier method, and the differences were assessed by the log-rank test.25 The chi square test or, when appropriate, the Fisher exact test (2-tailed) was used to compare qualitative data. All computations were performed by using the SPSS 10.0 Package (SPSS, Paris, France).
Results
Characteristics of the patients and results of treatment
From January 1990 to December 1996, a total of 393 patients with untreated early/intermediate HD were enrolled onto the H90-NM multicenter trial in 14 French centers. Two patients older than 65 years, 2 patients younger than 18 years, and 2 patients with concurrent serious illness (myocardial infarction and alcoholic liver cirrhosis) who were wrongly randomly assigned were excluded from the study, as well as 1 patient who decided not to be treated according to the protocol after having been randomly assigned. The remaining 386 patients included in the H90-NM trial were assessable.
Initial characteristics of the patients are listed in Table 2. Median age was similar in both arms at 30.5 years. Patients randomly assigned in each of the 2 arms had well-balanced characteristics. Two hundred seventy-six (71.5%) patients were at CS IA-IIA; of them, 211 were at supradiaphragmatic CS IA or IIA with an MMR less than 0.33. Fifty-nine patients with peripheral CS IA with one node of less than 2 cm diameter received only one course of CT (arm A, 37; arm E, 22). The median duration of the planned treatment was 6 months. CT was administered at full dose to 379 patients (98.2%); the remaining 6 patients received 75% or more of the planned doses. In January 2003, the median follow-up of living patients was 98 months (range, 72-140 months), and 5 patients, all in first CR, were lost to follow-up 91 to 116 months after completion of treatment.
Initial characteristics of the 386 adult patients with early/intermediate Hodgkin disease included in the H90-NM trial
. | All . | Arm E (EBVMm-RT) . | Arm A (ABVDm-RT) . |
|---|---|---|---|
| Patients | 386 | 186 | 200 |
| Sex | |||
| Male | 206 | 90 | 116 |
| Female | 180 | 96 | 84 |
| Age | |||
| Younger than 40 years | 279 | 132 | 147 |
| 40 years and older | 107 | 54 | 53 |
| Histology | |||
| Nodular sclerosis | 260 | 132 | 128 |
| Other subtypes | 126 | 54 | 72 |
| Clinical stages | |||
| IA | 81 | 33 | 48 |
| IIA | 195 | 95 | 100 |
| IIIA | 25 | 12 | 13 |
| IB-IIB | 65 | 36 | 29 |
| IIIB | 20 | 10 | 10 |
| Clinical presentation | |||
| Supradiaphragmatic CS I/II: | |||
| With MMR less than 0.33: | |||
| No symptoms | 211 | 96 | 115 |
| Symptoms | 46 | 26 | 20 |
| MMR 0.33 and greater and less than 0.45 | 64 | 34 | 30 |
| Infradiaphragmatic CS I/II | 20 | 8 | 12 |
| CS III | 45 | 22 | 23 |
| Contiguous extra nodal involvement | |||
| No | 371 | 179 | 192 |
| Yes | 15 | 7 | 8 |
. | All . | Arm E (EBVMm-RT) . | Arm A (ABVDm-RT) . |
|---|---|---|---|
| Patients | 386 | 186 | 200 |
| Sex | |||
| Male | 206 | 90 | 116 |
| Female | 180 | 96 | 84 |
| Age | |||
| Younger than 40 years | 279 | 132 | 147 |
| 40 years and older | 107 | 54 | 53 |
| Histology | |||
| Nodular sclerosis | 260 | 132 | 128 |
| Other subtypes | 126 | 54 | 72 |
| Clinical stages | |||
| IA | 81 | 33 | 48 |
| IIA | 195 | 95 | 100 |
| IIIA | 25 | 12 | 13 |
| IB-IIB | 65 | 36 | 29 |
| IIIB | 20 | 10 | 10 |
| Clinical presentation | |||
| Supradiaphragmatic CS I/II: | |||
| With MMR less than 0.33: | |||
| No symptoms | 211 | 96 | 115 |
| Symptoms | 46 | 26 | 20 |
| MMR 0.33 and greater and less than 0.45 | 64 | 34 | 30 |
| Infradiaphragmatic CS I/II | 20 | 8 | 12 |
| CS III | 45 | 22 | 23 |
| Contiguous extra nodal involvement | |||
| No | 371 | 179 | 192 |
| Yes | 15 | 7 | 8 |
MMR indicates mediastinal mass ratio (defined in “Patients and methods”).
Treatment results are summarized in Table 3. After completion of CT, 290 patients entered in CR, 159 in arm A (79.5%) and 131 in arm E (70.4%, P = .04). At completion of RT, which was given to the 372 CT-responding patients, 96% of patients in arm A and 94.6% of patients in arm E were in CR (P = NS). No severe toxicity occurred during CT or RT.
Treatment results of the 386 patients with early/intermediate Hodgkin's disease included in the H90-NM trial
. | All patients . | Arm E (EBVMm-RT) . | Arm A (ABVDm-RT) . |
|---|---|---|---|
| Patients submitted to CT | 386 | 186 | 200 |
| Complete remission after CT | 290 (75.1%) | 131 (70.4%) | 159 (79.5%) |
| Partial remission after CT | 82 | 48 | 34 |
| Failure to CT | 14 | 7 | 7 |
| Patients submitted to RT | 372 | 179 | 193 |
| Patients submitted to RT after CR/PR after CT | 290/82 | 131/48 | 159/34 |
| Complete remission after RT | 368 (95.3%) | 176 (94.6%) | 192 (96.0%) |
| Failure to RT | 4 | 3 | 1 |
| Patients submitted to salvage therapy | 18 | 10 | 8 |
| Patients submitted to salvage therapy after failure to CT/RT | 14/4 | 7/3 | 7/1 |
| Alive with sustained CR | 11 | 6 | 5 |
| Deceased in CR | 1 | 0 | 1 |
| Deceased with active disease | 6 | 4 | 2 |
| Patients followed after CR after RT | 368 | 176 | 192 |
| Alive with sustained first CR | 317 | 144 | 173 |
| Deceased in first CR | 17 | 6 | 11 |
| Relapsing after first CR | 34 | 26 | 8 |
| Alive with sustained second CR | 27 | 22 | 5 |
| Deceased in second CR | 2 | 1 | 1 |
| Deceased with active disease after relapse | 5 | 3 | 2 |
. | All patients . | Arm E (EBVMm-RT) . | Arm A (ABVDm-RT) . |
|---|---|---|---|
| Patients submitted to CT | 386 | 186 | 200 |
| Complete remission after CT | 290 (75.1%) | 131 (70.4%) | 159 (79.5%) |
| Partial remission after CT | 82 | 48 | 34 |
| Failure to CT | 14 | 7 | 7 |
| Patients submitted to RT | 372 | 179 | 193 |
| Patients submitted to RT after CR/PR after CT | 290/82 | 131/48 | 159/34 |
| Complete remission after RT | 368 (95.3%) | 176 (94.6%) | 192 (96.0%) |
| Failure to RT | 4 | 3 | 1 |
| Patients submitted to salvage therapy | 18 | 10 | 8 |
| Patients submitted to salvage therapy after failure to CT/RT | 14/4 | 7/3 | 7/1 |
| Alive with sustained CR | 11 | 6 | 5 |
| Deceased in CR | 1 | 0 | 1 |
| Deceased with active disease | 6 | 4 | 2 |
| Patients followed after CR after RT | 368 | 176 | 192 |
| Alive with sustained first CR | 317 | 144 | 173 |
| Deceased in first CR | 17 | 6 | 11 |
| Relapsing after first CR | 34 | 26 | 8 |
| Alive with sustained second CR | 27 | 22 | 5 |
| Deceased in second CR | 2 | 1 | 1 |
| Deceased with active disease after relapse | 5 | 3 | 2 |
CR indicates complete remission; CT, chemotherapy; RT, radiotherapy; PR, partial remission.
Freedom from progression and HD mortality
The 14 patients who failed to respond to CT and the 4 patients who did not respond to RT were given salvage therapies. Twelve of them obtained a sustained CR among whom one died; the 6 others achieved transient CR or even no CR and eventually died of HD (Table 3). Of the 368 patients who reached CR after the combined modality treatment, 34 relapsed (8 in arm A and 26 in arm E, P = 0.01, log rank) after 8 to 110 months (median, 26 months) of CR.
After retreatment, 29 entered in permanent CR (among whom 2 died), whereas the 5 others died of HD (Table 3). Of the 92 patients of both arms whose mediastinum was not irradiated, 2 suffered from a mediastinal relapse; both of them entered in sustained second CR after salvage CT and mediastinal RT.
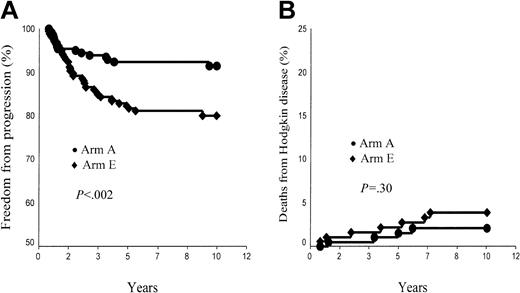
Overall, the FFP rate was 91.4% ± 2.1% in arm A and 80.0% ± 3.0% in arm E (P < .002; Figure 1A). In supradiaphragmatic CS IA or IIA with an MMR less than 0.33, FFP rates were 93.9% ± 2.2% in arm A and 82.5% ± 4.1% in arm B (P = .016). However, patients of each arm with CR after CT had a significantly higher 10-year FFP rate than those patients who did not reach CR after CT: 94.3% ± 2.1% versus 80.5% ± 6.2% in arm A (P = .001) and 85.4% ± 3.3% versus 67.2% ± 6.4% in arm E (P = .001).
Ten-year freedom from progression (FFP) and Hodgkin disease mortality (HDM) rates. (A) FFP, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) HDM, arm A (ABVD-RT) versus arm E (EBVM-RT).
Ten-year freedom from progression (FFP) and Hodgkin disease mortality (HDM) rates. (A) FFP, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) HDM, arm A (ABVD-RT) versus arm E (EBVM-RT).
A total of 11 patients died of HD, 6 after failing to initial CT or RT and 5 after a relapse (Table 3). HDM rate was, thus, 2.1% ± 1.0% and 3.9% ± 1.5% in arms A and E, respectively (P = NS; Figure 1B). In supradiaphragmatic CS IA or IIA with a MMR less than 0.33, HDM rates were 0% in arm A and 1.0% ± 1.0% in arm B (P = NS). In arm A, the post-CT status did not influence HDM rate (CR versus no CR, 1.3% ± 1% versus 4.9% ± 2.9%, P = .14), whereas it was a significant prognosis marker in arm E (CR versus no CR, 0.8% ± 0.8% versus 11.2% ± 4%, P < .001). In arm A, HDM was lower in patients aged younger than 40 years (0.7% ± 0.7%) than in patients older than this age (5.9% ± 3.2%, P = .02), whereas HDM of patients of arm E was identical in both age groups (3.8% ± 1.7% versus 4.1% ± 2.8%, respectively).
Life-threatening events and treatment mortality rates
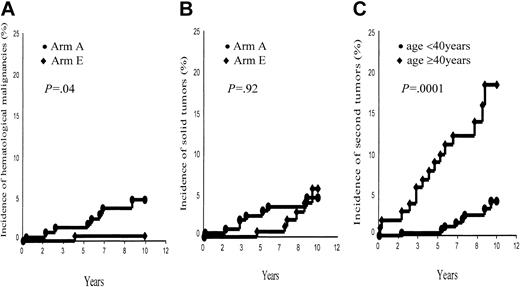
Second cancers. A total of 23 patients (arm A, 16; arm E, 7) developed a second cancer: 22 of them were in first CR when their second malignancy developed (Table 4). However, 2 second tumors (one breast cancer and one non-Hodgkin lymphoma [NHL]) occurred 2 and 3 months after completion of HD treatment and, thus, were not associated with HD treatment. Nine patients (all in first CR) suffered from a hematologic malignancy; after having excluded the above-mentioned NHL, this corresponds to a 10-year probability of 2.6% ± 0.9% for the whole group, higher in arm A (7 events, 4.5% ± 1.7%) than in arm E (1 event, 0.6% ± 0.6%, P = 0.04; Figure 2A). Sex of the patients was also predictive of the development of a hematologic malignancy (M, 8 events, 4.8% ± 1.7%, versus F, no event, P = .007).
Characteristics of the patients having developed a second primary neoplasm in the H90-NM trial
Arm . | Sex, age, y, and clinical stage at HD diagnosis . | Time from completion of therapy to tumor diagnosis, mo . | Type of malignancy . | Tumor developed in an irradiated area . | Survival from tumor diagnosis, mo . |
|---|---|---|---|---|---|
| Hematologic malignancies | |||||
| A | M, 25, IIA | 68 | Myelodysplasia | No | 55 |
| A | M, 38, IA | 106 | Chronic myelomonocytic leukemia | No | 12 |
| A | M, 38, IIIA | 77 | Acute nonlymphocytic leukemia | No | 23* |
| A | M, 39, IIA | 64 | Non-Hodgkin lymphoma | No | 30* |
| A | M, 43, IIA | 32 | Acute nonlymphocytic leukemia | No | 40 |
| A | M, 45, IIA | 75 | Non-Hodgkin lymphoma | No | 20 |
| A | M, 55, IIA | 23 | Non-Hodgkin lymphoma | Cervical | 70 |
| A† | F, 62, IIA | 3 | Non-Hodgkin lymphoma | No | 54* |
| E | M, 65, IIA | 51 | Non-Hodgkin lymphoma | No | 32 |
| Solid tumors | |||||
| A | M, 32, IA | 23 | Malignant melanoma | No | 100 |
| A† | F, 42, IIA | 2 | Breast | Mediastinal | 56* |
| A | M, 49, IIA | 44 | Esophagus | Mediastinal | 3* |
| A | M, 58, IIA | 107 | Lung | No | 1* |
| A | F, 59, IIA | 38 | Breast | Mediastinal | 44 |
| A‡ | M, 60, IIA | 38 | Bladder | No | 9* |
| A | M, 64, IA | 67 | Leiomyosarcoma | Axillary | 20* |
| A | M, 65, IIA | 61 | Colon | No | 10* |
| E | F, 18, IIA | 85 | Thyroid | Mediastinal | 24 |
| E | F, 23, IIA | 112 | Glioma | No | 3 |
| E | F, 39, IIA | 87 | Breast | Mediastinal | 17 |
| E | M, 46, IIA | 105 | Lung | Mediastinal | 7* |
| E | M, 46, IIIB | 56 | Larynx | Cervical | 8* |
| E | M, 50, IIIB | 97 | Lung | Mediastinal | 24* |
Arm . | Sex, age, y, and clinical stage at HD diagnosis . | Time from completion of therapy to tumor diagnosis, mo . | Type of malignancy . | Tumor developed in an irradiated area . | Survival from tumor diagnosis, mo . |
|---|---|---|---|---|---|
| Hematologic malignancies | |||||
| A | M, 25, IIA | 68 | Myelodysplasia | No | 55 |
| A | M, 38, IA | 106 | Chronic myelomonocytic leukemia | No | 12 |
| A | M, 38, IIIA | 77 | Acute nonlymphocytic leukemia | No | 23* |
| A | M, 39, IIA | 64 | Non-Hodgkin lymphoma | No | 30* |
| A | M, 43, IIA | 32 | Acute nonlymphocytic leukemia | No | 40 |
| A | M, 45, IIA | 75 | Non-Hodgkin lymphoma | No | 20 |
| A | M, 55, IIA | 23 | Non-Hodgkin lymphoma | Cervical | 70 |
| A† | F, 62, IIA | 3 | Non-Hodgkin lymphoma | No | 54* |
| E | M, 65, IIA | 51 | Non-Hodgkin lymphoma | No | 32 |
| Solid tumors | |||||
| A | M, 32, IA | 23 | Malignant melanoma | No | 100 |
| A† | F, 42, IIA | 2 | Breast | Mediastinal | 56* |
| A | M, 49, IIA | 44 | Esophagus | Mediastinal | 3* |
| A | M, 58, IIA | 107 | Lung | No | 1* |
| A | F, 59, IIA | 38 | Breast | Mediastinal | 44 |
| A‡ | M, 60, IIA | 38 | Bladder | No | 9* |
| A | M, 64, IA | 67 | Leiomyosarcoma | Axillary | 20* |
| A | M, 65, IIA | 61 | Colon | No | 10* |
| E | F, 18, IIA | 85 | Thyroid | Mediastinal | 24 |
| E | F, 23, IIA | 112 | Glioma | No | 3 |
| E | F, 39, IIA | 87 | Breast | Mediastinal | 17 |
| E | M, 46, IIA | 105 | Lung | Mediastinal | 7* |
| E | M, 46, IIIB | 56 | Larynx | Cervical | 8* |
| E | M, 50, IIIB | 97 | Lung | Mediastinal | 24* |
Patient deceased.
Not counted as an event in actuarial calculation of second malignancy risk.
Patient retreated after failure to initial chemotherapy.
Ten-year incidence of second tumors. (A) Second hematologic malignancies, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) Solid tumors, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (C) All second tumors, aged younger than 40 years versus aged 40 years and older.
Ten-year incidence of second tumors. (A) Second hematologic malignancies, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) Solid tumors, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (C) All second tumors, aged younger than 40 years versus aged 40 years and older.
However, a solid tumor developed in 14 patients (9 deaths); once having excluded the above-mentioned breast cancer, this corresponds to a 10-year incidence of 4.9% ± 1.4%, without any difference between arms A and E (4.2% ± 1.6% and 5.8% ± 2.4%, respectively, P = .92; Figure 2B). Sex was not predictive of a solid tumor (P = .5), whereas age at treatment (< 40 years versus ≥ 40 years) was a significant prognostic factor of the incidence of a second tumor (4.1% ± 1.5% versus 16.6% ± 4.6%, P = .0001; Figure 2C) in each of the 2 arms (arm A, 4.2% ± 1.9% versus 20.9% ± 6.6%, P = .0003; arm B, 4.2% ± 2.5% versus 12.3% ± 6.2%, P = .05).
Cardiac complications. Eighteen patients (8 in arm A and 10 in arm E) developed a cardiac event (2 deaths): 14 were in first CR, 2 were in CR after initial salvage treatment, and the other 2 were in second CR after a relapse (Table 5). The 10-year probability of developing a cardiac event was 5.6% ± 1.3% without any difference between both arms (A, 4.5% ± 1.6%; E, 7.0% ± 2.2%; P = .51).
Characteristics of the patients having developed a cardiac event or other life-threatening or fatal event in the H90-NM trial
Arm . | Sex, age, y, and clinical stage at HD diagnosis . | Time from completion of therapy to event, mo . | Event developed in an irradiated area . | Type of event . | Survival from event, mo . |
|---|---|---|---|---|---|
| Cardiac events | |||||
| A | F, 26, IIIA | 66 | Yes | Myocardial infarction | 63 |
| A | M, 34, IIB | 31 | No | Myocardial infarction | 107 |
| A | F, 35, IIA | 19 | Yes | Myocardial infarction | 76 |
| A | M, 36, IIA | 35 | Yes | Myocardial infarction | 81 |
| A | M, 36, IIA | 60 | No | Myocardial infarction | 28 |
| A | M, 37, IA | 94 | Yes | Myocardial infarction | 0* |
| A | F, 40, IIA | 73 | Yes | Myocardial infarction | 2 |
| +A | M, 57, IIA | 42 | Yes | Myocardial infarction | 55 |
| E | M, 19, IIIA | 104 | Yes | Myocardial infarction | 39 |
| E | F, 23, IIB† | 4 | Yes | Congestive heart failure | 96 |
| E | M, 36, IIB† | 99 | Yes | Myocardial infarction | 14 |
| E | M, 42, IIA | 75 | Yes | Angina pectoris | 37 |
| E | M, 45, IIA† | 47 | Yes | Angina pectoris | 59 |
| E | M, 46, IIIB | 46 | Yes | Myocardial infarction | 19‡ |
| E | M, 60, IIB | 74 | Yes | Dysrhythmia | 62 |
| E | M, 65, IIIB† | 31 | Yes | Myocardial infarction | 0* |
| E | M, 65, IIA | 90 | Yes | Angina pectoris | 15* |
| E | F, 65, IA | 2 | Yes | Myocardial infarction | 88 |
| Other events | |||||
| A | M, 45, IA† | 15 | No | Stroke | 0* |
| A | M, 49, IIIB | 36 | Yes | Postoperative sepsis | 1* |
| E | F, 58, IIIB | 8 | No | Multifocal leukoencephalitis | 1* |
Arm . | Sex, age, y, and clinical stage at HD diagnosis . | Time from completion of therapy to event, mo . | Event developed in an irradiated area . | Type of event . | Survival from event, mo . |
|---|---|---|---|---|---|
| Cardiac events | |||||
| A | F, 26, IIIA | 66 | Yes | Myocardial infarction | 63 |
| A | M, 34, IIB | 31 | No | Myocardial infarction | 107 |
| A | F, 35, IIA | 19 | Yes | Myocardial infarction | 76 |
| A | M, 36, IIA | 35 | Yes | Myocardial infarction | 81 |
| A | M, 36, IIA | 60 | No | Myocardial infarction | 28 |
| A | M, 37, IA | 94 | Yes | Myocardial infarction | 0* |
| A | F, 40, IIA | 73 | Yes | Myocardial infarction | 2 |
| +A | M, 57, IIA | 42 | Yes | Myocardial infarction | 55 |
| E | M, 19, IIIA | 104 | Yes | Myocardial infarction | 39 |
| E | F, 23, IIB† | 4 | Yes | Congestive heart failure | 96 |
| E | M, 36, IIB† | 99 | Yes | Myocardial infarction | 14 |
| E | M, 42, IIA | 75 | Yes | Angina pectoris | 37 |
| E | M, 45, IIA† | 47 | Yes | Angina pectoris | 59 |
| E | M, 46, IIIB | 46 | Yes | Myocardial infarction | 19‡ |
| E | M, 60, IIB | 74 | Yes | Dysrhythmia | 62 |
| E | M, 65, IIIB† | 31 | Yes | Myocardial infarction | 0* |
| E | M, 65, IIA | 90 | Yes | Angina pectoris | 15* |
| E | F, 65, IA | 2 | Yes | Myocardial infarction | 88 |
| Other events | |||||
| A | M, 45, IA† | 15 | No | Stroke | 0* |
| A | M, 49, IIIB | 36 | Yes | Postoperative sepsis | 1* |
| E | F, 58, IIIB | 8 | No | Multifocal leukoencephalitis | 1* |
Patient is deceased.
Patient has received a second-line anthracycline-based chemotherapy.
Patient died of larynx cancer (see Table 4).
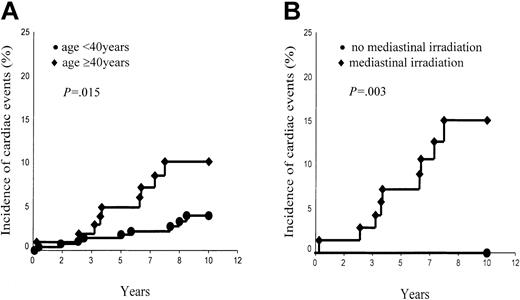
However, the incidence of cardiac events was significantly associated with the age of patients at HD treatment (< 40 years, 4.0% ± 1.3% versus ≥ 40 years, 10.2% ± 3.3%; P = .015; Figure 3A) and marginally with their sex (male, 7.9% ± 2.2% versus female, 2.9% ± 1.3%; P = .10). Of the 52 patients who received a second treatment (which included Adriamycin in most of the cases) 4 developed a cardiac event versus 14 among the 334 patients who had only one treatment (P = .93, log rank). However, the probability of a cardiac event was higher (without reaching statistical significance) in the group of patients whose mediastinum was irradiated (6.7% ± 1.6%) than in the group whose mediastinum was not irradiated (2.3% ± 1.6%, P = .20). However, this difference became statistically significant in the group of patients aged 40 years or older at treatment. Those patients who had their mediastinum irradiated had a 10-year risk of cardiac events as high as 15.0% ± 4.8%, whereas those patients whose mediastinum was not irradiated have not developed any cardiac event thus far (P = .03; Figure 3B).
Ten-year incidence of cardiac events. (A) Aged younger than 40 years versus aged 40 years and older. (B) Patients aged 40 years and older with mediastinal irradiation versus patients aged 40 years and older without mediastinal irradiation.
Ten-year incidence of cardiac events. (A) Aged younger than 40 years versus aged 40 years and older. (B) Patients aged 40 years and older with mediastinal irradiation versus patients aged 40 years and older without mediastinal irradiation.
Other complications. No patient has developed constrictive pericarditis, chronic dyspnea, severe lung infection, or systemic infection so far (Table 5). However, one patient died of a stroke with normal blood counts after having completed the treatment of his relapse. Another patient who had a limited pachypleuritis of the left costodiaphragmatic pleura (resulting from splenic RT) was (wrongly) operated on and died in first CR of a postoperative sepsis. Finally, one patient, who was splenectomized years before HD diagnosis after a car accident, died in first CR from a multifocal leukoencephalitis of unknown origin. Overall, 12 patients died of a second tumor (Table 4), 3 of a cardiac event, and 3 of the above-mentioned fatal events (Table 5).
The 10-year TRM probability was thus 7.5% ± 2.1% in arm A and 5.5% ± 2.4% in arm E (P = NS; Figure 4A). In supradiaphragmatic CS IA or IIA with an MMR less than 0.33, TRM rates were 9.8% ± 3.2% in arm A and 2.0% ± 1.9% in arm B (P = .02). The age of patients at HD treatment (< 40 years versus ≥ 40 years) was a strong predictor of the TRM incidence (all patients, 1.6% ± 0.9% versus 20.8% ± 5.5%, P = .0001; Figure 4B; arm A, 3.0% ± 1.7% versus 19.5% ± 6.7%, P = .0001; arm E, 0% versus 22.2% ± 9.5%, P < .0001).
Treatment-related mortality. (A) Arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) Age younger than 40 years versus age 40 years and older.
Treatment-related mortality. (A) Arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) Age younger than 40 years versus age 40 years and older.
Overall survival
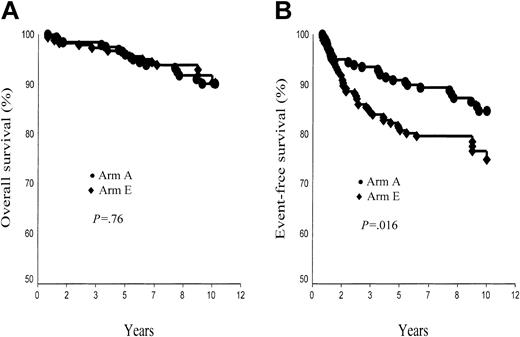
A total of 31 patients died: 11 of HD; 18 (15 in first CR) of a second tumor, a cardiac event, or another treatment-related or potentially treatment-related event; and the last 2 (both in first CR) of an unrelated cause (murder, car accident). Of note, the fact that in January 2003 all living patients were in CR, 10-year survival rate was thus 90.4% ± 2.3% in arm A and 90.3% ± 2.7% in arm E (P = NS; Figure 5A). In supradiaphragmatic CS IA or IIA with an MMR less than 0.33, corresponding figures were 89.4% ± 3.3% in arm A and 96.0% ± 2.4% in arm B (P = .10). Survival rate was strongly associated with the age of patients at treatment, higher in patients younger than 40 years than in patients 40 years or older (arm A, 95.7% ± 1.9% versus 74.3% ± 6.8%, P < .0001; arm E, 95.4% ± 1.8% versus 74.7% ± 9.6%, P = .01). However the post-CT status (CR versus no CR) was a significant predictor of survival in arm E (P = .01) but not in arm A (P = .3).
Ten-year overall survival (OS) and event-free survival (EFS) rates. (A) OS, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) EFS, arm A (ABVD-RT) versus arm E (EBVM-RT).
Ten-year overall survival (OS) and event-free survival (EFS) rates. (A) OS, arm A (ABVDm-RT) versus arm E (EBVMm-RT). (B) EFS, arm A (ABVD-RT) versus arm E (EBVM-RT).
Event-free survival
A total of 52 patients either failed to respond to CT or RT or relapsed after completion of treatment (arm A, 16; arm E, 36), and 17 other patients died in first CR (arm A, 11; arm E, 6) (Table 3). The 10-year probability of EFS was thus 84.6% ± 2.8% in arm A and 74.9% ± 3.6% in arm E (P = .016; Figure 5B). In supradiaphragmatic CS IA or IIA with MMR less than 0.33, EFS rates were 84.9% ± 3.7% in arm A and 79.7% ± 4.4% in arm B (P = .24).
The age of patients (< 40 years versus ≥ 40 years) was significantly correlated with EFS in arm A (88.9% ± 2.9% versus 72.2% ± 7%, P = .005) but not in arm E (80.6% ± 3.5% versus 58.7% ± 9.8%, P = .17). Moreover, patients of each arm who enjoyed CR after CT had higher EFS than those who did not enter in CR after CT: arm A, 87.5% ± 3.0% versus 73.3% ± 7.6%, P = .011; arm E, 79.7% ± 4.1% versus 63.6% ± 6.9%, P = .005.
Discussion
In this large group of patients with early or intermediate HD, we first explored in the setting of a randomized trial whether 3 courses of EBVMm or ABVDm, followed by the same RT, resulted in similar rates of CR and FFP. Our results showed that this was not the case. EBVMm induced a significantly lower rate of CR than ABVDm. Moreover, despite enjoying similar rates of CR after RT in both arms, patients of the EBVMm arm had higher relapse rate and lower FFP and EFS rates than patients of the ABVDm arm. EBVMm is thus clearly a less potent multiagent CT than ABVDm. A randomized trial comparing methotrexate cyclophosphamide, bleomycin, and prednisolone plus Adriamycin (50 mg/m2) or epirubicin (75 mg/m2, ie, + 50%) in a large group of intermediate- and high-grade non-Hodgkin lymphomas showed that both associations had the same incidence of adverse effects as well as the same CR rates (58.1% versus 58.5%).26 In our H90-NM trial, the dose of epirubicin (30 mg/m2) was only 20% higher than that of Adriamycin (25 mg/m2). The lower efficacy of EBVMm versus ABVDm was thus likely, resulting partly from an insufficient dose of epirubicin and possibly from the lower efficacy of methotrexate compared with dacarbazine.
In both arms of our trial, entering in CR after initial CT had also a favorable effect on FFP and EFS rates. The prognostic value of the result of the first 3 courses of CT has been shown 20 years ago in patients treated by MOPP (Mustargen, Oncovin, procarbazine, prednisone) followed by RT27 and was confirmed more recently by our group in patients treated by ABVD combined with RT.14,21 This information underlines the importance of taking into account this intermediate end point to direct the second part of the treatment whether it is RT or CT. It remains fortunate that most of the patients treated with EBVMm who did not enter in CR after CT or relapsed after completion of RT enjoyed a second sustained CR after salvage therapies; as a result, patients of both arms had finally the same HDM and OS rates.
The other important objective of this trial was to observe the long-term complications and the resulting mortality rates associated with this combined modality program. Concerning second tumors, studies gathering large numbers of patients with HD and with follow-up reaching sometimes more than 20 years have brought very robust information.6,7,11,28-32 However, the results of this trial are of particular interest because, besides that all severe complications were prospectively recorded, it is to our knowledge the first study comparing a CT (EBVMm) that did not contain any alkylating agent with the reference ABVDm combination that contained dacarbazine, which is an alkylating agent.18
Importantly, we showed that second hematologic malignancies, all of which occurred in male patients and in first CR, had a significantly lower incidence in the EBVMm arm than in the ABVDm arm (P = .04). No secondary leukemia occurred in the EBVMm-RT arm (no alkylating agent), which is in keeping with the very low rate (≤ 0.5%) observed after RT alone.29,30,33 In contrast, 4 secondary leukemias (2 acute nonlymphoblastic leukemias [ANLLs], 1 myelodysplastic syndrome, 1 chronic myelomonocytic leukemia) developed after ABVDm-RT, which corresponds to an incidence of 2.7% ± 1.4% (P = .05). Thus far, it was known that the combination of ABVDm plus RT was less leukemogenic than that of MOPP (an association including 2 alkylating agents, mechlorethamine and procarbazine) plus RT.34 This randomized study demonstrated that the leukemogenic effect of ABVDm plus RT was, however, significantly higher than that of EBVMm plus RT. ABVD alone is almost not leukemogenic,35 but, when alkylating agent(s) is (are) associated with RT, the rate of second leukemia has been shown to be dependent of the extent of RT.34
However, EBVMm combined with high-dose extended RT was associated with a very low 10-year rate of non-Hodgkin lymphomas (1 case, ie, 0.6% ± 0.6%), which is not in favor of the idea that NHLs could be part of the natural history of HD or of an underlying immunodeficiency as suggested by others.36,37 A review on the occurrence of NHLs after HD is in keeping with our observation; combined modality therapy such as MOPP-RT was shown to result in an increased risk of NHLs compared with RT alone.30 Moreover, it was also suggested that CTs comprising one alkylating agent resulted in lower risk of NHLs than CTs comprising 2 alkylating agents.30 Interestingly, no case of NHL has so far occurred after the 70th month following completion of treatment (344 patients still at risk). This finding suggests that the pattern of secondary NHL development would possibly resemble that of secondary ANLL. Longer follow-up of this randomized cohort of patients is needed to have a clearer answer to this question.
However, the incidence of solid tumors was similar in both arms of the trial; this incidence most likely reflects that the additional carcinogenic role of dacarbazine is very weak in comparison to the role of high-dose extended RT.38-42 The 10-year rate of solid tumors observed in this prospective trial is indeed in the range of what has been reported in a much larger cohort of patients with longer follow-up.32 As others, we observed the significant effect of the age of patients (< 40 years versus ≥ 40 years) at HD treatment on the development of solid tumors; this is, to a large extent, the result of the increasing incidence of background tumors with increasing age.31
Life-threatening or fatal cardiac events were suspected to be associated with mediastinal irradiation for decades.43 This suspicion was confirmed by our study in which all severe complications have been prospectively recorded. Overall, the cumulative rate of cardiac events approached 6% by 10 years. The age of the patients at HD treatment was predictive of a cardiac event with a lower risk when the treatment was applied before the age of 40 years (4.0% ± 1.4%) and a significantly higher risk after this age (10.2% ± 3.3%, P = .015), confirming previous findings.44 The increase of risk with age at treatment has been demonstrated to result at least partly from background cardiac events, the incidence of which increases with increasing age.12 However, the effect of RT, when given at 40 years or older, was clearly demonstrated in this trial in which the incidence of cardiac events was significantly higher in the patients whose mediastinum was irradiated (9 events, 5.0% ± 4.8%) than in those whose mediastinum was not irradiated (no event, P = .032), which is also in keeping with previous observations.4-12 Overall, our results confirm the predominant role of high-dose RT and the weak effect of anthracyclines (at the doses they were given in our trial) on the genesis of cardiac complications.
Importantly, most solid tumors as well as most cardiac events developed in the mediastinal irradiated area (40 Gy) and none in prophylactically irradiated areas (30 Gy). Limiting RT to involved fields without modifying its dose (40 Gy), thus, would probably not decrease the rate of second solid tumors or cardiac events. In contrast, it would certainly increase the rate of lumboaortic/splenic relapses, which were prevented to occur in this trial by prophylactic lumboaortic and splenic RT that sterilize occult infradiaphragmatic disease in supradiaphragmatic CS I/II HD.45
The preservation of the low rate of HDM observed in this trial and, at the same time, the decrease of the incidence of second tumors and/or cardiac events could only be achieved by increasing the efficacy of CT, either by increasing its number of cycles46 or by increasing its dose.47 In patients enjoying CR, this achievement would allow clinicians to sterilize occult disease and at the same time to decrease the dose of RT (limited to initially involved areas), or even to completely eliminate RT.48,49 These strategies should be adequately tested in the setting of large randomized trials.
Overall, it is of great concern to observe the high incidence of mortality resulting from second tumors, cardiac events, and other life-threatening events. Such an incidence reached 4.4% by 10 years, and the slope of the curve strongly suggests that this incidence should continue to increase with increasing lengths of follow-up as recently shown in cohorts of patients followed 20 or more years.12,32 Importantly, TRM was significantly associated with age of patients at treatment in both arms of the trial. In the group of patients treated at the age of 40 or older, the 10-year risk of dying of an event potentially attributable to the treatment reached 20% (19.5% in arm A and 22.2% in arm E), which reflects the increasing incidence of mortality from background events (which would have occurred in the absence of treatment) with increasing age, as it has been repetitively demonstrated.11,12,32 Thus, in this age group (28% of our patients), although HDM was low (5.9% in arm A and 4.1% in arm E), OS was only 74.3% in arm A and 74.7% in arm E. In contrast, in the group of patients treated before the age of 40 years (72% of our cohort), HDM rates were similarly low (0.7% in arm A and 3.8% in arm E), but TRM rates were very low (3% in arm A, 0% in arm E) because of the absence of background fatal events in this age group. On the basis of HDM and TRM, there was, thus, no final advantage of one arm over the other, which was also shown by 10-year survival rates of arms A and E, which were the same (95.7% and 95.4%, respectively). However, EFS (ie, no failure to CT or RT, no relapse, and no death in first CR), which is the most frequently used index of the success or failure of a treatment, is significantly higher in arm A (84.6%) than in arm E (74.9%, P = .016). Three courses of ABVDm plus RT remains, thus, the best available option to treat early/intermediate HD.
We have now reached a point in the history of CS I and II HD treatment at which it has become clear that combining CT and RT is a better option than RT alone. The validity of this combination has been shown by several groups50-52 and most recently by the 3-year FFS results of a large randomized trial comparing in early HD (supradiaphragmatic CS IA or IIA with a MMR < 0.33) 3 courses of Adriamycin plus vinblastine combined with subtotal RT (EFS, 94%) versus subtotal RT alone (EFS, 81%, P = .001).53 Three-year EFS rates of our H90-NM study for patients with similar characteristics were the following: arm A, 95%; arm E, 90% (P = NS). Improving these results will be a difficult task with currently available methods of treatment. For the present time, it remains that the only way to reduce the worrying rate of mortality resulting from cardiac events or second cancers is to disseminate specific guidelines to concerned people (patients, general practitioners, hematologists, oncologists, radiation therapists), indicating the necessity of a life-time follow-up for all patients with HD and particularly for those treated at the age of 40 or older. The guidelines should include systematic and iterative programs of early detection of cancers and heart diseases by appropriate means as well as the fight against smoking (which is known to increase background tumors and cardiac events).
Supported by a research grant from the Association de recherche sur les maladies tumorales et virales (AREMAS), Paris, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-05-1611.
We thank Dr Malika Djeridane for excellent assistance in data collection and technical help in several computer techniques.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal