Abstract
To investigate the role of Epstein-Barr virus (EBV) in the pathogenesis of primary effusion lymphoma (PEL), we infected human herpesvirus 8 (HHV-8+) but EBV- PEL lines BC-3, CRO-AP/6, and CRO-AP/3 cells with the recombinant Akata EBV strain. All EBV-infected clones expressed EBER-1, EBNA-1, and LMP2A. The expression of LMP1 and LMP2B was variable. None, however, expressed EBNA2-6. The surface markers CD30, CD74, and syndecan-1 were down-regulated in EBV convertants. EBV-infected BC-3 and CRO-AP/6 cells were highly tumorigenic in severe combined immunodeficiency (SCID) mice in contrast to their respective EBV- parental cells. However, neither the parental cells nor the virusconverted counterparts expressed TCL1. The results showing that PEL cells on in vitro EBV infection do not sustain latency III despite the absence of immune pressure indicate that the choice of EBV latent gene expression program is cell dependent. The data suggest an important role of EBV in the pathogenesis of PEL.
Introduction
Primary effusion lymphoma (PEL) is a unique lymphomatous tumor of body cavities. The hallmark of this tumor is its close association with human herpesvirus 8 (HHV-8).1 Other characteristics include a frequent but not absolute association with Epstein-Barr virus (EBV) and the lack of c-myc translocation. The PELs are of indeterminate immunophenotype (non-B, non-T); however, at the molecular level unequivocal immunoglobulin rearrangements have been observed thus classifying them as of B genotype.2 These tumors generally arise in patients with AIDS but infrequently also in HIV– individuals.3,4
The consistent presence of HHV-8 and of EBV in parallel in about 70% of PELs suggests an important role of these 2 herpesviruses in the development of this lymphoma.5 In EBV+ PELs, the expression of EBV-encoded growth transformation proteins is restricted to EBNA-1, EBER, and LMP2A.6,7 A very weak expression of LMP1 was also seen in a minority of the tumors.6 Among the EBV-encoded latent proteins, EBNA-2 and LMP1 are considered the major effectors of transformation. The EBV LMP1 transforms rodent cells8 and up-regulates bcl-2.9 EBNA-2 is essential for B-cell transformation and associates with the transcription factor RbPjK.10
EBV establishes 3 forms of latency in infected cells. The restricted type I latency is exemplified by the expression of EBNA-1 and LMP2A as observed in normal B lymphocytes.11 The phenotypically representative Burkitt lymphoma (BL) lines express EBNA-1 only.12 A majority of these lines drift toward an immunoblastic phenotype and express EBNA1-6, LMP1, LMP2A, and LMP2B. Such a viral latency program is known as latency III. The intermediate latency II, characterized by the expression of EBNA-1 and LMPs, is observed in nasopharyngeal carcinoma (NPC)13 and Hodgkin disease (HD).
In vitro infection of EBV– BLs with the prototype B95-8 strain has provided important clues as to the role of this virus in the pathogenesis of BLs.14 The contribution of EBV in the pathogenesis of PEL, however, is not clear. The very few HHV-8+/EBV– PEL cell lines described so far provide a valuable tool to study if EBV infection can phenotypically alter these cells. We used a recombinant Akata cell-derived NeoR EBV strain to address this question. Three cell lines resulted in stable EBV-infected convertants. We show that in vitro infection of PEL cells with EBV results in a restricted EBV latency program; down-regulation of CD30, CD74, and CD138 expression; and increased tumorigenicity in SCID mice without altering the expression of TCL1.
Study design
Cells
Infection with CD21 carrying retroviral vector and subsequently with the recombinant EBV
Immunoblotting
The expression of EBNA1-6 and LMP1 was checked by immunoblotting as described.21 Expression of TCL1, BCL-2, and BCLxL was verified on 15% sodium dodecyl sulfate (SDS)–polyacrylamide gel by using a respective monoclonal antibody.
RT-PCR
Surface marker analysis
One million cells were incubated with the respective R-phycoerythrin–conjugated primary antibodies on ice for 1 hour and analyzed with a Becton Dickinson CellQuest software (Heidelberg, Germany).
Tumorigenicity tests in SCID mice
SCID mice, aged 4 to 5 weeks, were purchased from Charles River Italia (Calco, Italy). Parental PEL cells and their EBV convertants were inoculated subcutaneously.
Results and discussion
The parental BC-3, BCBL-1, BCP-1, and CRO-AP/6 were infected with a CD21-containing retroviral vector pCLMFG CR2. The CD21 positivity in the infected PEL cells was verified by fluorescence-activated cell sorting (not shown).
The recombinant EBV-infected BC-3, CRO-AP/6, and CRO-AP/3 were selected with 2.3 mg/mL, 1 mg/mL, and 0.8 mg/mL G418, respectively. No drug-resistant clones were obtained from either BCBL-1 or BCP-1 cell lines.
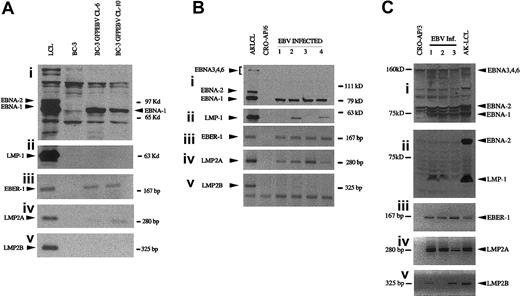
All EBV-infected clones of the PEL cells expressed EBER-1, EBNA-1, and LMP2A(Figure 1). EBNA2-6 was not expressed. Neither LMP1 nor LMP2B was expressed in BC-3 EBV convertants but CRO-AP/6 GFPEBV clones 2 and 4 were LMP1 expressors (Figure 1Bii). The expression of LMP1 and LMP2B was heterogeneous among the EBV-infected CRO-AP/3 (Figure 1C).
EBV latent gene expression in PEL cells superinfected with the recombinant EBV. Proteins were separated on 7.5% SDS-polyacrylamide gels. EBNAs were detected with a polyclonal serum. LMP1 was visualized by CS1-4 monoclonal antibodies. Akata virus–derived LCL was used as a positive control. EBER-1, LMP2A, and LMP2B were detected by RT-PCR. (A) BC-3; (B) CRO-APO/6, and (C) CRO-APO/3 and their respective EBV-infected clones. A mixture of CS1-4 and PE2 was used to detect LMP1 and EBNA-2 in CRO-AP/3 convertants.
EBV latent gene expression in PEL cells superinfected with the recombinant EBV. Proteins were separated on 7.5% SDS-polyacrylamide gels. EBNAs were detected with a polyclonal serum. LMP1 was visualized by CS1-4 monoclonal antibodies. Akata virus–derived LCL was used as a positive control. EBER-1, LMP2A, and LMP2B were detected by RT-PCR. (A) BC-3; (B) CRO-APO/6, and (C) CRO-APO/3 and their respective EBV-infected clones. A mixture of CS1-4 and PE2 was used to detect LMP1 and EBNA-2 in CRO-AP/3 convertants.
The surface markers tested are listed in Table 1. Whereas CD30 and CD74 expression was down-regulated in PEL EBV convertants, other markers were only marginally affected. In all CRO-AP/3 EBV clones, the typically PEL-associated marker CD138 (syndecan-1) was down-regulated.
Surface marker expression of parental and EBV-infected PEL cells
Cell line . | CD11a . | CD18 . | CD23 . | CD30 . | CD39 . | CD40 . | CD54 . | CD58 . | CD74 . | CD138 . |
|---|---|---|---|---|---|---|---|---|---|---|
| BC3 | 2.9 | 2.6 | 2.0 | 82.2 | 2.8 | 1.9 | 66.3 | 72.9 | 65 | 67.6 |
| BC-3 GFP EBV cl-6 | 9.3 | 8.2 | 8.0 | 6.2 | 5.1 | 9.2 | 66.0 | 67.3 | 5.0 | 60.9 |
| BC-3 GFP EBV cl-10 | 7.1 | 9.0 | 6.4 | 14.2 | 5.1 | 11.6 | 63.3 | 70.0 | 8.6 | 66.4 |
| CRO-AP/6 | 14.2 | 11.5 | 1.0 | 80.0 | 1.6 | 7.3 | 66.9 | 82.4 | 78.3 | 81.5 |
| CRO-AP/6 GFPEBV cl-1 | 11.3 | 14.9 | 11.1 | 25.3 | 19.0 | 11.1 | 69.7 | 71.7 | 12.3 | 78.3 |
| CRO-AP/6 GFPEBV cl-2 | 25.5 | 27.8 | 5.1 | 12.2 | 4.4 | 14.9 | 78.6 | 77.7 | 7.9 | 85.0 |
| CRO-AP/3 | 4.7 | 12.8 | 11 | 59.11 | 5 | 10.6 | 11.9 | 19.3 | 60 | 71.6 |
| CRO-AP/3 GFPEBV cl-1 | 4.9 | 9.2 | 6.5 | 12.8 | 4 | 5.3 | 4.3 | 5 | 11.2 | 17.9 |
| CRO-AP/3 GFPEBV cl-2 | 7 | 4.7 | 6.1 | 13.2 | 4.7 | 1 | 4.2 | 2.4 | 10.3 | 22 |
Cell line . | CD11a . | CD18 . | CD23 . | CD30 . | CD39 . | CD40 . | CD54 . | CD58 . | CD74 . | CD138 . |
|---|---|---|---|---|---|---|---|---|---|---|
| BC3 | 2.9 | 2.6 | 2.0 | 82.2 | 2.8 | 1.9 | 66.3 | 72.9 | 65 | 67.6 |
| BC-3 GFP EBV cl-6 | 9.3 | 8.2 | 8.0 | 6.2 | 5.1 | 9.2 | 66.0 | 67.3 | 5.0 | 60.9 |
| BC-3 GFP EBV cl-10 | 7.1 | 9.0 | 6.4 | 14.2 | 5.1 | 11.6 | 63.3 | 70.0 | 8.6 | 66.4 |
| CRO-AP/6 | 14.2 | 11.5 | 1.0 | 80.0 | 1.6 | 7.3 | 66.9 | 82.4 | 78.3 | 81.5 |
| CRO-AP/6 GFPEBV cl-1 | 11.3 | 14.9 | 11.1 | 25.3 | 19.0 | 11.1 | 69.7 | 71.7 | 12.3 | 78.3 |
| CRO-AP/6 GFPEBV cl-2 | 25.5 | 27.8 | 5.1 | 12.2 | 4.4 | 14.9 | 78.6 | 77.7 | 7.9 | 85.0 |
| CRO-AP/3 | 4.7 | 12.8 | 11 | 59.11 | 5 | 10.6 | 11.9 | 19.3 | 60 | 71.6 |
| CRO-AP/3 GFPEBV cl-1 | 4.9 | 9.2 | 6.5 | 12.8 | 4 | 5.3 | 4.3 | 5 | 11.2 | 17.9 |
| CRO-AP/3 GFPEBV cl-2 | 7 | 4.7 | 6.1 | 13.2 | 4.7 | 1 | 4.2 | 2.4 | 10.3 | 22 |
Reactivity with different monoclonal antibodies was measured by indirect immunofluorescence. One representative experiment of 3. Figures indicate percent of positive cells. Figures in bold show major changes.
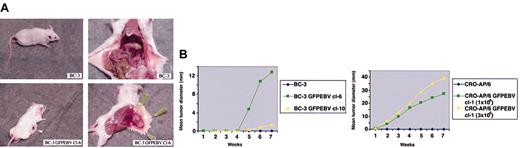
BC-3 parental cells were nontumorigenic in SCID mice. In contrast, BC-3 GFPEBV cl-6 was highly tumorigenic in a majority of mice inoculated (Figure 2A). The uninfected CRO-AP/6 were nontumorigenic at 2 different doses. At both doses, the EBV-converted CRO-AP/6 GFPEBV cl-1 was found highly tumorigenic (Table 2; Figure 2B). Table 2 summarizes the in vivo growth of EBV-infected PEL cells and only HHV-8+ parental lines. The rate of growth of the parental and EBV-converted PEL cell lines in mice is shown in Figure 2B.
Tumorigenicity of PEL cells and their EBV-converted sublines in SCID mice. (A) The SCID mice were subcutaneously injected with BC-3 and its EBV convertant. The mice were killed at the seventh week after inoculation. Note the highly vascularized tumor growth of BC-3 GFPEBV cl-6, whereas the mouse inoculated with the uninfected BC-3 is tumor free. The left and the right panels show the same mouse. (B) Kinetics of tumor growth in SCID mice. Summary of the rate of tumor growth in mice inoculated with parental BC-3 and BC-3 GFPEBV cl-6 and cl-10 and CRO-AP/6 and CRO-AP/6 GFPEBV cl-1. Two different doses of both the parental CRO-AP/6 and EBV-infected cl-1 were tested. The mean tumor load was calculated by dividing the sum of tumor diameters in all mice by the total number inoculated. Mice were kept under observation between 4 and 8 weeks.
Tumorigenicity of PEL cells and their EBV-converted sublines in SCID mice. (A) The SCID mice were subcutaneously injected with BC-3 and its EBV convertant. The mice were killed at the seventh week after inoculation. Note the highly vascularized tumor growth of BC-3 GFPEBV cl-6, whereas the mouse inoculated with the uninfected BC-3 is tumor free. The left and the right panels show the same mouse. (B) Kinetics of tumor growth in SCID mice. Summary of the rate of tumor growth in mice inoculated with parental BC-3 and BC-3 GFPEBV cl-6 and cl-10 and CRO-AP/6 and CRO-AP/6 GFPEBV cl-1. Two different doses of both the parental CRO-AP/6 and EBV-infected cl-1 were tested. The mean tumor load was calculated by dividing the sum of tumor diameters in all mice by the total number inoculated. Mice were kept under observation between 4 and 8 weeks.
Growth of parental PEL cells and their EBV convertants in SCID mice
Cell line . | Virus . | Dose . | Route . | No. of mice with tumors/total inoculated . |
|---|---|---|---|---|
| BC-3 | HHV-8 | 10 × 106 | SC | 0/9 |
| BC-3 GFP EBV cl-6 | HHV-8 + EBV | 10 × 106 | SC | 8/9 |
| BC-3 GFPEBV cl-10 | HHV-8 + EBV | 10 × 106 | SC | 1/6 |
| CRO-AP/6 | HHV-8 | 1 × 106 | SC | 0/6 |
| 3 × 106 | SC | 0/6 | ||
| 10 × 106 | SC | 6/6 | ||
| CRO-AP/6 GFPEBV cl-1 | HHV-8 + EBV | 1 × 106 | SC | 6/6 |
| 3 × 106 | SC | 6/6 | ||
| 10 × 106 | SC | 6/6 |
Cell line . | Virus . | Dose . | Route . | No. of mice with tumors/total inoculated . |
|---|---|---|---|---|
| BC-3 | HHV-8 | 10 × 106 | SC | 0/9 |
| BC-3 GFP EBV cl-6 | HHV-8 + EBV | 10 × 106 | SC | 8/9 |
| BC-3 GFPEBV cl-10 | HHV-8 + EBV | 10 × 106 | SC | 1/6 |
| CRO-AP/6 | HHV-8 | 1 × 106 | SC | 0/6 |
| 3 × 106 | SC | 0/6 | ||
| 10 × 106 | SC | 6/6 | ||
| CRO-AP/6 GFPEBV cl-1 | HHV-8 + EBV | 1 × 106 | SC | 6/6 |
| 3 × 106 | SC | 6/6 | ||
| 10 × 106 | SC | 6/6 |
SCID mice, aged 4 to 5 weeks, were inoculated with indicated tumor cells. SC indicates subcutaneous.
Immunohistochemistry of tumors from SCID mice revealed that BC-3 GFPEBV cl-6 consisted of a mixture of immunoblast-like cells to anaplastic and multinucleated large cells. The CRO-AP/6 EBVGFP cl-1 mainly consisted of immunoblast-like cells with plasmacytoid features (not shown).
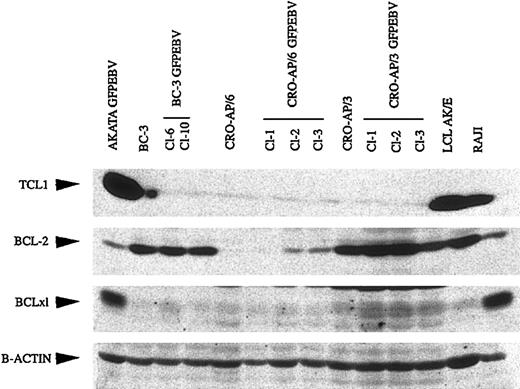
A recent study demonstrated that in vitro EBV infection of EBV-loss variant BLs leads to restoration of TCL1 expression.23 As seen in Figure 3, neither the parental PEL cells nor their respective EBV convertants expressed TCL1. The expression of antiapoptotic genes BCL-2 and BCLxL was neither significantly nor consistently affected in EBV-infected PEL cells. Interestingly, most PEL cells, regardless of the presence of EBV, expressed high levels of BCL-2 but low BCL-xL levels. In contrast, 2 BLs expressed low levels of BCL-2 but were strong BCLxL expressors.
TCL1, BCL-2, and BCLxL expression in PEL cells and EBV-infected counterparts. Total cell lysates were run on 15% SDS-polyacrylamide gels. Monoclonal antibodies against respective proteins were used to verify their expression.
TCL1, BCL-2, and BCLxL expression in PEL cells and EBV-infected counterparts. Total cell lysates were run on 15% SDS-polyacrylamide gels. Monoclonal antibodies against respective proteins were used to verify their expression.
The lack of EBNA2-6 and LMPs in most EBV-associated tumors on the one hand and the strong in vitro immunogenicity of these proteins on the other hand has led to a hypothesis that melds these 2 observations and suggests that EBV latent gene expression may be regulated by immunoselection. In that context, the restricted EBV latent gene expression in PEL cells is intriguing for 2 reasons. First, a vast majority of these tumors arise in patients with AIDS whose immune system is under HIV-induced suppression. Second, pathobiologically, PELs are a bridge between large cell immunoblastic and anaplastic large cell lymphomas.3 The latter lymphomas when associated with EBV generally express a latency III program. The fact that in vitro infected PELs in our study demonstrate type I/type II EBV latency is consistent with the suggestion that the pattern of EBV latent gene expression may depend on the cell type, regardless of whether infected in vivo or in vitro.
An interesting difference between EBV convertants of BC-3 and those of CRO-AP/3 and CRO-AP/6 is the expression of LMP1. BC-3 originated in a HIV– patient,16 but both CRO-AP/3 and CRO-AP/6 are derived from AIDS patients.18 It has been suggested that AIDS-associated PELs clinically and molecularly resemble immunoblastic lymphoma3 and over 90% of the immunoblastic lymphomas are EBV+ and LMP1+. It will be interesting to examine if the observed difference in LMP1 expression between these lines is related to differences in their origin.
A previous study showed that PELs, which were positive for both the viruses, are more tumorigenic in mice in contrast to only HHV-8+ cell lines.17 However, in that study the tumorigenicity comparison was carried out between 2 different PEL lines. We provide more direct evidence that HHV-8+ BC-3 and CRO-AP/6 cells are nontumorigenic in SCID mice but the same cells when infected with EBV become highly tumorigenic.
If EBV enhances tumorigencity of PELs, how does it do so? A recent study demonstrated that the expression of TCL1 oncogene was up-regulated in EBV-infected BLs.23 We investigated if EBV might have similar effect on TCL1 in PELs. The parental PEL cells and 9 EBV-infected clones derived from them were TCL1–. Taken together, this suggests that the effect of EBV on TCL1 expression seems to be highly differentiation specific and that in the B-cell tumors of postgerminal center (GC) derivation (as is the case with PELs in contrast to BLs, which are of GC origin), the virus may contribute to enhanced tumorigenicity in a TCL1-independent manner.
The BL cells proliferate due to activated c-myc expression resulting from the Ig/myc translocation but what drives the proliferation of PEL cells still remains to be elucidated. Our findings are consistent with the suggestion that cells containing both viruses may have a growth advantage. It will be important now to study if the prognosis of PELs containing both the viruses is worse than those that contain only HHV-8 in a clinical setting.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1710.
Supported by grants from the Ministero dell'istruzione, dell'universita e della ricerca (MUIR), Ministero della Sanità, Progetto AIDS, Associazione Italiana di ricerca sul Cancro (AIRC), and Istituto-Cenci-Bolognetti Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Mr Massimo Spada provided excellent technical help. Mr Sandro Valia and Giuseppe Lucania are gratefully acknowledged for photographic work. We thank E. Cesarman, P. S. Moore, and C. Boshoff for providing the cell lines.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal