Abstract
A new World Health Organization classification was recently proposed. However, classification of peripheral T-cell lymphomas remains to be clarified. Particularly, unspecified type was considered as a heterogeneous category. Here we studied the expressions of chemokine receptors, Th1-associated CXCR3 and CCR5 and Th2-associated marker ST2(L), and activated T-cell receptor OX40/CD134 in 185 patients with nodal T-cell lymphoma, and evaluated the relationship to prognosis. Their expression patterns correlated with the specific subtype of nodal T-cell lymphoma, such as angioimmunoblastic T-cell lymphoma (AILD), anaplastic large cell lymphoma (ALCL), and in peripheral T-cell lymphoma (PTCL), unspecified. In AILD, almost all cases were immunoreactive for OX40/CD134 (96%) and for CXCR3 (89%). In ALCL, all cases were immunonegative for OX40/CD134, and only a few cases (24%) were immunoreactive for CXCR3, whereas almost all cases (94%) were positive for ST2(L). Cases of PTCL, unspecified, were divided into 2 groups; group 1 (cases positive for either ST2(L), CCR5, or CXCR3) tended to show favorable prognosis compared with group 2 (cases negative for ST2(L), CCR5, and CXCR3). Our results indicate that further subtyping of PTCL, unspecified, into groups 1 and 2 could be significant for evaluating prognosis and understanding the functional role of these tumors.
Introduction
New insights into the pathogenesis of lymphoid malignancies have recently been made, thanks to the use of new techniques, such as genetic, molecular, and immunologic methods. Based on new insights obtained from these techniques, the new World Health Organization (WHO) classification was recently proposed. B-cell lymphomas and T-cell lymphomas of lymphoblastic type have been well described. However, the details of classification of peripheral T-cell lymphomas (PTCLs) remain to be clarified due to the small amount of available data.1
It has recently been demonstrated that the expression patterns of chemokine receptors in normal T-cell subsets correlate with the patterns of cytokine secretion by these cells. The expression of receptors CCR3, CCR4, and CCR8 is associated with Th2-polarized T cells, producing cytokines interleukin 4 (IL-4) and IL-5. In contrast, CXCR3 expression is highest in cells with a prominent Th1 pattern of cytokine secretion.2 In addition, CCR5 has recently been described as a surface marker of human T cells producing type 1 (Th1) cytokines.3 ST2(L) is a transmembrane form of a 61.5-kDa protein. This ST2(L) protein is very similar in structure to IL-1 receptor (IL-1R) types 1 and 2; thus, it is considered as a member of the IL-1R family.4,5 Several studies have shown that ST2(L) is expressed on the cell surface of Th2 cells but not on Th1 cells.3,5,6
To date, the classification of T-cell lymphoma has been largely based on morphologic or clinical criteria. For example, mycosis fungoides and intestinal T-cell lymphoma are defined based on the site of involvement and can have a wide range of histopathologic appearances. Other lymphomas, such as angioimmunoblastic lymphoma (AILD) and lymphoblastic lymphoma, are largely defined by morphologic criteria. Some lymphoma types are defined by specific phenotypic/genotypic criteria, such as the presence of human T-lymphocytic virus type 1 (HTLV-1) in adult T-cell leukemia/lymphoma (ATLL), and of CD30 and anaplastic lymphoma kinase (ALK) expression in anaplastic large cell lymphoma (ALCL).2 The unspecified T-cell lymphoma in the WHO classification was a wastebasket, forming a heterogeneous group with variable clinical features, histology, genetic alteration, response to treatment, and prognosis.
The aims of the present study were to substantiate the previous studies of OX40/CD134 and chemokine receptor expression in T-cell lymphoma (especially, Jones et al2,7 ) and to clarify the relationship between Th1/Th2 characteristics and prognosis of PTCL. For this purpose, we examined the expression of Th1 cell–associated chemokine receptors, CXCR3 and CCR5, and Th2 cell–associated marker, ST2(L), and OX40/CD134, which is a receptor with highly restricted expression to activated T cells.7 Furthermore, we also compared the expression of CXCR3, CCR5, ST2(L), and OX40/CD134 in T-cell lymphomas, including unspecified type, and also ATLL (lymphomatous type), ALCL, AILD, and lymphoblastic lymphoma.
Patients, materials, and methods
Patients
Over the 20-year period from 1975 to 1994, 185 patients with nodal T-cell lymphoma were identified in the lymph node registry files of the Department of Pathology, Fukuoka University. In all cases, clonal integration of HTLV-1 proviral DNA was examined, using Southern blot analysis, and the clinical follow-up data could be thus analyzed. Nodal lesions of mycosis fungoides were excluded because the cutaneous lesion was considered the primary site. The lymph nodes were fixed with buffered formalin, embedded in paraffin, and stained with hematoxylin-eosin (HE), Giemsa, periodic acid-Schiff (PAS), and Gomori stains for silver impregnation. All cases have been reported previously.1 These studies were approved by the patients or their legal guardians, as well as appropriate written consent from each participating institute or hospital.
Immunohistochemistry
The paraffin-embedded lymph nodes were used for immunohistochemical analysis of L26 (CD20) in B cells (Dakocytomation, Glostrup, Denmark), UCHL-1 (CD45RO) and CD3 in T cells (Dakocytomation), Leu-M1 (CD15; Becton Dickinson, Mountain View, CA), terminal deoxynucleotidyl transferase (TdT; Supertec, Bethesda, MD), EMA (epithelial membrane antigen; Dakocytomation) or Ber H2 (CD30; Dakocytomation), or both, ALK (Nichirei, Tokyo, Japan), ALK1 (Dakocytomation), ALKc (Immunotech, Marseille, France), CCR5 (Dakocytomation), CXCR3 (PharMingen, San Diego, CA), ST2(L) (MBL, Tokyo, Japan), and CD134 (OX40; PharMingen). A portion of each lymph node was kept at –80°C in a deep freezer and the nodes were examined using the monoclonal antibodies CD2, 3, 4, 7, 8, 10, 15, 19, 20, and 30 (Coulter, Hialeah, FL; Ortho, Raritan, NJ; Becton Dickinson; Dakocytomation).8
Immunohistologic scoring
The percentage of positive cells was averaged to yield an immunohistologic score of 0% to 100%, with paraffin-embedded lymph nodes by 3 well-trained hematopathologists. The following categories were defined: negative (< 10% positively stained tumor cells) and positive (≥ 10% positively stained tumor cells) for CCR5, CXCR3, OX40/CD134, and ST2(L), respectively. Negative and positive staining controls for CCR5, CXCR3, OX40/CD134, and ST2(L) were obtained by using fresh and paraffin-embedded tonsils with the histologic diagnosis of chronic tonsillitis and lymph nodes with the histologic diagnosis of nonspecific lymphadenitis.
DNA analysis
The other parts of the frozen materials were used for DNA isolation and gene analysis. Before performing DNA analysis, the samples were confirmed to contain lymphoma cells, which occupied more than 70% of the nucleated cells, using HE and immunologic staining.9,10 After the frozen materials were confirmed to contain lymphoma cells, T-cell–receptor (TCR) genes Cβ, Jγ, the immunoglobulin heavy-chain (JH) gene, and proviral DNA of HTLV-1 (full-length probe, including gag, pol, env, pX, and LTR) were also examined. The monoclonal integration of HTLV-1 DNA was examined by digestion with EcoRI, as reported previously.10
Definition of T-cell type: phenotype and genotype
Lymphoblastic lymphoma and other types of T-cell (peripheral T-cell) lymphoma showed slightly different phenotypes and genotypes. T-cell lymphoblastic lymphoma was diagnosed by the expression of one or more pan-T-cell antigens (CD45RO, CD2, CD3, and CD7) and the absence of pan-B-cell antigens (CD19 and CD20). The lymphoblastic cells were typically TdT+. Rearrangements of TCR β and γ genes were occasionally detected, whereas Ig JH rearrangements were rare. PTCL was also diagnosed by the expression of one or more pan-T-cell antigens (CD45RO, CD2, CD3, and CD7) and T cell–associated antigens (CD4 or CD8), as well as by the absence of pan-B-cell antigens (CD19 and CD20). Rearrangements of TCR β and γ genes were frequently detected, and Ig JH genes were always germline. In addition, ALCL expressed CD30, ALK (Nichirei), and partly ALK1 (Dakocytomation), ALKc (Immunotech), CD15, or EMA.
Morphologic classification
The morphologic classification was based on the WHO classification. In addition, based on the modified updated Kiel classification, PTCL, unspecified was further classified into diffuse large cell type, diffuse medium-sized cell type, pleomorphic medium-sized cell and large cell type, pleomorphic medium-sized cell type, pleomorphic small cell type, and Lennert type.
Statistical analysis
Statistical analysis (log-rank test by computer software Stat view version 5 [SAS Institute, Cary, NC]) of the Kaplan-Meier survival curves was performed in the present study.
Results
HTLV-1 analysis
Before performing DNA analysis, the lymph node samples were confirmed to contain mainly lymphoma cells, which occupied more than 70% of the nucleated cells. Based on these findings, the sensitivity of Southern blot was thus considered sufficient. All T-cell lymphomas could be classified into 2 groups as follows: (1) cases with ATLA and HTLV-1 clonal integration were defined as ATLL; and (2) cases without ATLA or pX amplification were defined as non-ATLL T-cell lymphoma. In the present study, 48 cases of ATLL (lymphomatous type), and 137 cases of non-ATLL T-cell lymphomas were analyzed.
Histopathologic classification
All cases described in the present study were morphologically classified. Large cell type was frequently detected in PTCL, unspecified in comparison with ATLL (lymphomatous type). In ATLL (lymphomatous type), pleomorphic type with giant cells was characteristic. AILD and lymphoblastic morphology was not seen in ATLL (lymphomatous type; Table 1).
Classification of nodal T-cell lymphoma based on expression of Th1-associated chemokine receptors, CXCR3 and CCR5, Th2-associated marker, ST2(L), and activated TCR OX40/CD134
. | OX40 (n = 162) . | . | CCR5 (n = 162) . | . | CXCR3 (n = 172) . | . | ST2(L) (n = 175) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | + . | - . | + . | - . | + . | - . | + . | - . | ||||
| Unspecified | 9 (17) | 43 (83) | 13 (25) | 38 (75) | 25 (42) | 35 (58) | 21 (34) | 40 (66) | ||||
| Large | 5 (19) | 22 (81) | 5 (20) | 20 (80) | 12 (44) | 17 (56) | 12 (44) | 17 (56) | ||||
| Medium | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 3 (38) | 5 (62) | 2 (29) | 5 (71) | ||||
| Pleo (L and M/M) | 4 (25) | 12 (75) | 6 (38) | 10 (62) | 6 (40) | 9 (60) | 5 (31) | 11 (69) | ||||
| Pleo (small) | 0 (0) | 5 (100) | 2 (40) | 3 (60) | 3 (60) | 2 (40) | 2 (40) | 3 (60) | ||||
| Lennert | 0 (0) | 4 (100) | 0 (0) | 4 (100) | 1 (33) | 2 (67) | 0 (0) | 4 (100) | ||||
| AILD | 25 (96) | 1 (4) | 8 (29) | 20 (71) | 25 (89) | 3 (11) | 0 (0) | 28 (100) | ||||
| ALCL | 0 (0) | 18 (100) | 16 (89) | 2 (11) | 4 (24) | 13 (76) | 17 (94) | 1 (6) | ||||
| Lymphoblastic | 0 (0) | 19 (100) | 0 (0) | 18 (100) | 4 (18) | 18 (82) | 0 (0) | 21 (100) | ||||
| ATLL (lymphomatous) | 5 (11) | 42 (89) | 9 (19) | 38 (81) | 11 (24) | 34 (76) | 23 (49) | 24 (51) | ||||
. | OX40 (n = 162) . | . | CCR5 (n = 162) . | . | CXCR3 (n = 172) . | . | ST2(L) (n = 175) . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | + . | - . | + . | - . | + . | - . | + . | - . | ||||
| Unspecified | 9 (17) | 43 (83) | 13 (25) | 38 (75) | 25 (42) | 35 (58) | 21 (34) | 40 (66) | ||||
| Large | 5 (19) | 22 (81) | 5 (20) | 20 (80) | 12 (44) | 17 (56) | 12 (44) | 17 (56) | ||||
| Medium | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 3 (38) | 5 (62) | 2 (29) | 5 (71) | ||||
| Pleo (L and M/M) | 4 (25) | 12 (75) | 6 (38) | 10 (62) | 6 (40) | 9 (60) | 5 (31) | 11 (69) | ||||
| Pleo (small) | 0 (0) | 5 (100) | 2 (40) | 3 (60) | 3 (60) | 2 (40) | 2 (40) | 3 (60) | ||||
| Lennert | 0 (0) | 4 (100) | 0 (0) | 4 (100) | 1 (33) | 2 (67) | 0 (0) | 4 (100) | ||||
| AILD | 25 (96) | 1 (4) | 8 (29) | 20 (71) | 25 (89) | 3 (11) | 0 (0) | 28 (100) | ||||
| ALCL | 0 (0) | 18 (100) | 16 (89) | 2 (11) | 4 (24) | 13 (76) | 17 (94) | 1 (6) | ||||
| Lymphoblastic | 0 (0) | 19 (100) | 0 (0) | 18 (100) | 4 (18) | 18 (82) | 0 (0) | 21 (100) | ||||
| ATLL (lymphomatous) | 5 (11) | 42 (89) | 9 (19) | 38 (81) | 11 (24) | 34 (76) | 23 (49) | 24 (51) | ||||
Data in parentheses represent the percentage of tumors. Pleo indicates pleomorphic.
Expression of chemokine receptors, OX40/CD134, and ST2(L) and prognosis
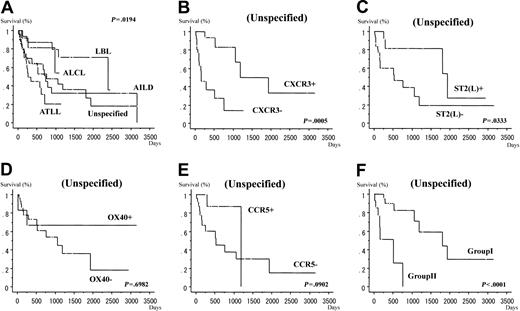
Tables 1 and 2 provide summaries of the expression of chemokine receptors (CCR5, CXCR3), ST2(L), and OX40/CD134. We examined the expression of the CCR5 by immunohistochemistry in 162 cases, CXCR3 in 172 cases, OX40/CD134 in 162 cases, and ST2(L) in 175 cases of nodal T-cell lymphoma, including PTCL lymphoma, unspecified, AILD, ALCL, lymphoblastic lymphoma, and ATLL (lymphomatous type). In PTCL, unspecified, 13 of 51 (25%) cases showed positive cytoplasmic CCR5 reactivity, and the survival curves were not significantly different between CCR5+ and CCR5– cases (P = .0902, log-rank test; Figure 1E). Eight of 28 (29%) cases with AILD, 16 of 18 (89%) cases with ALCL, and 0 of 18 (0%) cases with lymphoblastic lymphoma showed positive reaction for CCR5. Nine of 47 (19%) ATLL (lymphomatous type) cases were positive for CCR5. In PTCL, unspecified, 25 of 60 (42%) cases showed positive CXCR3 reactivity and the survival curves were significantly different (P = .0005, log-rank test) between CXCR3+ and CXCR3– lymphomas; the positive cases tended to show a more favorable prognosis than negative cases (Figure 1B). On the other hand, 25 of 28 (89%) cases of AILD, 4 of 17 (24%) cases of ALCL, and 4 of 22 (18%) cases of lymphoblastic lymphoma showed positive reaction for CXCR3. Eleven of 45 (24%) cases of ATLL (lymphomatous type) showed positive reaction for CXCR3. In PTCL, unspecified, 9 of 52 (17%) cases showed positive reactivity to OX40/CD134 and the survival curves were not significantly different between OX40/CD134+ and OX40/CD134– lymphomas (P = .6982, log-rank test; Figure 1D). Specifically, 25 of 26 (96%) cases of AILD, 0 of 18 (0%) cases of ALCL, and 0 of 19 (0%) cases of lymphoblastic lymphoma showed positive reaction for OX40/CD134. Furthermore, 5 of 47 (11%) cases of ATLL (lymphomatous type) showed positive reaction for OX40/CD134. In PTCL, unspecified, 21 of 61 (34%) cases showed positive ST2(L) reactivity and the survival curves were significantly different between ST2+ and ST2– lymphomas; the positive cases tended to show a more favorable prognosis than negative cases (P = .0333, log-rank test; Figure 1C). Specifically, 0 of 28 (0%) cases of AILD, 17 of 18 (94%) cases of ALCL, and 0 of 21 (0%) cases of lymphoblastic lymphoma showed positive reaction for ST2(L). Furthermore, 23 of 47 (49%) cases of ATLL (lymphomatous type) showed positive reactivity. The histologic features are shown in Figures 2 and 3.
Classification of PTCL, unspecified type, based on expression of Th1-associated chemokine receptors, CXCR3 and CCR5, and Th2-associated marker, ST2(L)
. | Group 1 (%) . | Group 2 (%) . |
|---|---|---|
| Unspecified | 22 (61) | 14 (39) |
| Large | 10 (59) | 7 (41) |
| Medium | 0 (0) | 0 (0) |
| Pleo (L and M/M) | 8 (67) | 4 (33) |
| Pleo (small) | 4 (80) | 1 (20) |
| Lennert | 0 (0) | 2 (100) |
. | Group 1 (%) . | Group 2 (%) . |
|---|---|---|
| Unspecified | 22 (61) | 14 (39) |
| Large | 10 (59) | 7 (41) |
| Medium | 0 (0) | 0 (0) |
| Pleo (L and M/M) | 8 (67) | 4 (33) |
| Pleo (small) | 4 (80) | 1 (20) |
| Lennert | 0 (0) | 2 (100) |
Group 1 is ST2(L)+ and/or CCR5+ and/or CXCR3+. Group 2 is ST2(L)-, CCR5-, and CXCR3-. Pleo indicates pleomorphic.
Survival curves. (A) Survival curves for all cases according to WHO classification. Survival curves for cases with PTCL, unspecified, according to the results of immunohistochemical staining for Th1-associated chemokine receptors, CXCR3 (B) and CCR5 (E), and Th2-associated marker, ST2(L) (C) and activated TCRr OX40/CD134 (D) and groups 1 and 2 (F).
Survival curves. (A) Survival curves for all cases according to WHO classification. Survival curves for cases with PTCL, unspecified, according to the results of immunohistochemical staining for Th1-associated chemokine receptors, CXCR3 (B) and CCR5 (E), and Th2-associated marker, ST2(L) (C) and activated TCRr OX40/CD134 (D) and groups 1 and 2 (F).
Histopathologic features of cells. (HE staining) (A) PTCL, unspecified type (diffuse large cell type); (B) lymphoblastic lymphoma; (C) AILD; (D) ALCL; (E) PTCL, unspecified type (diffuse medium-sized cell type); (F) ATLL; (G) ATLL; (H) PTCL, unspecified type (Lennert lymphoma). Original magnification, × 400.
Histopathologic features of cells. (HE staining) (A) PTCL, unspecified type (diffuse large cell type); (B) lymphoblastic lymphoma; (C) AILD; (D) ALCL; (E) PTCL, unspecified type (diffuse medium-sized cell type); (F) ATLL; (G) ATLL; (H) PTCL, unspecified type (Lennert lymphoma). Original magnification, × 400.
Histopathologic staining features. (A) CD45RO staining (positive) of ALCL. (B) CD20 staining (negative) of ALCL. (C) OX40/CD134 staining (positive) of AILD. (D) CXCR3 staining (positive) of AILD. (E) CCR5 staining (positive) of AILD. (F) ST2(L) staining (positive) of PTCL, unspecified type (diffuse large cell type). (G) CCR5 staining (positive) of PTCL, unspecified type (diffuse pleomorphic [L and M] cell type). (H) CXCR3 staining (positive) of PTCL, unspecified type (diffuse large cell type). (I) OX40/CD134 staining (positive) of PTCL, unspecified type (diffuse medium-sized cell type). Original magnification, × 400.
Histopathologic staining features. (A) CD45RO staining (positive) of ALCL. (B) CD20 staining (negative) of ALCL. (C) OX40/CD134 staining (positive) of AILD. (D) CXCR3 staining (positive) of AILD. (E) CCR5 staining (positive) of AILD. (F) ST2(L) staining (positive) of PTCL, unspecified type (diffuse large cell type). (G) CCR5 staining (positive) of PTCL, unspecified type (diffuse pleomorphic [L and M] cell type). (H) CXCR3 staining (positive) of PTCL, unspecified type (diffuse large cell type). (I) OX40/CD134 staining (positive) of PTCL, unspecified type (diffuse medium-sized cell type). Original magnification, × 400.
Combined expression of ST2(L), CCR5, and CXCR3, and prognosis
We also examined the combined expression of ST2(L), CCR5, and CXCR3. We defined the cases with positive reactivity for at least one of ST2(L), CCR5, or CXCR3 as group 1. We also defined the cases with the negative reactivity for ST2(L), CCR5, and CXCR3 expression as group 2. In PTCL, unspecified, 22 of 36 (61%) cases were classified as group 1, 14 (39%) cases were of group 2, and cases in group 1 tended to have favorable prognosis compared with those in group 2 (P < .0001, log-rank test; Figure 1F). When we applied the grouping to the other established subtypes of the nodal T-cell lymphoma, the results were as follows. In AILD, 26 of 28 (93%) cases were of group 1 and 2 (7%) cases were of group 2. In ALCL, 19 of 19 (100%) cases were of group 1. In lymphoblastic lymphoma, 4 of 20 (20%) cases were classified as group 1 and 16 (80%) cases were of group 2. In ATLL, 30 of 47 (64%) cases were of group 1, and 17 of 47 (36%) cases were classified as group 2.
Survival time and classification
The prognosis of ATLL (lymphomatous type) was poor, whereas unspecified type, AILD was intermediate, and those of the other types including lymphoblastic lymphoma, anaplastic large cell type, were all relatively favorable. In addition, in the cases of ALCL, 4 of 18 cases showed positive reactivity for both ALK1 and ALKc, and 14 of 18 cases showed positive reactivity for neither ALK1 nor ALKc. The former tended to have more favorable prognosis than the latter. Moreover, in the unspecified type, cases positive for CXCR3 or ST2(L) or both showed a more favorable prognosis than the other cases (P = .0005 and .0333, log-rank test, respectively). The survival curves of cases with positive reactivity for CCR5 or positive reactivity for OX40/CD134 or both were almost similar to those of other cases (P = .0902 and .6982, log-rank test, respectively), in the unspecified type. (But, in addition, at least we could say that, in the unspecified type, cases with positive reactivity for CCR5 tended to have favorable prognosis compared with cases with negative reactivity for CCR5, albeit statistically insignificant at P = .0902, log-rank test.) So, we used the markers of CXCR3, ST2(L), and CCR5 in our definition of group 1 and group 2. Moreover, in the unspecified type, cases of group 1 showed a more favorable prognosis than those of group 2 (P < .0001, log-rank test; Figure 1).
Discussion
In the Revised European-American Lymphoma (REAL) classification and new WHO classification, nodal T-cell lymphoma is generally classified into AILD, ALCL, ATLL, lymphoblastic lymphoma, and PTCL, unspecified. These are the established categories except for PTCL, unspecified, which is considered as a heterogeneous category.
According to the new WHO classification, the prognosis of ATLL (lymphomatous type) is very poor compared with B-cell lymphomas. Furthermore, the prognosis of T lymphoblastic lymphoma and PTCL, unspecified, is relatively poor, whereas ALCL exhibits a relatively favorable prognosis. In our previous study,1 we also reported that the prognosis of ATLL (lymphomatous type) was poor, whereas that of unspecified type was intermediate between ATLL (lymphomatous type) and other types including lymphoblastic lymphoma, AILD, and ALCL.
The nonneoplastic counterpart of PTCL, unspecified, is, in general, CD4+ T cells and rarely CD8+ T cells. In the present study, the cases of PTCL, unspecified, showed the CD4+CD8– phenotype or showed the CD4– phenotype, but the latter also showed negative reaction for TIA-1 (data not shown), so the normal counterparts of them were also considered as not cytotoxic T cell, but helper T cell. The down-regulation of the CD4 antigen is occasionally observed in the cases of ATLL. CD4+ T cell is functionally classified into functional Th1 cells, Th2 cells, and others (undifferentiated, nonfunctional T-cells). Th1 cells support T cell–mediated immunity, whereas Th2 cells support humoral immunity. Under normal conditions, the ratio between Th1 and Th2 cells is delicately regulated, and several human disorders (eg, allergic and rheumatic diseases) reflect or are due to a distortion of the Th1/Th2 ratio. The relationships between Th1 and Th2 cells and their secreted cytokines and their expressing chemokine receptors have been reported previously. In general, tumor necrosis factor α (TNF-α), IL-2, and interferon γ (IFN-γ) are Th1-type cytokines.3,11,12 On the other hand, IL-4, IL-5, IL-6, and IL-13 are Th2 cytokines.13-18 However, it is difficult to isolate cells solely based on their cytokine production. Thus, it is desirable to identify surface antigens associated with either Th1 or Th2 cytokine phenotype.
With regard to the expression of chemokine receptors on T cells, CXCR3 is commonly expressed on activated T cells and on a discrete proportion of resting T and natural killer (NK) cells.6 Th1 cells are characterized by the surface expression of lymphocyte activation gene 3 (LAG3) and CXCR3. Furthermore, CCR5 expression was reported to be a marker of human Th1 cytokine-producing cells. On the other hand, surface expression of CCR3, CCR4, CCR8, or ST2(L) has been associated with Th2 cells.3 However, the function of OX40/CD134 in T cells is not completely understood; yet, in the human immune system, OX40/CD134 expression is largely restricted to a subset of activated CD4+ T cells. Strong OX40/CD134 expression has been reported in lymph node T cells expressing Th1-type cytokines, IL-2 and IFN-γ.7
Recent studies reported CXCR3 (Th1-type chemokine receptor) expression in several lymphoma subtypes, including most cases of AILD. Furthermore, in AILD, the detection of CXCR3 correlated significantly with coexpression of TNF receptor OX40/CD134, supporting the Th1-like phenotype of AILD.2,7 In the present study, 25 of 26 (96%) cases with AILD showed positive reactivity for OX40/CD134, and 25 of 28 (89%) cases showed positive reactivity for CXCR3, which is expressed in Th1 phenotype.
ALCL generally expresses CD30 and ALK fusion protein.2,7 Previous studies also showed immunoreactivity for CCR4 in ALK+ ALCL, though such lymphomas were negative for CXCR3.2 Furthermore, a lack of OX40/CD134 reactivity was also characteristic of ALCL.2,7 The demonstration of CCR4 expression and lack of CXCR3 and OX40/CD134 provided evidence for Th2 phenotype. In the present study, 18 of 18 (100%) cases showed negative reactivity for OX40/CD134, and 13 of 17 (76%) cases showed negative reactivity for CXCR3, whereas 17 of 18 (94%) cases showed positive reactivity for ST2(L). Considered together, these findings suggest that ALCL can be regarded as Th2 phenotype lymphoma.
With regard to lymphoblastic lymphoma, it was reported previously that the lymphoma cells show negative reactivity to such chemokines as CXCR3, CCR4, as well as CD30 and OX40/CD134.2,7 In this study, none of the cases examined showed reactivity for OX40/CD134 and CCR5. Furthermore, 4 of 22 (18%) cases showed positive reactivity for CXCR3. The finding also supported the conclusion of Jones et al2 that lymphoblastic lymphoma has a native or immature phenotype.
Previous studies reported that ATLL was positive for OX40/CD134 and, in contrast, was largely negative for CD30. However, our study showed different results. There was a tendency for tumor cells to be negative for OX40/CD134, CXCR3, and CCR5 in ATLL (lymphomatous type). On the other hand, 23 of 47 (49%) cases were positive for ST2(L) among ATLL (lymphomatous type). Previous studies considered ATLL as a single disease entity, although its clinical features are quite diverse and increased production of cytokines, such as IL-6 (Th2 type), may explain the diversity of clinical features.19 Therefore, we believe that ATLL is quite diverse with regard to cytokine production.
With regard to PTCL, unspecified, previous reports showed high variability of chemokine receptor expression in these tumors and a lack of correlation with CD30 or OX40/CD134 expression. These findings were compatible with the evidence that this type of lymphoma is morphologically and immunophenotypically heterogeneous.2 In the present study, we identified 13 of 51 (25%), 25 of 60 (42%), 9 of 52 (17%), and 21 of 61 (34%) cases of PTCL, unspecified, to be immunoreactive for CCR5, CXCR3, OX40/CD134, and ST2(L), respectively.
In summary, the lymphoma cells of AILD and ALCL are considered to be Th1 and Th2 phenotypes, respectively, whereas those of lymphoblastic lymphoma and ATLL (lymphomatous type) are considered nonfunctional, non-Th1/Th2 phenotypes. Using immunohistochemistry, the PTCL lymphoma, unspecified, described in the present study could be classified into 2 groups: group 1, ST2(L)+ and/or CCR5+ and/or CXCR3+, and group 2, ST2(L)–, CCR5–, and CXCR3–. The group 1 lymphomas were considered as functional, whereas group 2 lymphomas were considered nonfunctional. The prognosis of ATLL (lymphomatous type) is very poor and that of ALCL is relatively favorable, whereas that of lymphoblastic lymphoma and AILD is intermediate. Moreover, ALCL and AILD are relatively close to group 1 in their expression of chemokine receptors, Th1-associated CXCR3 and CCR5, and Th2-associated marker ST2(L). It is interesting that AILD, which is considered as Th1-cell lymphoma, and ALCL, which is considered as Th2-cell lymphoma, are close to group 1. On the other hand, ATLL (lymphomatous type) and lymphoblastic lymphoma, which are considered as nonfunctional lymphomas, are close to group 2, in their expression of chemokine receptors, Th1-associated CXCR3 and CCR5, and Th2-associated marker ST2(L). For PTCL, unspecified, group 1 tended to have favorable prognosis compared with cases of group 2. Therefore, for PTCL, unspecified, further subtyping into groups 1 and 2 could be significant for evaluation of prognosis and understanding the functional role of these tumors. However, in the present study, functional analysis was difficult, for example, measurement of cytokine levels in each type of lymphoma. However, further functional studies are necessary to define the clinical prognosis of patients with these types of lymphomas.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2002-05-1352.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Histopathologic staining features. (A) CD45RO staining (positive) of ALCL. (B) CD20 staining (negative) of ALCL. (C) OX40/CD134 staining (positive) of AILD. (D) CXCR3 staining (positive) of AILD. (E) CCR5 staining (positive) of AILD. (F) ST2(L) staining (positive) of PTCL, unspecified type (diffuse large cell type). (G) CCR5 staining (positive) of PTCL, unspecified type (diffuse pleomorphic [L and M] cell type). (H) CXCR3 staining (positive) of PTCL, unspecified type (diffuse large cell type). (I) OX40/CD134 staining (positive) of PTCL, unspecified type (diffuse medium-sized cell type). Original magnification, × 400.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2002-05-1352/6/m_h80145420003.jpeg?Expires=1765917510&Signature=kAzRD9qndH0L9gdjfKhV5Eeo570SVWp1AZ5VdG4nTi9t5MlXHdMYb~wit57E7ZUPxf4EQCqNiJVIoevkJwpomFiycqoDkiD5u~87KS~5HtYMyL148KS6Cff1eT1bxXMyQH9TsJpVkkDj9ox55T5-S1Cen7DEw71Lskb8M1RELc9bMcqbf5iQ0Op9xgQDqvb~lzjTHv3C9l~U5geAqfFbJ8r5dalHvwQIuyEatumOIshNdLlRb7x-TGZ4OJ~Xr6rt6UgicVWfZT~Ul78XxW2sBOPTj7CNU02pPk5nPHJqvPLFapEOfS6uqNXLadgvhKK6cP91MLTf0-LjEv7M1~9jkw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal