Abstract
The effect of neonatal gene transfer on antibody formation was determined using a retroviral vector (RV) expressing human factor IX (hFIX). Normal mice from different strains injected intravenously with RV as newborns achieved therapeutic levels of hFIX without antibody production and were tolerant as adults to challenge with hFIX. Neonatal hemophilia B mice that received different amounts of RV achieved stable and dose-related expression of hFIX without anti-hFIX antibody formation. After protein challenge, antibody formation was markedly reduced for animals that expressed hFIX at levels higher than 14 ng/mL (0.3% of normal). However, antibodies developed for animals that received the lowest dose of RV and expressed hFIX at approximately 2 ng/mL before protein challenge. In dogs, neonatal injection of a high dose of RV resulted in 500 ng/mL hFIX in plasma without antibody formation. We conclude that neonatal gene transfer with RV does not induce antibody responses to hFIX in mice or dogs and that mice achieving levels greater than 3 × 10–10 M hFIX are usually tolerant to protein injection as adults. Low-dose gene therapy or frequent protein injections in the neonatal period might induce tolerance to subsequent injections of protein with a low risk for adverse effects.
Introduction
Hemophilia B (HB) is an X-linked disorder caused by deficient factor IX (FIX) activity that affects 1:30 000 males.1 Although gene therapy can result in therapeutic levels of FIX in blood by achieving continuous secretion of the 50-kDa FIX protein,2 antibody responses have occurred. Antibodies can reduce the coagulation function (referred to as inhibitors) or increase the clearance of protein from blood.
Anti-FIX antibodies often occur after gene transfer to adult immunocompetent mice. Antibodies developed after intramuscular (IM) injection of AAV23-8 or adenoviral9 vectors, intraperitoneal (IP) injection of transduced fibroblasts, 10 intravenous (IV) injection of a retroviral vector (RV), 11 or IV injection of adenoviral vectors in most strains except C57BL/6.12-15 Liver-restricted expression may reduce the chance of antibody formation because IV injection of AAV vectors, which are expressed primarily in the liver, failed to induce antibodies,3,4 though antibodies developed with varying frequency in other reports.16-18 Liver-restricted expression from an adenoviral vector may reduce antibody development,19,20 though differences in the level of expression observed with different vectors may affect the result. High expression is less likely than low expression to induce antibody formation after the delivery of AAV vectors to liver18 or muscle.8
Anti-FIX antibodies can also develop after gene transfer to adult large animals. Anti-FIX antibodies developed after IM injection of human FIX (hFIX)–expressing plasmid21 or AAV22 vectors in dogs or a canine FIX (cFIX)–expressing AAV vector in HB dogs from Auburn, which have a frameshift mutation and often develop inhibitors.23 However, anti-cFIX antibodies did not develop in an Auburn dog that received cyclophosphamide (Cytoxan) before IM injection of an AAV vector,24 and they only developed in 1 of 3 Auburn dogs that expressed an AAV vector in the liver.25 The Chapel Hill HB colony has a missense mutation and usually does not produce inhibitors to cFIX.26 In the Chapel Hill dogs, anti-cFIX antibodies did not develop after liver-directed gene therapy with retroviral27 or AAV17,25,28 vectors, and they were stable in only 1 of 9 dogs after IM injection of an AAV vector.29-31 In Rhesus macaques, antibodies to hFIX developed in 3 of 3 animals after IV injection of an adenoviral vector32 and in 1 of 5 animals after liver-directed, AAV-mediated gene transfer.33 Anti-hFIX antibodies did not develop in any of the humans who received muscle-directed AAV vector-mediated gene therapy.34 However, these patients were at low risk for antibody formation because they had prior exposure to hFIX without inhibitor development. Thus, inhibitor formation remains a concern for gene therapy approaches in humans, particularly those with null mutations.
Inhibitors also developed in approximately 3% of HB patients,35 but eradication with high doses of hFIX is expensive and not always successful.36 Identifying a method to prevent inhibitor formation after protein infusion would thus be an important advance.
Our hypothesis was that gene transfer into newborns with immature immune systems37,38 might prevent inhibitor formation. This could involve a high dose to achieve fully therapeutic levels of expression. Alternatively, low-dose gene therapy with subtherapeutic levels might induce tolerance to protein infusions with less chance for adverse effects. We recently reported that neonatal gene transfer with an RV did not induce antibody formation to cFIX in mice or HB dogs from Chapel Hill.11 We now examine the effect of neonatal delivery of an RV expressing the immunogenic hFIX protein. We conclude that neonatal gene transfer does not induce antibody formation in mice or dogs and that most mice are tolerized to subsequent infusions of protein.
Materials and methods
Reagents
Reagents were obtained from Sigma Chemical (St Louis, MO) unless otherwise stated.
Retroviral vector construction
A 1.5-kb hFIX cDNA39 with an optimal Kozak sequence40 and 48 nt of 3′-untranslated sequence was used. The hFIX cDNA was blunt-end ligated into the NotI site of hAAT-WPRE-76741 to generate hAAT-hFIX-WPRE. Generation of an amphotropic GP+AM12-based42 packaging cell line and large-scale production of the RV41 were as described previously. Titer in transducing unit (TU) per milliliter was determined by immunostaining after freezing once. Two days after NIH3T3 cells were infected, cells were fixed with formalin for 20 minutes at room temperature (RT) and were permeabilized for 10 minutes with methanol. Blocking buffer (Tris-buffered saline [40 mM Tris-HCl, 150 mM NaCl, pH 7.4] with 5% nonfat dry milk [TBS-milk; Schnucks Grocery, St Louis, MO]) was added at RT for 1 hour, and wells were incubated with a goat anti-hFIX antibody (GAFIX-AP; Enzyme Research Laboratories, South Bend, IN) at a 1:200 dilution for 1.5 hours. Cells were washed with TBS and then were incubated with a mouse anti-goat/sheep immunoglobulin G (IgG) antibody at a 1:100 dilution at RT for 1.5 hours. Staining was developed with 3,3′-diamobenzidine.43 The RV had fewer than 10 copies of replication-competent retrovirus by a vector rescue assay.41 Polybrene was added (final concentration, 8 μg/mL) before injection.
Animal procedures
National Institutes of Health and United States Department of Agriculture guidelines for the care and use of animals were followed. Inbred BALB/cByJ (referred to as BALB/c), C3H/HeJ (referred to as C3H), C;129S-Cd1tm1Gru (these are CD1 deficient and lack natural killer function, but have normal TH2 cell help and are referred to as BALB/c:129S), and C57BL/6J (referred to as C57BL/6) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). HB mice were in a mixed 129S×C57BL/6 background.44 Newborn mice were injected intravenously through the temporal vein with 100 μL RV at 2 to 3 days after birth. For protein challenge, animals were injected IP with 0.6 international units (IU) hFIX (BeneFix, specific activity 270 IU/mg; Wyeth Pharmaceutical, Cambridge, MA) in 300 μL phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4), which represented approximately 30 IU/kg. Some mice were injected IP with 0.6 IU BeneFix in 200 μL adjuvant RIBI MPL+TDM emulsion (Corixa, Hamilton, MT), which contains 0.5 mg/mL monophosphoryl lipid A, 0.5 mg/mL synthetic trehalose dicorynomycolate, 2% squalene, and 0.2% Tween 80. Plasma was collected through a nonheparinized capillary tube and was mixed with 0.1 vol of 3.2% sodium citrate.
Phenotypically normal puppies were identified by polymerase chain reaction (PCR) analysis of blood samples after breeding mucopolysaccharidosis VII dogs from the University of Pennsylvania colony.45 At 2 or 3 days after birth, 5 mL RV was injected as a single IV dose over 2 minutes.
Immunoassay for hFIX
Enzyme-linked immunosorbent assay (ELISA) plates were coated with mouse monoclonal anti-hFIX antibody (HIX-1, F2645) at a 1:500 dilution in PBS. Wells were blocked overnight with TBS-milk and then washed 6 times with TBS with 0.05% Tween 20 (TBS-Tween) after this and subsequent steps. Samples were diluted in TBS-milk to give values on the linear portion of the standard curve and were incubated at 37°C for 2 hours. A horseradish peroxidase (HRP)–conjugated goat anti-hFIX antibody (GAFIX-APHRP; Enzyme Research Laboratories, South Bend, IN) at a 1:500 dilution was incubated for 2 hours at 37°C, and the assay was developed with 3,3′, 5,5′-tetramethylbenzidine. Standards were dilutions of purified hFIX (Calbiochem, San Diego, CA).
Anti-hFIX IgG antibody assays
ELISA plates were coated with 5 μg/mL purified hFIX (Calbiochem) in PBS and were blocked with TBS-milk. Samples diluted 1:100 or higher in TBS-milk were incubated overnight at 4°C. For samples from mice, an HRP-conjugated goat anti-mouse IgG that recognizes all subclasses of IgG (Roche Molecular Biochemicals, Indianapolis, IN) was added at a 1:200 dilution at 37°C for 2 hours, and the plate was developed with 3,3′, 5,5′-tetramethylbenzidine. For each assay, standards with 2 μg/mL or less mouse IgG with a normal mixture of subtypes (no. 1-5381; Sigma Chemical, St Louis, MO) was used to calculate the relative amount of antibody in milligrams per milliliter. The titer was the highest dilution at which the optical density (OD) for a sample captured with hFIX was at least twice the background OD for the same sample captured with a PBS-coated well. For dog samples, an HRP-coupled sheep anti-canine IgG (Serotec, Raleigh, NC) was added at a 1:500 dilution. Standards were dilutions of dog plasma containing 3.5 μg/mL or less of dog IgG (RS10-105; Bethyl Laboratories, Montgomery, TX).
Bethesda assay
Samples were heat inactivated at 56°C for 60 minutes. For mouse samples, 10 μL mouse plasma, 30 μL PBS, and 10 μL normal human plasma (George King Biomedical, Overland, KS) were incubated for 2 hours at 37°C. Fifty microliters hFIX-deficient human plasma was added, and activated partial thromboplastin time (aPTT) assay was performed.11 Coagulation times were compared with standards containing 0 to 10 μL normal human plasma, 10 to 0 μL hFIX-deficient plasma, 10 μL heat-inactivated normal mouse plasma, and 30 μL PBS that were preincubated for 2 hours, after which aPTT was performed with 50 μL hFIX-deficient human plasma. The dilution factor was considered to be 1 if 10 μL undiluted mouse plasma was used. If necessary, samples were diluted in PBS, and values were compared with a standard curve with the same amount of normal mouse plasma. One Bethesda unit (BU) per milliliter inhibits 50% of the coagulation activity, and the limit of sensitivity was 1 BU/mL. Samples from dogs were assayed in a similar fashion except that 10 μL heat-inactivated dog plasma was used instead of mouse plasma for samples and standards.
Results
Generation of an RV-expressing hFIX
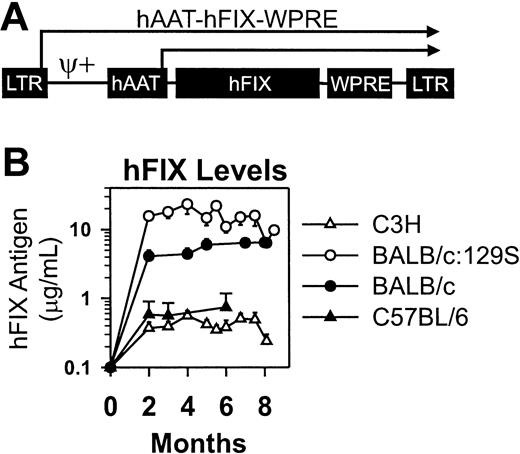
The goal of this project was to study immune responses after neonatal gene transfer of RV. The hFIX cDNA was used because mice and dogs usually make antibodies to the human protein, and reagents are available to characterize the response. The Moloney murine leukemia virus-based RV vector also contained the human α1-antitrypsin promoter and the woodchuck hepatitis virus posttranscriptional regulatory element (Figure 1A). The titer of the concentrated RV varied from 1.8 to 3.5 × 108 TU/mL.
Retroviral vector and expression levels in normal mice from different strains after neonatal transduction. (A) hAAT-hFIX-WPRE. The RV contains intact LTRs at the 5′ and 3′ ends, an extended packaging signal (ψ+), the 403-nt human α1-antitrypsin promoter (hAAT), the 1.5-kb hFIX cDNA (hFIX), and the 591-nt woodchuck hepatitis virus posttranscriptional regulatory element (WPRE). Transcription can initiate from the LTR or hAAT promoters, as indicated by the arrows. (B) Expression in normal mice from different strains after neonatal transduction. C3H (N = 5), BALB/c:129S (N = 5), BALB/c (N = 7), or C57BL/6 (N = 3) mice were injected with 1 × 1010 TU/kg at 2 or 3 days after birth. Average hFIX antigen levels ± SEM are shown at the indicated time in months after birth.
Retroviral vector and expression levels in normal mice from different strains after neonatal transduction. (A) hAAT-hFIX-WPRE. The RV contains intact LTRs at the 5′ and 3′ ends, an extended packaging signal (ψ+), the 403-nt human α1-antitrypsin promoter (hAAT), the 1.5-kb hFIX cDNA (hFIX), and the 591-nt woodchuck hepatitis virus posttranscriptional regulatory element (WPRE). Transcription can initiate from the LTR or hAAT promoters, as indicated by the arrows. (B) Expression in normal mice from different strains after neonatal transduction. C3H (N = 5), BALB/c:129S (N = 5), BALB/c (N = 7), or C57BL/6 (N = 3) mice were injected with 1 × 1010 TU/kg at 2 or 3 days after birth. Average hFIX antigen levels ± SEM are shown at the indicated time in months after birth.
Neonatal gene transfer results in stable expression of hFIX in normal mice
Mice from different strains received IV injections of high-dose (1 × 1010 TU/kg) RV as newborns. Expression was stable in all animals for the duration of evaluation (Figure 1B) and averaged 0.4 ± 0.1 μg/mL (mean ± SEM)] hFIX (9% of normal) for C3H, 17.0 ± 7.2 μg/mL for BALB/c:129S, 5.5 ± 0.5 μg/mL for BALB/c, and 0.6 ± 0.05 μg/mL for C57BL/6 mice. These levels are therapeutic because more than 10% of normal levels prevent most bleeding.
Neonatal gene transfer fails to induce antibody formation in normal mice
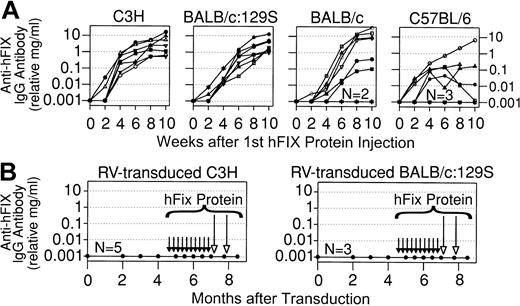
Plasma collected from the RV-treated mice was also evaluated for anti-hFIX antibodies. To determine whether these strains could produce antibodies, normal mice that did not receive gene transfer were injected with 30 IU/kg hFIX, which is the dose used for a minor bleed. The recombinant hFIX used contained an alanine at position 148 of the mature protein and was identical to that encoded by the RV. Protein was injected once a week, the frequency at which a patient with severe HB might be treated. Ten doses were given because inhibitors usually develop within 8 to 12 days of exposure.35 hFIX was injected IP because this method is easier than performing IV injections. Antibody levels were relative amounts in milligrams per milliliter after comparison with standards in which mouse IgG was bound to wells followed by incubation with the anti-mouse IgG antibody. However, the calculated value is not a correct measure of the amount of anti-hFIX antibody because not all the protein in the standards binds to the well (L.X., K.P.P., unpublished data, July 2001). C3H and BALB/c:129S mice that received protein injections without preceding gene transfer consistently made very high-titer antibodies to hFIX, with average relative IgG levels of 3.8 ± 1.4 and 12.2 ± 5.2 mg/mL, respectively, as shown in Figure 2A and as summarized in Table 1. Although those BALB/c mice that produced antibodies had high average relative IgG levels (13.8 ± 4.6 mg/mL), 25% failed to produce any antibodies. Only 5 of 8 C57BL/6 mice developed antibodies, and the average peak relative IgG level of 1.7 mg/mL was lower than that in animals from the other strains.
Anti-hFIX IgG antibodies in normal mice after protein injection or neonatal gene transfer. (A) Anti-hFIX antibody levels after protein injections. Mice of the indicated strain that did not receive gene transfer began to receive weekly IP injections of 30 IU/kg hFIX at 2 to 4 months after birth, for a total of 10 doses. The relative levels of anti-hFIX IgG antibody in milligrams per milliliter were determined by immunoassay and are plotted versus the time in weeks after the first dose of protein. Each line represents a single animal. For the BALB/c and C57BL/6 mice, 2 and 3 mice, respectively, failed to make antibodies (plotted as 0.001 mg/mL on this semilog scale) at any time of evaluation, as indicated by the N near the line at the bottom. (B) Anti-hFIX antibody levels in mice after neonatal gene transfer. These are the same C3H and BALB/c:129S mice that received neonatal injection of 1 × 1010 TU/kg of hAAT-hFIX-WPRE as described in Figure 1B. At 4.5 months after transduction, mice began to receive weekly injections of hFIX without adjuvant for a total of 10 doses, as indicated by the short black arrows. At 7 and 7.75 months after transduction, mice received hFIX in adjuvant, as indicated by the long open arrows. Anti-hFIX IgG antibody levels are shown at the indicated time in months after transduction. None of the C3H (N = 5) or BALB/c:129S (N = 3) mice made detectable antibodies at any time of evaluation.
Anti-hFIX IgG antibodies in normal mice after protein injection or neonatal gene transfer. (A) Anti-hFIX antibody levels after protein injections. Mice of the indicated strain that did not receive gene transfer began to receive weekly IP injections of 30 IU/kg hFIX at 2 to 4 months after birth, for a total of 10 doses. The relative levels of anti-hFIX IgG antibody in milligrams per milliliter were determined by immunoassay and are plotted versus the time in weeks after the first dose of protein. Each line represents a single animal. For the BALB/c and C57BL/6 mice, 2 and 3 mice, respectively, failed to make antibodies (plotted as 0.001 mg/mL on this semilog scale) at any time of evaluation, as indicated by the N near the line at the bottom. (B) Anti-hFIX antibody levels in mice after neonatal gene transfer. These are the same C3H and BALB/c:129S mice that received neonatal injection of 1 × 1010 TU/kg of hAAT-hFIX-WPRE as described in Figure 1B. At 4.5 months after transduction, mice began to receive weekly injections of hFIX without adjuvant for a total of 10 doses, as indicated by the short black arrows. At 7 and 7.75 months after transduction, mice received hFIX in adjuvant, as indicated by the long open arrows. Anti-hFIX IgG antibody levels are shown at the indicated time in months after transduction. None of the C3H (N = 5) or BALB/c:129S (N = 3) mice made detectable antibodies at any time of evaluation.
Summary of anti-hFIX IgG antibody formation in normal mice
Treatment group and mouse strain . | No. with antibodies* . | . | Average IgG, relative mg/mL (range)† . | . | Average inhibitor titer, BU/mL (range)§ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | ELISA . | Bethesda assay . | . | Average ELISA titer (range)‡ . | . | |||||
| Protein injections without gene transfer∥ | ||||||||||
| C3H | 7 of 7 | 7 of 7 | 3.8 ± 1.4 (0.5-10.6) | 263 314 ± 132 467 (102 400-409 600) | 16 ± 2 (6-20) | |||||
| BALB/c: 129S | 8 of 8 | 8 of 8 | 12.2 ± 5.2 (0.6-34.4) | 421 410 ± 169 746 (12 800-1 000 000) | 80 ± 30 (12-200) | |||||
| BALB/c | 6 of 8 | 6 of 8 | 13.8 ± 4.6 (0.2-25.9) | 1 097 600 ± 342 040 (6 400-1 638 400) | 92 ± 32 (1.8-180) | |||||
| C57BL/6 | 5 of 8 | 2 of 8 | 1.7 ± 1.3 (0.04-6.7) | 24 000 ± 19 622 (1 600-102 400) | 9 ± 8 (1 and 18) | |||||
| High-dose neonatal gene transfer followed by 10 hFIX injections without adjuvant and 2 hFIX injections with adjuvant¶ | ||||||||||
| C3H | 0 of 5 P = .0008 | 0 of 5 | 0 | < 1:100 | < 1 | |||||
| BALB/c: 129S | 0 of 3 P = .006 | 0 of 3 | 0 | < 1:100 | < 1 | |||||
| BALB/c | 0 of 7 P = .007 | 0 of 7 | 0 | < 1:100 | < 1 | |||||
| High-dose neonatal gene transfer without protein injections# | ||||||||||
| C57BL/6 | 0 of 3 NS | 0 of 3 | 0 | < 1:100 | < 1 | |||||
Treatment group and mouse strain . | No. with antibodies* . | . | Average IgG, relative mg/mL (range)† . | . | Average inhibitor titer, BU/mL (range)§ . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | ELISA . | Bethesda assay . | . | Average ELISA titer (range)‡ . | . | |||||
| Protein injections without gene transfer∥ | ||||||||||
| C3H | 7 of 7 | 7 of 7 | 3.8 ± 1.4 (0.5-10.6) | 263 314 ± 132 467 (102 400-409 600) | 16 ± 2 (6-20) | |||||
| BALB/c: 129S | 8 of 8 | 8 of 8 | 12.2 ± 5.2 (0.6-34.4) | 421 410 ± 169 746 (12 800-1 000 000) | 80 ± 30 (12-200) | |||||
| BALB/c | 6 of 8 | 6 of 8 | 13.8 ± 4.6 (0.2-25.9) | 1 097 600 ± 342 040 (6 400-1 638 400) | 92 ± 32 (1.8-180) | |||||
| C57BL/6 | 5 of 8 | 2 of 8 | 1.7 ± 1.3 (0.04-6.7) | 24 000 ± 19 622 (1 600-102 400) | 9 ± 8 (1 and 18) | |||||
| High-dose neonatal gene transfer followed by 10 hFIX injections without adjuvant and 2 hFIX injections with adjuvant¶ | ||||||||||
| C3H | 0 of 5 P = .0008 | 0 of 5 | 0 | < 1:100 | < 1 | |||||
| BALB/c: 129S | 0 of 3 P = .006 | 0 of 3 | 0 | < 1:100 | < 1 | |||||
| BALB/c | 0 of 7 P = .007 | 0 of 7 | 0 | < 1:100 | < 1 | |||||
| High-dose neonatal gene transfer without protein injections# | ||||||||||
| C57BL/6 | 0 of 3 NS | 0 of 3 | 0 | < 1:100 | < 1 | |||||
NS indicates not significant.
Number of animals with significant anti-hFIX IgG antibodies was determined from the total number of animals evaluated. P values were obtained by comparing the frequency of antibody formation using Fisher exact test for animals that received gene transfer with that in mice of the same strain that did not receive gene transfer but received 10 injections of hFIX without adjuvant.
Average relative levels of anti-hFIX IgG ± SEM were determined using the highest value obtained for each animal with detectable antibodies.
Average titer was determined using the highest value for each animal that was positive.
Bethesda titer was determined for the sample with the highest levels of anti-hFIX IgG antibody in the immunoassay.
Results are given for animals of the indicated strain that did not receive gene transfer and were treated with 10 injections of 30 IU/kg per dose hFIX without adjuvant. These are the same mice whose antibody levels are shown in Figure 2A.
Results are given for mice of the indicated strain that were injected with 1 × 1010 TU/kg hAAT-hFIX-WPRE at birth and then received 10 injections of 30 IU/kg per dose hFIX without adjuvant followed by 2 injections of 30 IU/kg hFIX with adjuvant. These are the same mice whose expression and antibody levels are shown in Figures 1B and 2B, respectively.
Results are given for mice of the indicated strain that were injected with 1 × 1010 TU/kg hAAT-hFIX-WPRE at birth and were not challenged with protein. These are the same mice whose expression levels are shown in Figure 1B.
Although all strains were capable of producing anti-hFIX antibodies after protein infusion, none of the mice that received neonatal gene transfer had anti-hFIX antibodies 2 months or later after birth (Table 1). To further test whether the neonatal gene transfer approach can induce tolerance, RV-treated mice of the strains with the most robust antibody response were challenged with hFIX beginning at 4.5 months after birth. They received 30 IU/kg hFIX, which increases plasma levels to approximately 1.5 μg/mL in humans. Given that the pharmacokinetics are similar in mice,46 this dose should increase blood levels by 3.4-, 0.1-, and 0.3-fold for C3H, BALB/c:129S, and BALB/c mice, respectively. None of the RV-treated mice developed anti-hFIX antibodies after 10 injections of protein, as shown in Figure 2B for C3H and BALB/c:129S mice and as summarized in Table 1 for BALB/c mice. As a final test of the ability of neonatal gene transfer to induce tolerance, these mice were injected twice with hFIX in adjuvant. All mice were tolerant as they continued to have stable expression of hFIX (Figure 1B) without antibody formation (Figure 2B; Table 1). We conclude that neonatal gene transfer with an RV dose that results in high-level expression induces tolerance to hFIX.
Induction of tolerance in HB mice
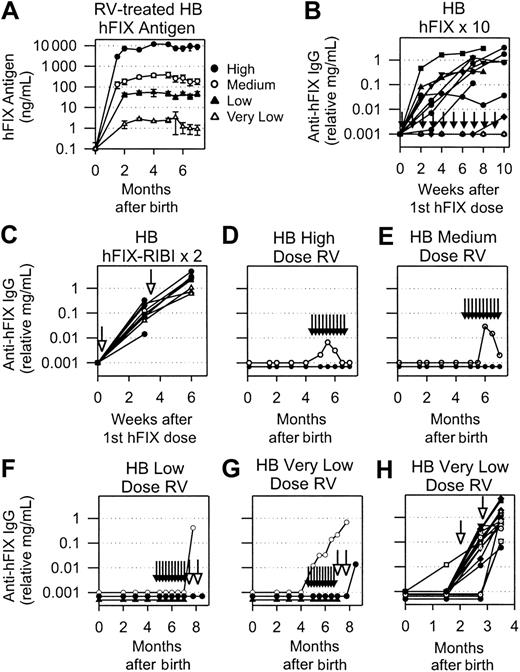
The ability of neonatal gene transfer to induce tolerance was also tested in HB mice that do not express detectable antigen.44 Because the human and mouse proteins are 80% identical, immune responses in null HB mice might differ from those in normal mice. Neonatal HB mice with a mixed 129S×C57BL/6 background were injected intravenously with high (1 × 1010 TU/kg), medium (1 × 109 TU/kg), low (1 × 108 TU/kg), or very low (1 × 107 TU/kg) doses of RV to determine whether expression level affected the ability to induce tolerance to the transgene. Expression was stable in most animals from 2 to 8 months after birth, with the exception of 1 animal in the very low-dose group (to be described at the end of this section). Average levels for those that maintained expression were 8204 ± 4993 ng/mL (164% of normal), 251 ± 188 ng/mL (5% of normal), 50 ± 30 ng/mL (1% of normal), and 2.2 ± 0.8 ng/mL (0.04% of normal) for the high, medium, low, and very low dose of RV, respectively, as shown in Figure 3A.
hFIX expression and anti-hFIX IgG antibody levels in HB mice. (A) hFIX levels in mice transduced as neonates. Neonatal 129S×C57BL/6 HB mice were injected IV with a high (1 × 1010 TU/kg), medium (1 × 109 TU/kg), low (1 × 108 TU/kg), or very low (1 × 107 TU/kg) dose of hAAT-hFIX-WPRE at 2 or 3 days after birth. Average plasma hFIX antigen levels ± SEM are shown. (B) Anti-hFIX IgG antibody levels after hFIX protein injection. Adult HB mice that never received gene transfer began to receive weekly IP injections of 30 IU/kg hFIX without adjuvant at 2 to 4 months after birth and continued for 10 injections total, as indicated by the short vertical arrows in this and subsequent panels. Plasma anti-hFIX antibody levels were determined at the indicated time in weeks after the first dose of hFIX. Each line indicates an individual mouse. Values are plotted as 0.001 mg/mL for the 5 mice that failed to make antibodies at any time of evaluation. (C) Anti-hFIX IgG antibody levels after hFIX protein injection with adjuvant. Adult HB mice that never received gene transfer received 2 injections of 30 IU/kg hFIX in adjuvant separated by 3 weeks. Long open arrows indicate the time of injection of protein with adjuvant in this and subsequent panels. Plasma anti-hFIX IgG antibody levels are plotted versus the time after the first dose of hFIX. Each line indicates an individual mouse. (D-H) Anti-hFIX IgG antibody levels in HB mice that were transduced as neonates. Plasma from mice that were treated at birth with a high (D), medium (E), low (F), or very low (G-H) dose of hAAT-hFIX-WPRE and began to receive hFIX protein injections at 4.5 months after birth was tested for anti-hFIX–specific IgG antibodies at the indicated time after birth. These are the same animals whose hFIX levels are shown in panel A. For panels D and E, the line with open circles represents an individual mouse with low and transient levels of an antibody, whereas the line with closed circles represents 5 mice that did not have detectable antibodies at any time of evaluation. For panel F, the line with open circles represents an animal that developed an antibody after administration of 1 dose of hFIX in adjuvant. The line with closed triangles represents 3 mice that did not develop antibodies after 10 injections of hFIX without adjuvant. The line with closed circles represents 3 mice that did not develop antibodies after 10 injections of hFIX without adjuvant and 1 or 2 injections of hFIX with adjuvant. (G) Anti-hFIX IgG antibody levels in HB mice that were transduced with the very low dose of RV as neonates and were challenged with hFIX as indicated. Each line represents an individual animal. Neonatal mice were injected at birth with the very low dose of RV and were challenged at 2 and 2.75 months with hFIX in adjuvant. Each line indicates an individual animal.
hFIX expression and anti-hFIX IgG antibody levels in HB mice. (A) hFIX levels in mice transduced as neonates. Neonatal 129S×C57BL/6 HB mice were injected IV with a high (1 × 1010 TU/kg), medium (1 × 109 TU/kg), low (1 × 108 TU/kg), or very low (1 × 107 TU/kg) dose of hAAT-hFIX-WPRE at 2 or 3 days after birth. Average plasma hFIX antigen levels ± SEM are shown. (B) Anti-hFIX IgG antibody levels after hFIX protein injection. Adult HB mice that never received gene transfer began to receive weekly IP injections of 30 IU/kg hFIX without adjuvant at 2 to 4 months after birth and continued for 10 injections total, as indicated by the short vertical arrows in this and subsequent panels. Plasma anti-hFIX antibody levels were determined at the indicated time in weeks after the first dose of hFIX. Each line indicates an individual mouse. Values are plotted as 0.001 mg/mL for the 5 mice that failed to make antibodies at any time of evaluation. (C) Anti-hFIX IgG antibody levels after hFIX protein injection with adjuvant. Adult HB mice that never received gene transfer received 2 injections of 30 IU/kg hFIX in adjuvant separated by 3 weeks. Long open arrows indicate the time of injection of protein with adjuvant in this and subsequent panels. Plasma anti-hFIX IgG antibody levels are plotted versus the time after the first dose of hFIX. Each line indicates an individual mouse. (D-H) Anti-hFIX IgG antibody levels in HB mice that were transduced as neonates. Plasma from mice that were treated at birth with a high (D), medium (E), low (F), or very low (G-H) dose of hAAT-hFIX-WPRE and began to receive hFIX protein injections at 4.5 months after birth was tested for anti-hFIX–specific IgG antibodies at the indicated time after birth. These are the same animals whose hFIX levels are shown in panel A. For panels D and E, the line with open circles represents an individual mouse with low and transient levels of an antibody, whereas the line with closed circles represents 5 mice that did not have detectable antibodies at any time of evaluation. For panel F, the line with open circles represents an animal that developed an antibody after administration of 1 dose of hFIX in adjuvant. The line with closed triangles represents 3 mice that did not develop antibodies after 10 injections of hFIX without adjuvant. The line with closed circles represents 3 mice that did not develop antibodies after 10 injections of hFIX without adjuvant and 1 or 2 injections of hFIX with adjuvant. (G) Anti-hFIX IgG antibody levels in HB mice that were transduced with the very low dose of RV as neonates and were challenged with hFIX as indicated. Each line represents an individual animal. Neonatal mice were injected at birth with the very low dose of RV and were challenged at 2 and 2.75 months with hFIX in adjuvant. Each line indicates an individual animal.
Sixty-nine percent of control adult HB mice that did not receive gene transfer developed moderate levels of antibodies (1.2 ± 0.3 relative mg/mL IgG) after 8 to 10 injections of hFIX without adjuvant (Figure 3B; Table 2). Anti-hFIX antibody formation occurred in 100% of mice that received 2 doses of hFIX in adjuvant, and the average levels were 1.8-fold those in animals that received injections without adjuvant (Figure 3C; Table 2). Approximately 50% of the HB mice designated to receive 10 doses of hFIX protein and 25% of those designated to receive 2 doses of hFIX with adjuvant died because of bleeding complications.
Summary of anti-hFIX IgG antibody formation in HB mice
Treatment group . | . | No. with antibodies* . | . | Average anti-hFIX IgG, mg/mL (range)† . | Average anti-hFIX antibody titer (range)‡ . | Average inhibitor titer, BU/mL (range)§ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose of RV . | hFIX protein injections . | ELISA . | Bethesda assay . | . | . | . | ||||||
| hFIX protein injections into HB mice that did not receive gene transfer∥ | ||||||||||||
| None | 8-10 without adjuvant (HB × 10) | 11 of 15 | 9 of 15 | 1.2 ± 0.3 (0.05-3.2) | 20 436 ± 6 140 (800-51 200) | 7.5 ± 2.2 (1.6-18) | ||||||
| None | 2 with adjuvant (HB × 2) | 10 of 10 | 10 of 10 | 2.1 ± 0.5 (0.6-4.6) | 143 000 ± 34 800 (40 000-320 000) | 9.1 ± 2.2 (1-18) | ||||||
| Neonatal gene transfer to HB mice before hFIX protein injections¶ | ||||||||||||
| High-dose RV, 1 × 1010 TU/kg | 10 without adjuvant | 0 of 6 P = .004 vs HB × 10 | 0 of 6 | 0 | < 1:100 | < 1 | ||||||
| Medium-dose RV, 1 × 109 TU/kg | 10 without adjuvant | 0 of 6 P = .004 vs HB × 10 | 0 of 6 | 0 | < 1:100 | < 1 | ||||||
| Low-dose RV, 1 × 108 TU/kg | None | 0 of 10 | 0 of 10 | 0 | < 1:100 | < 1 | ||||||
| 10 without adjuvant | 0 of 7 P = .004 vs HB × 10 | 0 of 7 | 0 | < 1:100 | < 1 | |||||||
| 10 without adjuvant and 1-2 with adjuvant | 1 of 4 P = .01 vs HB × 2 | 0 of 4 | 0.4 | 3 200 | < 1 | |||||||
| Very-low-dose RV, 1 × 107 TU/kg | None | 2 of 24 | 0 of 24 | 0.009 ± 0.003 (0.006 and 0.012) | 300 ± 100 (200 and 400) | < 1 | ||||||
| 10 without adjuvant | 1 of 3 NS vs HB × 10 | 0 of 3 | 0.21 | 6 400 | < 1 | |||||||
| 10 without adjuvant and 1-2 with adjuvant | 2 of 2 NS vs HB × 2 | 1 of 2 | 0.355 ± 0.345 (0.01-0.7) | 3 400 ± 3 000 (400 and 6 400) | 18 | |||||||
| 2 with adjuvant | 17 of 17 NS vs HB × 2 | 17 of 17 | 1.25 ± 0.4 (0.06-5.8) | 61 412 ± 18 017 (2 000-200 000) | 21.9 ± 10.3 (1.8-180) | |||||||
Treatment group . | . | No. with antibodies* . | . | Average anti-hFIX IgG, mg/mL (range)† . | Average anti-hFIX antibody titer (range)‡ . | Average inhibitor titer, BU/mL (range)§ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose of RV . | hFIX protein injections . | ELISA . | Bethesda assay . | . | . | . | ||||||
| hFIX protein injections into HB mice that did not receive gene transfer∥ | ||||||||||||
| None | 8-10 without adjuvant (HB × 10) | 11 of 15 | 9 of 15 | 1.2 ± 0.3 (0.05-3.2) | 20 436 ± 6 140 (800-51 200) | 7.5 ± 2.2 (1.6-18) | ||||||
| None | 2 with adjuvant (HB × 2) | 10 of 10 | 10 of 10 | 2.1 ± 0.5 (0.6-4.6) | 143 000 ± 34 800 (40 000-320 000) | 9.1 ± 2.2 (1-18) | ||||||
| Neonatal gene transfer to HB mice before hFIX protein injections¶ | ||||||||||||
| High-dose RV, 1 × 1010 TU/kg | 10 without adjuvant | 0 of 6 P = .004 vs HB × 10 | 0 of 6 | 0 | < 1:100 | < 1 | ||||||
| Medium-dose RV, 1 × 109 TU/kg | 10 without adjuvant | 0 of 6 P = .004 vs HB × 10 | 0 of 6 | 0 | < 1:100 | < 1 | ||||||
| Low-dose RV, 1 × 108 TU/kg | None | 0 of 10 | 0 of 10 | 0 | < 1:100 | < 1 | ||||||
| 10 without adjuvant | 0 of 7 P = .004 vs HB × 10 | 0 of 7 | 0 | < 1:100 | < 1 | |||||||
| 10 without adjuvant and 1-2 with adjuvant | 1 of 4 P = .01 vs HB × 2 | 0 of 4 | 0.4 | 3 200 | < 1 | |||||||
| Very-low-dose RV, 1 × 107 TU/kg | None | 2 of 24 | 0 of 24 | 0.009 ± 0.003 (0.006 and 0.012) | 300 ± 100 (200 and 400) | < 1 | ||||||
| 10 without adjuvant | 1 of 3 NS vs HB × 10 | 0 of 3 | 0.21 | 6 400 | < 1 | |||||||
| 10 without adjuvant and 1-2 with adjuvant | 2 of 2 NS vs HB × 2 | 1 of 2 | 0.355 ± 0.345 (0.01-0.7) | 3 400 ± 3 000 (400 and 6 400) | 18 | |||||||
| 2 with adjuvant | 17 of 17 NS vs HB × 2 | 17 of 17 | 1.25 ± 0.4 (0.06-5.8) | 61 412 ± 18 017 (2 000-200 000) | 21.9 ± 10.3 (1.8-180) | |||||||
Number of animals with significant anti-hFIX IgG antibodies was determined out of the total number of animals evaluated. P values were obtained by comparing the frequency of antibody formation using Fisher exact test for animals that received gene transfer with that in HB mice that received 10 injections of hFIX without adjuvant (HB × 10) or 2 injections of hFIX with adjuvant (HB × 2).
Average relative levels of anti-hFIX IgG ± SEM were determined using the highest value obtained for each animal with detectable antibodies.
Average anti-hFIX IgG titer was determined using the highest value for each animal that was positive.
Bethesda titer was determined for the sample with the highest anti-hFIX IgG antibody level and was averaged for all the animals that were positive.
Results are given for HB mice that did not receive gene transfer and were treated with 10 injections of 30 IU/kg per dose hFIX without adjuvant (HB × 10) or 2 doses of 30 IU/kg hFIX with adjuvant (HB × 2). These are the same mice whose antibody levels are shown in Figure 3B-C, respectively.
Results are given for HB mice that were injected with different doses of hAAT-hFIX-WPRE at birth. These are the same mice whose expression and antibody levels are shown in Figure 3A, D-H, respectively.
At 4.5 months after birth, RV-treated mice began to receive weekly IP injections of 0.6 IU BeneFix without adjuvant, which was continued for 10 weeks. This dose should have increased hFIX plasma levels to 0.2-, 6-, 30-, and 680-fold those of the pre-hFIX protein injection levels for mice that received the high, medium, low, and very low doses of RV, respectively. Five of 6 mice that received the high RV dose had no detectable antibodies, whereas 1 had a very low level (relative IgG, 0.007 mg/mL) of an anti-hFIX antibody at 4 weeks after the first dose of hFIX that subsequently disappeared (Figure 3D); this was considered biologically insignificant. The frequency of antibody formation in mice that received the high RV dose was statistically lower than it was in HB mice that did not receive gene transfer but received 8 to 10 hFIX injections without adjuvant (P = .004, Fisher exact test). Similarly, 5 of 6 animals that received the medium RV dose had no detectable antibodies after 10 hFIX injections. One mouse had a low level (relative IgG, 0.03 mg/mL) at 6 weeks, which fell to barely detectable levels by 10 weeks and was considered biologically insignificant (Figure 3E). There was no loss of animals in either group because of bleeding complications.
For animals that received the low dose of RV, 0 of 7 mice that completed 8 or 10 doses of hFIX made anti-hFIX antibodies (P = .004 [Fisher exact test] compared with HB mice after 10 hFIX injections without adjuvant), as shown in Figure 3F. Three mice in this group died early of bleeding complications, suggesting that a plasma hFIX level of 50 ng/mL (1% of normal) does not achieve hemostasis. These mice were further challenged with 2 injections of hFIX in adjuvant. Of the mice that survived 1 or 2 injections, 1 developed an anti-hFIX antibody of a moderate level (relative IgG, 0.4 mg/mL), and the others remained negative (Figure 3F). The frequency of antibody formation after the administration of hFIX in adjuvant (1 of 4) remained lower than it was in HB mice that did not receive gene transfer and received hFIX in adjuvant (P = .01, Fisher exact test).
One mouse that received the very low RV dose developed an anti-FIX antibody with a relative IgG level of 0.21 mg/mL after 10 injections of hFIX without adjuvant (Figure 3G); this was associated with a decrease in plasma hFIX antigen to undetectable levels. The antibody level increased further after 1 dose of hFIX in adjuvant. Although antibodies did not develop in the other 2 animals that completed 10 injections of hFIX without adjuvant, the frequency of antibody formation in this group (1 of 3) was not statistically different from that in control HB mice that received 8 to 10 hFIX injections without preceding gene transfer. Mice that did not develop antibodies after injections of protein without adjuvant were then injected with hFIX with adjuvant. One developed an antibody, but the other did not survive the first injection. Because of the low survival rate in this group attributed to bleeding, additional HB mice were injected with the very low RV dose at birth. This resulted in average hFIX levels of 1.6 ± 0.6 ng/mL at 6 weeks (data not shown), which was similar to the level observed in the initial study. One of 18 mice developed an antibody in response to gene transfer, but the level (less than 0.012 relative mg/mL IgG) was low. However, all 17 mice that completed 2 injections of hFIX in adjuvant developed anti-hFIX antibodies, with an average relative IgG level of 1.25 ± 0.4 mg/mL. We concluded that the high, medium, and low doses of RV result in tolerance to hFIX protein injections but that the very low dose does not.
Inhibitor formation in normal and HB mice
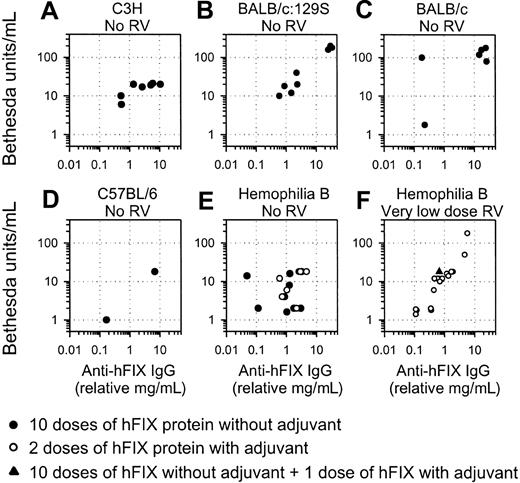
Samples with the highest IgG levels were also tested for inhibitor activity. All C3H and BALB/c:129S mice that did not undergo the preceding gene transfer and were challenged with hFIX without adjuvant developed inhibitors, which correlated reasonably well with the relative levels of anti-hFIX IgG (Figure 4A-B; Table 1). Similarly, all BALB/c mice with anti-hFIX antibodies detectable by immunoassay had inhibitors (Figure 4C), though those that were negative by immunoassay were also negative by the Bethesda assay (data not shown). Inhibitor titers were generally low or undetectable for the C57BL/6 mice (Figure 4D). Inhibitor levels were similar for HB mice that did not receive gene transfer regardless of whether they were stimulated with hFIX with or without adjuvant (Figure 4E).
Inhibitor formation in normal and HB mice. The inhibitor activity for the sample from each mouse with the highest antibody level is plotted versus the anti-hFIX IgG level for that sample. (A-D) Values are shown for mice of the indicated strain that did not receive gene transfer and were challenged with 10 doses of 30 IU hFIX without adjuvant. (E) HB mice that did not receive gene transfer were challenged with 10 injections of 30 IU/kg hFIX without adjuvant (•) or 2 doses of 30 IU/kg hFIX with adjuvant (○). (F) HB mice were treated with the very low dose of RV. One mouse (the time course of antibody levels for this mouse is shown as a ○ in Figure 3G) was challenged with 10 doses of hFIX without adjuvant and 1 dose of hFIX with adjuvant (▴). The other mice (shown in Figure 3H) were stimulated with 2 doses of hFIX with adjuvant and are shown as ○ here.
Inhibitor formation in normal and HB mice. The inhibitor activity for the sample from each mouse with the highest antibody level is plotted versus the anti-hFIX IgG level for that sample. (A-D) Values are shown for mice of the indicated strain that did not receive gene transfer and were challenged with 10 doses of 30 IU hFIX without adjuvant. (E) HB mice that did not receive gene transfer were challenged with 10 injections of 30 IU/kg hFIX without adjuvant (•) or 2 doses of 30 IU/kg hFIX with adjuvant (○). (F) HB mice were treated with the very low dose of RV. One mouse (the time course of antibody levels for this mouse is shown as a ○ in Figure 3G) was challenged with 10 doses of hFIX without adjuvant and 1 dose of hFIX with adjuvant (▴). The other mice (shown in Figure 3H) were stimulated with 2 doses of hFIX with adjuvant and are shown as ○ here.
Inhibitors were also evaluated in mice that received neonatal gene transfer. None of the C3H, BALB/c:129S, or BALB/c mice treated with gene transfer at birth and challenged with hFIX developed inhibitors (Table 1), which is consistent with the absence of anti-hFIX antibodies by immunoassay. Similarly, none of the C57BL/6 mice developed inhibitors, though protein stimulation was not performed. Inhibitors were also absent from all HB mice that received the high, medium, or low dose of RV and were challenged with protein (Table 2). However, most mice that received the very low dose of RV developed inhibitors in response to protein administration (Figure 4F; Table 2). We conclude that administering a higher dose of RV to newborns results in tolerance to protein infusion but that administering the very low dose does not.
Neonatal gene transfer in normal dogs
Five normal dogs were injected with hAAT-hFIX-WPRE at 2 or 3 days after birth. The platelet counts were normal at 183 000 ± 26 000 and 175 000 ± 104 000 at 24 and 48 hours after injection, respectively, which suggests that the modest decrease in the platelet count noted previously with a 3-fold higher dose of RV11 was dose related. All dogs had stable expression of hFIX, which varied from 223 to 914 ng/mL in individual animals and averaged 494 ± 132 ng/mL (Figure 5A). No animals developed anti-hFIX antibodies as assessed by immunoassay or Bethesda assay (Figure 5C; Table 3).
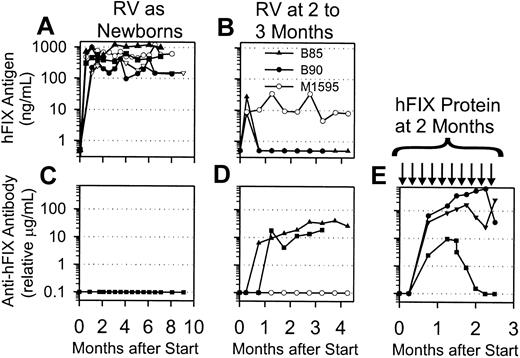
Expression of hFIX and anti-hFIX IgG levels in normal dogs after gene transfer or protein injection. (A) hFIX antigen levels after neonatal gene transfer. Newborn normal dogs (N = 5) were injected intravenously with 3.2 × 109 TU/kg hAAT-hFIX-WPRE at 2 days after birth, and plasma was tested for hFIX antigen levels at the indicated time in months after transduction. (B) hFIX antigen levels after gene transfer to young dogs. Two 8-week-old dogs (B85 and B90) were injected intravenously with 5 × 108 TU/kg hAAT-hFIX-WPRE, whereas one 11-week-old dog (M1595) was injected intravenously with 2 × 108 TU/kg. The plasma was tested for hFIX antigen levels at the indicated time in months after transduction. Antigen levels that were undetectable were plotted as 0.5 ng/mL on this semilog scale. (C-E) Anti-hFIX IgG antibody levels in dogs. Anti-hFIX IgG antibody levels were determined by immunoassay. If antibody was undetectable, it was plotted as 0.1 μg/mL on this semilog scale. (C) Plasma was from the dogs that were transduced as newborns and whose antigen levels are shown in panel A. Time of evaluation varied from 6 to 9 months after birth. (D) Plasma was from the dogs that were transduced as juveniles and whose antigen levels are shown in panel B, and the values are plotted at the indicated times after transduction. (E) Plasma was from dogs that began to receive weekly IV injections of 30 IU/kg hFIX at 8 weeks after birth, which was continued for 10 weeks, as indicated by the black arrows. Antibody levels are plotted versus the time after the first dose of hFIX protein.
Expression of hFIX and anti-hFIX IgG levels in normal dogs after gene transfer or protein injection. (A) hFIX antigen levels after neonatal gene transfer. Newborn normal dogs (N = 5) were injected intravenously with 3.2 × 109 TU/kg hAAT-hFIX-WPRE at 2 days after birth, and plasma was tested for hFIX antigen levels at the indicated time in months after transduction. (B) hFIX antigen levels after gene transfer to young dogs. Two 8-week-old dogs (B85 and B90) were injected intravenously with 5 × 108 TU/kg hAAT-hFIX-WPRE, whereas one 11-week-old dog (M1595) was injected intravenously with 2 × 108 TU/kg. The plasma was tested for hFIX antigen levels at the indicated time in months after transduction. Antigen levels that were undetectable were plotted as 0.5 ng/mL on this semilog scale. (C-E) Anti-hFIX IgG antibody levels in dogs. Anti-hFIX IgG antibody levels were determined by immunoassay. If antibody was undetectable, it was plotted as 0.1 μg/mL on this semilog scale. (C) Plasma was from the dogs that were transduced as newborns and whose antigen levels are shown in panel A. Time of evaluation varied from 6 to 9 months after birth. (D) Plasma was from the dogs that were transduced as juveniles and whose antigen levels are shown in panel B, and the values are plotted at the indicated times after transduction. (E) Plasma was from dogs that began to receive weekly IV injections of 30 IU/kg hFIX at 8 weeks after birth, which was continued for 10 weeks, as indicated by the black arrows. Antibody levels are plotted versus the time after the first dose of hFIX protein.
Summary of anti-hFIX IgG antibody formation in dogs
Treatment group . | Dogs with antibodies* . | Identifying no. . | Peak relative IgG, μg/mL . | Peak immunoassay titer . | Inhibitor titer, BU/mL† . |
|---|---|---|---|---|---|
| Dogs transduced at birth‡ | 0 of 5 | — | 0 | < 1:100 | < 1 |
| Dogs transduced at 8-11 wk§ | 2 of 3 | M1595 | 0 | < 1:100 | < 1 |
| B85 | 41 | 1:800 | < 1 | ||
| B90 | 19 | 1:400 | < 1 | ||
| Dogs injected with 10 doses hFIX protein starting at 8 wk∥ | 3 of 3 | M1641 | 631 | 1:102 400 | 2 |
| M1644 | 252 | 1:25 600 | 5 | ||
| M1645 | 10 | 1:200 | < 1 |
Treatment group . | Dogs with antibodies* . | Identifying no. . | Peak relative IgG, μg/mL . | Peak immunoassay titer . | Inhibitor titer, BU/mL† . |
|---|---|---|---|---|---|
| Dogs transduced at birth‡ | 0 of 5 | — | 0 | < 1:100 | < 1 |
| Dogs transduced at 8-11 wk§ | 2 of 3 | M1595 | 0 | < 1:100 | < 1 |
| B85 | 41 | 1:800 | < 1 | ||
| B90 | 19 | 1:400 | < 1 | ||
| Dogs injected with 10 doses hFIX protein starting at 8 wk∥ | 3 of 3 | M1641 | 631 | 1:102 400 | 2 |
| M1644 | 252 | 1:25 600 | 5 | ||
| M1645 | 10 | 1:200 | < 1 |
Number of animals with antibodies was determined out of the total number evaluated.
Bethesda titer was determined for the sample with the highest levels of anti-hFIX IgG or for the sample collected at the last time point.
Dogs were injected with 3.2 × 109 TU/kg hAAT-hFIX-WPRE at 2 or 3 days after birth and were never stimulated with hFIX protein injections. These are the same dogs whose hFIX antigen and anti-hFIX antibody levels are shown in Figure 5A,C.
Dogs were injected with 5 × 108 TU/kg hAAT-hFIX-WPRE at 8 weeks after birth (B85 and B90) or 2 × 108 TU/kg hAAT-hFIX-WPRE at 11 weeks after birth (M1595) and were never stimulated with hFIX protein. These are the same dogs whose hFIX antigen and anti-hFIX antibody levels are shown in Figure 5B,D.
Dogs that did not receive gene transfer were injected intravenously with 10 doses of 30 IU/kg hFIX beginning at 8 weeks after birth. These are the same dogs whose anti-hFIX antibody levels are shown in Figure 5E.
Two experiments documented that this colony of dogs could produce anti-hFIX antibodies. Three dogs injected with hAAT-hFIX-WPRE at 8 to 11 weeks after birth exhibited low-level expression at 1 week (Figure 5B), which averaged 14.3 ± 6.3 ng/mL. Two dogs had subsequent decreases in their plasma hFIX antigen levels in conjunction with the development of anti-hFIX antibodies that were of relatively low titer (Figure 5D), whereas the third dog maintained hFIX levels at approximately 8 ng/mL for 6 months and never developed anti-hFIX antibodies. In addition, 2 dogs injected intravenously with 10 doses of 30 IU/kg hFIX starting at 8 weeks after birth developed high-titer anti-hFIX antibodies (Figure 5E) with Bethesda titers of 2 and 5 BU/mL (Table 3). A third dog developed a low-titer antibody without inhibitory activity that disappeared with time.
Discussion
Neonatal gene transfer does not induce anti-hFIX antibodies in mice or dogs
This study demonstrates that neonatal gene transfer with a high dose (1 × 1010 TU/kg) of an amphotropic RV expressing hFIX does not induce anti-hFIX antibody formation in C3H, BALB/c: 129S, BALB/c, C57BL/6, or HB mice. In contrast, mice from these strains produce anti-hFIX antibodies after protein infusion as adults, albeit with varying efficiency. Similarly, none of 5 dogs that received neonatal gene transfer with hAAT-hFIX-WPRE developed antibodies, though clinically significant anti-hFIX antibodies developed after protein infusion in 2 of 3 normal dogs in this study and in 6 of 8 normal47 and 6 of 6HB48 dogs in previous studies. The frequency of anti-hFIX antibody formation in dogs is statistically lower after neonatal gene transfer than after protein infusion if these historical controls are included (P = .002, Fisher exact test).
These results are consistent with our previous study in which significant levels of anti-cFIX antibodies did not develop after neonatal transfer of an RV expressing cFIX to mice and dogs11 and the absence of anti-hFIX antibodies after neonatal gene transfer with AAV49 or adenoviral46 vectors. Our results differ from those of VandenDrissche et al,50 who found inhibitors in 50% of hemophilia A mice that received neonatal IV injections of a VSV-G–pseudotyped RV expressing human factor VIII (hFVIII). This discrepancy could be attributed to a greater immunogenicity of hFVIII, induction of inflammatory responses by VSV-G or to other causes.
Although others have suggested that liver-restricted expression can reduce or prevent an antibody response after gene transfer with AAV3,4,18 or adenoviral19,20 vectors, it is unlikely that this is the mechanism here. We previously found that expression was high in the spleen from the long terminal repeat (LTR) of an RV at 5 days after neonatal transfer in dogs,41 and the LTR of our RV (Figure 1A) can direct expression of hFIX in nonhepatic cells. In addition, spleen mRNA levels were approximately 1% those in liver at 6 months after neonatal injection of a similar vector into mice.51 Studies are in progress to confirm that expression occurs in the spleen shortly after neonatal gene transfer in mice.
Anti-hFIX antibodies were still not observed in most mice after neonatal gene transfer with progressively lower doses (1 × 109, 1 × 108, or 1 × 107 TU/kg) of hAAT-hFIX-WPRE in HB mice. This result differs from that of Mingozzi et al,18 who reported that lower expression of the transgene after an AAV vector was delivered to the livers of adult mice was more likely to result in antibody formation to FIX than was higher expression. Possible explanations for this discrepancy include differences in the ages or the genetic backgrounds of the mice.
Neonatal gene transfer induces dose-dependent tolerance to hFIX
Mice that received neonatal injections of hAAT-hFIX-WPRE were tested for tolerance to hFIX for 2 reasons. First, some patients will probably not achieve fully therapeutic levels of hFIX after gene transfer and would have to be treated intermittently with factor. Second, low-dose neonatal gene therapy might be used to induce tolerance to factor infusion, which should have a proportionately lower risk for adverse effects. C3H, BALB/c:129S, and BALB/c mice that received a high dose (1 × 1010 TU/kg) of RV failed to develop anti-hFIX IgG antibodies after 10 IP injections of hFIX protein without adjuvant. It is possible that different results would have been obtained with IV injections, which is the route used in humans. However, this is unlikely because most proteins rapidly reach the blood after IP injection, and that was used in this study because it is easier to perform. These mice also failed to make antibodies after injections of 2 doses of hFIX in adjuvant, which is a more stringent test of tolerance. Similarly, HB mice that received a high or medium (1 × 109 TU/kg) dose of RV failed to develop anti-hFIX antibodies in response to 10 injections of hFIX without adjuvant.
In contrast to the results with the high and medium doses of RV, some HB mice that received lower doses of RV as newborns developed antibodies after challenge with hFIX. For the low-dose (1 × 108 TU/kg) group, the frequency was statistically different from that in HB mice that did not receive gene transfer. Thus, although induction of tolerance was incomplete, it was still markedly reduced, and the antibody that developed was of low titer. For the very low dose (1 × 107 TU/kg), the frequency of antibody formation with simple protein injection was harder to assess, given the small number of animals that survived, because of bleeding, but it was not statistically different from that in HB mice that did not receive gene transfer before protein challenge. All animals that received the very low RV dose developed antibodies after hFIX injection with adjuvant.
We conclude that continuous expression of more than 14 ng/mL (3 × 10–10 M) hFIX starting shortly after birth results in tolerance to the administration of protein in adulthood. This is consistent with the observed tolerance in transgenic mice that express antigen at 10–8 to 10–10 M, though lower expression was insufficient to induce tolerance.52-60 In these studies of transgenic mice, the absence of antibodies in vivo is attributed to T-cell tolerance; B cells remain capable of responding when incubated with T cells from nontransgenic mice. Future studies will determine whether the induction of tolerance after neonatal RV gene transfer is caused by a similar mechanism.
Implications for patients with hemophilia
Neonatal gene therapy might be used to reduce bleeding in patients with HB if long-term preclinical data demonstrate safety. These data suggest that this neonatal RV-mediated gene therapy approach will not induce antibody formation, regardless of the expression level. However, the immune system of newborn humans is relatively more mature than that of newborn mice, though immune responses in newborn humans are still markedly blunted relative to that of adult humans.37,38 It will, therefore, be necessary to confirm in future studies that neonatal gene therapy does not induce immune responses in large animals, including primates, before this approach is used in humans with HB.
One use for neonatal gene therapy for hemophilia would be to induce tolerance to the subsequent infusion of protein with a relatively low dose of RV that should have a reduced risk for adverse effects. Although inhibitors develop in only 3% of patients with HB, they occur in 35% of patients with hemophilia A with large deletions or early truncations.61 Future studies will determine whether the expression of more than 3 × 10–10 M hFIX induces tolerance in larger animals and whether tolerance to hFVIII occurs. Implementing this approach for inducing tolerance in patients will also require long-term evaluation of the safety of neonatal gene transfer.
A final implication of this study is that patients might be tolerized to hFIX (or hFVIII) by achieving a relatively stable level of protein in blood with frequent protein injections during the first several months after birth. Indeed, injecting hFVIII into newborn mice resulted in tolerization to protein challenge when they became adults,62 whereas initiating frequent injections of hFIX at birth led to the development of tolerance in HB dogs from Chapel Hill.63 These results provide a rationale for testing whether frequent administration of factor immediately after birth can reduce the frequency of inhibitor formation in patients at high risk for their development.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-06-2181.
Supported by the National Institutes of Health grants DK48028 (K.P.P.) and RR02512 (M.E.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Donna Armentano and Savio Woo for the modified hFIX cDNA, Wyeth Pharmaceutical for BeneFix, Hui-Feng Lin and Darrel Stafford for HB mice, Paul Monahan and Chris Walsh for a canine anti-hFIX antibody, Roland Herzog for advice on immunoassays, and Patty O'Donnell and Karyn Cullen for assistance with dog studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal