Abstract
The murine adult hematopoietic stem cell is able to function as a hemangioblast, contributing both to blood reconstitution and to blood vessel repair in response to ischemic injury. We developed a novel mouse xenotransplantation model of retinal neovascularization to test human hematopoietic cell plasticity. Immunocompromised nonobese diabetic (NOD)/scid mice underwent myeloablative conditioning and transplantation with human CD34+ umbilical cord blood. After multilineage reconstitution was established, retinal ischemia was induced to promote neovascularization. Our results demonstrate human retinal neovascularization, thus revealing the functional hemangioblast activity of human hematopoietic cells.
Introduction
Using a unique model of murine vasculogenesis, we found that the adult hematopoietic stem cell (HSC) provides functional hemangioblast activity, producing both blood and blood vessels.1 These experiments provided proof that an adult HSC can function as a hemangioblast in response to vascular endothelial growth factor (VEGF) and ischemic injury. Furthermore, this study was the first to prove that an adult stem cell can clonally exhibit functional plasticity in the transplantation setting. However, primate and human HSC plasticity have been difficult to reproduce, bringing into question the clinical relevance of stem cell plasticity.2,3 This difficulty prompted us to test whether human hematopoietic cells also exhibit functional hemangioblast activity.
To study adult human hematopoiesis, the nonobese diabetic (NOD)/scid strain has been used because it is tolerant of human chimerism.4 Cell enrichment and gene marking studies have shown that the repopulating cells, termed scid mouse–repopulating cells (SRCs), are primitive and distinct.5 SRCs express a CD34+CD38– phenotype and are the best surrogate for testing HSCs.
Study design
Generation of human chimeric mice
Chimeric mice were generated by irradiating recipient NOD/scid mice with 325 rads followed by intravenous injection of 2 × 105 human CD34+ cells greatly enriched for HSC/hematopoietic progenitor cell (HPC) from umbilical cord blood (UCB) by magnetic bead positive selection using Miltenyi magnetically activated cell sorter (MACS; Miltenyi Biotech, Auburn, CA). The CD34+ cells were then stained for CD45 to confirm predominant hematopoietic origin (Figure 1A). Typical CD34+ purity was more than 95%. At 4 to 6 weeks after xenotransplantation, approximately 50% of NOD/scid mice receiving xenotransplants had human hematopoietic reconstitution in peripheral blood and 80% had multilineage human hematopoietic reconstitution in bone marrow (Figure 1B). Production of diploid circulating endothelial progenitor cells (EPCs)6 was confirmed by sorting either murine or human VEGF receptor 2+ (VEGFR-2+) cells from the peripheral blood and staining for DNA content with propidium iodide (Figure 1C). Both murine-only control animals and xenograft recipients who underwent the neovascularization model had significant levels of circulating diploid EPCs (3%-5% of total peripheral blood mononuclear cells [PBMNCs] versus > 0.5% in uninjured control animals).
FACS analysis of enriched human hematopoietic stem cells (HSCs) and multilineage reconstitution. (A) Pretransplantation umbilical cord blood (UCB) HSC/HPC enrichment as identified by antibodies to CD34 (phycoerythrin [PE]) and CD45 (allophycocyanin [APC]). Positive fraction (right) shows more than 90% purity of CD34+CD45+ cells. Negative fraction (left) contains no CD34+ cells. (B) Multilineage human hematopoietic reconstitution in NOD/scid mouse bone marrow seen 4 weeks after human CD34+ cell transplantation. Bone marrow cells stained positive with human specific antibodies against leukocytes (CD45), B cells (CD19), T cells (CD3), and myelomonocytic cells (CD15). All panels shown are gated on live cells. (C) Fluorescence-activated cell sorting (FACS)–sorted control murine EPCs (VEGR-2+) from mice undergoing retinal ischemia, stained for DNA content with propidium iodide (left panel); sorted xenograft-derived human EPCs stained for DNA content with propidium iodide (right panel). Both panels exhibit a classic diploid staining profile.
FACS analysis of enriched human hematopoietic stem cells (HSCs) and multilineage reconstitution. (A) Pretransplantation umbilical cord blood (UCB) HSC/HPC enrichment as identified by antibodies to CD34 (phycoerythrin [PE]) and CD45 (allophycocyanin [APC]). Positive fraction (right) shows more than 90% purity of CD34+CD45+ cells. Negative fraction (left) contains no CD34+ cells. (B) Multilineage human hematopoietic reconstitution in NOD/scid mouse bone marrow seen 4 weeks after human CD34+ cell transplantation. Bone marrow cells stained positive with human specific antibodies against leukocytes (CD45), B cells (CD19), T cells (CD3), and myelomonocytic cells (CD15). All panels shown are gated on live cells. (C) Fluorescence-activated cell sorting (FACS)–sorted control murine EPCs (VEGR-2+) from mice undergoing retinal ischemia, stained for DNA content with propidium iodide (left panel); sorted xenograft-derived human EPCs stained for DNA content with propidium iodide (right panel). Both panels exhibit a classic diploid staining profile.
Induction of retinal neovascularization
After multilineage human hematopoietic reconstitution was established (1 month after transplantation), chimeras were subjected to the retinal vessel injury model as previously described.1 In brief, a combination of site-specific VEGF administration followed by retinal vessel photocoagulation by way of an argon laser was applied to one eye of the animal that received a xenotransplant.
Data collection and analysis
Two weeks after photocoagulation, peripheral blood mononuclear cells were collected from all injured mice that received xenotransplants and analyzed for human leukocyte engraftment. The first cohort of mice that received xenotransplants (n = 5) were anesthetized and subsequently perfused with Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) followed by antihuman CD31 (fluorescein isothiocyanate [FITC]), and 2 wash perfusions. After staining, retinas were mounted flat for confocal microscopy using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) to inhibit photobleaching. Two additional cohorts of mice that received xenotransplants (n = 5 each) were anesthetized and perfused with 4% paraformaldehyde, and eyes were harvested for sectioning. Also at the time of death, bone marrow was collected in all animals that received xenotransplants to analyze for human hematopoietic engraftment.
Immunohistochemistry
After fluorescence imaging, retinas were fixed in paraformaldehyde, cryopreserved, and sectioned at 5 μ. Immunohistochemical staining was performed by using monoclonal antibodies to human CD31 (Dako, Carpinteria, CA) and lysosome associate membrane protein 1 (LAMP-1; Developmental Studies Hybridoma Bank at the University of Iowa, Ames, IA). Detection of primary antibody was carried out by using a labeled streptavidin biotin–horseradish peroxidase (LSAB-HRP) kit (Dako) following manufacturer's instructions. Diaminobenzidene (DAB) was used as the chromogen, and slides were counterstained with hematoxylin quantity sufficient (QS; Vector; Vector Laboratories). Human uterus samples were used as positive control.
Results and discussion
We recently developed a murine model of retinal neovascularization that mimics pathology observed in proliferative diabetic retinopathy.1 In the present study we sought to determine if human hematopoietic cells are also capable of functional hemangioblast activity.
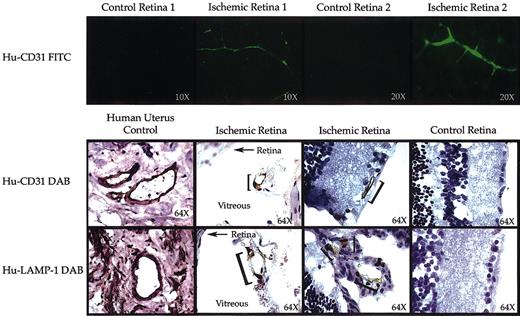
The pattern of new blood vessel development induced in our xenotransplantation model is depicted in Figure 2. Representative flat mounts of ischemic eyes from 2 animals show areas with newly regenerated blood vessels that have endothelial cells derived from donor human HSC/HPC (regions of FITC-green). The noninjured control eyes from the same animals have no staining for human CD31, indicating no human contribution to endothelial cells and demonstrating the specificity of the staining protocol. Additionally, immunohistochemistry was performed on sections from fixed eyes to identify human endothelial cell contribution to the newly formed blood vessels within the retina and vitreous of the ischemic eyes. Staining for human-specific CD31 expression was used to detect endothelial cells, whereas staining for human LAMP-1 was used to detect all cells of human origin. Human cells lining blood vessels and expressing endothelial proteins are clearly detected in the ischemic retinas of animals that received xenotransplants. Human endothelial cells were still detectable up to 5 months after xenotransplantation. Overall level of human contribution to neovascularization was in the 1% to 5% range. Control eyes from the same animals demonstrate very low background staining and a complete lack of human cell contribution. The level of human endothelial engraftment in the ischemic eyes roughly correlated with degree of bone marrow chimerism. Without human hematopoietic engraftment we never saw human endothelial contribution or circulating EPCs, strongly suggesting that human HSCs make endothelial progenitor cells. These findings mimic the results of the original murine model.
Induction of retinal neovascularization containing human endothelial cells. (Top) Mice that received xenotransplants were perfused with anti-human CD31 (FITC) followed by 2 wash perfusions. Flat mounts of the retina were examined by confocal fluorescence microscopy. Panels show fluorescence micrographs of noninjured control retinas versus injured ischemic retinas isolated from the same animal. Only injured eyes stained positive (green) for human CD31. Original magnification for control and ischemic retina 1, × 10; for control and ischemic retina 2, × 20. (Bottom) Immunohistochemistry staining for human CD31 and human LAMP-1 was performed on human uterus as a positive control (original magnification, × 64), noninjured retinas from the same animal that received xenotransplants served as negative controls (control eye, original magnification × 64), and injured ischemic retinas (original magnification, × 64). Human endothelial cells (brown stained cells demarcated with a bracket) were detected only in the injured retinas. Some of the new vessels were formed within the vitreous of the injured eyes. In those sections the retina is annotated with an arrow and the vitreous in clearly labeled.
Induction of retinal neovascularization containing human endothelial cells. (Top) Mice that received xenotransplants were perfused with anti-human CD31 (FITC) followed by 2 wash perfusions. Flat mounts of the retina were examined by confocal fluorescence microscopy. Panels show fluorescence micrographs of noninjured control retinas versus injured ischemic retinas isolated from the same animal. Only injured eyes stained positive (green) for human CD31. Original magnification for control and ischemic retina 1, × 10; for control and ischemic retina 2, × 20. (Bottom) Immunohistochemistry staining for human CD31 and human LAMP-1 was performed on human uterus as a positive control (original magnification, × 64), noninjured retinas from the same animal that received xenotransplants served as negative controls (control eye, original magnification × 64), and injured ischemic retinas (original magnification, × 64). Human endothelial cells (brown stained cells demarcated with a bracket) were detected only in the injured retinas. Some of the new vessels were formed within the vitreous of the injured eyes. In those sections the retina is annotated with an arrow and the vitreous in clearly labeled.
This study demonstrates that UCB-derived human CD34+CD45+ cells are the source of circulating EPCs that play a physiologic role in blood vessel repair. Thus, human HSCs display a similar hemangioblast activity to that of adult murine HSCs. Human endothelial cell contributions persist to the normal limits of human hematopoietic engraftment in the xenograft model. The classic definitive assay for HSC function is serial long-term engraftment, which is very difficult to reproduce in the xenotransplantation model. We are currently pursuing serial xenotransplantation to address this shortcoming in the best manner possible. Additionally, it is plausible that different human hematopoietic cell progenitors contribute to blood reconstitution versus blood vessel repair. Lentiviral tagging studies are, thus, being performed to determine if the described hemangioblast activity is polyclonal response in the setting of injury or an oligoclonal phenomenon of select HSCs responding much like that observed in hematopoietic studies performed by Guenechea et al7 and Dick et al.8
Recent reports demonstrate that cell fusion can play a major role in liver regeneration.9,10 Other studies have clearly demonstrated that HSCs can contribute to the pancreas,11 brain (C. R. C., A. T. Yachnis, E. D. Laywell, et al, unpublished observations, October 27, 2003), and vascular endothelium12 without fusion. Several striking differences exist between the systems. The FAH–/– (fumarylacetoacetase hydrolase–/–) liver regeneration system uses a tremendous selective pressure in favor of wild-type (WT) contributions. The liver is also an organ that normally contains large numbers of functional hepatocytes that are tetraploid or greater. In the other systems, the tagged cells had no selective advantage, and the tissues tend to be exclusively diploid. The results of this study demonstrate that human hematopoietic cells, like their murine counterparts, produce both hematopoietic cells and circulating endothelial progenitor cells. These circulating EPCs are diploid cells (Figure 1C) that can form new vessels both in vitro and in vivo (Figure 2) in both mice and man.1,13 These data strongly suggest that cell fusion does not play a major role in the endothelial cell engraftment we detected.
Therefore, factors that affect human hematopoietic homing and migration are ideal targets to modulate EPC-derived vasculogenesis/angiogenesis. For example, the chemokine, stromal-derived factor 1 (SDF-1), whose functions include lymphocyte homing and stem cell growth has recently been found to modulate hemangioblast activity in the murine system (J. Butler, S. M. G., S. Caballero, et al, unpublished observations, October 27, 2003). By identifying other critical factors affecting human hemangioblast activity, it may be possible to more effectively treat vasculopathic conditions such as diabetic retinopathy, wound healing, and tumor neoangiogenesis.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-06-2101.
Supported by grants HL70813, CA72769, and HL70738 (E.W.S.) and by National Institutes of Health Training Grant T32CA09126-25 (C.R.C.). E.W.S. is a scholar of the Leukemia and Lymphoma Society of America.
E.W.S. is a co-founder and shareholder of RegenMed, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jason Butler for his kind help on this manuscript.

![Figure 1. FACS analysis of enriched human hematopoietic stem cells (HSCs) and multilineage reconstitution. (A) Pretransplantation umbilical cord blood (UCB) HSC/HPC enrichment as identified by antibodies to CD34 (phycoerythrin [PE]) and CD45 (allophycocyanin [APC]). Positive fraction (right) shows more than 90% purity of CD34+CD45+ cells. Negative fraction (left) contains no CD34+ cells. (B) Multilineage human hematopoietic reconstitution in NOD/scid mouse bone marrow seen 4 weeks after human CD34+ cell transplantation. Bone marrow cells stained positive with human specific antibodies against leukocytes (CD45), B cells (CD19), T cells (CD3), and myelomonocytic cells (CD15). All panels shown are gated on live cells. (C) Fluorescence-activated cell sorting (FACS)–sorted control murine EPCs (VEGR-2+) from mice undergoing retinal ischemia, stained for DNA content with propidium iodide (left panel); sorted xenograft-derived human EPCs stained for DNA content with propidium iodide (right panel). Both panels exhibit a classic diploid staining profile.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-06-2101/6/m_h80145464001.jpeg?Expires=1769125386&Signature=u-6SjINRNmdYCZeZUkq4jc4VTodzOA6pe9yA4dovBMrZe3VoAkp7VwuXbfzswVWveYimFqbCFQUJz9LPvH1ZDlFtZtiO0kjXfpEbdXmUxbihem1IZ-xsiPL6oSSnupfBSPUMJPHi2dfqaQk-y3VbCpzijL3GHsDA~kDKU-xq0sHoIMwpcN5JuGlekffojaVuzFdISAd0hDmRj6THMfBR6XwlanmUA53ciTlSnLqtR-BCH3pLve8TOmUicHHSf-pBwBbNoi08AX-rsmp3nq-R~TBpUPXjWZ9gXtRdukAHUn3d7Z1CjQHxLsPEBMO9KCbuUMyWKGoMJLBF3ZiliTve9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal