Peripheral blood (PB) stem cell harvests are reported to contain less tumor burden than bone marrow from the same patient,1 making them a more attractive source of hematopoietic progenitors for transplantation and improving tolerance to intensive therapy. Although more intensive therapies frequently induce enhanced response rates, this does not necessarily result in an increased overall survival. This may be explained by drug-resistant disease or poor immune response, or tumor cells reinfused during transplantation may induce relapse. That reinfused tumor cells contribute to relapse in children with neuroblastoma (NBL) is supported by the presence of gene-marked reinfused tumor cells at the sites of disease relapse.2,3
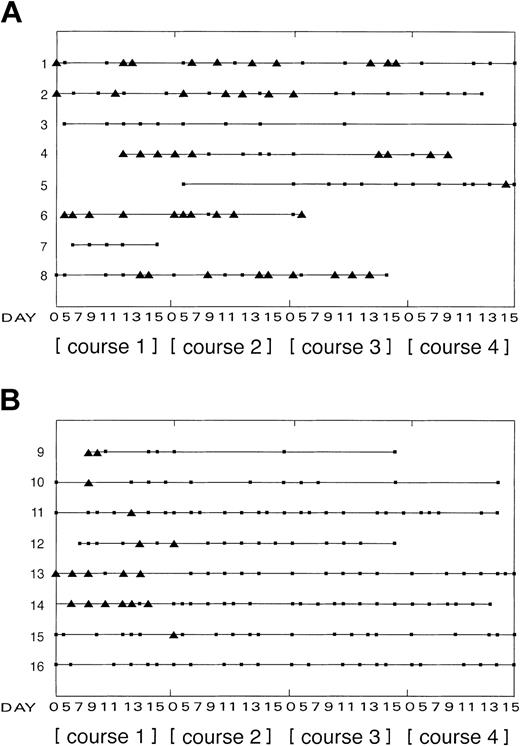
Using reverse-transcriptase polymerase chain reaction (RTPCR)4 for tyrosine hydroxylase (TH) mRNA, tumor cells in PB samples from 6 of 8 children with NBL have been detected following mobilization of stem cells with chemotherapy and granulocyte colony-stimulating factor (G-CSF) (Figure 1A), and EWS-FLI1 fusion transcripts in samples from 7 of 7 children with tumors of the Ewing sarcoma family (ESFT), including 5 of 5 Ewing sarcomas (ES) and 2 of 2 peripheral primitive neuroectodermal tumors (pPNETs) (Figure 1B). No EWS-WT1 fusion transcripts were identified in PB from one child with a desmoplastic small round-cell tumor (DSRCT) (Figure 1B, child 16). All children were entered into multicenter trials using recommended chemotherapy. On day 5 after the start of chemotherapy, children were treated with recombinant G-CSF (5 μg/kg per day) for 10 days to mobilize stem cells into PB over 4 courses. PB samples taken at intervals (between days 5 and 15 of each course) from the central venous line were analyzed for contaminating tumor cells by RT-PCR: all primary neuroblastomas expressed TH mRNA, all ESFTs expressed EWS-FLI1 fusion transcripts, and the EWS-WT1 fusion product was confirmed in the DSRCT.
Summary of tumor cells detected in PB from children with high-risk disease following chemotherapy and G-CSF over 4 courses. Results from all 16 children are summarized: 1 to 8 indicate children with neuroblastoma (A); 9 to 15, children with ESFT; and 16, child with DSRCT (B). Patients 9 to 13 were diagnosed with ES; patients 14 and 15, with pPNETs. At diagnosis all patients had metastatic disease detected by conventional imaging, with the exception of patients 13 and 14 who presented with localized disease. Only patients 1 and 5 had bone marrow metastases. Recombinant G-CSF (5 μg/kg per day) was administered for 10 days to mobilize stem cells into PB over 4 courses after chemotherapy, commencing on day 5 after the start of chemotherapy. There were two 2-mL PB samples taken from the central venous line from day 5 after chemotherapy at intervals up to day 15 for analysis by RT-PCR. There was no unexpected toxicity associated with the chemotherapy or G-CSF treatment. ▪ indicates PB sample analyzed, negative by RT-PCR for contaminating tumor cells; ▴, PB sample analyzed, positive by RT-PCR for contaminating tumor cells. The length of the line for each patient shows the period over which PB samples were collected for analysis.
Summary of tumor cells detected in PB from children with high-risk disease following chemotherapy and G-CSF over 4 courses. Results from all 16 children are summarized: 1 to 8 indicate children with neuroblastoma (A); 9 to 15, children with ESFT; and 16, child with DSRCT (B). Patients 9 to 13 were diagnosed with ES; patients 14 and 15, with pPNETs. At diagnosis all patients had metastatic disease detected by conventional imaging, with the exception of patients 13 and 14 who presented with localized disease. Only patients 1 and 5 had bone marrow metastases. Recombinant G-CSF (5 μg/kg per day) was administered for 10 days to mobilize stem cells into PB over 4 courses after chemotherapy, commencing on day 5 after the start of chemotherapy. There were two 2-mL PB samples taken from the central venous line from day 5 after chemotherapy at intervals up to day 15 for analysis by RT-PCR. There was no unexpected toxicity associated with the chemotherapy or G-CSF treatment. ▪ indicates PB sample analyzed, negative by RT-PCR for contaminating tumor cells; ▴, PB sample analyzed, positive by RT-PCR for contaminating tumor cells. The length of the line for each patient shows the period over which PB samples were collected for analysis.
In 5 of 8 children with neuroblastoma, TH mRNA was detected in PB throughout treatment (Figure 1A); there was no apparent pattern relating to course number, day in course, hematopoietic cell number, or total RNA isolated from sample. However, in children with ESFT, tumor cells were not detected in PB collected after 2 courses of chemotherapy; this is consistent with a reduced tumor load in courses 2, 3, and 4 compared with that in course 1 (Figure 1B). This restricted pattern may explain the conflicting frequency of tumor contamination reported in PB and PB stem cell harvest from patients with ESFT.5-9 From this study it is not possible to evaluate the clinical significance of reinfused tumor cells; indeed any association between tumor contamination of PB with outcome might purely reflect the disease status of children at the time of PB collection. However, the profiles of tumor contamination in PB after mobilization of stem cells in the group of children with NBL compared with those with ESFT suggest that judicious timing of PB stem cell collection can influence tumor contamination in some cancers. Whether this may reduce the potential risk of secondary disease from reinfused tumor cells in children with ESFT requires further investigation, particularly in view of the recent speculation that reinfused tumor cells might elicit a protective antitumor immune response after autologous transplantation.10
We are indebted to the clinical and nursing staff of the Manchester Children's Hospital, Manchester, and the Paediatric Oncology Department, St James's University Hospital, Leeds, United Kingdom, who collected samples for this study. Institutional ethical approval was obtained and parental consent given for all patients from whom blood was taken. This study was supported by Chugai Pharma UK Ltd.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal