Abstract
Iron regulatory proteins (IRP1 and IRP2) are RNA-binding proteins that affect the translation and stabilization of specific mRNAs by binding to stem-loop structures known as iron responsive elements (IREs). IREs are found in the 5′-untranslated region (UTR) of ferritin (Ft) and mitochondrial aconitase (m-Aco) mRNAs, and in the 3′-UTR of transferrin receptor (TfR) and divalent metal transporter-1 (DMT1) mRNAs. Our previous studies show that besides iron, IRPs are regulated by hypoxia. Here we describe the consequences of IRP regulation and show that iron homeostasis is regulated in 2 phases during hypoxia: an early phase where IRP1 RNA-binding activity decreases and iron uptake and Ft synthesis increase, and a late phase where IRP2 RNA-binding activity increases and iron uptake and Ft synthesis decrease. The increase in iron uptake is independent of DMT1 and TfR, suggesting an unknown transporter. Unlike Ft, m-Aco is not regulated during hypoxia. During the late phase of hypoxia, IRP2 RNA-binding activity increases, becoming the dominant regulator responsible for decreasing Ft synthesis. During reoxygenation (ReO2), Ft protein increases concomitant with a decrease in IRP2 RNA-binding activity. The data suggest that the differential regulation of IRPs during hypoxia may be important for cellular adaptation to low oxygen tension.
Introduction
Periods of low O2 concentration or hypoxia occur during a broad range of biologic conditions from early development to tumorigenesis and heart disease. Under such situations, cells adapt to the low O2 environment by increasing the expression of a number of genes including vascular endothelial growth factor, erythropoietin, glycolytic enzymes,1 and genes involved in iron homeostasis, such as ceruloplasmin, transferrin (Tf), and transferrin receptor (TfR).2-5 The expression of these genes requires activation by hypoxia-inducible factor-1 (HIF-1), a transcription factor consisting of hypoxia-inducible HIF-1α and constitutively expressed HIF-1β subunits. During normoxia, HIF-1α is destabilized by a mechanism involving prolyl hydroxylation and targeted for proteasomal degradation.6 During hypoxia, prolyl hydroxylase activity is reduced and the nonhydroxylated form of HIF-1α is stabilized. HIF-1α then binds to the constitutively expressed HIF-1β subunit to activate transcription of genes that allow for adaptation to hypoxia.
When the O2 concentration returns to normal, the production of toxic reactive oxygen species (ROS), such as the hydroxyl radical (·OH), superoxide, and hydrogen peroxide (H2O2) increases. Iron contributes to ROS formation by catalyzing the generation of ·OH from H2O2 by Fenton chemistry.7 ROS, especially ·OH, can damage proteins, DNA, and lipids, and are thought to be responsible for much of the cellular and tissue injury associated with reperfusion disorders.8,9 In both animal and cell culture models, iron chelation has been shown to decrease the damage caused during reperfusion.10-12
Due to the dual nature of iron as essential for both cellular growth and survival, yet toxic when present in excess, cells have evolved a mechanism to maintain iron homeostasis via iron regulatory protein 1 (IRP1) and IRP2.13-15 When the cellular-free iron pool is low, IRPs bind to specific RNA stem loop structures, called iron-responsive elements (IREs), located in the 5′-untranslated region (UTR) of mRNAs such as ferritin heavy chain (FtH) and ferritin light chain (FtL) subunits and mitochondrial aconitase (m-Aco), thereby preventing their translation. IRPs also bind to IREs located in the 3′-UTR of TfR mRNA, stabilizing the message from endonucleolytic cleavage and increasing the uptake of Tf-bound iron. Conversely, when the cellular free iron pool is high, IRP2 is degraded by the proteasome in an iron-dependent manner, and IRP1 is converted from an RNA-binding protein to a [4Fe-4S] cluster-containing protein that displays cytosolic aconitase (c-Aco) activity. These changes result in a decrease in TfR synthesis with a corresponding increase in Ft translation, leading to a decrease in the cellular-free iron pool. By constantly responding to changes in the cellular-free iron pool, IRPs maintain iron homeostasis by regulating iron uptake and sequestration.
In addition to iron, IRP activities are influenced by other effectors, including ROS and reactive nitrogen species,16-19 phosphorylation,20,21 hypoxia/reoxygenation (ReO2),22-26 and ischemia/reperfusion.27,28 ROS such as superoxide inactivate aconitases by disassembly of the [4Fe-4S] cluster.29,30 Nitric oxide (NO), produced in cells stimulated with cytokines or in cells treated with NO-generating compounds, activates IRP1 RNA-binding activity by causing cluster disassembly19,31-33 while decreasing IRP2 RNA-binding activity.33,34 Others have shown that phosphorylation of a critical serine in IRP1 results in the destabilization of the [4Fe-4S] cluster of IRP1, suggesting an iron-independent mechanism of IRP1 regulation.21
Our previous work has shown that hypoxia regulates IRP RNA-binding activities in opposing directions such that IRP2 RNA-binding activity increases while that of IRP1 decreases.23 However, the cellular consequences of this regulation remain unclear. Here, we investigate the effects of IRP regulation during hypoxia in human embryonic kidney 293 (HEK293) cells and describe the mechanisms responsible for regulation of iron homeostasis during hypoxia.
Materials and methods
Cell culture and reagents
HEK293 cells were cultured in complete medium consisting of Dulbecco modified eagle medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) supplemented with 100 units/mL penicillin (Invitrogen) and 100 μg/mL streptomycin (Invitrogen) (pen/strep) at 37°C in 5% CO2 and atmospheric air (ie, normoxia). To increase cellular adhesion, culture dishes were treated with 15 μg/mL poly-lysine (Sigma, St Louis, MO). For hypoxia time-course experiments, cells were plated in 60-mm culture dishes containing 2.5 mL complete medium, and subsequently placed in a humidified incubator containing 1% O2, 5% CO2, balance N2 for the indicated times. For iron and iron-chelation experiments, cells were treated with 50 to 150 μg/mL ferric ammonium citrate (FAC) for 5 hours or 100 to 200 μM deferoxamine mesylate (Df; Sigma) for 16 hours under normoxic conditions as indicated. For experiments with high-molecular-weight (MW) Df (a gift from Dr Bo Hedlund, Biomedical Frontiers), complete medium was treated overnight with 600 μM high-MW Df before being added to cells for various lengths of time under normoxic or hypoxic conditions as indicated. This concentration was found to elicit the maximal increase in both IRP1 and IRP2 RNA-binding activities after 16 hours under normoxic conditions (data not shown).
Cytosolic extract preparation and RNA supershift analysis
HEK293 cells were washed with phosphate-buffered saline (PBS) and lysed with 140-μL lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 25 mM KCl, 0.5% nonidet P-40 [NP-40], and 1 mM dithiothreitol [DTT]). Cells were scraped and cell lysates cleared by centrifugation at 15 000g for 15 minutes at 4°C. Protein concentration was determined using Coomassie reagent (Pierce, Rockford, IL). RNA supershift assays were performed as described previously.23,35 Band intensity was quantified with a PhosphorImager (Molecular Dynamics, Piscataway, NJ).
Immunoblot analysis
Cytosolic cell extracts (20 μg) of HEK293 cells were separated on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (15% for Ft detection), and proteins were transferred to a nitrocellulose membrane. Blots were probed with the following primary antibodies: chicken antirat IRP1 polyclonal antibody,36 rabbit antirat IRP2 polyclonal antibody,37 sheep antihuman Ft antibody (The Binding Site, San Diego, CA), and mouse antihuman TfR monoclonal antibody (Zymed, South San Francisco). The m-Aco polyclonal antibody used was made by resolving porcine heart aconitase (Sigma) over two 8% SDS-PAGE gels. The protein band was excised and used as antigen for injection in rabbits. Proteins were detected with a horseradish peroxidase-conjugated secondary antibody followed by chemiluminescence (NEN Life Science Products, Boston, MA).
Northern analysis and preparation of RNA
HEK293 cells were grown in either hypoxic or normoxic conditions for the indicated times, and total RNA was prepared using Trizol reagent (Invitrogen). Total RNA or poly(A)+ RNA, purified from total RNA using an Oligotex purification system (Qiagen, Valencia, CA), was resolved on 0.9% (poly(A)+ RNA) or 1.2% (total RNA) agarose formaldehyde gels. RNA was transferred to a nylon membrane and hybridized with the following 32P-labeled DNA probes: human TfR,38 porcine m-Aco,39 rat FtH subunit,40 rat FtL pseudogene 66,35 human 18S rRNA, mouse skeletal muscle β-actin (Stratagene, La Jolla, CA), and an IRP1 mouse/human hybrid.41 Blots were prehybridized for one hour, hybridized overnight with 1 to 1.25 × 106 cpm/mL of the indicated probe, washed, and subjected to autoradiography. Band intensity was quantified with a PhosphorImager (Molecular Dynamics).
Iron uptake
For uptake of 55Fe-Tf, HEK293 cells were washed with serum- and pen/strep-free DMEM and incubated with 1 nM 55Fe-Tf in this medium for the last one hour of normoxic or hypoxic treatment. Cells were then washed with PBS and harvested in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [tris(hydroxymethyl)aminomethane, pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid [DOC], 0.1% SDS) and the lysates centrifuged at 15 000g for 15 minutes at 4°C. Protein concentration of cell lysates was determined as described for cytosolic extract preparation, and radioactivity was counted using a scintillation counter. Radioactive counts were normalized to protein concentration and compared relative to the normoxic control. For cold Fe-Tf experiments, 1 μM human holo-Tf (Sigma) was added to cells immediately before 55Fe-Tf labeling.
55Fe-NTA was prepared by complexing 55Fe (55FeCl3 from NEN Life Science Products) with a 4-fold molar excess of NTA (nitrilotriacetic acid) in 20 mM HEPES, and the mixture was adjusted to pH 7 with 4 M NaOH. To measure Tf-independent iron uptake, HEK293 cells were washed with serum- and pen/strep-free DMEM or Hanks balanced salt solution (HBSS) and incubated with 1 μM 55Fe-NTA in this medium for the last one hour of normoxic or hypoxic treatment. As a control, 1 mM cold Fe-NTA was added to cells immediately before 55Fe-NTA labeling. Cells were washed with PBS and harvested in RIPA buffer. Cell lysates were centrifuged at 15 000g for 10 minutes at 4°C. Protein concentration and radioactivity of cell lysates was determined as described above. For the experiments in Figure 6A, complete DMEM was treated with 600 μM high-MW Df overnight at 4°C, while for Figure 6B, serum- and pen/strep-free DMEM was treated with 1 μM 55Fe-NTA and 600 μM high-MW Df overnight at 4°C before being added to cells.
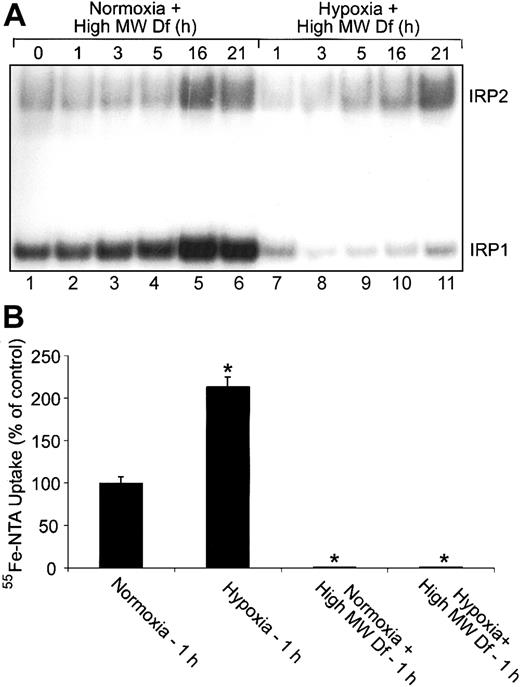
Extracellular iron is not responsible for the changes in IRP1 RNA-binding activity during hypoxia. (A) RNA supershift analysis of cytosolic HEK293 cell extracts as described in Figure 1A. Complete DMEM was treated with 600-μM high-MW Df overnight to chelate iron. Cells were treated with this high-MW Df-containing medium under normoxic or hypoxic conditions for the indicated times. Experiments were performed 3 times with a representative blot shown. (B) Bar graph shows the mean percent radioactivity ± SEM, n = 3, of hypoxia-treated cell extracts relative to the normoxic control. High-MW Df-containing serum-free DMEM was treated with 55Fe-NTA overnight. Cells were labeled with 55Fe-NTA in serum-free DMEM only or with high-MW Df (600 μM)/55Fe-NTA-containing serum-free DMEM for one hour under hypoxic or normoxic conditions. The * indicates points that differ from the control with P < .03 using a Student t test.
Extracellular iron is not responsible for the changes in IRP1 RNA-binding activity during hypoxia. (A) RNA supershift analysis of cytosolic HEK293 cell extracts as described in Figure 1A. Complete DMEM was treated with 600-μM high-MW Df overnight to chelate iron. Cells were treated with this high-MW Df-containing medium under normoxic or hypoxic conditions for the indicated times. Experiments were performed 3 times with a representative blot shown. (B) Bar graph shows the mean percent radioactivity ± SEM, n = 3, of hypoxia-treated cell extracts relative to the normoxic control. High-MW Df-containing serum-free DMEM was treated with 55Fe-NTA overnight. Cells were labeled with 55Fe-NTA in serum-free DMEM only or with high-MW Df (600 μM)/55Fe-NTA-containing serum-free DMEM for one hour under hypoxic or normoxic conditions. The * indicates points that differ from the control with P < .03 using a Student t test.
Separation of mitochondrial and cytosolic fractions and aconitase assays
HEK293 cells were grown in DMEM (5% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin) for the indicated times under normoxic or hypoxic conditions. Cells were treated with 0.01% digitonin for 15 minutes, scraped, and spun at 600g for 1 minute at 4°C to pellet cells. The supernatant was removed and spun at 15 000g for 5 minutes at 4°C. The cleared lysate was saved as the cytosolic fraction. The cell pellet was washed with PBS and solubilized by the addition of Triton X buffer (0.2% Triton X-100, 150 mM NaCl, 20 mM HEPES, pH 7.5) for 10 minutes on ice. Cellular debris was removed by centrifugation at 15 000g, and the cleared lysate was saved as the mitochondrial fraction. Protein concentration was determined as described for cytosolic extract preparation. Aconitase activity was determined in each fraction as described previously.42 Briefly, 0.2 mM cis-aconitate was added to 50 μg protein extract in 500 μL of 50 mM Tris-HCl, pH 7.5. The disappearance of cis-aconitate was followed at 240 nm over time.
35S-labeling and Ft immunoprecipitation
HEK293 cells grown in 35-mm culture dishes were washed with DMEM (Cys-, Met-, serum-, and pen/strep-free) followed by the addition of 100 μCi/mL (3.7 MBq/mL) 35S-Met/Cys (ICN Pharmaceuticals, Costa Mesa, CA) in 500 μL of this medium for the last one hour of normoxic or hypoxic treatment. Cells were lysed in RIPA buffer and cell extracts were centrifuged at 15 000g for 25 minutes at 4°C. For immunoprecipitations, 200 μg cell lysate was incubated with 1.0 μL rabbit antihuman Ft polyclonal antibody (Dako, Carpinteria, CA) in 1 mL RIPA buffer at 4°C for one hour. Then, 50 μL of a 50% slurry of protein-A agarose beads (Invitrogen) was added for one hour at 4°C. The protein-agarose beads were spun and washed with 1 mL RIPA buffer and then boiled for 10 minutes in SDS-loading buffer (350 mM Tris-base, pH 6.8, 10% SDS, 600 mM DTT, 36% glycerol, 0.01% bromophenol blue). Samples were centrifuged and proteins resolved on 15% SDS-PAGE gels. Gels were dried and analyzed by autoradiography.
Results
Iron homeostasis exhibits a biphasic response during hypoxia
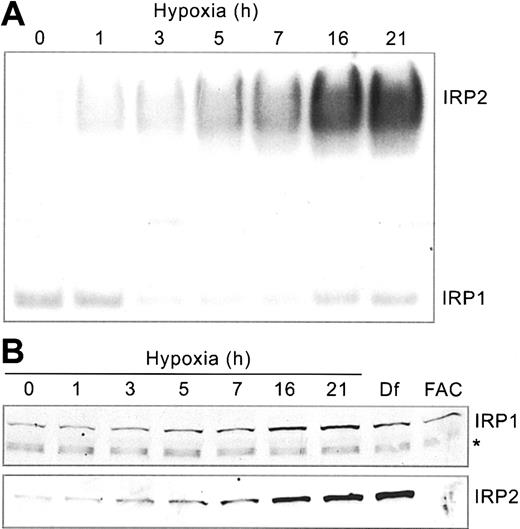
Our previous studies showed that IRP1 and IRP2 RNA-binding activities respond differently to hypoxia.22,23 To determine how this affects iron homeostasis, we first carried out a detailed time course to analyze IRP RNA-binding in HEK293 cells subjected to hypoxia (1% O2) for 0 to 21 hours. Cells were harvested at various times following hypoxic exposure, and protein extracts were subjected to RNA supershift analysis using a 32P-labeled Ft IRE RNA. Supershift assays of IRP2 were necessary because human IRP1/IRP2 comigrate.43 Figure 1A demonstrates that IRP2 RNA-binding activity increases more than 5-fold over control conditions by 21 hours of hypoxia. Immunoblot analysis shows that this increase correlates with an increase in the amount of IRP2 protein (Figure 1B), which is due to protein stabilization.23 As controls, treatment with Df, an iron chelator, and FAC increase and decrease, respectively, the amounts of IRP2 protein (Figure 1B).
Hypoxia differentially regulates IRP RNA-binding activities. HEK293 cells were grown for the indicated times under hypoxic (1% O2) or normoxic (21% O2, control) conditions. (A) RNA supershift analysis of cytosolic cell extracts using a 32P-labeled Ft IRE and anti-IRP2 antibodies. Experiment was performed 5 times with a representative blot shown. (B) Immunoblot analysis of cytosolic cell extracts with chicken anti-IRP1 antibodies36 or rabbit anti-IRP2 antibodies.37 Df, 100 μM for 16 hours; FAC, 50 μg/mL for 5 hours. The * represents a nonspecific band. IRP1 and IRP2 Western analysis experiments were performed 3 and 4 times, respectively, with representative blots shown.
Hypoxia differentially regulates IRP RNA-binding activities. HEK293 cells were grown for the indicated times under hypoxic (1% O2) or normoxic (21% O2, control) conditions. (A) RNA supershift analysis of cytosolic cell extracts using a 32P-labeled Ft IRE and anti-IRP2 antibodies. Experiment was performed 5 times with a representative blot shown. (B) Immunoblot analysis of cytosolic cell extracts with chicken anti-IRP1 antibodies36 or rabbit anti-IRP2 antibodies.37 Df, 100 μM for 16 hours; FAC, 50 μg/mL for 5 hours. The * represents a nonspecific band. IRP1 and IRP2 Western analysis experiments were performed 3 and 4 times, respectively, with representative blots shown.
Unlike IRP2, IRP1 RNA-binding activity exhibits biphasic regulation, decreasing to approximately 45% of control at 3 to 7 hours followed by an increase to approximately 70% of control by 21 hours of hypoxia (Figure 1A). The change in IRP1 RNA-binding activity cannot be accounted for by a decrease in protein (Figure 1B). In fact, there may be a slight increase in the amounts of IRP1 protein during this time course. These data are consistent with studies in other cell lines,5,22 demonstrating the differential regulation of IRPs during hypoxia.
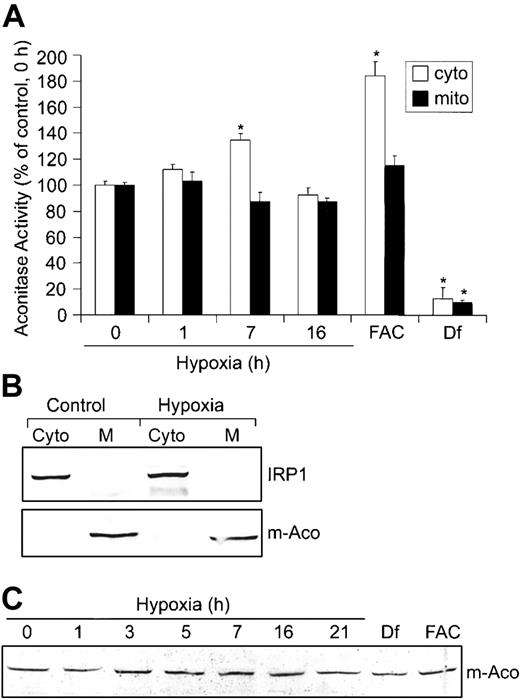
Based on the mutually exclusive functions of IRP1 as either an RNA-binding protein or c-Aco,44 we determined whether the decrease in IRP1 RNA-binding activity during hypoxia resulted in a corresponding increase in c-Aco activity. HEK293 cells were grown under hypoxic conditions for 0, 1, 7, and 16 hours, and aconitase activities were measured in fractionated cytosol and mitochondria (Figure 2A). The purity of these fractions was determined by immunoblot analysis using m-Aco and IRP1 antibodies, respectively (Figure 2B). Figure 2A shows that hypoxia increases c-Aco activity approximately 35% after 7 hours of hypoxia, corresponding to a time when IRP1 RNA-binding activity is at a minimum. By 16 hours of hypoxia, c-Aco activity returns to about control levels, correlating with the increase in IRP1 RNA-binding activity (Figure 1A). m-Aco activity did not change significantly over the hypoxia time course. As expected, FAC increases c-Aco activity approximately 84% and Df decreases it by approximately 87%. FAC did not significantly affect m-Aco activity, although Df decreases its activity by approximately 90%. These results suggest that m-Aco activity is not as sensitive as c-Aco to hypoxia and excess intracellular iron. Because m-Aco mRNA contains a 5′-IRE that is regulated by the IRPs,13 we asked whether the differential regulation of IRP1 and IRP2 during hypoxia influenced m-Aco regulation by measuring m-Aco protein. Figure 2C shows that the amount of m-Aco protein does not change during 21 hours of hypoxia, consistent with the lack of change in m-Aco activity. Df and FAC also do not change the amounts of m-Aco protein, although Df dramatically reduces m-Aco activity. Taken together, these data show that the differential regulation of IRPs during hypoxia does not affect the amounts of m-Aco protein, despite the presence of a 5′-IRE.
Hypoxia increases c-Aco activity but does not regulate m-Aco protein accumulation or activity. (A-C) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with Df (200 μM for 16 hours) or FAC (150 μg/mL for 5 hours). (A) Bar graph shows the mean aconitase activity of mitochondrial and cytosolic cell fractions ± SEM, n = 6 (0, 1, 7, and 16 hours hypoxia) or n = 4 (FAC and Df). The * indicates points that differ from the control group (0 hour hypoxia) with P < .007 using a Student t test. Aconitase activity was assayed by measuring the change in absorbance at 240 nm over time as cis-aconitate is converted to isocitrate.42 (B) Immunoblot analysis with chicken anti-IRP136 or rabbit anti-m-Aco antibodies to determine the purity of the mitochondrial and cytosolic fractions. Western analysis was performed for all experiments with a representative blot of the 16-hour hypoxia time point shown. Cyto indicates cytosolic fraction; M, mitochondrial fraction. (C) Immunoblot analysis of cytosolic cell extracts with rabbit anti-m-Aco antibodies. Experiment was performed 3 times with a representative blot shown.
Hypoxia increases c-Aco activity but does not regulate m-Aco protein accumulation or activity. (A-C) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with Df (200 μM for 16 hours) or FAC (150 μg/mL for 5 hours). (A) Bar graph shows the mean aconitase activity of mitochondrial and cytosolic cell fractions ± SEM, n = 6 (0, 1, 7, and 16 hours hypoxia) or n = 4 (FAC and Df). The * indicates points that differ from the control group (0 hour hypoxia) with P < .007 using a Student t test. Aconitase activity was assayed by measuring the change in absorbance at 240 nm over time as cis-aconitate is converted to isocitrate.42 (B) Immunoblot analysis with chicken anti-IRP136 or rabbit anti-m-Aco antibodies to determine the purity of the mitochondrial and cytosolic fractions. Western analysis was performed for all experiments with a representative blot of the 16-hour hypoxia time point shown. Cyto indicates cytosolic fraction; M, mitochondrial fraction. (C) Immunoblot analysis of cytosolic cell extracts with rabbit anti-m-Aco antibodies. Experiment was performed 3 times with a representative blot shown.
Unlike m-Aco, Ft synthesis and protein levels are regulated during hypoxia
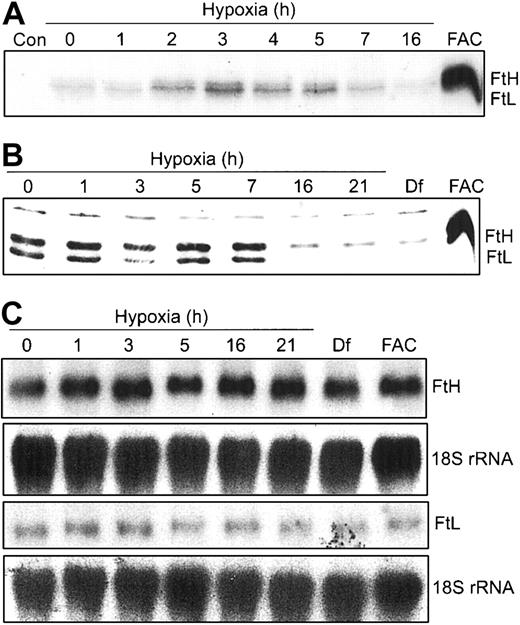
Our data in Figure 2 show that m-Aco activity and protein do not change during hypoxia. Since both IRPs bind to 5′-IREs in FtH and FtL mRNAs, we wanted to determine how the differential regulation of IRP RNA-binding activities during hypoxia would influence Ft synthesis. HEK293 cells were grown under hypoxic conditions for 0 to 16 hours and radiolabeled for the last one hour of treatment with 35S-Met/Cys. FtH and FtL subunits were immunoprecipitated with an antihuman Ft antibody and subjected to SDS-PAGE and autoradiography. Figure 3A shows that FtH and FtL subunit synthesis exhibits a biphasic response, increasing by 2 hours of hypoxia, peaking at 3 hours, and declining to barely detectable levels by 16 hours of hypoxia. As a control, FAC treatment for 3 hours greatly enhances synthesis of both Ft subunits. The changes in protein synthesis during hypoxia are not due to a decrease in uptake of the radiolabel or a decrease in global protein synthesis as determined by radioactive counts of cell lysates and trichloroacetic acid (TCA)-precipitable protein, respectively (data not shown). Western blot analysis shows that the steady-state amounts of both Ft protein subunits remain constant through 7 hours but then dramatically decrease by 16 to 21 hours of hypoxia (Figure 3B). As expected, Df and FAC treatment of HEK293 cells decreases and increases, respectively, the amounts of both Ft protein subunits (Figure 3B). The increase in Ft synthesis at 3 hours of hypoxia correlates with the maximal decrease in IRP1 RNA-binding activity at 3 hours, while the decrease after 7 hours of hypoxia correlates with the robust increase in IRP2 RNA-binding activity at this time (Figure 1A). Figure 3C indicates that the biphasic response of Ft synthesis during hypoxia correlates with IRP regulation, since Northern blots show no change in the amounts of FtH and FtL mRNA during 21 hours of hypoxia. This further indicates that the changes in Ft synthesis and protein during hypoxia occur by a posttranscriptional mechanism. Taken together, these data suggest that, unlike m-Aco, IRP2 does regulate Ft translation during hypoxia.
Ft synthesis exhibits a biphasic response during hypoxia. (A-B) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with Df (16 hours) or FAC. (A) Synthetic rate of Ft during hypoxia. For the last one hour of hypoxic or normoxic treatment, cells were labeled with 35S-Met/Cys in serum-free DMEM. Cell extracts were immunoprecipitated with rabbit antihuman Ft antibodies and proteins subjected to SDS-PAGE analysis and autoradiography. FAC, 50 μg/mL for 3 hours. Con indicates control immunoprecipitation lacking antibody. Experiment was performed 4 times with a representative blot shown. (B) Immunoblot analysis of cytosolic cell extracts with antihuman Ft antibodies. Df, 100 μM; FAC, 50 μg/mL for 5 hours. Experiment was performed 4 times with a representative blot shown. (C) Northern analysis of total cellular RNA. RNAs were transferred to a nylon membrane and hybridized with a 32P-labeled FtH, FtL, or 18S rRNA probe (control). Df, 200 μM; FAC, 150 μg/mL for 5 hours. Experiments were performed twice with representative blots shown.
Ft synthesis exhibits a biphasic response during hypoxia. (A-B) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with Df (16 hours) or FAC. (A) Synthetic rate of Ft during hypoxia. For the last one hour of hypoxic or normoxic treatment, cells were labeled with 35S-Met/Cys in serum-free DMEM. Cell extracts were immunoprecipitated with rabbit antihuman Ft antibodies and proteins subjected to SDS-PAGE analysis and autoradiography. FAC, 50 μg/mL for 3 hours. Con indicates control immunoprecipitation lacking antibody. Experiment was performed 4 times with a representative blot shown. (B) Immunoblot analysis of cytosolic cell extracts with antihuman Ft antibodies. Df, 100 μM; FAC, 50 μg/mL for 5 hours. Experiment was performed 4 times with a representative blot shown. (C) Northern analysis of total cellular RNA. RNAs were transferred to a nylon membrane and hybridized with a 32P-labeled FtH, FtL, or 18S rRNA probe (control). Df, 200 μM; FAC, 150 μg/mL for 5 hours. Experiments were performed twice with representative blots shown.
Hypoxia increases Tf-independent iron uptake
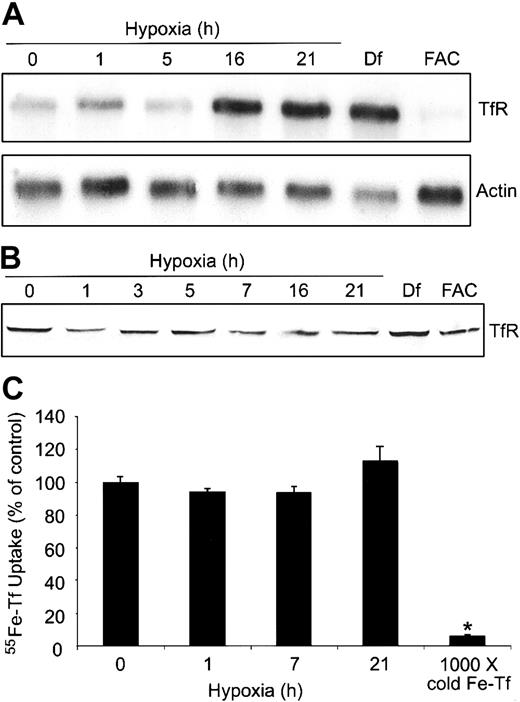
To determine how the differential regulation of IRPs might alter the expression of TfR and possibly the cellular free iron pool, we measured TfR mRNA and protein during a 0- to 21-hour hypoxia time course in HEK293 cells. Figure 4A shows that TfR mRNA levels increase approximately 2-fold by 16 hours of hypoxia and are most likely a combination of both transcriptional activation of TfR by HIF-1 as well as increased mRNA stability via IRP2, although the contribution of each cannot be discerned here. As expected, Df and FAC treatment result in an approximately 5-fold increase and an approximately 3-fold decrease, respectively, in the amounts of TfR mRNA. Although Df causes a slight decrease in the amounts of actin mRNA, the mechanism for this is not known. The increase in the amounts of TfR mRNA during hypoxia does not result in a significant change in the amount of TfR protein (Figure 4B), although there is a slight increase in Df-treated cells. In FAC-treated cells, TfR mRNA levels decrease, but this does not result in a decrease in TfR protein, probably due to the more than 24-hour half-life of the protein.45 These data indicate that an increase in TfR mRNA does not always correlate with an increase in protein or Tf-dependent iron uptake.
Hypoxia does not change Tf-dependent iron uptake. (A-B) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with Df (16 hours) or FAC (5 hours). (A) Northern analysis of poly(A)+ mRNA. RNAs were transferred to a nylon membrane and hybridized with a 32P-labeled TfR or actin probe as a control. Df, 200 μM; FAC, 150 μg/mL. Experiment was performed 4 times with a representative blot shown. (B) Immunoblot analysis of cytosolic cell extracts with antihuman TfR antibodies. Df, 100 μM; FAC, 50 μg/mL. Experiment was performed 5 times with a representative blot shown. (C) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with 1000 × cold Fe-Tf. Cells were incubated with 55Fe-Tf in serum-free DMEM for the last one hour of treatment, washed, harvested, and radioactivity quantified by counting cell extracts. The bar graph shows the mean percent radioactivity ± SEM, n = 9 (0, 1, 7, and 21 hours hypoxia) or n = 3 (1000 × cold Fe-Tf) of hypoxia-treated cell extracts. The * indicates point that differs from the control group with P < .005 using a Student t test.
Hypoxia does not change Tf-dependent iron uptake. (A-B) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with Df (16 hours) or FAC (5 hours). (A) Northern analysis of poly(A)+ mRNA. RNAs were transferred to a nylon membrane and hybridized with a 32P-labeled TfR or actin probe as a control. Df, 200 μM; FAC, 150 μg/mL. Experiment was performed 4 times with a representative blot shown. (B) Immunoblot analysis of cytosolic cell extracts with antihuman TfR antibodies. Df, 100 μM; FAC, 50 μg/mL. Experiment was performed 5 times with a representative blot shown. (C) HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with 1000 × cold Fe-Tf. Cells were incubated with 55Fe-Tf in serum-free DMEM for the last one hour of treatment, washed, harvested, and radioactivity quantified by counting cell extracts. The bar graph shows the mean percent radioactivity ± SEM, n = 9 (0, 1, 7, and 21 hours hypoxia) or n = 3 (1000 × cold Fe-Tf) of hypoxia-treated cell extracts. The * indicates point that differs from the control group with P < .005 using a Student t test.
Although other studies have shown that TfR mRNA increases during hypoxia,4,5 it was unclear in these studies if Tf-dependent iron uptake also increased. To determine if Tf-dependent iron uptake changed during hypoxia, we measured 55Fe-Tf uptake in HEK293 cells exposed to hypoxia for 0, 1, 7, and 21 hours. Figure 4C shows that the uptake of 55Fe-Tf in serum-free DMEM was not significantly altered during hypoxia, consistent with an undetectable change in TfR protein during this time course (Figure 4B). As a control, labeling cells in the presence of 1000 × unlabeled Fe-Tf significantly decreased the uptake of 55Fe-Tf during hypoxia. Although this experiment does not address the possible mobilization of iron from internal stores, it suggests that the changes in IRP1 RNA-binding activity and Ft synthesis cannot be accounted for by an increase in Tf-dependent iron uptake.
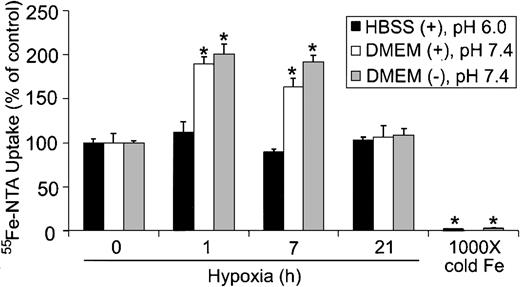
Because cells can take up iron by both Tf-dependent and Tf-independent mechanisms,46,47 we determined whether Tf-independent iron uptake was altered during hypoxia. Tf-independent iron uptake in the gut occurs through the proton-coupled Fe2+ transporter known as divalent metal transporter-1/divalent cation transporter-1 (DMT1/DCT1), which functions optimally at acidic pH.48-50 To determine if hypoxia altered Tf-independent iron uptake, HEK293 cells were subjected to hypoxia for 0, 1, 7, and 21 hours, and the uptake of 55Fe-NTA in serum-free DMEM (pH 7.4) or HBSS (pH 6.0) was measured in the presence of ascorbic acid. Figure 5 shows that 55Fe-NTA uptake did not significantly change during hypoxia when cells were assayed in serum-free HBSS at pH 6.0. When 55Fe-NTA uptake was measured in serum-free DMEM at pH 7.4, in either the presence or absence of ascorbic acid, approximately 2-fold increase in iron uptake was observed by one hour of hypoxia, which remained elevated until 7 hours (Figure 5). By 21 hours of hypoxia, 55Fe-NTA uptake returned to control levels. Since DMT1 is more active in transporting iron at acidic pH,49 these data indicate that the increase in 55Fe-NTA uptake is independent of DMT1. Because there is relatively little change in IRP RNA-binding activity by one hour of hypoxia, the data also suggest that the initial increase is independent of IRP regulation yet stimulated by hypoxia. Finally, these data indicate that Tf-independent iron uptake exhibits a biphasic response during hypoxia that parallels Ft synthesis.
Tf-independent iron uptake displays a biphasic response during hypoxia. HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with 1000 × cold Fe-NTA. Cells were incubated with 55Fe-NTA in serum-free DMEM (pH 7.4) or HBSS (pH 6.0) for the last one hour of treatment, washed, harvested, and radioactivity quantified by counting cell extracts. The bar graph shows the mean percent radioactivity ± SEM, n = 9 (0, 1, 7, and 16 hours hypoxia) or n = 6 (1000 × cold Fe) of hypoxia-treated cell extracts. The * indicates points that differ from the control group with P < .005 using a Student t test. The ± refers to the presence or absence, respectively, of 20 μM ascorbic acid in serum-free DMEM or HBSS.
Tf-independent iron uptake displays a biphasic response during hypoxia. HEK293 cells were grown for the indicated times under hypoxic conditions or under normoxia with 1000 × cold Fe-NTA. Cells were incubated with 55Fe-NTA in serum-free DMEM (pH 7.4) or HBSS (pH 6.0) for the last one hour of treatment, washed, harvested, and radioactivity quantified by counting cell extracts. The bar graph shows the mean percent radioactivity ± SEM, n = 9 (0, 1, 7, and 16 hours hypoxia) or n = 6 (1000 × cold Fe) of hypoxia-treated cell extracts. The * indicates points that differ from the control group with P < .005 using a Student t test. The ± refers to the presence or absence, respectively, of 20 μM ascorbic acid in serum-free DMEM or HBSS.
Extracellular iron is not responsible for the changes in IRP1 RNA-binding activity during hypoxia
The increase in Tf-independent iron uptake during early hypoxia prompted us to determine whether extracellular iron is responsible for the decrease in IRP1 RNA-binding activity at this time. HEK293 cells were cultured in complete medium that had been pretreated with 600-μM high-MW Df (to chelate iron) and subjected to a normoxia or hypoxia time course. RNA supershift analysis shows that IRP1 RNA-binding activity displays the same biphasic regulation under hypoxia with high-MW Df compared with hypoxia alone (compare Figure 6A, lanes 7-11 with Figure 1A). Figure 6A demonstrates that the high-MW Df was chelating iron, since cells treated with the high-MW Df-containing medium under normoxia increase both IRP1 and IRP2 RNA-binding activities in a time-dependent fashion as expected (Figure 6A, lanes 1-6). To ensure that high-MW Df prevents Tf-independent iron uptake, we labeled cells for one hour under normoxia or hypoxia with 55Fe-NTA in only serum-free DMEM or with 55Fe-NTA-containing serum-free DMEM that had been pretreated with high-MW Df (Figure 6B). Figure 6B shows that pretreating 55Fe-NTA-containing serum-free DMEM with high-MW Df prevents the increase in Tf-independent iron uptake at one hour of hypoxia. These data show that the increase in Tf-independent iron uptake at one hour of hypoxia is not responsible for the decrease in IRP1 RNA-binding activity during early hypoxia.
Hypoxic regulation of Ft protein is reversible upon ReO2
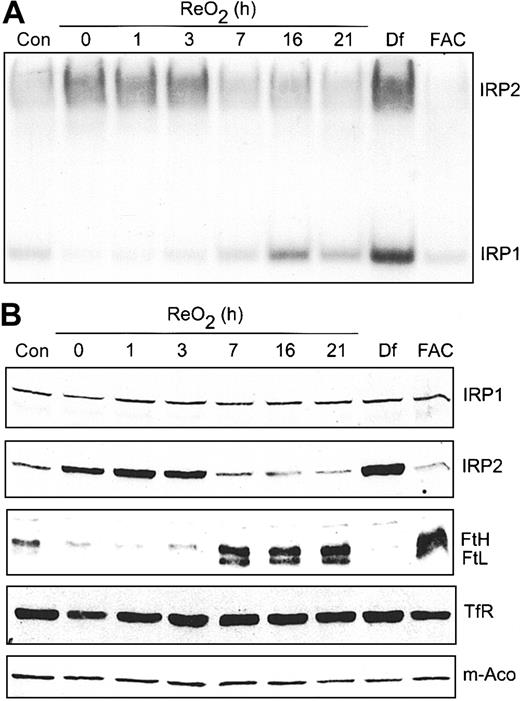
Our previous work showed that hypoxic regulation of IRP1 RNA-binding activity is reversible upon ReO2.22 However, in that study, cells were exposed to 3% O2 rather than 1% O2 used here. At 3% O2, IRP1 RNA-binding activity decreased, as shown here, but IRP2 RNA-binding activity was not affected. To determine if the hypoxic increase in IRP2 RNA-binding activity is reversible upon ReO2, cells were grown under hypoxia for 21 hours followed by growth under normoxia for 0 to 21 hours. RNA supershift analysis shows that IRP1 RNA-binding activity starts to increase at 3 hours of ReO2, reaching normoxic amounts by 16 hours without any changes in IRP1 protein (Figure 7A-B). In contrast, IRP2 RNA-binding activity decreases to normoxic amounts after 7 hours of ReO2, paralleling a decrease in IRP2 protein (Figure 7A-B). As controls, Df and FAC treatment of HEK293 cells maximally increases and decreases, respectively, the RNA-binding activity of both IRP1 and IRP2. To determine if the reversibility in both IRP1 and IRP2 RNA-binding activity following ReO2 influenced the expression of Ft, m-Aco, and TfR, cell extracts used in the supershift analysis (Figure 7A) were used for immunoblotting. Figure 7B shows that the amount of both Ft protein subunits increases by 7 hours of ReO2, correlating with the decrease in IRP2 RNA-binding activity, while the amounts of TfR and m-Aco protein remain unchanged throughout the ReO2 time course, as during hypoxia.
Hypoxic regulation of Ft and IRP2 protein levels is reversible upon ReO2. (A-B) HEK293 cells were grown under hypoxic conditions for 21 hours followed by ReO2 for the indicated times or under normoxia with Df (200 μM for 16 hours) or FAC (150 μg/mL for 5 hours). (A) RNA supershift analysis of cytosolic cell extracts as described in Figure 1A. Con indicates normoxic control. Experiment was performed 3 times with a representative blot shown. (B) Immunoblot analysis of the cytosolic cell extracts used in panel A using antibodies against the indicated proteins. Con indicates normoxic control. Experiments were performed 2 (IRP1, IRP2, TfR, and m-Aco) or 3 (Ft) times with representative blots shown.
Hypoxic regulation of Ft and IRP2 protein levels is reversible upon ReO2. (A-B) HEK293 cells were grown under hypoxic conditions for 21 hours followed by ReO2 for the indicated times or under normoxia with Df (200 μM for 16 hours) or FAC (150 μg/mL for 5 hours). (A) RNA supershift analysis of cytosolic cell extracts as described in Figure 1A. Con indicates normoxic control. Experiment was performed 3 times with a representative blot shown. (B) Immunoblot analysis of the cytosolic cell extracts used in panel A using antibodies against the indicated proteins. Con indicates normoxic control. Experiments were performed 2 (IRP1, IRP2, TfR, and m-Aco) or 3 (Ft) times with representative blots shown.
Discussion
Here we report on the cellular consequences of the differential regulation of IRPs during hypoxia and ReO2. Our studies show that iron homeostasis is regulated in 2 distinct phases during hypoxia. The early phase (0-7 hours) is marked by a decrease in IRP1 RNA-binding activity and an increase in Ft synthesis and Tf- and DMT1-independent iron uptake. The late phase (16-21 hours) is marked by greatly enhanced IRP2 RNA-binding activity, a corresponding decrease in Ft synthesis and protein levels, and a return of Tf-independent iron uptake to control levels.
Our results indicate that Ft has a major role in iron homeostasis during hypoxia. During early hypoxia, the decrease in IRP1 RNA-binding activity correlates with the derepression of Ft synthesis, since Ft mRNA remains unchanged and IRP2 RNA-binding activity is only slightly increased during this time. The increase in Ft synthesis during early hypoxia shown here is consistent with studies in oligodendrocytes where Ft synthesis increased after 6 hours of hypoxia.51 Because these investigators did not perform a longer hypoxia time course, it is unknown if Ft synthesis would be repressed at 16 hours as we report here. Others have also detected an increase in Ft synthesis during hypoxia in mouse peritoneal macrophages.25 However, Ft synthesis at 16 hours of hypoxia was not addressed and there was no detectable IRP2 in these cells, making it difficult to draw conclusions as to the contribution of IRP2 in Ft synthesis during hypoxia. Interestingly, the initial increase in Ft synthesis is not reflected in a corresponding increase in protein at this time. One explanation could be a decreased half-life of Ft during hypoxia; however, we do not believe this is the case since pulse-chase experiments showed that the half-life of Ft during hypoxia was comparable with normoxia (data not shown). We believe that the initial increase in Ft synthesis is insufficient to detect by Western analysis. During the late phase of hypoxia, IRP2 RNA-binding activity is greatly elevated and represses Ft synthesis and protein. During this time, cells may have limited iron-storage capacity, leaving them vulnerable to iron-catalyzed ROS production. Similar to hypoxia, H2O2 has been shown to decrease the amount of Ft protein in cells yet increase its capacity to store iron.52 Increased NADPH (nicotinamide adenine dinucleotide phosphate) levels during hypoxia may also help maintain iron in a reduced state, facilitating its incorporation into Ft.53 Whether the reduced level of Ft during late-phase hypoxia has an increased capacity to store iron has not been determined.
During ReO2, amounts of both Ft protein subunits increase to above control levels, demonstrating the reversibility of Ft regulation. Such an increase would allow cells to sequester free iron, and consequently, limit iron-catalyzed ROS production via Fenton chemistry. Support for this includes studies in erythroid cells that demonstrate a correlation between increasing FtH levels with lower levels of ROS and cellular-free iron and protection from ROS-induced cell death.54 Several other studies also implicate a role for Ft in response to oxidative stress and illustrate the significance of IRPs in its regulation under such conditions. For example, Ft synthesis is induced in response to phorone,55 H2O2,56 and NO.57 The increase in Ft synthesis by NO and phorone correlates with decreasing IRP2 RNA-binding activity,55,57 while one study shows that the response to H2O2 is biphasic.56 In this latter study, upon H2O2 treatment, there is an initial decrease in Ft synthesis, correlating with an initial increase in IRP1 RNA-binding activity, followed by increasing Ft synthesis and a corresponding decrease in IRP1 RNA-binding activity. Transcription of Ft genes is also regulated by ischemia/reperfusion58 and other oxidants,55,56,59 further emphasizing the importance of both transcriptional and translational regulation during oxidative stress.
Our data show that not all 5′-IRE mRNAs are regulated equally by the IRPs during hypoxia. Whereas the synthesis and steady-state amounts of Ft protein decrease after 7 hours of hypoxia, no change is observed in the steady-state amounts of m-Aco protein. Although we do not know whether m-Aco synthesis changes during hypoxia because our antibody was not effective in immunoprecipitation, our data are consistent with studies showing that structural differences between Ft and m-Aco IREs influence the differential binding affinities of IRP1 and IRP2.60,61 Other studies show that treatment of HL-60 cells with hemin causes more than 2-fold increase in m-Aco synthesis versus more than 20-fold increase in Ft synthesis,62 suggesting that IRPs can differentially regulate translation of 5′-IRE-containing mRNAs in response to iron. These data suggest that an increased affinity of IRP2 for the 5′-IRE of Ft versus m-Aco IRE is responsible for the differential regulation of Ft and m-Aco during the late phase of hypoxia.
We show that Tf-dependent iron uptake does not significantly change during hypoxia. Although both IRPs can bind to the 3′-IREs of TfR mRNA,63 the early decrease in IRP1 RNA-binding activity does not significantly alter TfR mRNA levels. As hypoxia progresses, the increase in IRP2 RNA-binding activity may be capable of stabilizing the message, although TfR is also transcriptionally regulated by HIF1.4,5 Interestingly, the stabilization of TfR mRNA is not reflected in the steady-state amounts of TfR protein during hypoxia. Similarly, others have shown that H2O2 treatment of B6 fibroblasts increases the amount of TfR mRNA approximately 4-fold, although the amount of protein increases only approximately 2-fold, suggesting additional mechanism(s) of TfR regulation.52 Nonetheless, our result is consistent with the lack of any significant change in Tf-dependent iron uptake during hypoxia. Rather, hypoxia induces an early increase in Tf-independent iron uptake, a response that has been shown to occur in rat myocardial cells.64 DMT1 functions optimally at acidic pH.49,50 As shown here, Tf-independent iron uptake shows little change when assayed at pH 6.0, but increases approximately 2-fold during hypoxia when measured at pH 7.4 indicating the increase is DMT1 independent. This increase in iron uptake during hypoxia is not blocked by actinomycin D or cycloheximide, suggesting that transcription and translation, respectively, are not required (data not shown). The initial increase is also independent of IRP regulation since it occurs before any significant change in IRP RNA-binding activity. These data suggest that hypoxia may induce the rapid localization of an unknown transporter to the plasma membrane. Iron uptake during hypoxia may also occur through a recently defined pathway involving a lipocalin.65 Such a rapid response to hypoxia may allow cells to sequester sufficient iron to maintain enzyme function and cellular survival during a potentially extended period of low oxygen concentration.
What is the mechanism regulating IRPs during hypoxia? One explanation to account for IRP1 regulation during hypoxia is an increase in the cellular free iron pool. We show that Tf-independent iron uptake increases by one hour of hypoxia. This increase, however, is not responsible for the inactivation of IRP1 RNA-binding activity, since blocking iron uptake with high-MW Df did not prevent the decrease in IRP1 RNA-binding activity during hypoxia (Figure 6). Furthermore, this increase in iron uptake does not signal IRP2 degradation, and in fact, IRP2 steadily accumulates during hypoxia. This suggests that either the increase in iron is insufficient to signal IRP2 degradation or that the iron is rapidly incorporated into proteins or sequestered by Ft. Finally, we cannot rule out the possibility of an increase in the cellular free iron pool during hypoxia by mobilization of iron from internal stores that could drive the formation of the [4Fe-4S] cluster in IRP1.
Our favored explanation for the regulation of IRP1/c-Aco during hypoxia is reduced ROS production. Although there are conflicting reports regarding how ROS levels change during hypoxia,66,67 we believe our data are consistent with a decrease in cytosolic ROS levels during hypoxia. We show that c-Aco activity is increased by approximately 35% at 7 hours of hypoxia, while FAC increases c-Aco activity approximately 84%, indicating that this is the maximal amount of c-Aco activity that can be expected under these conditions. Since [4Fe-4S] clusters are extremely sensitive to ROS-induced disassembly,32,68,69 reduced ROS production would favor the stabilization of the [4Fe-4S] cluster and increase c-Aco activity, consistent with our data shown here. Surprisingly, m-Aco activity was unaffected by hypoxia. Some studies have shown that mitochondrial ROS production increases during hypoxia,66,70 which might prevent any hypoxic stabilization of the m-Aco [4Fe-4S] cluster. Analysis of our data shows that m-Aco activity is always lower than control by 16 hours of hypoxia, although this did not reach statistical significance, suggesting that the effects on c-Aco and m-Aco activity during hypoxia could be explained by the compartmentalization of ROS production. Although the physiologic relevance of elevated c-Aco during hypoxia is unclear, one suggestion is that increases in c-Aco activity would increase NADPH levels, thereby providing reducing equivalents for regeneration of glutathione and allowing cells to maintain redox balance.71 Increased NADPH levels may also favor the Fe(II) state, aiding in the incorporation of iron into Ft.53 During the late phase of hypoxia, IRP2 RNA-binding activity steadily increases due to the accumulation of IRP2 protein.23 Although the mechanism regulating IRP2 accumulation during hypoxia is not clear, our studies suggest that this is due to an oxygen-dependent decrease in IRP2 ubiquitination.72
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-02-0433.
Supported by grants from the National Institutes of Health (grant GM45201) and the American Heart Association Western States Affiliate (E.A.L.), and a University of Utah Graduate Research Fellowship (B.D.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Drs Jerry Kaplan, Dennis Winge, and Eric Hanson for reading the manuscript. We also thank Dr Bo Hedlund and Greg Hanson for technical assistance with the high-MW Df and Dr Aniko Szabo at the HCI Biostatistics Core Facility for help with the statistical analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal