Abstract
Recent publications have highlighted the chemotactic activities of antimicrobial proteins derived from the granules of neutrophils and basophils. Eosinophil granules also contain antimicrobial proteins. One of them is eosinophil-derived neurotoxin (EDN), a protein belonging to the ribonuclease A (RNase A) superfamily, which has recently been found to have antiviral activity in vitro. We found that EDN was selectively chemotactic for dendritic cells (DCs). The DC chemotactic activity of EDN was inhibited by either pretreatment of DCs with pertussis toxin or by simultaneous addition of placental RNase inhibitor to inhibit the activity of EDN. EDN was not chemotactic for leukocytes other than DCs. Mouse eosinophilassociated RNase 2 (mEAR2), one of a cluster of divergent orthologs of human EDN, was also chemotactic for human as well as mouse DCs. Sequence and mutational analysis demonstrated the importance of the N-terminal region of mEAR2 in mediating its chemotactic effect on DCs. EDN also induced the activation of p42/44 mitogen-activated protein kinase (MAPK) in DCs. Furthermore, injection of mEAR2 into the air pouches of mice resulted in the recruitment of DCs into the air pouches. Thus, EDN and its mouse ortholog, mEAR2, are eosinophil granule–derived antimicrobial RNases that function as chemoattractants for DCs in vitro and in vivo.
Introduction
Antimicrobial proteins of neutrophil granules have long been considered as important elements of innate host defense.1 In recent years, evidence has been accumulating indicating that some neutrophil granule–derived antimicrobial proteins also act as chemoattractants for various types of leukocytes. For example, neutrophil-derived α-defensins have been shown to be chemotactic for human monocytes, T cells, and immature dendritic cells (iDCs).2-4 Azurocidin and cathelicidins, 2 antimicrobial proteins predominantly stored in neutrophil granules, are chemotactic for neutrophils, monocytes, T cells, and mast cells.3,5-9 Cathepsin G, on the other hand, is chemotactic for neutrophils and monocytes.5 Furthermore, in mouse experimental models, neutrophil granule–derived defensins are capable of enhancing antigen-specific immune responses when administered simultaneously with antigens.10,11 Thus, these neutrophil granule–derived antimicrobial proteins have the capacity to participate in mobilizing and amplifying adaptive immunity by functioning as leukocyte chemoattactants and/or activators.12
In addition to neutrophil granule–derived antimicrobial proteins, chymase, a serine protease found in the granules of basophils and mast cells, has been shown to induce neutrophil and monocyte chemotaxis.13 Human eosinophil granules contain several antimicrobial proteins including eosinophil-derived neurotoxin (EDN),14,15 which together with eosinophil cationic protein belongs to what has come to be recognized as the RNase A superfamily.16 EDN was purified in 198114 and its gene was cloned in 1989.17 Its 3-dimensional structure has also been solved by X-ray crystallography.18 Aside from its neurotoxic effect,14 EDN has been shown to have antiviral activity, in particular against respiratory syncytial virus in vitro,19,20 suggesting that EDN may contribute to host antiviral defense against single-strand RNA viruses.21 Very recently, EDN has also been shown to be responsible in part for the HIV-1 inhibitory activities in the supernatant of allogeneic mixed lymphocyte reaction.22 However, it is not known whether EDN, like several other antimicrobial proteins derived from the granules of neutrophils and basophils/mast cells, may also have chemotactic activity for leukocytes. To test this possibility, we have investigated the capacity of EDN to induce the migration of various types of human leukocytes and found that EDN and its divergent mouse ortholog, mouse eosinophil-associated RNase 2 (mEAR2),23 can act as selective chemoattractants for DCs.
Materials and methods
Reagents
Recombinant human (rh) stromal cell–derived factor-1α (SDF-1α), tumor necrosis factor α (TNFα), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 4 (IL-4), Flt3-L, stem cell factor (SCF), and thrombopoietin (TPO) were purchased from PeproTech (Rocky Hill, NJ). N-formyl-Met-Leu-Phe (fMLP), bovine pancreatic ribonuclease A (bpRNase), ribonuclease inhibitor (RI; from human placenta, 30 000-50 000 units/mL), and pertussis toxin (PTX) were purchased from Sigma (St Louis, MO). Human angiogenin (hANG) was purchased from R & D Systems (Minneapolis, MN). Recombinant human pancreatic ribonuclease A (hPR) was kindly provided by Drs Dianne L. Newton and Susan M. Rybak (SAIC Frederick, NCI at Frederick, National Institutes of Health [NIH]).24
Human EDN (in this case the -4 form of EDN that contains 4 additional amino acids on the N-terminus originally considered as part of the signal sequence)25 was purified from commercial preparations of human urinary gonadotrophin as described.26 For the production of recombinant mEAR2, the culture supernatants of Spodoptera frugiperda (Sf9) insect cells infected with recombinant baculovirus–encoding mEAR2 were used. Briefly, both purifications involving resuspended urinary gonadotrophin preparations or supernatant containing recombinant mEAR2 were dialyzed extensively at 4°C against 50 mM Tris (tris(hydroxymethyl)aminomethane, pH 8.0) containing 1 mM NaCl and were then fractionated by heparin-Sepharose column chromatography (Pharmacia Biotech, Piscataway, NJ) equilibrated with 50 mM Tris (pH 8.0) containing 1 mM NaCl using a salt-gradient (1 mM to 1 M NaCl in 50 mM Tris, pH 8.0) for the elution. The fractions containing the ribonuclease in question were pooled, concentrated (Centricon 10; Amicon, Beverly, MA), and subjected to purification by Superdex 200 (Pharmacia Biotech) chromatography. The concentrations of purified EDN and mEAR2 were determined by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL) and confirmed in the latter case by quantitative Western blotting with anti-FLAG monoclonal antibody.26 The hPR/mEAR2 chimera was prepared by polymerase chain reaction (PCR) mutagenesis, with a 5′ primer including sequence encoding the first 10 amino acids of hPR followed by sequence encoding mEAR amino acids 10 to 15, thus creating the appropriately overlapping chimeric sequence. The chimeric sequence was subcloned into the pFCTS-FLAG bacterial expression vector as described for other RNase A ribonucleases26 and confirmed by sequence analysis. Recombinant protein was prepared from a lysate of 5 L bacterial culture and purified, as described for the purification of mEAR2, with heparin-Sepharose chromatography (1 mM to 2 M NaCl gradient) followed by dialysis against phosphate-buffered saline (PBS) and final affinity purification with anti-FLAG Sepharose resin (Sigma).26
Cell isolation and purification
Human neutrophils (PMNs) were isolated and purified to more than 99% as described previously.27 Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque density gradient centrifugation from leukopacks supplied by the Department of Transfusion Medicine (Clinical Center, National Institutes of Health, Bethesda, MD). Monocytes were purified (> 95%) from human PBMCs with magnetic cell sorting (MACS) CD14 monocyte isolation kit (Miltenyi Biotech, Auburn, CA). Human peripheral blood T cells were purified (> 90%) from PBMCs by the use of CD3+ T-cell–negative selection columns (R & D Systems) following the manufacturer's recommendation. Human peripheral blood eosinophils were isolated from peripheral blood granulocytes by the use of an Eosinophil Purification Kit (Miltenyi Biotech) following the manufacturer's protocol. Human cord blood CD34+ progenitors (> 90%) were purchased from Poietics (Gaithersburg, MD). The DC precursors were amplified from CD34+ hematopoietic progenitor cells (HPCs) exactly as described by culturing the cells at 5 × 104 cells/mL in Iscove modified Dulbecco medium (IMDM) supplemented with 20% fetal bovine serum (FBS), 10-5 M dithiothreitol, 25 ng/mL rhFlt3-L, 10 ng/mL rhTPO, and 20 ng/mL rhSCF for 4 weeks.28 The amplified DC precursors were cryopreserved in IMDM containing 20% FBS and 10% dimethyl sulfoxide until later usage. Murine HPCs were prepared from the bone marrow of C57BL/6 mice (female, 7 weeks) as described.29 Briefly, bone marrow cells flushed from femur and tibia were depleted of red blood cells by ammonium chloride treatment. For depletion of lymphocytes and Ia-positive cells, the remaining cells were incubated with a cocktail of monoclonal antibodies (mAbs) for 1 hour at 4°C followed by depletion with immunomagnetic beads coated with antirat immunoglobulin G (IgG; Dynal, Lake Success, NY). The mAbs used were anti-B220/CD45R (Pharmingen, San Diego, CA), anti–major histocompatibility complex (MHC) class II (M5/114.15.2 anti–I-Ab, d, q and I-Ed, k; American Type Culture Collection [ATCC], Manassas, VA), and anti-CD90 (Thy1; Pharmingen). The resulting cells consisted of murine HPCs.
DC culture
DCs were generated as described previously.28,30 In brief, monocytederived immature DCs (Mo-iDCs) were generated by incubating purified monocytes at 1 × 106/mL in G4 medium (RPMI 1640 containing 10% FBS, 2 mM glutamine, 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 100 U/mL penicillin, 100 μg/mL streptomycin, 50 ng/mL rhGM-CSF, and 50 ng/mL rhIL-4) at 37°C in a CO2 (5%) incubator for 7 days. To generate CD34+ HPC-derived immature DCs (CD34-iDCs), the amplified DC precursors were cultured in G4 medium for 48 hours at 37°C in a CO2 (5%) incubator. Murine iDCs were generated by incubation of murine HPCs at 1 × 106/mL in RPMI 1640 medium in the presence of recombinant murine GM-CSF (rmGM-CSF; 50 U/mL) and rmIL-4 (10 ng/mL) at 37°C in a CO2 (5%) incubator for 5 days.29 All of the cultures were fed with the same cytokine-containing medium every 2 to 3 days. To induce DC maturation, iDCs were cultured in the same cytokine cocktails with the addition of rTNFα (50 ng/mL) for 2 days. All the iDCs used were CD86-/+, MHC class II++, and unable to stimulate allogeneic mixed lymphocyte reaction, whereas all mature DCs (mDCs) used were CD86++, MHC class II++++, and highly capable of stimulating marked allogeneic mixed lymphocyte reaction.
Chemotaxis assay
Cell migration was assessed using a 48-well microchemotaxis chamber.30 The cells were washed 3 times and resuspended in chemotaxis medium (CM; RPMI 1640 containing 1% bovine serum albumin [BSA]). SDF-1α, fMLP, and various test factors diluted with CM were placed in wells of the lower compartment of the chamber (Neuro Probe, Cabin John, MD), and cells suspended in CM (1 × 106 cells/mL for neutrophils, monocytes, DC precursors, and DCs; 5 × 106 cells/mL for T cells) were added into wells of the upper compartment. The lower and upper compartments were separated by a 5-μm polycarbonate filter (Osmonics, Livermore, CA). For T-cell chemotaxis, the filter was coated overnight at 4°C in RPMI 1640 containing 10 μg/mL fibronectin (Sigma) and air-dried just before use. For checkerboard analysis, EDN was simultaneously added into the top compartments. To test whether the chemotactic activity of EDN could be specifically inhibited, RI at concentrations indicated was also included in the lower compartments. In some experiments, DCs were pretreated for 30 minutes at 37°C in the absence or presence of PTX (final concentration = 200 ng/mL) before usage. After incubation at 37°C for 1 hour (for PMNs), 1.5 hours (for monocytes, DC precursors, iDCs, and mDCs), or 3 hours (for T cells) in humidified air with 5% CO2, the filters were removed, scraped, and stained. The cells that migrated across the filter were counted with the use of a Bioquant semiautomatic counting system (which objectively determines the average cell number of 6 defined microscopic fields of each spot on the filter membrane) and presented as the number of cells per high power field (No./HPF). Chemotaxis was tested at least twice on cells from an individual donor and the results of one representative experiment are shown.
Detection of mitogen-activated protein kinase (MAPK) activation
Mo-iDCs were cultured in G4 medium with reduced FBS (0.5%) overnight in order to make the cells quiescent. Subsequently, DCs (4 × 106/4 mL) were stimulated at 37°C with EDN at 1 μg/mL for a period of time as specified. For positive control, fMLP at 10-7 M was used. At the end of stimulation, 36 mL ice-cold PBS was added and the treated DCs were spun down to stop the stimulation. The supernatant was immediately removed and the DCs were lysed by adding 200 μL of 1 × SDS (sodium dodecyl sulfate) sample buffer (62.5 mM Tris-HCl, pH 6.8, at 25°C, 2% wt/vol SDS, 10% glycerol, 50 mM dithiothretol, 0.01% bromophenol blue). After transfer into a microcentrifuge tube, the extract was sonicated for 10 seconds on ice to shear DNA for the purpose of reducing sample viscosity. Thereafter, the extract was boiled for 5 minutes, cooled on ice, and centrifuged at 17 500g for 5 minutes. The extracts were loaded (20 μL/lane) and separated on a 4% to 12% NuPAGE Bis-Tris Gel (Invitrogen, Carlsbad, CA) using 1 × NuPAGE MES (2-(N-morpholino)ethane sulfonic acid) SDS running buffer (Invitrogen) as the electrode buffer. SeeBlue Plus2 (Invitrogen) was used as the molecular size marker. After the electrophoresis, proteins in the gel were electrotransferred (30 V constant for 1 h) onto a piece of Immobilon membrane (Millipore, Bedford, MA) with an Xcell II Blot Module (Novex, San Diego, CA) using 1 × NuPAGE transfer buffer (12 mM Tris, 96 mM glycine, pH 8.3, 0.1% vol/vol antioxidant, 10% vol/vol methanol).
Phosphorylated MAPK was detected by Western blotting. The blotting was carried out at room temperature unless specified otherwise. The polyvinylidene fluoride (PVDF) membrane was sequentially washed with 25 mL of 1 × TBS (Tris-buffered saline) for 5 minutes, incubated for 1 hour in 25 mL blocking buffer (1 × TBS, 0.1% Tween-20, 5% wt/vol nonfat milk), washed 3 times for 5 minutes each with TBS/T (1 × TBS, 0.1% Tween-20) and incubated at 4°C overnight in blocking buffer containing 1:1000 dilution of rabbit anti–phospho-p44/42 (Cell Signaling, Beverly, MA; catalog no. 9101). On the next day, the membrane was washed 3 times for 5 minutes each with 15 mL TBS/T and incubated with 10 mL of 1:2000 dilution of horseradish peroxidase–conjugated antirabbit IgG (Cell Signaling, catalog no. 7074) for 1 hour. After washing 3 times for 5 minutes each with 15 mL TBS/T, the membrane was incubated with 10 mL working solution of ECL Plus Detection Reagents (Amersham, Piscataway, NJ) for 5 minutes, immediately wrapped with Saran wrap, and exposed to a piece of BioMax X-ray film (Kodak, Rochester, NY) for 5 seconds. The X-ray film was developed using an automatic processor (Kodak X-OMAT 200A). The same membrane was incubated in stripping solution (62.5 mM Tris-HCl, pH 6.7, 2% SDS, 100 mM 2-ME [mercaptoethanol]) at 70°C for 30 minutes. After washing 3 times for 5 minutes each with 15 mL TBS/T, the stripped membrane was probed for p44/42 protein essentially in the same manner except using rabbit anti-p44/42 (Cell Signaling, catalog no. 9102) as the primary antibody.
Air pouch experiments
Six- to 8-week-old Balb/c mice were obtained from the Animal Production Facility of the National Cancer Institute at Frederick (Frederick, MD) and maintained under specific pathogen-free conditions in the Experimental Animal Facility at the National Cancer Institute at Frederick for 2 weeks to allow acclimation before the experiments. The animal studies were conducted in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Animals. Air pouches were raised on the dorsum by subcutaneous injection of 3 mL sterile air on days 0 and 3. On day 6, mice with well-formed air pouches were randomized into 3 different groups. Each mouse was injected with 1 mL endotoxin-free PBS alone or PBS containing 1 μg/mL hANG or mEAR2 into the air pouches. Four hours after the injection, the mice were killed by CO2 asphyxiation, the air pouches were washed once with 2 mL PBS containing 5 mM EDTA (ethylenediaminetetraacetic acid) and 20 U/mL heparin. The exudates were centrifuged at 500g for 5 minutes at room temperature. Cells were counted with a hematocytometer following Trypan Blue staining. Characterization of leukocyte subpopulations migrating into the pouch space was performed by eosin–methylene blue staining of cytospins. To evaluate the migration of DCs (CD11c and I-A/I-E double positive31 ) into the air pouch, a portion of the cells were double-stained with a combination of fluorescein isothiocyanate (FITC)–conjugated rabbit antimouse I-A/I-E (Clone 2G9; PharMingen) and phycoerythrin (PE)–conjugated hamster antimouse CD11c (Clone HL3; PharMingen). Cells were stained with isotype-matched FITC-conjugated rabbit IgG (2a, κ; PharMingen) and PE-conjugated hamster IgG (group 1, κ; PharMingen) for the determination of quadrant setting. The stained samples were analyzed by a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Results
EDN induces chemotaxis of CD34+ cell–derived iDCs in a Giα-protein–dependent manner
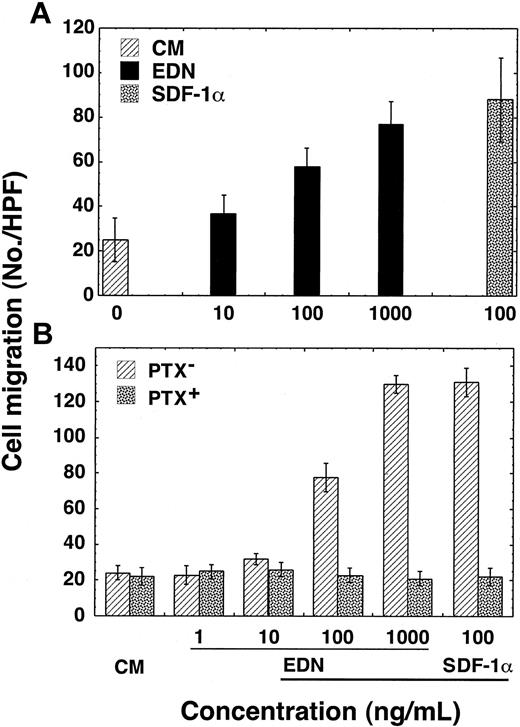
The capacity of EDN to induce the migration of CD34+ cell–derived iDCs was examined using a 48-well microchemotaxis chamber assay. EDN was tested over a range of 10 to 1000 ng/mL (corresponding to 0.6-60 nM; molecular mass of EDN ∼15.5 kDa) and was found to induce the migration of human CD34+ cell–derived iDCs (Figure 1A). To address the question as to whether EDN-induced migration of iDCs was due to chemotaxis or chemokinesis, checkerboard analysis was performed (Table 1). EDN, in a dose-dependent manner, induced the migration of human CD34+ cell–derived iDCs when added to the lower wells of the chemotaxis chamber. However, increasing concentrations of EDN added simultaneously to the cells of the upper wells of the chamber abrogated iDC migration induced by EDN in the lower wells. Thus, EDN-induced migration of iDCs was based on chemotaxis rather than chemokinesis.
EDN induction of human iDC migration in a PTX-sensitive manner. (A) Dose-dependent migration of CD34+ cell–derived iDCs in response to EDN. (B) Inhibition of EDN-induced migration of CD34+ cell–derived iDCs by PTX. Immature DCs were preincubated at 37°C for 30 minutes in the absence (PTX-) and presence (PTX+) of PTX (200 ng/mL) before applying to the upper wells of chemotaxis chamber. DC migration is shown as the average number of migrated cells (mean ± SD) in triplicate wells.
EDN induction of human iDC migration in a PTX-sensitive manner. (A) Dose-dependent migration of CD34+ cell–derived iDCs in response to EDN. (B) Inhibition of EDN-induced migration of CD34+ cell–derived iDCs by PTX. Immature DCs were preincubated at 37°C for 30 minutes in the absence (PTX-) and presence (PTX+) of PTX (200 ng/mL) before applying to the upper wells of chemotaxis chamber. DC migration is shown as the average number of migrated cells (mean ± SD) in triplicate wells.
Checkerboard analysis of EDN-induced DC migration
EDN in the lower wells (ng/mL) . | EDN in the upper wells, ng/mL . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | 0 . | 10 . | 100 . | 1000 . | |||
| 0 | 22 ± 3 | 24 ± 5 | 24 ± 5 | 22 ± 4 | |||
| 10 | 31 ± 5* | 22 ± 3 | 22 ± 5 | 22 ± 5 | |||
| 100 | 51 ± 5† | 35 ± 6* | 23 ± 4 | 20 ± 4 | |||
| 1000 | 105 ± 11† | 51 ± 7† | 36 ± 6* | 28 ± 4 | |||
EDN in the lower wells (ng/mL) . | EDN in the upper wells, ng/mL . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | 0 . | 10 . | 100 . | 1000 . | |||
| 0 | 22 ± 3 | 24 ± 5 | 24 ± 5 | 22 ± 4 | |||
| 10 | 31 ± 5* | 22 ± 3 | 22 ± 5 | 22 ± 5 | |||
| 100 | 51 ± 5† | 35 ± 6* | 23 ± 4 | 20 ± 4 | |||
| 1000 | 105 ± 11† | 51 ± 7† | 36 ± 6* | 28 ± 4 | |||
CD34+ cell-derived iDCs were used at 1 × 106/mL. EDN at specified concentrations was added to the lower wells of the chemotaxis chamber and DCs in the absence or presence of specified concentrations of EDN were added to the top wells of the chemotaxis chamber. The results are shown as the average (mean ± SD) of migrated DCs of triplicate wells (No./HPF).
P < .05 when compared with background DC migration (22 ± 3) by Mann-Whitney test
P < .001 when compared with background DC migration (22 ± 3) by Mann-Whitney test
To investigate whether EDN-induced iDC chemotaxis was mediated by a Giα-protein–coupled receptor (GPCR), we next examined whether EDN-induced iDC chemotaxis could be inhibited by PTX, a toxin that specifically inhibits GPCR signaling by adenosine diphosphate (ADP)–ribosylating Giα protein.32 Incubation of iDC at 37°C for 30 minutes in the presence of PTX (200 ng/mL) prior to chemotaxis assay completely inhibited the migration of iDCs in response to EDN (Figure 1B). Pretreatment with PTX did not affect the motility of iDCs as we observed no difference in spontaneous migration (in response to CM) between PTX-pretreated or control iDCs. Furthermore, the inhibition was not due to the preincubation per se, since control incubation of iDCs in the absence of PTX did not inhibit migration in response to EDN (Figure 1B). Thus, it can be concluded that EDN induces chemotaxis of iDCs in a dose- and Giα-protein–dependent manner.
The chemotactic activity of EDN for CD34+ cell–derived iDCs is reduced in the presence of RNase inhibitor
The RNase activity of EDN is inhibitable by RI and so is its antiviral activity.19,20 Addition of RI (10-1000 units/mL) simultaneously with 1000 ng/mL EDN into the lower wells of the chemotaxis chamber inhibited the migration of iDCs (Figure 2A). As anticipated, RI did not inhibit the migration of iDCs induced by 100 ng/mL SDF-1α (Figure 2A), indicating that the chemotactic activity of the EDN sample was actually due to the ribonuclease, EDN, and not a trace contaminant. Furthermore, the pattern of EDN-induced iDC chemotaxis is a bell-shaped dose-response curve that is typical of most GPCR-dependent chemotactic factors (Figure 2B).
Chemotaxis of human dendritic cells in response to EDN and mEAR2. (A) Specific abrogation of EDN-induced chemotaxis CD34+ cell–derived iDCs by RI. RI was mixed with EDN (1000 ng/mL) or SDF-1α (100 ng/mL) at concentrations as specified and the mixtures were added to the lower wells of chemotaxis chambers. (B) DC chemotactic activities of several members of the RNase A superfamily. Chemotaxis of iDCs is shown as the average cell migration (mean ± SD) of triplicate wells. hPR and bPR indicate human and bovine pancreatic RNase, respectively; hANG, human angiogenin; and mEAR2, murine eosinophil–associated RNase 2.
Chemotaxis of human dendritic cells in response to EDN and mEAR2. (A) Specific abrogation of EDN-induced chemotaxis CD34+ cell–derived iDCs by RI. RI was mixed with EDN (1000 ng/mL) or SDF-1α (100 ng/mL) at concentrations as specified and the mixtures were added to the lower wells of chemotaxis chambers. (B) DC chemotactic activities of several members of the RNase A superfamily. Chemotaxis of iDCs is shown as the average cell migration (mean ± SD) of triplicate wells. hPR and bPR indicate human and bovine pancreatic RNase, respectively; hANG, human angiogenin; and mEAR2, murine eosinophil–associated RNase 2.
As EDN is a member of the RNase A superfamily,16 we then investigated whether other members of this superfamily might also have iDC chemotactic activity similar to EDN. Of the additional 4 RNases tested, neither human or bovine pancreatic RNase A nor human angiogenin could induce iDC chemotaxis. Interestingly, mEAR2, a member of the mouse EAR cluster that, as a group, are the highly divergent orthologs of human EDN, demonstrated chemotactic activity for human iDCs at a level very similar to EDN in terms of dose response and optimal concentration. As all 5 ribonucleases are catalytically active but only EDN and mEAR2 are chemotactic for iDCs, we can conclude that ribonuclease activity, if involved at all, is not the only feature of these proteins contributing to the observed chemotactic activity.
EDN is selectively chemotactic for DCs
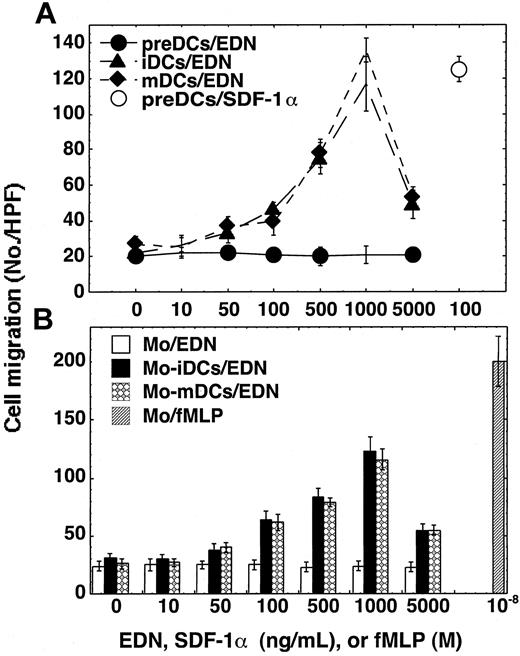
To identify the spectrum of target cells for EDN's chemotactic activity, we examined the changes in chemotaxis response to EDN in the course of DC differentiation and maturation. Precursors of DCs (preDCs) amplified from CD34+ HPCs failed to respond chemotactically to EDN (Figure 3A). This failure was not due to the lack of capacity of the preDCs to migrate since preDCs migrated toward SDF-1α in the same experiment (Figure 3A). As preDCs differentiated into iDCs they developed the capacity to migrate in response to EDN (Figure 3A). Furthermore, mDCs still maintained the capacity to migrate chemotactically to EDN (Figure 3A).
Target cell spectrum for EDN. The migration of CD34+ cell–derived DC precursors (preDCs), iDCs, or mDCs (A) and monocytes (Mo), Mo-derived DCs (Mo-iDCs and Mo-mDCs) (B) in response to EDN was evaluated by chemotaxis assay. Cell migration is shown as the average (mean ± SD) number of cells migrated in triplicate wells. The error bars are not evident if they are smaller than the size of the symbols.
Target cell spectrum for EDN. The migration of CD34+ cell–derived DC precursors (preDCs), iDCs, or mDCs (A) and monocytes (Mo), Mo-derived DCs (Mo-iDCs and Mo-mDCs) (B) in response to EDN was evaluated by chemotaxis assay. Cell migration is shown as the average (mean ± SD) number of cells migrated in triplicate wells. The error bars are not evident if they are smaller than the size of the symbols.
We next investigated whether EDN was also chemotactic for dendritic cells generated from human peripheral blood monocytes.30 Both monocyte-derived iDCs and mDCs migrated chemotactically in response to EDN with optimal doses at 1000 ng/mL (Figure 3B). EDN failed to chemoattract monocytes from which DCs were generated, although those monocytes were fully capable of migrating to fMLP (Figure 3B). Screening of human peripheral blood leukocytes revealed that EDN over a wide concentration range (10-5000 ng/mL) did not induce the migration of purified neutrophils, eosinophils, or T cells (data not shown). Therefore, EDN is selectively chemotactic for human iDCs and mDCs but not chemotactic for any other leukocytes examined.
EDN and mEAR2 are also chemotactic for murine DCs
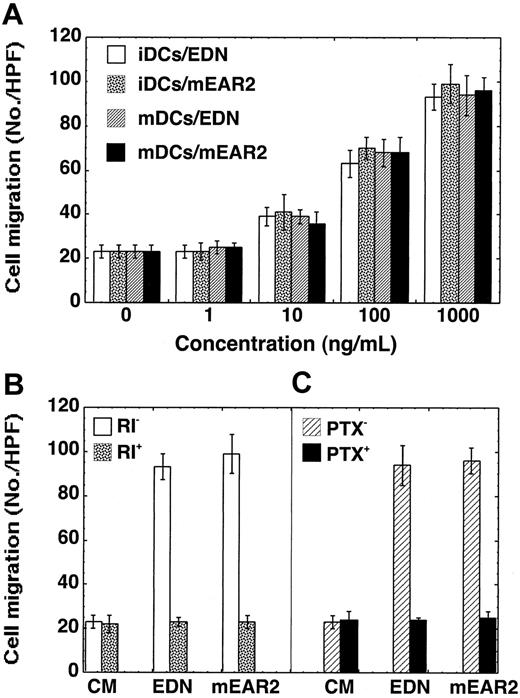
We further investigated whether EDN or mEAR2 could chemoattract mouse dendritic cells (Figure 4). Both EDN and mEAR2 induced chemotaxis of iDCs and mDCs generated from mouse bone marrow HPCs (Figure 4A). As for human DCs, the migration of mouse DCs in response to EDN or mEAR2 was inhibited by the presence of placental RI (Figure 4B) or the pretreatment of DCs with 200 ng/mL PTX at 37°C for 30 minutes (Figure 4C). Collectively, EDN and mEAR2, despite their sequence divergence, are both selective chemoattractants for both human and mouse DCs.
EDN and mEAR2 as chemoattractants for mouse DCs. The migration of iDCs and mDCs generated from mouse bone marrow HPCs in response to EDN or mEAR2 was investigated by chemotaxis assay and the results are shown as the average cell migration (mean ± SD) in triplicate wells. (A) Dose-responses. (B) Blockade of EDN- or mEAR2-induced migration of iDCs by the presence of placental RI at 1000 U/mL. (C) Inhibition of EDN-induced mDC migration by PTX. Mature DCs were preincubated at 37°C for 30 minutes in the absence (PTX-) and presence (PTX+) of PTX (200 ng/mL) prior to chemotaxis assay. In panels B and C, EDN or mEAR2 was used at 1000 ng/mL.
EDN and mEAR2 as chemoattractants for mouse DCs. The migration of iDCs and mDCs generated from mouse bone marrow HPCs in response to EDN or mEAR2 was investigated by chemotaxis assay and the results are shown as the average cell migration (mean ± SD) in triplicate wells. (A) Dose-responses. (B) Blockade of EDN- or mEAR2-induced migration of iDCs by the presence of placental RI at 1000 U/mL. (C) Inhibition of EDN-induced mDC migration by PTX. Mature DCs were preincubated at 37°C for 30 minutes in the absence (PTX-) and presence (PTX+) of PTX (200 ng/mL) prior to chemotaxis assay. In panels B and C, EDN or mEAR2 was used at 1000 ng/mL.
The N-terminal region of mEAR2 contributes to its chemotactic effect
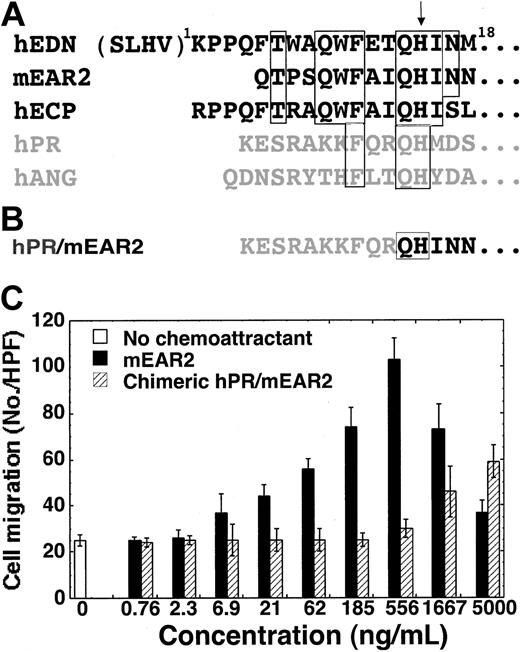
The chemotactic activities of EDN and mEAR2 for DCs can be inhibited by placental RI (Figures 2A and 4B). While this provided necessary confirmation of the role of EDN in promoting chemotaxis, RI's interaction with EDN is such that all regions of the ribonuclease are covered, and as such, we cannot conclude anything regarding the role of ribonuclease activity per se. At the same time, 3 other RNase A superfamily members, including 2 highly efficient ribonucleases (hpRNase and bpRNase) displayed no chemotactic activity, suggesting that ribonuclease activity, if involved at all, contributes in conjunction with other specific elements of amino acid sequence present only in EDN and mEAR2. Since human EDN and mEAR2 are chemotactic for dendritic cells and human angiogenin and pancreatic ribonuclease are not, their sequences were compared in an attempt to identify the region(s) of sequence most likely to play a significant role in this activity. The results of such an analysis (Figure 5A) pointed to the amino terminal sequences of EDN and mEAR2 as having signifi-cant sequence homology to each other (8/18 conserved) and at the same time very limited homology to either pancreatic ribonuclease (3/18) or angiogenin (also 3/18; P < .02 by χ2 test). To address the role of the N-terminal region in mediating the chemotactic effect, we generated a chimeric hPR/mEAR2 protein in which the first 9 amino acid residues of mEAR2 were substituted by the first N-terminal 10 amino acid residues of hPR as per the alignment (Figure 5A) and as illustrated in Figure 5B. When tested in parallel with mEAR2, the chimeric hPR/mEAR2 protein exhibited markedly reduced DC chemotactic activity compared with mEAR2 (Figure 5C). The mEAR2 and hPR/mEAR2 began to show significant chemotactic activity at 21 and 1667 ng/mL, respectively. The numbers of DC migration in response to 62 ng/mL mEAR2 and 5000 ng/mL hPR/mEAR2 were comparable. By rough estimation, the chimeric hPR/mEAR2 showed less than one tenth of the DC chemotactic activity of mEAR2.
Critical role of the N-terminal region of mEAR2 for its DC chemotactic effect. (A) Alignment of the amino terminal sequences of hEDN, mEAR2, human eosinophil cationic protein (hECP), human pancreatic ribonuclease (hPR), and human angiogenin (hANG). Regions of identical sequence shared by EDN and mEAR2 are identified within the open boxes and, if shared by ECP, hPR and hANG, the boxes are extended to include them. The additional amino-terminal residues of the “-4” form of EDN are included in parentheses. An arrow indicates the position of the universally conserved histidine that serves as a crucial catalytic residue in all RNase A superfamily ribonucleases. The RNases predominantly produced by eosinophils are shown in black. (B) The N-terminal sequence of chimeric hPR/mEAR2. (C) Chemotaxis of human Mo-iDCs in response to mEAR2 and hPR/mEAR2 chimera. Chemotaxis of iDCs is shown as the average cell migration (mean ± SD) of triplicate wells.
Critical role of the N-terminal region of mEAR2 for its DC chemotactic effect. (A) Alignment of the amino terminal sequences of hEDN, mEAR2, human eosinophil cationic protein (hECP), human pancreatic ribonuclease (hPR), and human angiogenin (hANG). Regions of identical sequence shared by EDN and mEAR2 are identified within the open boxes and, if shared by ECP, hPR and hANG, the boxes are extended to include them. The additional amino-terminal residues of the “-4” form of EDN are included in parentheses. An arrow indicates the position of the universally conserved histidine that serves as a crucial catalytic residue in all RNase A superfamily ribonucleases. The RNases predominantly produced by eosinophils are shown in black. (B) The N-terminal sequence of chimeric hPR/mEAR2. (C) Chemotaxis of human Mo-iDCs in response to mEAR2 and hPR/mEAR2 chimera. Chemotaxis of iDCs is shown as the average cell migration (mean ± SD) of triplicate wells.
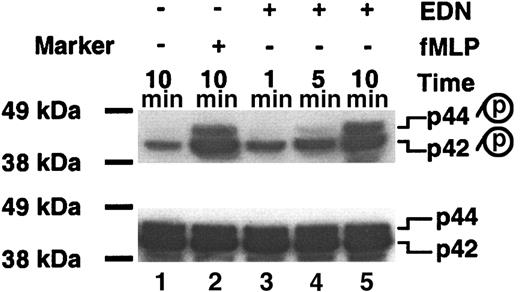
EDN activates p44/42 MAPK in DCs
To look for additional signaling event(s) induced by EDN in DCs, we investigated whether EDN could induce the activation of p44/42 MAPK by measuring the level of phospho p44/42 MAPK (Figure 6). Untreated DCs displayed basal level of phospho-p42 with phospho-p44 undetectable (Figure 6; lane 1, top). Treatment of DCs with fMLP at 10-7 M for 10 minutes (as a positive control) resulted in a marked increase of both phospho-p42 and phosphop44 as expected (Figure 6; lane 2, top). Interestingly, treatment of Mo-iDCs with 1 μg/mL of EDN enhanced the levels of both phospho-p42 and phospho-p44 MAPK (Figure 6; lanes 3-5, top). Phosphorylation of p42 was increased upon EDN treatment for as short as 1 minute, whereas the increase of phospho-p44 did not become evident until 5 minutes. Probing the same membrane with anti-p44/42 antibody after stripping revealed identical amounts of p44/42 MAPK on each lane (Figure 6, bottom), indicating both equal loading and no significant up-regulation of p44/42 MAPK proteins in response to EDN. These results demonstrate that EDN, similar to other chemotactic factors, is capable of inducing the activation of p44/42 MAPK in DCs.
EDN induces the activation of p44/42 MAPK in Mo-iDCs. Mo-iDCs untreated or treated with fMLP (10-7 M) or EDN (1 μg/mL) for a period of time as specified were lysed, separated on a 4% to 12% gradient SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to PVDF membrane, and Western blotted with anti–phospho-p44/42 (top). p, phosphorylated. The same membrane was stripped and blotted with anti-p44/42 (bottom). The exposure time for the top or bottom autoradiograph was 5 seconds. One experiment representative of 3 is shown.
EDN induces the activation of p44/42 MAPK in Mo-iDCs. Mo-iDCs untreated or treated with fMLP (10-7 M) or EDN (1 μg/mL) for a period of time as specified were lysed, separated on a 4% to 12% gradient SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to PVDF membrane, and Western blotted with anti–phospho-p44/42 (top). p, phosphorylated. The same membrane was stripped and blotted with anti-p44/42 (bottom). The exposure time for the top or bottom autoradiograph was 5 seconds. One experiment representative of 3 is shown.
mEAR2 induces the accumulation of DCs in vivo
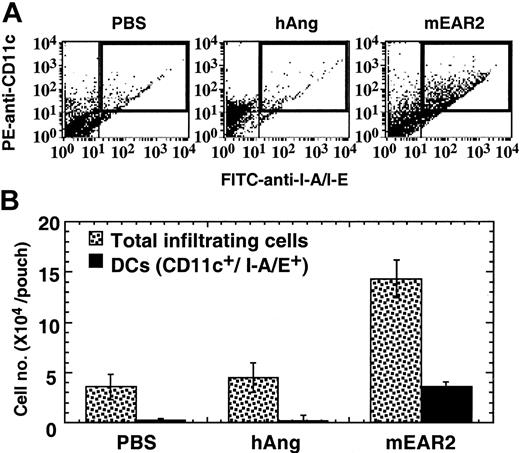
To confirm chemoattractant activity in vivo, we determined the number and identity of cells accumulating in response to injection of mEAR2 into the air pouches of mice. Four hours after injection of either PBS, hANG, or mEAR2 into the air pouches, cells infiltrating into the air pouches were washed out, counted, and analyzed for the presence of CD11c (PE) and I-A/I-E (FITC), markers identifying double-positive DCs. Figure 7A shows the representative dot plots of infiltrating cells recovered from injected air pouches. The proportion of PE/FITC double-positive cells (Figure 7A, top right quadrant) in mEAR2-treated group was much higher than in either the PBS- or hANG-treated group, suggesting that injection of mEAR2 into the air pouch resulted in recruitment of DCs into the air pouches. The average number of leukocytes and DCs recruited into the air pouches of each group is shown in Figure 7B. Injection of hANG did not enhance the recruitment of either total leukocytes or DCs into the air pouches. In agreement with the in vitro DC chemotactic activity of EDN and mEAR2, injection of mEAR2 promoted the recruitment of DCs (Figure 7B, ▪) into the air pouches. Notably, in the mEAR2-treated group, DCs accounted for only approximately 50% of the increase in total infiltrating cells, suggesting that leukocytes other than DCs were also recruited into the air pouches by mEAR2 injection. Staining of the cytospin of cells recovered from mEAR2-injected air pouches revealed that more than 95% of the cells were of mononuclear morphology, indicating that there was no apparent granulocyte recruitment (data not shown). These data suggest that mEAR2 acts as a DC chemoattractant in vivo.
In vivo chemotactic activity of mEAR2. Balb/c mice with well-formed air pouches were randomized into 3 groups (n = 3-5). The air pouches of mice were injected with 1 mL PBS alone or containing 1 μg/mL hANG or mEAR2, respectively. After 4 hours, the air pouches were washed with PBS containing 5 mM EDTA and 20 U/mL heparin. Cells recovered from the air pouches were counted and analyzed by FACScan after staining with PE–anti-CD11c and FITC–anti–I-A/I-E or isotypematched control antibodies. (A) Representative dot plots showing the PE and FITC double-positive DCs (top right quadrant highlighted by a rectangle) in the infiltrating cells recovered from PBS-, hANG-, or mEAR2-injected air pouches. (B) The average (mean ± SD) of total infiltrating cells or DCs of each group, which was calculated by multiplying total cell number with percentage of DCs of individual air pouches.
In vivo chemotactic activity of mEAR2. Balb/c mice with well-formed air pouches were randomized into 3 groups (n = 3-5). The air pouches of mice were injected with 1 mL PBS alone or containing 1 μg/mL hANG or mEAR2, respectively. After 4 hours, the air pouches were washed with PBS containing 5 mM EDTA and 20 U/mL heparin. Cells recovered from the air pouches were counted and analyzed by FACScan after staining with PE–anti-CD11c and FITC–anti–I-A/I-E or isotypematched control antibodies. (A) Representative dot plots showing the PE and FITC double-positive DCs (top right quadrant highlighted by a rectangle) in the infiltrating cells recovered from PBS-, hANG-, or mEAR2-injected air pouches. (B) The average (mean ± SD) of total infiltrating cells or DCs of each group, which was calculated by multiplying total cell number with percentage of DCs of individual air pouches.
Discussion
In the present study, we have demonstrated that EDN can induce the in vitro migration of iDCs and mDCs but not neutrophils, monocytes, T cells, or even DC precursors. EDN-mediated DC migration showed a bell-shaped dose-response curve typical of most chemotactic factors and could be homologously desensitized (Table 1). EDN-induced migration of DCs could be inhibited by pretreatment of target cells with PTX, suggesting it uses a GPCR–dependent mechanism. In addition, mEAR2, a member of a cluster of ribonuclease genes that as a group are distant orthologs of EDN in mice,16,23,33 was also chemotactic for both human and mouse DCs. Furthermore, EDN also induced the activation of p44/42 MAPK (Figure 6), a characteristic shared by many chemotactic factors.34,35 By every criterion, EDN and mEAR2 are clearly chemoattractants selective for DCs. Many chemotactic factors including chemokines and classical chemoattractants are active on dendritic cells;36 however, only 3, including SDF-1α,36 plateletactivating factor,37 and complement factor C5a,38 are chemotactic for both iDCs and mDCs. However, the DC chemotactic activities of EDN and mEAR2 are unlikely to be based on induction of any of these factors for the following reasons. First, we have evidence demonstrating that EDN treatment of DCs does not induce the production of either SDF-1α or platelet factor (data not shown). Furthermore, C5a is generated as a product of complement activation and thus was likely to be produced in our in vitro experimental system. In addition to their chemotactic activities for DCs, SDF-1α, platelet-activating factor, and C5a are also chemotactic for diverse types of leukocytes. For example, SDF-1α is chemotactic for DC precursors (Figure 3A) and lymphocytes.39 Platelet-activating factor and C5a are chemotactic for neutrophils and monocytes.40 In contrast, we have shown that EDN and mEAR2 are unique chemoattractants for DCs as they do not act on neutrophils, eosinophils, monocytes, or T cells, at least in vitro.
EDN exists in 2 forms in vivo due to alternative splicing.25,26,41 The -4 form of EDN has 4 additional amino acid residues (SLHV) on the N-terminus (Figure 5A). Although the -4 form of EDN was used in our study, it is highly possible that EDN without the N-terminal 4 additional residues may also be chemotactic since both forms have essentially identical tertiary structures.18,42 Additional support for this notion comes from the fact that, mEAR2, which lacks the corresponding N-terminal 8 residues of -4 form of EDN (Figure 5A), is nevertheless similarly chemotactic as the -4 form of EDN. Sequence comparison of EDN and mEAR2 (chemotactic for DCs), and hANG and hPR (not chemotactic for DCs) and remarkably decreased DC chemotactic activity of the hPR/mEAR2 chimera indicate that the N-terminal region (1Q-9I) of mEAR2 is important for its DC chemotactic activity (Figure 5). The 1Q-9I region of mEAR2 corresponds to the 5F-13T region of EDN, indicating that the 4 additional N-terminal amino acid residues of the -4 form of EDN are not critical for its chemotactic effect (Figure 5). According to the sequence analysis, one would also predict that human ECP may also act as a chemoattractant for DCs, given the sequence identity with both EDN and mEAR2, specifi-cally at the amino terminus (7/18 and 9/18 conserved when compared with EDN and mEAR2, respectively). This possibility awaits investigation.
Injection of 1 μg mEAR2 into air pouches resulted in the recruitment of DCs (CD11c and I-A/I-E double positive) within 4 hours (Figure 7), indicating that mEAR2 also acts as a DC chemoattractant in vivo. However, mononuclear cells other than DCs were also recruited in response to mEAR2 injection (Figure 7B). Since EDN and mEAR2 are selectively chemotactic for DCs in vitro, the recruitment of mononuclear cells other than DCs into mEAR2-administered air pouches was most likely due to the induction of chemokines active on mononuclear cells by recruited DCs. In support of this notion, we very recently found that treatment of human iDCs with EDN induces the production of several chemokines active on mononuclear cells (D.Y., Q.C., J.J.O., unpublished results, May 2003). Whether mEAR2 would, similar to EDN, stimulate DCs to produce cytokines active on mononuclear cells needs further investigation.
What might be the pathophysiologic implication(s) for the chemotactic activity of EDN? There is no definitive answer at present, however, based on its chemotactic activity for DCs and the distinct roles of iDCs and mDCs in controlling immunity, EDN may play a role in promoting adaptive immunity. Immature DCs are responsible for antigen uptake and processing, whereas mDCs are the most potent professional antigen-presenting cells.31 Although EDN can be expressed by liver, spleen, and neutrophils21,43 as well as peripheral blood mononuclear cells (D.Y., Q.C., J.J.O., unpublished results, August 2002), it is predominantly produced during eosinophilopoeisis in the bone marrow and released upon eosinophil activation.15,21 Therefore, EDN is potentially present at sites of helminth and viral infection and allergic reactions.15,21 In its role as an iDC chemotactic factor, EDN may contribute to the recruitment of DCs to sites of helminth, virus, and allergen entry, thereby enhancing antigen-specific immunity by promoting antigen uptake, processing, and ultimately presentation. Recruitment and activation of both iDCs and mDCs at sites of helminth and allergic antigen entry may promote the initiation of the immune response in situ, resulting in the formation of so-called “tertiary lymphoid tissue,”44 such as granuloma formation around the helminth eggs. Additional studies are currently under way to define the receptor for EDN as well as additional effects of EDN on dendritic cells. The results of the present study provide additional evidence for the notion that EDN is a multifunctional mediator with cytotoxic, antiviral, antihelminth, and DC chemotactic activities.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-01-0151.
Supported in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The publisher or recipient acknowledges right of the US Government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs D. Newton and S. Rybak (Intramural Research Support Program, SAIC Frederick, NCI at Frederick, NIH) for providing us with recombinant human pancreatic RNase.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal