Abstract
We have identified a novel gene MEL1 (MDS1/EVI1-like gene 1) encoding a zinc finger protein near the breakpoint of t(1; 3)(p36;q21)-positive human acute myeloid leukemia (AML) cells. Here, we studied the structure, expression pattern, and function of MEL1 in leukemia cells. In this study, we have identified 3 transcription start sites, 1 in exon 1 and 2 in exon 2, and 2 kinds of translation products, 170 kDa (MEL1) and 150 kDa (MEL1S). Notably, the 150-kDa band of MEL1S was detected mainly in the t(1;3)(p36;q21)-positive AML cells. By immunoblot analysis and proteolytic mapping, it is suggested that the 150-kDa band of MEL1S in the leukemia cells is translated from the internal initiation codon ATG597 in exon 4 and is mostly lacking the amino-terminal PR domain of MEL1. By the cyclic amplification and selection of targets (CASTing) method for identifying consensus sequences, it was shown that the consensus sequences of MEL1 were included in 2 different consensus sequences for DNA-binding domain 1 and 2 (D1-CONS and D2-CONS) of EVI1. In reporter gene assays, MEL1S activated transcription via binding to D2-CONS; however, the fusion of MEL1 or MEL1S to GAL4 DNA-binding domain (DBD) made them GAL4 binding site–dependent transcriptional repressors. Moreover, overexpression of MEL1S blocked granulocytic differentiation induced by granulocyte colony-stimulating factor (G-CSF) in interleukin-3 (IL-3)–dependent murine myeloid L-G3 cells, while MEL1 could not block the differentiation. Thus, it is likely that overexpression of the zinc finger protein lacking the PR domain (EVI1 and MEL1S) in the leukemia cells is one of the causative factors in the pathogenesis of myeloid leukemia.
Introduction
The PRDI-BF1-RIZ1 homologous (PR) domain is a newly recognized amino-terminal module1 and was first noted for the homologous 100–amino acid region shared between positive regulatory domain I binding factor 1 (PRDI-BF1) (PRDM1)2 and retinoblastoma-interacting zinc finger protein (RIZ) (PRDM2).1 PR domain members (PRDMs) are also known to be in the suppressor of var, enhancer of zeste and trithorax (SET) domain superfamily.3 RIZ is isolated by protein-protein interaction with the retinoblastoma tumor suppressor protein (Rb).1 Consistent with a potential role in the Rb pathway, RIZ may play an important role in the pathogenesis of human cancer. Interestingly, RIZ produces 2 different gene products, a full-length RIZ1 and a short-form RIZ2 lacking a PR domain at the amino-terminus, which is generated by an alternative transcript derived from an internal promoter.4 Both transcripts are widely expressed in many organs. However, recent study revealed that the expression rate of RIZ1 is decreased or several point mutations in the RIZ1 coding region exist in many kinds of tumors, suggesting that RIZ1 with a PR domain is a candidate tumor suppressor gene.5,6
Murine ecotropic virus integration 1 site (Evi-1) zinc finger protein was isolated from common sites of viral integration in murine myeloid leukemia.7 The human homolog, EVI1 at chromosomal position 3q26, is also transcriptionally activated by several recurrent chromosomal aberrations in acute myeloid leukemia (AML).8 The most frequent rearrangements are t(3;3)(q21;q26) and inv(3)(q21q26) associated with AML (3q21q26 syndrome) and myelodysplastic syndrome (MDS).8 A PR domain was later found in the MDS1/EVI1 gene product, which is derived from one of the EVI1 alternative transcripts.9 The genomic region of MDS1 is located around 300 kilobases (kb) upstream from the EVI1 gene, and the transcripts of MDS1/EVI1 were found in normal tissues including hematopoietic cells.10 Because EVI1 lacking the PR domain is overexpressed in leukemia cells with 3q26 abnormalities,8 EVI1 is thought to be the same oncogenic form as MLL, whose PR/SET domain is deleted by chromosomal translocation. Therefore, it is suggested that the PR domain is related to the inhibition of tumor formation or to suppression of the growth advantages of a tumor.
EVI1 has 2 different specific DNA consensus sequences, which are GA(C/T)AAGA(T/C)AAGATAA for DNA-binding domain 1 (D1-CONS)11 and GAAGATGAG for DNA-binding domain 2 (D2-CONS).12 It was shown that EVI1 is a bifunctional regulator of transcription: EVI1 can repress transcriptional activation by GATA-1 D1-CONS dependently13 and also weakly activate transcription D2-CONS dependently.14 MDS1/EVI1 was demonstrated to be a new type of GATA-binding transactivator via binding to D1-CONS.15 However, GAL4-EVI1 and GAL4-MDS1/EVI1 fusion proteins showed transcription repressor activity that was dependent on GAL4 DNA-binding capability.16 Moreover, EVI1 can bind to transcriptional repressor cofactors (adenovirus E1A C-terminal binding protein 1 or 2 [CtBP1 or CtBP2])17,18 and activator cofactors (cyclic adenosine 3′,5′ monophosphate [cAMP]–responsive element-binding protein-binding protein [CBP] or p300/CBP-associated factor [P/CAF]),19 suggesting that EVI1 can function as a dual transcription regulator. On the other hand, it is reported that overexpression of EVI1 transforms rat fibroblast RAT1 cells,20 and it blocks transforming growth factor β (TGF-β) signal transduction in mink lung cells21 and also blocks granulocytic differentiation of interleukin-3 (IL-3)–dependent myeloid cells induced by granulocyte colony-stimulating factor (G-CSF).22 However, no apparent target genes of EVI1 were found, and it is not known how EVI1 overexpression is involved in leukemogenesis.
Recently, we have identified MEL1 (MDS1/EVI1-like gene 1) as a member of the EVI1 gene family and also as a PR domain member (PRDM16).23 MEL1 was found in t(1;3)(p36;q21)-positive AML, which has similar clinicopathological features to 3q21q26 syndrome, as a transcriptionally activated gene near the chromosomal breakpoint. The structure of the MEL1 gene product is very similar to that of MDS1/EVI1. Both products have the PR domain at the amino-terminus, 2 separated DNA-binding domains with 7 and 3 tandem zinc finger repeats, CtBP-binding motifs, and other possible transcriptional regulatory domains. In this study, we here identify 3 transcription start sites of MEL1 mRNA and 2 kinds of MEL1 gene products; one is the full-length MEL1 protein with a PR domain, and the other is a short form lacking the PR domain designated MEL1S. The MEL1S gene product was detected mainly in t(1;3)(p36;q21)-positive AML cells by immunoblot analysis. We examined its DNA-binding activity by the cyclic amplification and selection of targets (CASTing) method and identified the consensus sequences of MEL1 as very similar to those of EVI1. Therefore, the DNA-binding consensus sequence of EVI1 was shared with MEL1 protein, suggesting that MEL1 and MEL1S have a similar DNA-binding capability and transcriptional regulation activity to EVI1. Moreover, overexpression of MEL1S blocked G-CSF–induced myeloid differentiation in murine IL-3–dependent L-G3 cells, suggesting that MEL1S has the same transforming capability for hematopoietic cells as EVI1. In this paper, we discuss the molecular mechanism underlying leukemogenesis caused by overexpression of the EVI1 gene family and also the role of the PR domain in tumorigenicity.
Materials and methods
cDNA and plasmid constructions
MEL1 cDNA clones (N207, N2094, N1210, and N1163) were isolated from mRNA of t(1;3)(p36;q21)-positive AML cells.23 The nucleotide sequence of human MEL1 (nucleotide nos. [nt.] 1 to 5429) was submitted to SAKURA sequence entry program of DNA Data Bank of Japan (DDBJ) (sequence submission no. AB078876) with coding region (nt. 45 to 3818). All cDNA clones were inserted into pSKII plasmid (Stratagene, La Jolla, CA) as pSK207 (nt. 1 to 1555), pSK2094 (nt. 282 to 2829), pSK1210 (nt. 1535 to 5429), or pSK1163 (nt. 257 to 3938). A putative full-length human MEL1 cDNA (pSKMEL1) covered a 3938–base pair (bp) sequence (nt. 1 to 3938), and the short-form MEL1S cDNA (pSKMEL1S) covered a 3657-bp sequence (nt. 282 to 3938). Δ13MEL1 was deleted the first 482 bp (exons 1 to 3) from MEL1 cDNA and was amplified with primers 5′MEL1 (5′-GCAAGCTTATCTCCGAAGACCTGGGCA-3′) and 3′MEL1 (5′-GCTCTAGGGTGGGGAGGATGATGCTGAGG-3′) (nt. 483 to 3938). To express MEL1, MEL1S, and Δ13MEL1 in mammalian cells, the human MEL1 (MEL1S or Δ13MEL1) cDNA was digested from pSKMEL1 (pSKMEL1S or pSKΔ13MEL1) and inserted into HindIII and XbaI sites of cytomegalovirus mammalian expression vector pRc/CMV (pCMV) (Invitrogen, Carlsbad, CA) to generate pCMV-MEL1 (pCMV-MEL1S or pCMV-Δ13MEL1).
To produce and purify the MEL1 gene products in bacteria cells for antibody production and electrophoretic mobility shift assay (EMSA), MEL1 DNA-binding domains 1 and 2 were used for generating glutathione-S-transferase (GST) fusion proteins as described previously.14 A DNA fragment of human MEL1 DNA-binding domain 1 was amplified by the polymerase chain reaction (PCR) using pSKMEL1 as a template with primers MELD1N (5′-CGTGGATCCATTGAGCCAGGTGAGGAG-3′) and MELD1C (5′-CGAATTCAGGCTGGCGTGATTGAGGCT-3′). A DNA fragment of the DNA domain 2 was amplified with primers MELD2F (5′-GGTGGATCCAAGCCCTCGCCCTTCTTCATG-3′) and MELD2R (5′-AAGGAATTCTGGTGCGTTCTCGTGCTCGTG-3′). Each PCR fragment was inserted into BamHI and EcoRI sites of pGEX-1λT (Amersham Biosciences, Piscataway, NJ) to generate pGEX-MELDBD1 (amino acid residues [aa.] 200 to 483) or pGEX-MELDBD2 (aa. 857 to 1036), respectively.
For reporter gene assay, firefly luciferase reporter vector, pGL3-p-D1 (or pGL3-p-D2), was generated to insert the 3 tandem repeats of D1-CONS (or D2-CONS) into NheI and XhoI sites of pGL3-promoter vector (pGL3-p) (Promega, Madison, WI).
To generate human MEL1 (or MEL1S) fused with GAL4 DNA-binding domain (DBD), MEL1 (or MEL1S) was inserted into an EcoRI site of the pM GAL4 DBD cloning vector (Clontech, Palo Alto, CA), resulting in GAL4 DBD-MEL1 (or GAL4 DBD-MEL1S). The GAL4 binding site in the pG5-CAT vector (Clontech) was inserted into the region before the promoter of pGL3-promoter vector (Promega) to generate pG5proLuc. pM3-VP16 control vector (Clontech) was used as a positive control for the GAL4 transcription assay, and pRL-TK Renilla luciferase control reporter vector (Promega) was used as an internal control.
A MEL1 cDNA fragment contained with Flag sequences at the amino-terminus was amplified by PCR from pSKMEL1 using 2 primers, 5′EcoNFLAGMEL1 (5′-GCGAATTCGCCACCATGGATTACAAGGATGACGACGATAAGCGATCCAAGGCGAGGGCG-3′) and 3′BamMEL (5′-GCGGATCCTCAGAGGTGGTTGATGGGGT-3′). To create pLXSN-NFLAG-MEL1, the Flag-tagged MEL1 cDNA fragment was digested and ligated into EcoRI and BamHI sites of the retroviral vector pLXSN (Clontech). Similarly, pLXSN-NFLAG-MEL1S was created using primers 5′EcoNFLAGMEL1S (5′-GCGAATTCGCCACCATGGATTACAAGGATGACGACGATAAGGAAGCCGGGGAGAGGCTG-3′) and 3′BamMEL as above.
Cell culture, patient samples, and transfection
Murine kidney COS7,24 human cervical carcinoma HeLa,25 human embryonic kidney BOSC23,26 and murine teratocarcinoma P1927 cells were cultured in Dulbecco modified minimum essential medium (DMEM) with 10% fetal calf serum (FCS) (Biofluids, Camarillo, CA). MOLT15,28 U937,29 and K56230 were cultured in RPMI 1640 with 10% FCS, and UCSD/AML131 cells were cultured in RPMI 1640 with 10% FCS and 10 ng/mL human granulocyte-macrophage (GM)–CSF. Murine IL-3–dependent L-G3 cells32 were cultured in RPMI 1640 with 10% FCS and 5 ng/mL murine IL-3 (mIL-3) (Kirin Brewery, Tokyo, Japan). FuGENE 6 transfection reagent (Roche, Mannheim, Germany) was used for transfection of the cells according to the manufacturer's instructions. Cells were processed 36 hours after transfection for immunoblotting and luciferase analyses. Informed consent was obtained from all blood donors according to the Declaration of Helsinki.
Retroviral infections
To generate L-G3 clones stably expressing human MEL1 and MEL1S, infectious retroviral particles encoding Flag-tagged MEL1 or MEL1S were prepared by transfection to the producer BOSC23 cells with pLXSN-NFLAG-MEL1, pLXSN-NFLAG-MEL1S, or the empty retroviral vector (pLXSN). Neomycin-resistant clones were screened for expression of Flag-tagged proteins by immunoblotting analysis using anti-Flag M2 monoclonal antibody (Sigma, St Louis, MO). For each experiment, 3 independent clones with comparable expression were used for further assays.
Proliferation and differentiation assay
For proliferation and differentiation assays, the stable L-G3 transformants were seeded at a density of 1 × 105 cells per well in 6-well culture plates. Twenty-four hours later, the cells were washed twice in RPMI 1640 and transferred into the medium supplemented with 5 ng/mL mIL-3, 5 ng/mL G-CSF (Kirin Brewery), or no growth factor. For the measurement of cell numbers and the morphologic observation, the cells were incubated for 6 days at 37°Cina5%CO2 incubator. After the culture, the cells were stained with May-Grünwald-Giemsa solution (Merck, West Point, PA) for a microscopic examination of differentiation. Murine IL-3 and G-CSF were kindly provided from Kirin Brewery.
Antibody production, immunoprecipitation, and immunoblotting analysis
To generate antibody, standard procedure was followed as described.33 A polyclonal antibody, anti-MELDBD1, was produced by injecting a rabbit with purified GST-MELDBD1 fusion protein. Cell extracts from 1 × 108 leukemia cells were immunoprecipitated by the anti-MELDBD1 antibody and eluted from the gel of protein A–Sepharose 4B (Amersham Biosciences). The immunoprecipitated products and control samples extracted from COS7 cells transfected with a mock (pCMV), pCMV-MEL1, or pCMV-MEL1S were subjected to 6% sodium dodecyl sulfate (SDS)–polyacrylamide gel and transferred to an Immobilon P filter (Millipore, Bedford, MA). The transferred membrane was incubated with rabbit anti-MELDBD1 antibody, biotinylated F(ab′)2 fragment of affinity-isolated swine antirabbit immunoglobulins (Dako, Glostrup, Denmark), and streptavidin-biotinylated horseradish peroxidase complex (Amersham Biosciences) as described previously.14 As an internal control, the same membrane was incubated with anti–β-actin monoclonal antibody, clone AC-15 (Sigma). Immunoreactive proteins were visualized by LAS-1000 (Fuji-film, Tokyo, Japan) using Lumi-Light Western blotting substrate (Roche).
The cell extracts from L-G3 cells infected by retrovirus containing FLAG-tagged MEL1 or MEL1S were immunoprecipitated with anti-FLAG M2 affinity gel (Sigma).
RNase protection assay
RNase protection assay was carried out with BD RiboQuant multiprobe ribonuclease protection assay system (BD PharMingen, San Diego, CA). Briefly, 1 μg of the BglII-digested MEL1 cDNA (clone N207) was transcribed by T3 RNA polymerase with [32P]-uridine 5′-triphosphate ([32P]UTP). A 562-bp antisense RNAprobe was hybridized with 30 μg total RNAfrom t(1;3)(p36;q21)-positive AML cells, t(3;3)(q21;q26)-positive AML cells (Kasumi-3),34 or 30 μg yeast tRNA as control samples and was digested by S-1 endonuclease. The digested RNA mixtures were subjected to a denaturing gel of 5% polyacrylamide with 7 M urea after heat denaturing. After drying the gel, the gel was exposed to a phosphorimaging plate, and the latent image was developed in the phosphorimager BAS-1000 (Fuji-film).
Analysis of genome database
Genomic structure of the MEL1 gene was investigated in National Center for Biotechnology Information (NCBI) and European Molecular Biology Laboratory (EMBL) genome databases.
In vitro transcription and translation analysis
In vitro transcription and translation reactions were carried out with TNT T7Quick Coupled Transcription/Translation System (Promega) using rabbit reticulocytes with [35S]methionine according to the manufacturer's instructions.
Reporter gene assay
Dual-luciferase reporter assay system (Promega) was used for reporter gene assay according to the manufacturer's instructions. Firefly luciferase activity was measured as relative light units (RLUs). The RLUs from individual transfection were normalized by measurement of Renilla luciferase activity in the same samples. Individual transfection experiments were performed in triplicate, and the results were reported as mean RLUs and standard deviations (± SD).
Nucleotide sequencing
Nucleotide sequences were determined by dideoxynucleotide chain termination reaction. BigDye terminator cycle sequencing kit was used with AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA).
Binding and amplification reactions
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed as described previously.14 Briefly, double-strand binding oligonucleotides (D1-CONS or D2-CONS) were end-labeled using T4 polynucleotide kinase and [γ-32P]-adenosine triphosphate ([γ-32P]ATP). The binding buffer contains 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.6), 100 mM KCl, 100 μM EDTA (ethylenediaminetetraacetic acid), 10% glycerol, 1 mM dithiothreitol (DTT), 25 μg/mL poly(dIdC), and 1 mg/mL bovine serum albumin.
Primer extension and 5′-rapid amplification of cDNA ends (5′-RACE)
Primer extension and 5′-rapid amplification of cDNA ends (5′-RACE) was performed utilizing the 5′-full RACE core set (Takara, Shiga, Japan) according to the manufacturer's instructions. Briefly, cDNA was synthesized from 1 μg polyA+ RNA from t(1;3)-positive AML cells with 5′-phosphorylated reverse transcriptase (RT) primer 5pMrtp (5′-pCACACGGATGTACTTGAGCCAGCT-3′, corresponding to nt. 543 to 566) by avian myeloblastosis virus (AMV) reverse transcriptase. After degradation of RNA in RNA-DNA hybrid by treatment with RNase H, the single-strand cDNA was self-ligated with T4 RNA ligase. Circularized or concatemerized cDNAs were amplified by primers M5gsp1 forward (5′-AGAAGTTCTGCGTGGATGCAAATCAG-3′, nt. 505 to 530) and M5gap1 reverse (5′-CACTGCCCAGGTCTTCGGAGATCT-3′, nt. 481 to 504) for first-round PCR and then amplified by nested primers M5gsp2 forward (5′-AATCAGGCGGGGGCTGGCAGCT-3′, nt. 525 to 546) and M5gap2 reverse (5-AGATCTTTGTGATGCAGCCTTCCTG-3, nt. 461 to 486) for second-round PCR. PCR products were subcloned into a pCR4-TOPO vector (Invitrogen) and sequenced.
Partial proteolytic mapping treated with V8 protease or cyanogen bromide (CNBr)
After immunoprecipitation the MEL1 protein from t(1;3)(p36;q21)-positive AML cells, and COS7 cells transfected with a mock (pCMV), pCMV-MEL1S, or pCMV-Δ13MEL1 by anti-MELDBD1 antibody, each 150-kDa band was separated on 6% polyacrylamide gel. After transfer to a nitrocellulose membrane (Bio-Rad, Hercules, CA) and each piece of the membrane was cut out with the band, the band was cleavaged in situ by CNBr (Nacalai Tesque, Kyoto, Japan) in 70% formic acid.36 On the other hand, the protein band was excised and partially digested by V8 protease (Pierce, Rockford, IL) according to the procedure of Cleveland et al.37 The proteolytic polypeptides were separated on 20% polyacrylamide gel and detected with Silver Stain II Kit (Wako, Osaka, Japan) according to the manufacturer's instructions.
Results
Identification of 3 transcription start sites of MEL1
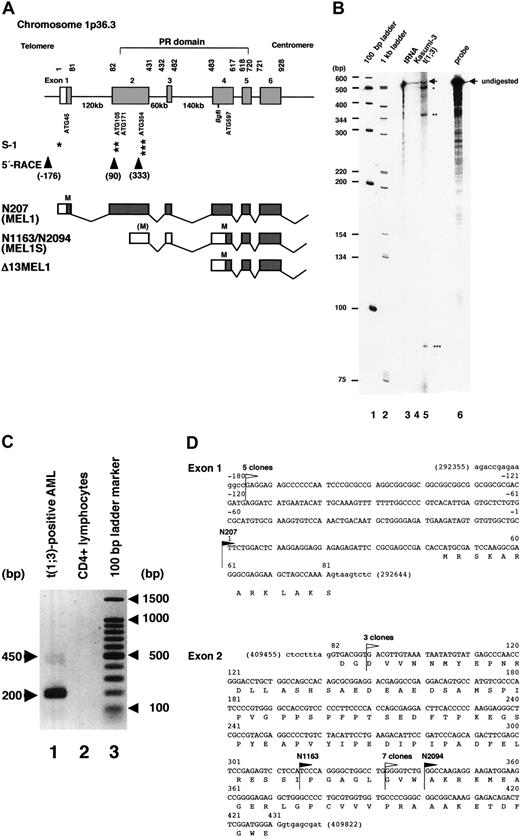
We have reported that the novel zinc finger protein MEL1 (PRDM16) with a PR domain at the amino-terminus was isolated from a cDNA library of t(1;3)(p36;q21)-positive AML cells (sequence shown submission no. AB078876).23 The N207 cDNA had 44 bp of the 5′ noncoding sequence before the putative first methionine (ATG45). However, 2 cDNA clones, N1163 and N2094, lacked 256 or 281 bp at the 5′ end of the clone N207, respectively (Figure 1A). Based on the genomic sequences from the human genome DNA database, the entire genomic region of MEL1 spans about 400 kb with 17 coding exons on chromosome band 1p36.3 (data not shown). The 5′ end of clone N207 is in exon 1, and those of N1163 and N2094 are in the middle of exon 2. To identify a possible transcription starting point in the 5′ region of the full-length MEL1 cDNA, we performed an RNase protection assay and 5′-rapid amplification of cDNA ends (5′-RACE) by the primer extension method. In the RNase protection assay, an antisense MEL1 probe was synthesized by T3 RNA polymerase on the BglII-restricted plasmid pSK207 and incubated with RNAs from t(1;3)(p36;q21)-positive AML cells. As shown in Figure 1B, 3 protected bands were detected. The longest protected fragment of about 500 nucleotides represents the putative full-length MEL1 mRNA, and the short fragments of about 360 (about nt. 120) and about 90 (about nt. 410) nucleotides possibly represent the shorter transcripts designated MEL1S, the 5′ ends of which were in exon 2 (Figure 1A-B). To confirm the results, the 5′ region of the MEL1 cDNA was synthesized with a 5′-phosphorylated sequence-specific primer 5pMrtp at nt. 543 to 566 and AMV reverse transcriptase according to the manufacturer's instructions. For 5′-RACE, the synthesized cDNA mixture was self-ligated and amplified by the first-round primers and then nested second primers as described in “Materials and methods.” As shown in Figure 1C, 450- and 200-bp cDNA bands were amplified, and 15 independent cDNA clones were subcloned and sequenced. Based on the sequence analysis, 3 kinds of cDNA fragments were isolated by 5′-RACE. The 5 longest clones started from nt. –176(G) before putative exon 1, 3 clones from nt. 90 (G) and 7 clones from nt. 333 (G) in exon 2 (Figure 1D). Therefore, it is suggested that shorter transcripts from exon 2 were expressed mainly in the t(1;3)-positive leukemia cells and that clones N1163 and N2094 may represent the short form MEL1S. Thus, a putative full-length MEL1 cDNA (pSKMEL1) and the shortform MEL1S cDNA (pSKMEL1S) covering nt. 1 to 3948 and nt. 283 to 3948, respectively, were used for the subsequent experiments.
Expression of multiple forms of MEL1 gene products. (A) Schematic representation of the amino-terminus of MEL1, RNase protection, and primer extension mapping with 5′-rapid amplification of cDNA ends (5′-RACE) analysis. Part of the human MEL1 genomic structure is schematically shown as a bar with the predicted domain structure as indicated. The nucleotide and exon numbers, the position of 5 in-frame ATG sites, the Bgl II restriction site, and the PR domain are indicated. From the RNase protection assay (indicated as S-1), 3 transcription initiation sites were identified and are indicated by asterisks, as in panel B. From the primer extension and 5′-RACE (indicated as 5′-RACE), 3 transcription initiation sites with nucleotide numbers were identified (arrowheads). The lower 3 lanes show the 5′ end of cDNA clones (N207, N1163/N2094, and Δ13MEL1, respectively) with exon structures and the position of ATG (indicated as M). (B) RNase protection assay. An antisense MEL1 RNA probe was generated and radiolabeled with T3 RNA polymerase using the Bgl II-linearized plasmid template SK207. The RNA probe was hybridized with 30 μg yeast tRNA (lane 3), or total RNA from Kasumi-3 with t(3;7)(q26;q22) (lane 4), or t(1;3)(p36;q21)-positive leukemia cells (lane 5). The positions of major protected fragments or undigested probes (lane 6) are marked with stars and arrows, respectively. Lanes 1 and 2 are 100-bp and 1-kb ladder markers, respectively, and the numbers of base pairs are given as bp. (C) Detection of cDNA products by primer extension and 5′-RACE of MEL1. Two major bands, 450 and 200 bp, are shown in cDNA from poly(A)+ RNA from t(1;3)(p36;q21)-positive AML cells (lane 1), but no cDNA was amplified from control CD4+ lymphocytes (lane 2). The 100-bp ladder markers are presented in lane 3. (D) Nucleotide sequences of exons 1 and 2 with 5′ end of the MEL1 cDNA clones. Black flags indicate the 5′ end of 3 cDNA clones (N207, N1163, and N2094). White flags indicate the 5′ end of 5′-RACE cDNAs, including 5 clones from nt –176, 3 clones from nt 90, and 7 clones from nt 333.
Expression of multiple forms of MEL1 gene products. (A) Schematic representation of the amino-terminus of MEL1, RNase protection, and primer extension mapping with 5′-rapid amplification of cDNA ends (5′-RACE) analysis. Part of the human MEL1 genomic structure is schematically shown as a bar with the predicted domain structure as indicated. The nucleotide and exon numbers, the position of 5 in-frame ATG sites, the Bgl II restriction site, and the PR domain are indicated. From the RNase protection assay (indicated as S-1), 3 transcription initiation sites were identified and are indicated by asterisks, as in panel B. From the primer extension and 5′-RACE (indicated as 5′-RACE), 3 transcription initiation sites with nucleotide numbers were identified (arrowheads). The lower 3 lanes show the 5′ end of cDNA clones (N207, N1163/N2094, and Δ13MEL1, respectively) with exon structures and the position of ATG (indicated as M). (B) RNase protection assay. An antisense MEL1 RNA probe was generated and radiolabeled with T3 RNA polymerase using the Bgl II-linearized plasmid template SK207. The RNA probe was hybridized with 30 μg yeast tRNA (lane 3), or total RNA from Kasumi-3 with t(3;7)(q26;q22) (lane 4), or t(1;3)(p36;q21)-positive leukemia cells (lane 5). The positions of major protected fragments or undigested probes (lane 6) are marked with stars and arrows, respectively. Lanes 1 and 2 are 100-bp and 1-kb ladder markers, respectively, and the numbers of base pairs are given as bp. (C) Detection of cDNA products by primer extension and 5′-RACE of MEL1. Two major bands, 450 and 200 bp, are shown in cDNA from poly(A)+ RNA from t(1;3)(p36;q21)-positive AML cells (lane 1), but no cDNA was amplified from control CD4+ lymphocytes (lane 2). The 100-bp ladder markers are presented in lane 3. (D) Nucleotide sequences of exons 1 and 2 with 5′ end of the MEL1 cDNA clones. Black flags indicate the 5′ end of 3 cDNA clones (N207, N1163, and N2094). White flags indicate the 5′ end of 5′-RACE cDNAs, including 5 clones from nt –176, 3 clones from nt 90, and 7 clones from nt 333.
Identification of 2 translated products, MEL1 and MEL1S
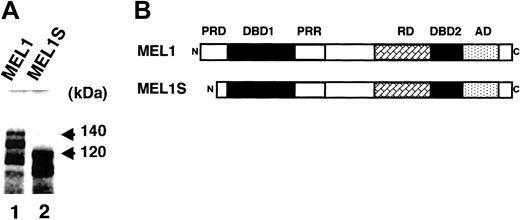
To investigate the molecular weights of the MEL1 and MEL1S gene products, 2 cDNA clones were transcribed and translated in vitro in reticulocyte lysates containing [35S]methionine. It was found that the gene product from pSKMEL1 cDNA migrated with a major 140-kDa band; however, the gene product from pSKMEL1S cDNA migrated with a major 120-kDa band (Figure 2A). Along with the main upper bands, lower-molecular-weight products were also seen in the gel. It is speculated that the lower bands were probably derived from immature terminations of translation, aberrant initiations at internal AUG, or proteolysis. Based on the MEL1 nucleotide sequences, 5 in-frame internal translation initiation codons (ATGs) were identified in the PR domain at the amino-terminus, namely ATG45, ATG105, ATG171, ATG354, and ATG597, as shown in Figure 1A. Within these putative initiation codons, the first ATG45 shows the best match to a Kozak consensus sequence: ACACC AUG C (CCRCC AUG G),38 and the second best match is ATG597: UCACC AUG U. Within 3 other ATG sites in exon 2, ATG105 (AUAAU AUG U), ATG171 (GUGCC AUG U), and ATG354 (GGAAG AUG G), ATG354 might be the major translation initiation site for mRNAs within exon 2, because A–3 and G+4 flanking the ATG contributes to a strong initiation site.39 The 20-kDa molecular weight difference between the 2 gene products (140- and 120-kDa bands) approximately corresponds to 190 amino acid residues (around 570 nucleotides). The MEL1 gene product from the first methionine ATG45 in exon 1 has 1257 amino acid residues with a deduced molecular weight of 133 567.89. The gene product from ATG354 in exon 2 has 1154 amino acid residues (127 019.63), and that from ATG597 has 1073 amino acid residues (118 285.96). Therefore, the results suggest that the 140-kDa band was probably derived from a gene product of the full-length MEL1, which starts from the first translation initiation ATG45. However, it could not be clearly determined whether the 120-kDa band was derived from the initiation at ATG354 (127 kDa) or ATG597 (118 kDa). If the MEL1S protein was initiated from either ATG354 or ATG597, MEL1S protein may be deleted of most of the amino-terminal portion of the PR domain (Figure 2B). Therefore, we performed the following experiments to confirm the translation initiation site of the 120-kDa band.
Structure of human MEL1 and the 2 gene products. (A) In vitro transcription and translation products of MEL1 and MEL1S. MEL1 and MEL1S cDNAs were transcribed and translated using an in vitro transcription and translation system with rabbit reticulocyte lysates. The products were fractionated on 10% SDS–polyacrylamide gel by electrophoresis. Molecular weights are given in kilodaltons. (B) Comparison of the predicted domain structures between MEL1 and MEL1S. PRD indicates PR domain; DBD1, DNA-binding domain 1; PRR, proline-rich domain; RD, repressor domain; DBD2, DNA-binding domain 2; AD, acidic domain.
Structure of human MEL1 and the 2 gene products. (A) In vitro transcription and translation products of MEL1 and MEL1S. MEL1 and MEL1S cDNAs were transcribed and translated using an in vitro transcription and translation system with rabbit reticulocyte lysates. The products were fractionated on 10% SDS–polyacrylamide gel by electrophoresis. Molecular weights are given in kilodaltons. (B) Comparison of the predicted domain structures between MEL1 and MEL1S. PRD indicates PR domain; DBD1, DNA-binding domain 1; PRR, proline-rich domain; RD, repressor domain; DBD2, DNA-binding domain 2; AD, acidic domain.
MEL1S gene products translated from ATG597 were expressed mainly in the t(1;3)(p36;q21)-positive AML cells
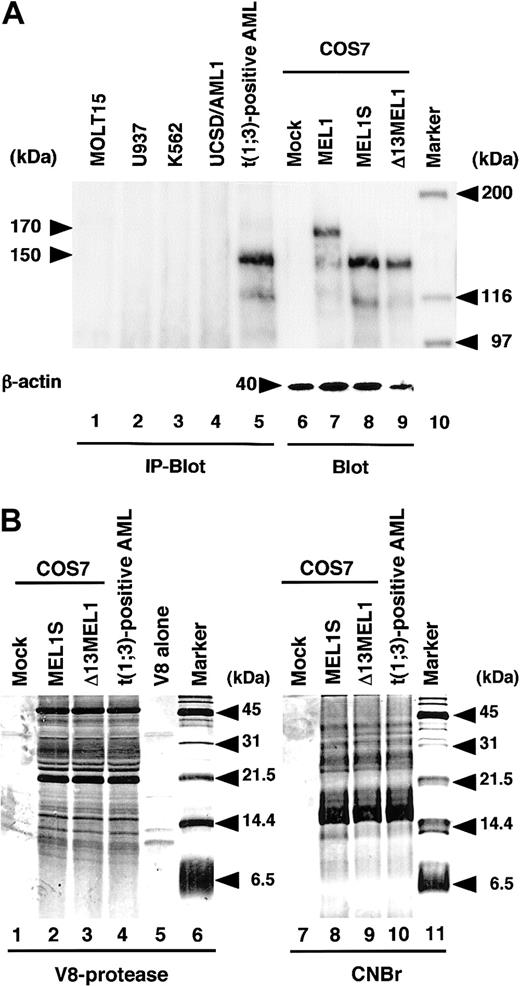
To identify MEL1 and MEL1S gene products in mammalian cells, cell extracts from t(1;3)(p36;q21)-positive or t(1;3)(p36;q21)-negative leukemia cells (MOLT15, U937, K562, and UCSD/AML1) were immunoprecipitated with rabbit anti-MELDBD1 antibody directed against a recombinant GST-MEL1 fusion protein as described in “Materials and methods.” MOLT15 was derived from a lymphocytic lineage, and U937, K562, and UCSD/AML1 were derived from myeloid lineages. The EVI1 transcripts were highly expressed in the t(3;3)(q21;q26)-positive leukemia cells (UCSD/AML1). As controls, COS7 cells transfected with a mock vector (pCMV), pCMV-MEL1, or pCMV-MEL1S were extracted. The immunoprecipitated products and the control samples were separated on SDS–polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting analysis using the anti-MELDBD1 antibody. As shown in Figure 3A, the protein product derived from MEL1 in COS7 cells comigrated with a major 170-kDa band (Figure 3A, lane 7), and protein products from MEL1S comigrated with a major 150-kDa band (Figure 3A, lane 8). A major protein product of 150 kDa was specifically recognized by the anti-MELDBD1 antibody in the cell extracts from t(1;3)-positive AML (Figure 3A, lane 5) but not from other cell lines (Figure 3A, lanes 1 to 4). To confirm whether the 150-kDa band in the leukemia cells is translated from ATG597 in exon 4, a cDNA fragment spanning MEL1 exon 4 to the end (nt. 483 to 3938) was amplified by PCR, which lacks the first 4 ATG sites (Figure 1A). The cDNA fragment (Δ13MEL1) inserted in the expression vector pCMV (pCMV-Δ13MEL1) was transfected into COS7 cells. As shown in Figure 3A, the same sized band of 150 kDa was detected in the lanes of MEL1S (Figure 3A, lane 8) and Δ13MEL1 (Figure 3A, lane 9). If MEL1S reflected translation from ATG354, the resulting protein should be approximately 9 kDa larger than the protein generated from the Δ13MEL1 construct. Therefore, it was suggested that the 150-kDa band is translated from ATG597. To confirm whether the 150-kDa protein in t(1;3)-positive leukemia cells is the same polypeptide as those of MEL1S and Δ13MEL1, the proteins were purified by immunoprecipiation and the bands excised from the SDS-PAGE gel were treated with V8 protease or cyanogen bromide (CNBr). After the separation of the partially digested polypeptides by SDS-PAGE, the gel was stained with silver staining reagent. As shown in Figure 3B, the cleavage patterns of the 3 polypeptides were almost identical after both treatments, suggesting that the 3 different proteins originate from the same sequence. Thus, these results demonstrated that the 150-kDa protein, MEL1S, was expressed mainly in the t(1;3)-positive leukemias and translated fromATG597. BecauseATG597 is in the PR domain, unlike EVI1, which lacks the entire PR domain, MEL1S retains the carboxyl-terminal portion of the PR domain. We have not examined whether MEL1 or MEL1S protein is expressed in healthy organs, but each mRNA was detected in the kidney by RT-PCR (data not shown). Also, we could detect MEL1S protein in another case of t(1;3)-positive leukemia (data not shown). It is noticeable that the molecular weights of MEL1 and MEL1S in vivo (170 kDa and 150 kDa, respectively) were slightly larger than those of in vitro–translated products (140 kDa and 120 kDa, respectively), presumably due to posttranslational modifications.
Expression of MEL1S in the t(1;3)(p36;q21)-positive leukemia cells. (A) Cell extracts from t(1;3)(p36;q21)-positive or -negative leukemia immunoprecipitated with anti-MELDBD1 antibody were separated by 6% SDS–polyacrylamide gel electrophoresis followed by immunoblotting with the anti-MELDBD1 antibody (IP-Blot). Also, extracts from COS7 cells with each CMV expression vector were used as controls for immunoblotting analysis (Blot). Lanes represent MOLT15 (lane 1); U937 (lane 2); K562 (lane 3); UCSD/AML1 (lane 4); the t(1;3)(p36;q21)-positive leukemia cells (lane 5); and COS7 cells with a mock vector (pCMV; lane 6), MEL1 (pCMV-MEL1; lane 7), or MEL1S (pCMV-MEL1S; lane 8); and Δ13MEL1 (pCMV-Δ13MEL1; lane 9). As an internal control for immunoblot, a 40-kDa band of β-actin was shown at the bottom (lanes 6-9). Molecular weight markers (lane 10) are given in kilodaltons. (B) SDS-PAGE of polypeptides derived from V8 protease digestion or CNBr cleavage of MEL1S, Δ13MEL1, and the 150-kDa band from t(1;3)-positive leukemia. The first 5 lanes are V8 protease–digested samples from COS7 cells transfected by a mock vector (lane 1), MEL1S (lane 2), or Δ13MEL1 (lane 3), the 150-kDa band from t(1;3)-positive leukemia cells (lane 4), and V8 protease alone (lane 5). The last 4 lanes are CNBr cleavage samples indicated at the top of each lane. Molecular weight markers (lanes 6 and 11) are given in kilodaltons.
Expression of MEL1S in the t(1;3)(p36;q21)-positive leukemia cells. (A) Cell extracts from t(1;3)(p36;q21)-positive or -negative leukemia immunoprecipitated with anti-MELDBD1 antibody were separated by 6% SDS–polyacrylamide gel electrophoresis followed by immunoblotting with the anti-MELDBD1 antibody (IP-Blot). Also, extracts from COS7 cells with each CMV expression vector were used as controls for immunoblotting analysis (Blot). Lanes represent MOLT15 (lane 1); U937 (lane 2); K562 (lane 3); UCSD/AML1 (lane 4); the t(1;3)(p36;q21)-positive leukemia cells (lane 5); and COS7 cells with a mock vector (pCMV; lane 6), MEL1 (pCMV-MEL1; lane 7), or MEL1S (pCMV-MEL1S; lane 8); and Δ13MEL1 (pCMV-Δ13MEL1; lane 9). As an internal control for immunoblot, a 40-kDa band of β-actin was shown at the bottom (lanes 6-9). Molecular weight markers (lane 10) are given in kilodaltons. (B) SDS-PAGE of polypeptides derived from V8 protease digestion or CNBr cleavage of MEL1S, Δ13MEL1, and the 150-kDa band from t(1;3)-positive leukemia. The first 5 lanes are V8 protease–digested samples from COS7 cells transfected by a mock vector (lane 1), MEL1S (lane 2), or Δ13MEL1 (lane 3), the 150-kDa band from t(1;3)-positive leukemia cells (lane 4), and V8 protease alone (lane 5). The last 4 lanes are CNBr cleavage samples indicated at the top of each lane. Molecular weight markers (lanes 6 and 11) are given in kilodaltons.
Consensus sequences of MEL1 are included within those of EVI1
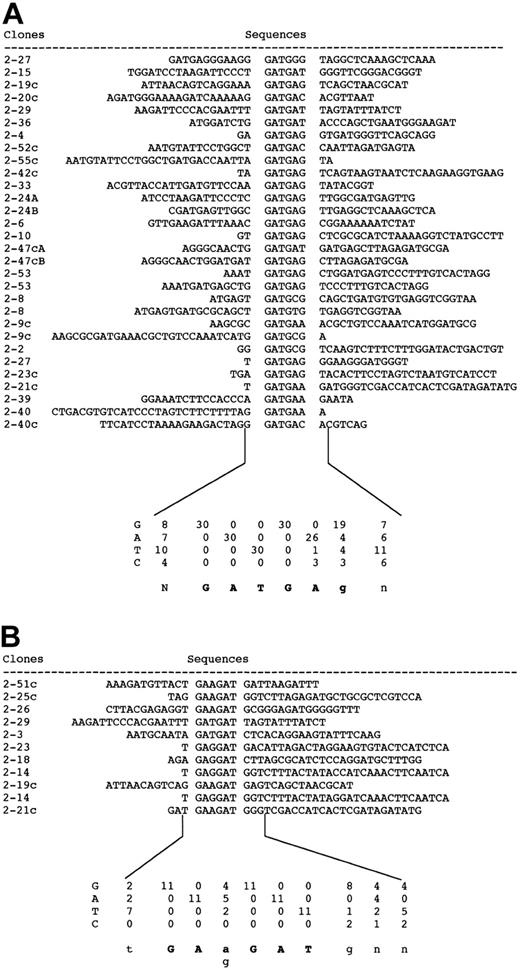
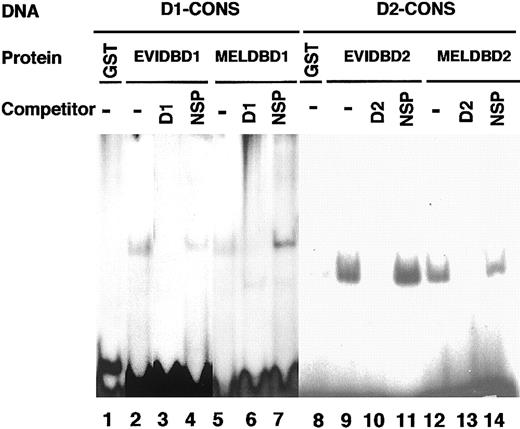
To identify the consensus sequences of MEL1 DNA-binding domain 1 and 2, a GST fusion protein with MEL1 DNA-binding domain 1 or 2 was used for binding and amplification reactions with random oligonucleotides as described in “Materials and methods.” Each DNA-binding domain of EVI1 or MEL1 was inserted into a 1 λ T vector to generate GST fusion proteins (EVIDBD1, EVIDBD2, MELDBD1, or MELDBD2) (data not shown). After 7 cycles, each of 30 and 41 independent selected targets was sequenced. As shown in Figures 4 and 5, the consensus sequence of DNA-binding domain 1 is AGAT/cAAGATAaga (Figure 4) and that of domain 2 is GATGAg from 30 clones (Figure 5A) or GAa/gGATg from 11 clones (Figure 5B). By comparison between consensus sequences of EVI1 and MEL1, the consensus sequence for domain 1 of EVI1 (GAC/TAAGAT/CAAGATAA) covered that of MEL1 (AGAT/cAAGATAaga). The consensus sequence for domain 2 of EVI1 (GAGGATGAA) also covered that of MEL1 (GATGAg or GAa/gGATg). To confirm the results of consensus sequences, D1-CONS (GACAAGATAAGATAA) and D2-CONS (GAAGATGAG) of EVI1 were used for EMSA to detect the specific DNA-binding activity of MEL1. End-labeled D1-CONS and D2-CONS were used as DNA probes. As shown in Figure 6B, both EVIDBD1 and MELDBD1 could bind to D1-CONS (Figure 6B, lanes 2 and 5). Both EVIDBD2 and MELDBD2 also bound to D2-CONS (Figure 6B, lanes 9 and 12). The sequence specificity of DNA binding was examined by adding an unlabeled excess amount of cold D1- or D2-CONS to compete for binding. Binding of the radiolabeled D1- or D2-CONS oligonucleotide to each GST fusion protein was not interfered with by addition of an excess amount of random oligonucleotides (Figure 6B, lanes 4, 7, 11, and 14), while cold D1- or D2-CONS well competed the binding (Figure 6B, lanes 3, 6, 10, and 13). These results indicated that MEL1 or MEL1S protein retains the capability to bind to the same consensus sequences as EVI1 in vitro.
Aligned nucleotide sequences of PCR clones selected with MEL1-DNA binding domain 1. Positions of the consensus sequence in each clone are in the middle of the sequence with a blank on each side. The numbers under the sequence represent numbers of each nucleotide at each position in the aligned sequences. The consensus sequence is shown at the bottom of the numbers.
Aligned nucleotide sequences of PCR clones selected with MEL1-DNA binding domain 1. Positions of the consensus sequence in each clone are in the middle of the sequence with a blank on each side. The numbers under the sequence represent numbers of each nucleotide at each position in the aligned sequences. The consensus sequence is shown at the bottom of the numbers.
Aligned nucleotide sequences of PCR clones selected with MEL1-DNA binding domain 2. Clones that contained D2-CONS are divided into 2 groups. Group A showed the consensus sequence GATGAg and group B showed GAa/gGAT.
Aligned nucleotide sequences of PCR clones selected with MEL1-DNA binding domain 2. Clones that contained D2-CONS are divided into 2 groups. Group A showed the consensus sequence GATGAg and group B showed GAa/gGAT.
DNA-binding activities of GST-fused DNA-binding domain 1 and 2 of MEL1 against DNA-binding consensus sequences. DNA-binding activities of each GST fusion protein were detected by electrophoretic mobility shift assay (EMSA). End-labeled consensus sequences of DNA-binding domain 1 (D1-CONS) and DNA-binding domain 2 (D2-CONS) were used for EMSA; 100 ng of each purified GST fusion protein was incubated with 32P-labeled D1- or D2-CONS oligonucleotides. 32P-labeled D1-CONS was incubated with control GST (lane 1), EVI1DBD1 (lane 2), or MEL1DBD1 (lane 5) and 32P-labeled D2-CONS was incubated with control GST (lane 8), EVI1DBD2 (lane 9), or MEL1DBD2 (lane 12). For the competition assay, a 100-times higher concentration of double-stranded D1-CONS (D1; lanes 3 and 6) or D2-CONS (D2; lanes 10 and 13) or else the same amount of double-stranded random oligonucleotides (NSP; lanes 4, 7, 11, and 14) was incubated in each reaction.
DNA-binding activities of GST-fused DNA-binding domain 1 and 2 of MEL1 against DNA-binding consensus sequences. DNA-binding activities of each GST fusion protein were detected by electrophoretic mobility shift assay (EMSA). End-labeled consensus sequences of DNA-binding domain 1 (D1-CONS) and DNA-binding domain 2 (D2-CONS) were used for EMSA; 100 ng of each purified GST fusion protein was incubated with 32P-labeled D1- or D2-CONS oligonucleotides. 32P-labeled D1-CONS was incubated with control GST (lane 1), EVI1DBD1 (lane 2), or MEL1DBD1 (lane 5) and 32P-labeled D2-CONS was incubated with control GST (lane 8), EVI1DBD2 (lane 9), or MEL1DBD2 (lane 12). For the competition assay, a 100-times higher concentration of double-stranded D1-CONS (D1; lanes 3 and 6) or D2-CONS (D2; lanes 10 and 13) or else the same amount of double-stranded random oligonucleotides (NSP; lanes 4, 7, 11, and 14) was incubated in each reaction.
Weak transcriptional activation of MEL1S protein against D2-CONS
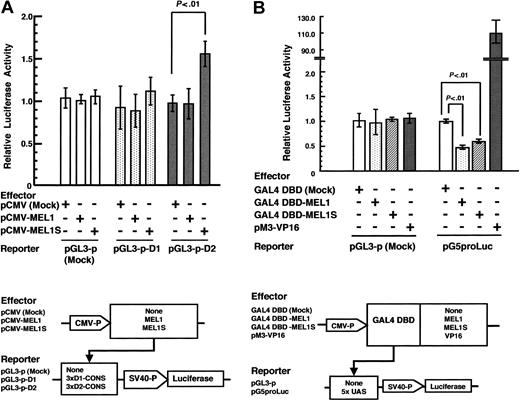
To investigate the transcriptional regulatory functions of the human MEL1 and MEL1S gene products, we performed transient reporter gene assays using the consensus sequences of MEL1 combined with the firefly luciferase gene as a reporter. Three tandem repeats of D1-CONS or D2-CONS were inserted upstream of the SV40 promoter with the luciferase gene (pGL3-p-D1 or pGL3-p-D2). These reporter plasmids were cotransfected with a mock expression vector (pCMV), MEL1 (pCMV-MEL1), or MEL1S (pCMV-MEL1S) into murine teratocarcinoma P19 cells. As shown in Figure 7A, MEL1 showed neither transcriptional activation nor repression of luciferase reporter plasmids with D1-CONS or D2-CONS. To the contrary, MEL1S showed around a 1.5-fold increase in transcriptional activation against the reporter plasmid with D2-CONS (P< .01) but not with D1-CONS. The same result was obtained using the SV40 promoter with enhancer vector (pGL3-control), and MEL1S activated both reporters in a dose-dependent manner (data not shown). Thus, these results indicated that MEL1S protein could weakly enhance transcription, which is dependent on DNA binding to D2-CONS. Based on the data, it is speculated that MEL1S could have different conformational states when it binds to either D1-COS or D2-CONS.
Transcription activity of MEL1 and MEL1S. (A) Transactivation of the luciferase gene via binding of MEL1 and MEL1S proteins to DNA consensus sequences. Transient transcriptional activation of the luciferase gene was measured with dual-luciferase reporter assay system. pGL3-promoter vector containing D1-CONS or D2-CONS was cotransfected into P19 cells along with either mock expression vector (pCMV), pCMV-MEL1, or pCMV-MEL1S and Renilla luciferase expression vector (pRL-TK). Relative firefly luciferase activity was measured in cell extracts and normalized with respect to Renilla luciferase activity. Each value represents the average obtained from 3 independent experiments. Bars indicate standard deviation errors. (B) Repressor activities of GAL4 DBD-fused MEL1 and MEL1S. P19 cells were cotransfected with the luciferase reporter vector of pGL3-p or pG5proLuc with 5 × the consensus GAL4 binding site (UAS) and with the mock expression vector (GAL4 DBD), GAL4 DBD-MEL1, GAL4 DBD-MEL1S, or positive control vector (pM3-VP16), respectively. After 48 hours of incubation, the cell extracts were analyzed for firefly luciferase activity, which was normalized using Renilla luciferase activity according to the manufacturer's instructions. Values of relative luciferase activity and error bars represent the means and standard deviations, respectively, of 3 separate experiments.
Transcription activity of MEL1 and MEL1S. (A) Transactivation of the luciferase gene via binding of MEL1 and MEL1S proteins to DNA consensus sequences. Transient transcriptional activation of the luciferase gene was measured with dual-luciferase reporter assay system. pGL3-promoter vector containing D1-CONS or D2-CONS was cotransfected into P19 cells along with either mock expression vector (pCMV), pCMV-MEL1, or pCMV-MEL1S and Renilla luciferase expression vector (pRL-TK). Relative firefly luciferase activity was measured in cell extracts and normalized with respect to Renilla luciferase activity. Each value represents the average obtained from 3 independent experiments. Bars indicate standard deviation errors. (B) Repressor activities of GAL4 DBD-fused MEL1 and MEL1S. P19 cells were cotransfected with the luciferase reporter vector of pGL3-p or pG5proLuc with 5 × the consensus GAL4 binding site (UAS) and with the mock expression vector (GAL4 DBD), GAL4 DBD-MEL1, GAL4 DBD-MEL1S, or positive control vector (pM3-VP16), respectively. After 48 hours of incubation, the cell extracts were analyzed for firefly luciferase activity, which was normalized using Renilla luciferase activity according to the manufacturer's instructions. Values of relative luciferase activity and error bars represent the means and standard deviations, respectively, of 3 separate experiments.
Transcriptional repression by GAL4-MEL1 and -MEL1S fusion proteins
We have reported that EVI1 is a weak transcriptional activator via DNA-binding domain 2.14 However, GAL4-EVI1 fusion protein has been reported to show transcriptional repression via the GAL4 DNA-binding capacity.40 To determine whether MEL1 or MEL1S has repressive activity via the GAL4 DNA-binding domain (DBD), we constructed a GAL4 DBD fusion protein with MEL1 or MEL1S (GAL4 DBD-MEL1 or -MEL1S). As reporters, the GAL4 DNA-binding sites (UAS) were inserted into the pGL3-p plasmid under the SV40 promoter with the luciferase gene (pG5proLuc). These effector and reporter plasmids were cotransfected into murine teratocarcinoma P19 cells; the luciferase activity was measured 48 hours after transfection. Both MEL1 and MEL1S suppressed about 50% of luciferase activity (Figure 7B) but neither activated nor repressed as compared with pGL3-p mock vector without the UAS binding site (pGL3-p). Therefore, these results indicated that both GAL4 DBD-MEL1 and -MEL1S suppressed transcription, dependent on the GAL4 DBD. Because MEL1 and MEL1S showed almost the same repressive activity, it is suggested that the PR domain of MEL1 has almost no effect on the transcriptional suppression by MEL1.
Forced expression of MEL1S in L-G3 cells blocks granulocyte differentiation induced by G-CSF
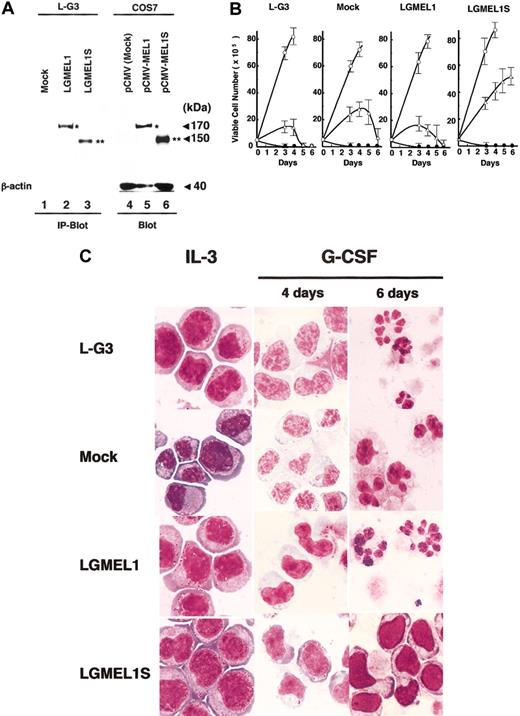
We here show that the full-length human MEL1 and the short-form MEL1S have almost the same DNA-binding and transcriptional activities. To investigate which type of gene product is related to the leukemogenesis, we examined whether forced expression of MEL1 or MEL1S affects the growth, differentiation, and apoptosis of IL-3–dependent myeloid cells. Retrovirus harboring Flag-tagged MEL1 (pLXSN-NFLAG-MEL1), MEL1S (pLXSN-NFLAG-MEL1S), or a mock vector (pLXSN) was introduced into murine L-G3 cells, which are completely dependent on IL-3 for their growth and have the ability to differentiate into granulocytes when treated with G-CSF.32 After infection and selection using G-418, individual G418-resistant clones expressing MEL1 or MEL1S (LGMEL1 or LGMEL1S) or no expression (mock) were established. Cell extracts after immunoprecipitation with anti-Flag M2 affinity gel were subjected to SDS–polyacrylamide gel electrophoresis followed by immunoblotting analysis with the anti-MELDBD1 antibody. Also, the extracts from COS7 cells transfected with a mock vector (pCMV), pCMV-MEL1, or pCMV-MEL1S were used as controls for the immunoblotting analysis. As shown in Figure 8A, a 170-kDa band was detected in the clone LGMEL1 and pCMV-MEL1–transfected COS7 cells (Figure 8A, lanes 2 and 5). A 150-kDa band was detected in the clone LGMEL1S and pCMV-MEL1S–transfected COS7 cells (Figure 8A, lanes 3 and 6). These stable transformants were used for further biologic assays. In parental L-G3 cells, intrinsic murine mel1 and/or mel1S expression levels were below detectable limits using the antibody against human MEL1 (Figure 8A, lane 1). To confirm that there is no expression of the murine mel1 gene in L-G3 cells, the murine mel1 cDNA clones were isolated by RT-PCR from murine kidney mRNA (sequence accession no. AB078338). The nucleotide sequence of murine mel1 cDNA showed 85% identity to that of the human MEL1 gene, and the amino acid sequence of murine mel1 showed 86% identity to that of human MEL1. According to RT-PCR analysis, no murine mel1 cDNA bands were present in the mRNA from L-G3 cells (data not shown).
Forced expression of MEL1S blocks G-CSF–induced granulocytic differentiation. (A) Expression of MEL1 and MEL1S in retroviral infected L-G3 cell lines. Cell extracts from L-G3 cells with retrovirus containing Flag-tagged MEL1 or MEL1S were immunoprecipitated using anti-Flag M2 affinity gel followed by immunoblotting as described in “Materials and methods” (IP-Blot). Also, the extracts from COS7 cells with each CMV expression vector were used as controls for the immunoblotting analysis (Blot). Lanes 1, 2, and 3 represent L-G3 cells infected with a mock retrovirus (pLXSN), FLAG-tagged MEL1, or MEL1S, respectively. Lanes 4, 5, and 6 indicate control COS7 cells transfected with a mock vector (pCMV, lane 4), pCMV-MEL1 (lane 5), or pCMV-MEL1S (lane 6). As an internal control for immunoblot, a 40-kDa band of β-actin was shown at the bottom (lanes 4-6). Molecular weights are given in kilodaltons. (B) Growth curve of L-G3 cells infected by retrovirus with MEL1 or MEL1S. The indicated cells in the figures were subcloned by G418 selection after infection with the retrovirus containing FLAG-tagged MEL1 (LGMEL1 in the figure) or MEL1S (LGMEL1S). The mock construct (Mock) was used as a vector control. L-G3 cells (L-G3) were derived from parental cells as a control. Viable cells were counted by the trypan blue exclusion method at each time point. ○ or • indicates the culture conditions using medium with or without IL-3, respectively, and ▵ indicates medium with G-CSF. The error bars represent the standard deviations of 3 independent experiments. (C) Parental and infected L-G3 cells at IL-3, 4 or 6 days after the addition of G-CSF. The cells were stained with May-Grünwald-Giemsa solution. The parental L-G3, mock, and LGMEL1 cells show a change in the nuclear-cytoplasmic ratio and nuclear indentations signifying differentiation in response to G-CSF. In comparison, LGMEL1S cells expanded in the presence of G-CSF with an immature nuclear morphology. Original magnification, × 100.
Forced expression of MEL1S blocks G-CSF–induced granulocytic differentiation. (A) Expression of MEL1 and MEL1S in retroviral infected L-G3 cell lines. Cell extracts from L-G3 cells with retrovirus containing Flag-tagged MEL1 or MEL1S were immunoprecipitated using anti-Flag M2 affinity gel followed by immunoblotting as described in “Materials and methods” (IP-Blot). Also, the extracts from COS7 cells with each CMV expression vector were used as controls for the immunoblotting analysis (Blot). Lanes 1, 2, and 3 represent L-G3 cells infected with a mock retrovirus (pLXSN), FLAG-tagged MEL1, or MEL1S, respectively. Lanes 4, 5, and 6 indicate control COS7 cells transfected with a mock vector (pCMV, lane 4), pCMV-MEL1 (lane 5), or pCMV-MEL1S (lane 6). As an internal control for immunoblot, a 40-kDa band of β-actin was shown at the bottom (lanes 4-6). Molecular weights are given in kilodaltons. (B) Growth curve of L-G3 cells infected by retrovirus with MEL1 or MEL1S. The indicated cells in the figures were subcloned by G418 selection after infection with the retrovirus containing FLAG-tagged MEL1 (LGMEL1 in the figure) or MEL1S (LGMEL1S). The mock construct (Mock) was used as a vector control. L-G3 cells (L-G3) were derived from parental cells as a control. Viable cells were counted by the trypan blue exclusion method at each time point. ○ or • indicates the culture conditions using medium with or without IL-3, respectively, and ▵ indicates medium with G-CSF. The error bars represent the standard deviations of 3 independent experiments. (C) Parental and infected L-G3 cells at IL-3, 4 or 6 days after the addition of G-CSF. The cells were stained with May-Grünwald-Giemsa solution. The parental L-G3, mock, and LGMEL1 cells show a change in the nuclear-cytoplasmic ratio and nuclear indentations signifying differentiation in response to G-CSF. In comparison, LGMEL1S cells expanded in the presence of G-CSF with an immature nuclear morphology. Original magnification, × 100.
All clones proliferated in the presence of mIL-3 and died in the culture medium deprived of mIL-3 within 3 days. When cultured with G-CSF instead of mIL-3, parental L-G3 cells, the mock clone, and the LGMEL1 cells remained viable for 3 and 4 days and died within 6 days. In contrast, the LGMEL1S clone proliferated in the presence of G-CSF (Figure 8B). Moreover, the parental L-G3, mock, and LGMEL1 cells showed a change in the nuclear-cytoplasmic ratio and nuclear indentations signifying differentiation in response to G-CSF, but the LGMEL1S clone remained immature with myeloid cells stained by May-Grünwald-Giemsa solution (Figure 8C). These observations suggested that overexpression of MEL1S blocks the granulocytic differentiation of L-G3 myeloid cells induced by G-CSF. Interestingly, EVI1 lacking the PR domain has been reported to show the same blocking effect on the G-CSF–induced differentiation in myeloid cells.22 Thus, it is likely that MEL1S contributes mainly to the leukemogenesis in the myeloid cells and that both EVI1 and MEL1S lacking the PR domain share a common molecular mechanism underlying the leukemogenic transformation.
Discussion
In this paper, we showed that the human MEL1 gene generates a full-length protein product of 170 kDa and an alternative shortform product of 150 kDa, designated MEL1 and MEL1S, respectively, and the short form is expressed mainly in t(1;3)(p36;q21)-positive leukemia cells. Because MEL1 is part of the EVI1 gene family and also a PR domain member, we investigated whether the 150-kDa band might be derived from an amino-terminal–truncated version of a MEL1 gene product lacking the PR domain. Based on this study, the MEL1S gene product is a zinc finger protein mostly lacking a PR domain at the amino-terminus of MEL1 (Figure 2B). Because MEL1S has the same structure as the PR(–) product EVI1 expressed in leukemia with 3q26 abnormalities (3q21q26 syndrome), it is probable that overexpression of the zinc finger protein lacking the PR domain (EVI1 and MEL1S) in the leukemia cells is one of the causes in the pathogenesis of myeloid leukemia.
It was reported by Soderholm et al that the PR(+) product MDS1/EVI1 acted as a strong transcriptional activator when used with a genomic fragment containing GATA repeats in the promoter region of the indicator plasmid.15 However, it was also reported that MDS1/EVI1 fused with GAL4 DNA-binding domain (DBD) presented repressor activity.16 When the consensus sequences of EVI1 were used for the reporter gene assay, MEL1 did not show any transcriptional activity. However, GAL4 DBD-MEL1 suppressed transcription of the GAL4 indicator plasmid in the same manner as GAL4 DBD-MEL1S. On the other hand, the PR(–) product MEL1S weakly activated the transcription of the indicator plasmid via D2-CONS, but the PR(+) product MEL1 did not show any activity in the reporter gene assay with D2-CONS. In addition, because the suppression of GAL4 DBD-MEL1 is usually around 10% more than that of GAL4 DBD-MEL1S, it is possible that the PR domain in MEL1 works as a suppressive domain. In terms of biologic functions, overexpression of MEL1S could block the differentiation of L-G3 cells induced by G-CSF, but MEL1 could not. On the other hand, it is reported that overexpression of EVI1 blocks granulocytic differentiation of myeloid cells induced by G-CSF,22,41 but MDS1/EVI1 has no effect.41 Therefore, the PR domain in MEL1 or MDS1/EVI1 may regulate its own biologic effect of MEL1S or EVI1. In addition, it was reported that the PR(+) product RIZ1 works as a tumor suppressor gene in RIZ1-deficient mice, and many genetic frameshift mutations were found in the RIZ1 genomic DNA in human solid tumors.5 Accordingly, it is likely that the PR domain is one of the most important domains suppressive for tumorigenesis. Recently, it was reported that the human SET domain–containing gene, SUV39H1, functions as a histone H3 methyltransferase and is important in chromatin condensation during mitosis.42 Moreover, 2 members of the SET/PR superfamily, NSD1 (nuclear receptor-binding SET domain–containing protein) and RIZ, can also function as coactivators or corepressors of nuclear hormone receptors,43,44 suggesting that the SET/PR domain may function as a novel class of transcriptional coregulator. If the PR domain blocks the ability of EVI1 family members to contribute to leukemia, it will be important for future investigations to assess the mechanisms through which the PR domain has this effect.
MDS1/EVI1 and MEL1 with highly homologous amino acid sequences in DNA-binding domains showed the capacity to bind the same consensus sequences. In functional assays, EVI1 and MEL1S were indistinguishable. Nevertheless, differential expression patterns in organs may be reflected in their activities. For example, GATA proteins are members of a zinc finger subfamily of DNA-binding proteins that recognize the same consensus motif (T/A)GATA(A/G).45 Six GATA family members have been identified in vertebrates, and each GATA protein is expressed in different organs or different stages of fetal development.45 The question of how the different GATA proteins are able to function differentially in activating individual genes also arises in the cases of EVI1 and MEL1S. To answer this question, further investigation of the expression patterns in various adult or fetal organs by in situ hybridization, the DNA-binding selectivity of the finger regions, and potential for transcriptional activation are necessary. It is also important to generate gene-targeted mice to elucidate the intrinsic functions of MEL1. Notably, a comparison of mice that lack both MEL1 and MEL1S with mice that lack only MEL1 may illuminate the intrinsic functions of both proteins. This information will help us resolve how EVI1 or MEL1S is involved in transformation in hematopoietic cells.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2002-12-3944.
Supported by Grants-in-Aid for Scientific Research of Priority Area and for 21st Century COE program (Life Science) from the Ministry of Education, Culture, Sports, Science and Technology in Japan; TAKEDA Science Foundation; and NAITO Foundation (K.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr T. Uetsuki (Osaka University) for helpful suggestions, observations, and a critical reading of the manuscript and Dr K. Hatakeyama (Miyazaki Medical College) for his technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal