Abstract
To elucidate the role of CD4+CD25+ regulatory T cells in oral tolerance, we used the model of contact hypersensitivity (CHS) to 2,4-dinitrofluorobenzene (DNFB), which is mediated by CD8+ Tc1 effector cells independently of CD4+ T-cell help. Conversely to normal mice, invariant chain knock-out (KO) (Ii°/°) mice, which are deficient in CD4+ T cells, cannot be orally tolerized and develop a chronic hapten-specific CHS response. Transfer of naive CD4+ T cells before hapten gavage into Ii°/° mice restores oral tolerance by a mechanism independent of interleukin-10 (IL-10) production by CD4+ T cells. That naturally occurring CD4+CD25+ T cells are critical for oral tolerance induction is demonstrated by the finding that (1) transfer of CD4+CD25+ but not CD4+CD25– T cells into Ii°/° recipients completely prevents the CHS response and skin infiltration by CD8+ T cells, by blocking development of hapten-specific CD8+ T cells; (2) in vivo depletion of CD4+CD25+ cells by antibody treatment in normal mice impairs oral tolerance; and (3) CD4+CD25+ T cells inhibit hapten-specific CD8+ T-cell proliferation and interferon γ (IFNγ) production, in vitro. These data show that naturally occurring CD4+CD25+ T cells are instrumental for orally induced tolerance and are key actors for the control of antigen-specific CD8+ T-cell effectors mediating skin inflammation.
Introduction
Oral tolerance has long been recognized as a physiologic mechanism of immune unresponsiveness to dietary antigens and bacterial microflora antigens, which maintain tissue integrity by preventing harmful delayed-type hypersensitivity (DTH) responses in the intestine and may also limit the efficiency of oral vaccination. Indeed, antigen encounter in the intestine triggers an active inhibitory process preventing the onset of CD4+ and CD8+ T-cell antigen-specific immune responses to subsequent systemic immunization with the same antigen (reviewed in Garside and Mowat1 ). Several mechanisms have been proposed to explain peripheral tolerance induced by antigen feeding. These include anergy2,3 or deletion of antigen-specific T cells,4,5 immune deviation to Th2-biased immune response, and induction of regulatory Th3 (transforming growth factor β [TGFβ]–producing) cells.6,7
The naturally occurring regulatory subset of CD4+CD25+ T cells accounting for 5% to 10% of peripheral CD4+ T cells has been extensively reported to exert potent immunosuppressive function in vivo and in vitro toward CD4+ T-cell effectors8 and may represent regulatory T cells responsible for orally induced peripheral tolerance. Indeed, CD4+CD25+ T cells, which arise from the thymus as early as day 3 of life,9 are characterized by a memory phenotype; low proliferative capacity and interleukin-2 (IL-2) production; secretion of high levels of the immunosuppressive cytokines IL-10 and TGF-β; and expression of cytotoxic T-lymphocyte antigen 4 (CTLA-4),10-13 a molecule that contributes to orally induced tolerance.14 These cells have been described in a variety of experimental models to protect from autoimmune diseases, as well as colitis and allograft rejection.8 Reminiscent to these cells, IL-10–producing ovalbumin (OVA)–specific CD4+ T clones (Tr1) generated in vitro after repeated stimulation with antigen in the presence of IL-10 were shown to prevent colitis when cotransferred with naive CD4+CD45RBhigh T cells in OVAfed immunocompromised severe combine immunodeficiency (SCID) or Nude mice.15 Interestingly, mice genetically deficient for either IL-2, IL-2R, T-cell receptor αβ (TcRαβ), TGFβ, IL-10, or major histocompatibility complex (MHC) class II were shown to develop spontaneous colitis,16-20 compatible with a shared physical or functional defect in the regulatory CD4+CD25+ subset. Although recent studies in TcR transgenic models have reported that oral antigen delivery can induce activation and/or differentiation of regulatory CD4+CD25+ T cells,21,22 evidence that they are instrumental for in vivo induction of oral tolerance has not been provided. Moreover, evidence as to whether CD4+CD25+ cells are responsible for peripheral suppression of antigen-specific CD8+ cytotoxic T-lymphocyte (CTL) responses is still sparse.23
In this study we examined whether CD4+CD25+ T cells contribute to oral tolerance in normal nonlymphopenic hosts, using a pathophysiologic model of antigen-specific skin inflammation mediated by CD8+ CTL effectors. Contact hypersensitivity (CHS) to the hapten 2,4-dinitrofluorobenzene (DNFB) provides a unique model to address this issue because (1) skin inflammation generated upon skin challenge with the DNFB in sensitized mice is mediated by specific MHC class I–restricted interferon γ (IFNγ)–producing CD8+ CTL effectors, independently of CD4+ T-cell help24-28 ; (2) feeding mice once with the hapten prior to skin sensitization completely abrogates the CHS response by blocking development of specific IFNγ-producing CD8+ CTLs29,30 ; and (3) oral tolerance cannot be induced in mice deficient in CD4+ T cells. We now show that CD4+CD25+ T cells are mandatory for orally induced tolerance and block in vivo development of hapten-specific CD8+ T-cell–mediating skin inflammation.
Materials and methods
Mice
All mice were used at 6 to 10 weeks of age and were on a C57BL/6 background (H-2b). Female C57BL/6 mice were purchased from Charles Rivers Laboratories (l'Arbresle, France). MHC class II (Aβ°/°)31 and invariant chain (Ii°/°)32 –deficient mice were kindly provided by D. Mathis and C. Benoist. IL-10–deficient mice were obtained from Dr W. Mueller (Institute for Genetics, University of Cologne, Germany).20 All mice were bred as homozygotes in Charles Rivers Laboratories.
Contact sensitivity assay
CHS to DNFB was determined by the mouse ear-swelling test.33 Briefly, mice were sensitized epicutaneously on day 0 by application of 25 μL of 0.5% 2,4-dinitrofluorobenzene (DNFB; Sigma, St Quentin Fallavier, France) diluted in acetone–olive oil (4:1, vol/vol) onto 2 cm2 of shaved abdominal skin. Mice were challenged on day 5 with 4 μL of a nonirritant concentration of 0.25% DNFB applied onto each side of the right ear. The left ear received the vehicle alone. Ear thickness was measured using a caliper (J15 Blet; Lyon, France) before and at various times after challenge. The ear swelling (micrometers) was calculated as (T–T0 of the right ear) – (T–T0 of the left ear), where T0 and T represent the values of ear thickness before and after the challenge, respectively. Ear swelling in unsensitized but ear-challenged mice was usually less than 20 μm. Statistical significance was calculated by the Mann-Whitney-Wilcoxon ranking test.
Induction of oral tolerance
Mice were orally tolerized by a single intragastric administration of 300 μL of either 0.1% DNFB in acetone–olive oil (1:10, vol/vol) or vehicle alone (as control), 7 days before sensitization with DNFB (day –7), as previously described.29 For adoptive transfer experiments, nonfractionated CD4+ T cells or purified CD4+ T-cell subsets were transferred intravenously into Ii°/° or Aβ°/° recipient mice, 16 hours before feeding (day –8). For depletion experiments, mice were injected with either a control rat monoclonal antibody (mAb) or a depleting anti-CD25 mAb (clone PC61) on days –10, –7, –3, and 0.
Immunohistochemical staining of CD8+ T cells
Cryostat sections of the ears were incubated for one hour with anti-CD8 rat mAb (clone 53-6.7 from Pharmingen, San Diego, CA) or an irrelevant rat mAb as control, followed by a biotinylated mouse adsorbed goat anti–rat immunoglobulin G (IgG) Ab. Specific binding was revealed with a streptavidin-peroxidase kit (Dako, Glostrup, Denmark) and AEC (3-amino-9-ethylcarbazole) as previously described.30 Sections were counterstained with hematoxylin.
Purification of T-cell subsets
Spleens and lymph nodes (mesenteric, inguinal, and axillary) were used to prepare single cell suspensions. For most experiments, CD4+ and CD8+ T cells were isolated by positive selection using anti-CD4– or anti-CD8–coated microbeads and selection columns (Miltenyi Biotec, Bergish Gladbach, Germany). Purity was routinely more than 95%. For certain experiments, CD4+ and CD8+ T cells were enriched by negative selection using columns coated with a goat anti–mouse Ig, a goat anti–rat IgG, and a rat antimouse CD4 or CD8 mAbs (Biotex, Edmonton, AB, Canada). In this case, a purity of more than 80% was routinely obtained. CD4– cells were purified using LD depletion columns (Miltenyi Biotec).
For isolation of CD4+ T-cell subsets, CD4+ T cells were first enriched from spleen, mesenteric lymph nodes (MLNs), and peripheral lymph node cell suspensions by negative selection using anti–MHC class II, anti-CD11b, and anti-CD8 mAbs and magnetic beads. Enriched CD4+ T cells were then incubated with biotin-conjugated anti-CD25 mAb (7D4) and PE–anti-CD4 followed by fluorescein isothiocyanate (FITC)–conjugated streptavidin. CD4+CD25+ and CD4+CD25– cells were then purified by flow cytometry using a FACStar cell sorter (BD Biosciences, San Jose, CA). Alternatively, for most in vivo transfer experiments, cell suspensions were sequentially incubated with biotin-conjugated anti-CD25 mAb (7D4) (15μg/108 cells) (Pharmingen), FITC-conjugated streptavidin, and anti-FITC microbeads (Miltenyi Biotec). CD4+CD25+ cells were isolated by 2 runs on LS selection columns and were routinely more than 90% pure. For isolation of CD4+CD25– cells, stained suspensions were first depleted of CD25+ cells using depletion columns, and CD4+ T cells were purified using anti-CD4 microbeads and positive selection columns. CD4+CD25– cells were always more than 90% CD4+ and less than 1% CD25+.
Preparation of APCs
Bone marrow–derived dendritic cells (BM-DCs) and syngeneic naive spleen cells were used as antigen-presenting cells (APCs) for in vitro experiments. DCs were generated from bone marrow cells as previously described,34 with some modifications. In brief, bone marrow was flushed from tibias and femurs prior to red blood cell depletion. Cells were cultured at 37°C in 24-well culture (2 × 105/mL/well) in complete RPMI medium supplemented with 40 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ). Half of the medium was renewed every other 2 days by fresh medium and GM-CSF. Cells were collected after 7 days and were routinely more than 70% CD11c+. Spleen cells or BM-DCs were first incubated for 20 minutes at 37°C with 4 mM 2,4-dinitrobenzenesulfonate (DNBS) or medium alone (serum-free RPMI) and washed 3 times in complete medium. Hapten-pulsed APCs were then treated with mitomycin C (25 μg/mL) for 25 minutes at 37°C in complete medium and thoroughly washed before use.
Hapten-specific CD8+ T-cell proliferation and IFNγ production
CD8+ T cells were isolated from spleen and abdominal skin draining lymph nodes from mice 5 days after DNFB skin sensitization. CD8+ cells (1-2 × 105) were cultured in round-bottom 96-well plates in the presence of DNBS-pulsed mitomycin C–treated splenocytes (APCs/CD8 = 2.5:1) or BM-DCs (APCs/CD8 = 1:5). CD4+CD25+ cells were purified from naive or orally tolerized mice and added at various numbers into cultures. The proliferative response was assessed after 3 days of culture by [3H]thymidine incorporation (1 μCi/well [0.037 MBq/well]) during the last 8 hours. The cultures were harvested and the amount of [3H]thymidine uptake was counted using a β-plate liquid scintillation counter. The results are expressed as Δcpm ± SD, where Δcpm = (cpm in cultures of T cells with DNBS-pulsed APCs)–(cpm in cultures of T cells with untreated APCs). Cell-free supernatants were harvested after 48 hours, and IFNγ production was titrated by enzyme-linked immunosorbent assay (ELISA) using rat antimouse γ-IFN clone R46-A2 as the capture mAb and biotin-conjugated rat antimouse γ-IFN clone XMG1.2 as secondary mAb (both from Pharmingen).
Results
Experimental model
We have previously reported oral tolerance breakdown in 3 distinct experimental models of CD4+ T-cell deficiency: (1) anti-CD4 mAb–treated C57BL/6 mice (with a complete defect in CD4+ T cells), (2) MHC class II knock-out (Aβ°/°) mice (with complete defect in MHC class II–restricted CD4+ T cells), and (3) invariant-chain–deficient (Ii°/°) mice (with partial defect in MHC class II–restricted CD4+ T cells). We previously reported that conversely to normal C57BL/6 mice, in which a single oral administration of the hapten (DNFB) can block development of the CHS response and of hapten-specific CD8+ effector cells, mice deficient in CD4+ T cells in each of these models are refractory to tolerance induction,29,30 suggesting a role for regulatory CD4+ T cells in oral tolerance. In this study we used Ii°/° mice, which have residual MHC class II–expressing APCs32 and develop preferential Th1 immune response35 as well as strong and chronic CHS to DNFB,29 as a model to investigate the ability of various CD4+ T-cell subsets to restore oral tolerance.
CD4+ T cells can restore oral tolerance
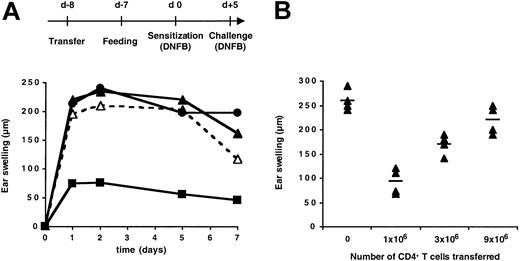
As previously observed,29 oral administration of DNFB prior to skin sensitization in Ii°/° mice failed to induce tolerance and affected neither the magnitude nor the kinetics of the skin inflammatory response to hapten challenge (Figure 1A). Adoptive transfer before hapten gavage of CD4+ T cells (but not CD4-depleted cells) from naive C57BL/6 mice restored oral tolerance in Ii°/° mice and prevented skin inflammation (Figure 1A). This effect was correlated with the number of CD4+ T cells transferred, inasmuch as 106 CD4+ T cells were inefficient, while 9 × 106 CD4+ T cells induced up to 80% inhibition of the CHS response (Figure 1B). Transfer of CD4+ T cells in control vehicle–fed skin-sensitized Ii°/° mice did not affect the CHS response (data not shown). Thus, CD4+ T cells and orally administered hapten are both required to block the onset of hapten-induced skin inflammation in invariant chain–deficient mice.
Adoptive transfer of CD4+ T cells restored oral tolerance in invariant chain–deficient mice. Ii°/° mice were either left untreated or transferred intravenously on day –8 with (A) 10 × 106 CD4+ T cells or CD4-depleted cells from naive C57BL/6 mice or (B) graded numbers of CD4+ T cells. All mice were fed either vehicle or DNFB one day later, sensitized epicutaneously with 0.5% DNFB on day 0, and ear challenged with 0.25% DNFB on day +5. The CHS response was determined by ear swelling at various times (A) or 48 hours (B) after hapten challenge. Standard errors were less than 15% (A). Mean increases in ear thickness are indicated by horizontal bars (B). In panel A, ▵ indicates untreated mice fed with vehicle; ▴, untreated mice fed with DNFB; ▪, mice transferred with CD4+ T cells and fed with DNFB; and •, mice transferred with CD4-depleted cells and fed with DNFB.
Adoptive transfer of CD4+ T cells restored oral tolerance in invariant chain–deficient mice. Ii°/° mice were either left untreated or transferred intravenously on day –8 with (A) 10 × 106 CD4+ T cells or CD4-depleted cells from naive C57BL/6 mice or (B) graded numbers of CD4+ T cells. All mice were fed either vehicle or DNFB one day later, sensitized epicutaneously with 0.5% DNFB on day 0, and ear challenged with 0.25% DNFB on day +5. The CHS response was determined by ear swelling at various times (A) or 48 hours (B) after hapten challenge. Standard errors were less than 15% (A). Mean increases in ear thickness are indicated by horizontal bars (B). In panel A, ▵ indicates untreated mice fed with vehicle; ▴, untreated mice fed with DNFB; ▪, mice transferred with CD4+ T cells and fed with DNFB; and •, mice transferred with CD4-depleted cells and fed with DNFB.
Contribution of MHC class II molecules and IL-10 in restoration of oral tolerance by CD4+ T cells
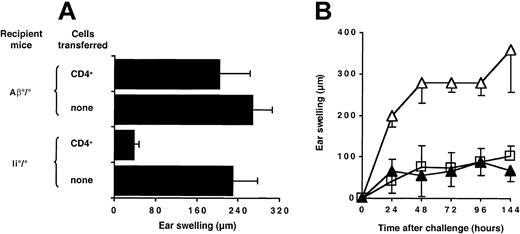
Ii°/° mice have residual MHC class II molecules, which might be required for oral tolerance induction through activation of CD4+ regulatory cells, expressed on the cell surface of APCs.32 To address this hypothesis, we performed adoptive transfer experiments in MHC class II–deficient (Aβ°/°) recipient mice. As shown in Figure 2A and in contrast to Ii°/° mice, MHC class II–deficient mice were not tolerized when naive CD4+ T cells were adoptively transferred prior to hapten feeding. Thus, CD4+ T cells required the presence of host MHC class II–positive APCs in order to restore oral tolerance.
Restoration of oral tolerance by CD4+ T cells is MHC class II–dependent but is not mediated by IL-10 secretion. (A) Naive CD4+ T cells (10 × 106) were transferred intravenously into Ii°/° or Aβ°/° recipient mice on day –8. (B) CD4+ T cells (10 × 106) purified from either naive wild-type C57BL/6 (▴) or IL-10–deficient (□) mice were adoptively transferred in Ii°/° mice on day –8. Groups of mice without cell transfer (▵) were used as control. All mice were then fed DNFB on day –7, sensitized on day 0 with DNFB, and ear challenged on day +5. Ear swelling was determined at 48 hours (A) or at various times after challenge (B). Results are expressed as mean values ± SD and are representative of 3 independent experiments.
Restoration of oral tolerance by CD4+ T cells is MHC class II–dependent but is not mediated by IL-10 secretion. (A) Naive CD4+ T cells (10 × 106) were transferred intravenously into Ii°/° or Aβ°/° recipient mice on day –8. (B) CD4+ T cells (10 × 106) purified from either naive wild-type C57BL/6 (▴) or IL-10–deficient (□) mice were adoptively transferred in Ii°/° mice on day –8. Groups of mice without cell transfer (▵) were used as control. All mice were then fed DNFB on day –7, sensitized on day 0 with DNFB, and ear challenged on day +5. Ear swelling was determined at 48 hours (A) or at various times after challenge (B). Results are expressed as mean values ± SD and are representative of 3 independent experiments.
Oral tolerance of the CHS response to DNFB is greatly impaired both in IL-10°/° mice and in normal mice treated with neutralizing anti–IL-10 antibody (data not shown). To examine whether IL-10 produced by CD4+ T cells contributed to oral tolerance induction, we compared the ability of CD4+ T cells from either control or IL-10–deficient mice to restore susceptibility to oral tolerance upon transfer into Ii°/° recipient mice. As depicted in Figure 2B, CD4+ T cells from either naive IL-10°/° or C57BL/6 mice transferred one day before feeding were equally efficient at restoring oral tolerance in Ii°/° recipient mice, indicating that IL-10 production by CD4+ T cells was not involved in their regulatory function in vivo.
In vivo depletion of CD25+ T cells by antibody treatment abrogates oral tolerance
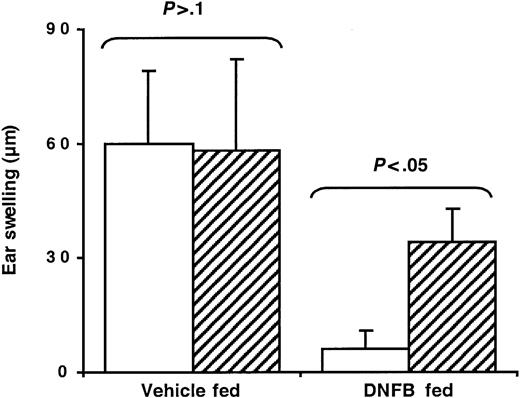
To address the role of CD4+CD25+ cells in oral tolerance, normal C57BL/6 mice were treated with a depleting anti-CD25 mAb (PC61) or a rat IgG mAb as control and tested for oral tolerance induction. Flow cytometry analysis using an anti-CD4 mAb and the anti-CD25 clone 7D4 (directed against an epitope of the molecule distinct from that recognized by PC61) confirmed depletion of more than 95% of CD4+CD25+ cells from both blood and spleen (data not shown). In hapten-fed mice, depletion of CD25+ cells resulted in the abrogation of tolerance as indicated by the appearance of a significant CHS response after skin sensitization (Figure 3). These data suggest that naturally occurring CD4+CD25+ T cells represent a critical regulatory cell subset during oral tolerance induction in vivo.
Anti-CD25 mAb treatment impairs oral tolerance in normal mice. C57BL/6 mice were injected intraperitoneally with either a control rat mAb (□) or a depleting anti-CD25 mAb (▨) on days –10, –7, –3, and 0, with respect to day 0 of DNFB sensitization as illustrated in Figure 1. Mice were fed either vehicle or DNFB, sensitized, and ear challenged with DNFB. Ear-swelling responses were determined at 48 hours after challenge.
Anti-CD25 mAb treatment impairs oral tolerance in normal mice. C57BL/6 mice were injected intraperitoneally with either a control rat mAb (□) or a depleting anti-CD25 mAb (▨) on days –10, –7, –3, and 0, with respect to day 0 of DNFB sensitization as illustrated in Figure 1. Mice were fed either vehicle or DNFB, sensitized, and ear challenged with DNFB. Ear-swelling responses were determined at 48 hours after challenge.
CD4+ CD25+ T cells restore oral tolerance in Ii°/° mice by preventing hapten-specific skin inflammation
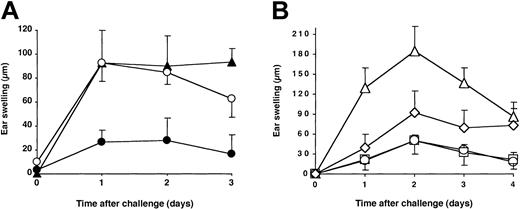
To further demonstrate that CD4+CD25+ T cells are required for oral tolerance induction, we examined whether CD4+CD25+ T-cell transfer could prevent CHS only when transferred before DNFB gavage in Ii°/° mice. As shown in Figure 4A, CD4+CD25+ T cells were unable to suppress CHS in vehicle-fed Ii°/° recipients but achieved complete suppression of CHS in DNFB-fed mice. This confirms that concomitant hapten feeding is mandatory for the ability of CD4+CD25+ T-cell transfer to restore tolerance. To determine whether CD4+CD25+ T cells represented the major regulatory subset able to restore oral tolerance, we compared the capacity of CD4+CD25+ and CD4+CD25– T cells to restore oral tolerance in Ii°/° mice. To this end, both subsets were purified from naive C57BL/6 mice by cell sorting and transferred before gavage into Ii°/° mice, in cell number representing the relative proportion of each subset among total unfractionated CD4+ T cells. As shown in Figure 4B, as few as 1 × 106 CD4+CD25+ cells prevented the development of the skin inflammatory response as efficiently as 10 × 106 total CD4+ T cells. In contrast, transfer of 9 × 106 CD4+CD25– cells resulted in only partial and transient downregulation of the CHS. These results demonstrate that only CD4+CD25+ T cells allowed maximal and stable restoration of oral tolerance in Ii°/° mice.
CD4+ CD25+ regulatory cells restore oral tolerance in Ii°/° mice. (A) Ii°/° mice were left untreated (▴) or transferred intravenously with 1.106 naive CD4+CD25+ T cells one day before gavage (ie, day –8) with either DNFB (•) or vehicle alone (○). (B) Ii°/° mice were left untreated (▵) or transferred intravenously with either naive total CD4+ T cells (10 × 106; □), CD4+CD25– T cells (9 × 106; ⋄), or CD4+CD25+ T cells (1 × 106; ○) one day before gavage with DNFB. All mice were sensitized epicutaneously on day 0, and the CHS response was measured as described in Figure 1 legend. The data are expressed as mean values ± SD and are representative of at least 3 independent experiments.
CD4+ CD25+ regulatory cells restore oral tolerance in Ii°/° mice. (A) Ii°/° mice were left untreated (▴) or transferred intravenously with 1.106 naive CD4+CD25+ T cells one day before gavage (ie, day –8) with either DNFB (•) or vehicle alone (○). (B) Ii°/° mice were left untreated (▵) or transferred intravenously with either naive total CD4+ T cells (10 × 106; □), CD4+CD25– T cells (9 × 106; ⋄), or CD4+CD25+ T cells (1 × 106; ○) one day before gavage with DNFB. All mice were sensitized epicutaneously on day 0, and the CHS response was measured as described in Figure 1 legend. The data are expressed as mean values ± SD and are representative of at least 3 independent experiments.
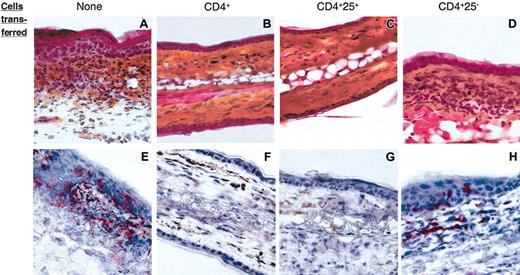
We have previously reported that skin inflammation in CHS is mediated by hapten-specific cytolytic CD8+ T cells recruited at the site of hapten challenge27,28 and that lack of CHS in orally tolerized mice correlated with absence of CD8+ T cells in the challenged skin.30 Immunohistochemical analysis was thus carried out to examine the relative outcome of CD4+ T-cell subset transfer on skin inflammation and infiltration with CD8+ T cells upon challenge in the same Ii°/° recipients as those shown in Figure 4. As expected, untransferred Ii°/° mice had an intense skin inflammatory reaction manifested by dermal edema and fibrosis associated with a massive cellular infiltration of both dermis and epidermis (Figure 5A) containing many CD8+ T cells (Figure 5E). Similar skin inflammatory infiltrate and CD8+ T-cell recruitment (Figure 5D,H) occurred in Ii°/° recipients of CD4+CD25– T cells. In contrast, Ii°/° mice tolerized by transfer of either CD4+ T cells (Figure 5B,F) or CD4+CD25+ T cells (Figure 5C,G) exhibited a normal skin histology with no sign of inflammation and complete lack of CD8+ T cells. Thus, the efficacy of CD4+CD25+ but not CD4+CD25– cells to restore oral tolerance and prevent CHS correlated with lack of CD8+ T-cell effectors recruited in the skin.
CD4+ CD25+ T cells prevent CD8+-mediated skin inflammation. Ears from the same Ii°/° recipients as those in Figure 3 were harvested 96 hours after DNFB challenge. Cryostat sections of ears were either stained with hematoxylin/phloxin/safran (A-D) or with an anti-CD8 mAb (red) and counter-stained with hematoxylin (E-G). Original magnification, × 40. No CD8-specific staining or local inflammatory reaction was detected in sections of ears from nonsensitized animals (not shown).
CD4+ CD25+ T cells prevent CD8+-mediated skin inflammation. Ears from the same Ii°/° recipients as those in Figure 3 were harvested 96 hours after DNFB challenge. Cryostat sections of ears were either stained with hematoxylin/phloxin/safran (A-D) or with an anti-CD8 mAb (red) and counter-stained with hematoxylin (E-G). Original magnification, × 40. No CD8-specific staining or local inflammatory reaction was detected in sections of ears from nonsensitized animals (not shown).
CD4+ CD25+ cells tolerize hapten-specific CD8+ T cells in vivo
Because CD4+CD25+ T-cell–mediated restoration of oral tolerance in Ii°/° mice could result from impaired priming/expansion of hapten-specific CD8+ effector cells, as observed in orally tolerized normal mice,30 we next analyzed the presence of hapten-specific CD8+ T cells in secondary lymphoid organs of hapten-fed Ii°/° recipients on day 5 after skin sensitization. As expected, hapten feeding in Ii°/° mice prior to skin sensitization was unable to inhibit the development of hapten-specific CD8+ effector T-cell response in secondary lymphoid organs (Figure 6). Likewise, adoptive transfer of the CD4+CD25– T-cell subset did not affect the hapten-specific CD8+ T-cell response. Alternatively, adoptive transfer of either 10 × 106 unfractionated CD4+ T cells or 106 CD4+CD25+ T cells completely prevented hapten-specific CD8+ T-cell proliferation within secondary lymphoid organs (Figure 6). Altogether, these data demonstrated that CD4+CD25+ T cells restored oral tolerance in Ii-deficient mice by preventing the priming/expansion of hapten-specific CD8+ CHS effector T cells.
CD4+ CD25+ cells prevent expansion of hapten-specific CD8+ T cells. Ii°/° mice were either left untreated or were transferred intravenously on day –8 with either naive total CD4+ T cells (10 × 106), CD4+CD25– T cells (9 × 106), or CD4+CD25+ T cells (1 × 106), fed with DNFB on day –7 and skin sensitized on day 0 with DNFB. Purified CD8+ T cells (2 × 105) from spleen and lymph nodes, harvested on day 5 after sensitization, were restimulated in vitro for 3 days with syngeneic mitomycin C–treated spleen cells (5 × 105) either untreated or pulsed with DNBS. T-cell proliferation was determined by [3H]thymidine uptake during the last 8 hours. Results are expressed as Δcpm values (ie, cpm from hapten-derivatized spleen cells – cpm from untreated spleen cells) ± SD of triplicate wells.
CD4+ CD25+ cells prevent expansion of hapten-specific CD8+ T cells. Ii°/° mice were either left untreated or were transferred intravenously on day –8 with either naive total CD4+ T cells (10 × 106), CD4+CD25– T cells (9 × 106), or CD4+CD25+ T cells (1 × 106), fed with DNFB on day –7 and skin sensitized on day 0 with DNFB. Purified CD8+ T cells (2 × 105) from spleen and lymph nodes, harvested on day 5 after sensitization, were restimulated in vitro for 3 days with syngeneic mitomycin C–treated spleen cells (5 × 105) either untreated or pulsed with DNBS. T-cell proliferation was determined by [3H]thymidine uptake during the last 8 hours. Results are expressed as Δcpm values (ie, cpm from hapten-derivatized spleen cells – cpm from untreated spleen cells) ± SD of triplicate wells.
CD4+ CD25+ T cells inhibit hapten-specific CD8+ T-cell responses in vitro
We next examined whether CD4+CD25+ T cells could directly inhibit hapten-specific CD8+ T-cell responses and whether hapten feeding potentiated their suppressive function. For this purpose, hapten-primed CD8+ T cells were purified from spleen and draining lymph nodes of DNFB-sensitized mice and were restimulated in vitro with hapten-pulsed bone marrow–derived DCs in the presence of graded numbers of CD4+CD25+ T cells purified from either naive or tolerant mice. CD4+CD25+ T cells from naive or tolerant mice were hyporesponsive to in vitro stimulation and did not proliferate in response to hapten-pulsed DCs (data not shown). As shown in Figure 7, CD4+CD25+ T cells from both naive and tolerant mice suppressed in a dose-dependent manner CD8+ T-cell proliferation and γ-IFN production, both resulting in more than 70% inhibition when used at a 1:1 ratio with effector CD8+ T cells. CD4+CD25+ T cells isolated from tolerant mice were reproducibly found to have slightly higher suppressive activity, compared with CD4+CD25+ cells isolated from naive donors, especially when used at lower cell ratios. Thus, naturally occurring CD4+CD25+ cells can control hapten-specific CD8+ T-cell responses, and oral exposure to antigen enhanced their suppressive function.
Innate CD4+ CD25+ cells inhibit hapten-specific CD8+ expansion in vitro. CD8+ T cells (105) were isolated from day-5 DNFB-sensitized C57BL/6 mice and stimulated with hapten-pulsed and mitomycin C–treated BM-DCs (2 × 104) (▦). Graded numbers of CD4+CD25+ T cells, purified from spleen and lymph nodes of either naive (□) or orally tolerant C57BL/6 mice (▨), were added to cultures. Proliferation was determined after 3 days by [3H]thymidine uptake (A) and γ-IFN production was titrated by ELISA in 48 hours cell free supernatants (B). Results are expressed as mean values ± SD and are representative of 3 independent experiments.
Innate CD4+ CD25+ cells inhibit hapten-specific CD8+ expansion in vitro. CD8+ T cells (105) were isolated from day-5 DNFB-sensitized C57BL/6 mice and stimulated with hapten-pulsed and mitomycin C–treated BM-DCs (2 × 104) (▦). Graded numbers of CD4+CD25+ T cells, purified from spleen and lymph nodes of either naive (□) or orally tolerant C57BL/6 mice (▨), were added to cultures. Proliferation was determined after 3 days by [3H]thymidine uptake (A) and γ-IFN production was titrated by ELISA in 48 hours cell free supernatants (B). Results are expressed as mean values ± SD and are representative of 3 independent experiments.
Discussion
This study demonstrates that CD4+ T cells are MHC class II–dependent regulatory cells responsible for orally induced tolerance of CD8+ T-cell–mediated CHS responses and that CD4+CD25+ cells represent the major subset responsible for this effect. Remarkably, adoptive transfer of naive CD4+ T cells can restore complete oral tolerance in otherwise refractory invariant chain–deficient mice. CD4+ T-cell transfer in the absence of hapten feeding failed to inhibit the CHS response, indicating that CD4+ T cells and oral hapten were both required to achieve oral tolerization.
We showed that within CD4+ T lymphocytes, the naturally occurring CD4+CD25+ T-cell subset is responsible for restoration of oral tolerance. Indeed, in vivo transfer of as few as 106 purified CD4+CD25+ T cells to Ii°/° recipient mice before hapten feeding completely prevented the CHS response induced by subsequent skin sensitization, while 10.106 CD4+ T cells were required to achieve similar suppression. The suppressive effect of transferred CD4+CD25+ T cells on the CHS response required concomitant antigen feeding, since CD4+CD25+ cell transfer did not affect the CHS response of vehicle-fed Ii°/° recipients. That CD4+CD25+ but not CD4+CD25– T cells were responsible for the in vivo regulatory effect of unfractionated CD4+ T cells was confirmed by the finding that only CD4+CD25+ T-cell transfer was able to prevent (1) the development and differentiation of hapten-specific CD8+ T cells in secondary lymphoid organs and (2) CD8+ T-cell infiltration in the hapten-challenged skin of the Ii°/° recipient. In addition, in vivo depletion of CD25+ T cells by specific antibody treatment impaired oral tolerance in normal C57BL/6 mice, although not completely as compared with anti-CD4 mAb depletion.30 This could be explained by either (1) incomplete depletion of CD4+CD25+ cells, especially from tissues such as the intestine, (2) concomitant depletion of activated CD8+ effectors that have up-regulated CD25, or (3) the ability of CD4+CD25– cells to exert some level of regulation, as reported in other models.21,36-38 Indeed, CD4+CD25– cells were found to inhibit wasting or autoimmune disease in lymphopenic host to the same extent as CD4+CD25+ cells.36,37 In addition, TcR transgenic CD4+CD25– T cells were reported to differentiate in vivo into CD4+CD25+ regulatory T cells upon activation with antigen expressed in peripheral tissues38 or encountered following oral delivery.21 Although transfer of CD4+CD25– T cells induced a partial and transient decrease in the CHS response in some mice, these cells were unable to prevent skin infiltration by CD8+ T cells or development of the hapten-specific CD8+ T-cell response in secondary lymphoid organs. It has recently been emphasized that the relative homeostatic expansion capacity of T-cell subsets transferred in immunocompromised SCID or RAG°/° (recombination activating genes) hosts plays an important role in their suppressive properties.39 Although Ii°/° mice are not immunocompromised, it is possible that the partial and transient protection induced by CD4+CD25– cells might relate to the large number of cells transferred and/or to the homeostatic expansion that may occur to some extent, resulting in competition with hapten-specific CD8+ effector T cells.
Our finding that naive regulatory CD4+CD25+ cells could block in vivo development and/or differentiation of antigen-specific CD8+ T-cell effectors during orally induced tolerance confirms and extends to a pathophysiologic situation, previous data showing that CD8+ T cells can be targets of regulatory CD4+CD25+ T cells in vitro, via direct T-T interaction.40 This raises the question of the mechanism(s) by which regulatory CD4+CD25+ T cells prevent CD8+ T-cell expansion during oral tolerance and control CD8+ T-cell–mediated inflammatory responses in Ii°/° mice. It is possible that hapten penetration through the gut mucosa activates, or favors the differentiation of, a pool of regulatory CD4+CD25+ T cells that recirculate via blood or lymph and are readily present in skin draining lymph nodes at the time of skin sensitization, thus preventing priming/expansion of hapten-specific CD8+ effector cells during the afferent phase of the CHS response. Alternatively, hapten feeding may prime hapten-specific CD8+ T cells, which are inactivated or deleted by regulatory CD4+CD25+ T cells, thus resulting in lack of functional hapten-specific CD8+ T cells available at the time of skin sensitization. This latter hypothesis is supported by studies reporting that orally induced systemic tolerance to protein antigens is preceded by rapid activation of specific CD8+ CTLs in Peyer patches and mesenteric lymph nodes41,42 and by our finding that CD4+CD25+ T cells can block IFNγ production and proliferation of hapten-primed CD8+ T cells upon in vitro restimulation with the hapten.
It may be proposed that MHC class II–dependent interaction between CD4+CD25+ T cells and the APCs that have captured the hapten from the gut is required to trigger their suppressive function. Indeed, the inability of CD4+CD25+ T cells to restore oral tolerance in Aβ°/° recipients indicates that CD4+CD25+ cells need MHC class II molecule expression by host APCs to exert their regulatory function and is reminiscent of a recent study showing that peripheral MHC class II molecules allow maintenance of regulatory CD4+CD25+ T cells in lymphopenic hosts.43 In addition, CD4+CD25+ T cells harvested from orally tolerized normal mice exhibited a more potent regulatory effect on hapten-specific CD8+ T-cell responses in vitro, suggesting that hapten feeding could affect the size, regulatory activity, or migratory capacity of CD4+CD25+ T cells. In this respect, recent studies reported that oral antigen administration in recipients receiving TcR transgenic T cells could increase the size of the regulatory CD4+CD25+ T-cell pool at the periphery.21,22
Whether regulatory T cells, and CD4+CD25+ T cells in particular, exert peripheral suppression via a bystander or an antigen-specific mechanism is still debated. Numerous studies have described oral tolerance as an antigen-specific mechanism, because systemic immune response to a nominal antigen (either a protein or a hapten) could be prevented only by prior oral administration of the same antigen. Likewise, our previous studies of oral tolerance in the model of CHS showed that even when mice were double sensitized with 2 non–cross-reacting haptens (ie, DNFB and OXA) to generate effector cells specific for both, tolerance was induced exclusively by feeding with the same hapten as the one used for challenge.30 It is possible that hapten feeding activates and/or expands antigen-specific CD4+CD25+ T cells recognizing complexes of MHC class II/hapten-modified peptides. Alternatively, the apparent antigen specificity of T-cell regulation may relate to the fact that the APCs may simultaneously present the oral hapten to CD8+ T cells and activate regulatory CD4+CD25+ T cells via self-peptide/MHC class II complexes. Such bridging could allow tolerization of hapten-specific CD8+ T cells by naturally occurring CD4+CD25+ T cells.
Whether IL-10 plays a role in oral tolerance and whether it is responsible for the regulatory function of CD4+CD25+ T cells are of major importance. This latter issue has yielded divergent results that may be related to differences in the experimental systems used. Thus, IL-10 production appears crucial for the ability of CD4+CD25+ cells to prevent colitis in immunocompromised mice44 but not for their inhibitory effect on gastritis.45 Furthermore, IL-10 production by CD4+CD25+ cells was shown to be mandatory for controlling the inflammatory response induced by bacterial superantigen in CD25-deficient mice.46 In our model, IL-10 production by CD4+CD25+ T cells did not account for induction of CD8+ T-cell tolerance. Indeed, (1) CD4+ T cells from IL-10–deficient mice were as efficient as wild-type CD4+ T cells to restore oral tolerance to CHS in Ii°/° mice, (2) hapten feeding in normal mice did not potentiate IL-10 production by CD4+CD25+ T cells, and (3) neutralizing anti–IL-10 or anti–IL-10 receptor mAbs did not affect their ability to inhibit the hapten-specific CD8+ T-cell response in vitro (data not shown). Nevertheless, we found that IL-10 is critical for efficient induction of oral tolerance, inasmuch as oral tolerance of the CHS response to DNFB is greatly impaired both in IL-10°/° mice as well as in normal mice treated with neutralizing anti–IL-10 antibody (B.D., D.K., unpublished observations, 2003). Thus, IL-10, which is constitutively produced by intestinal epithelial cells47 and Peyer patch dendritic cells,48 may be a critical factor in the gut microenvironment at the time of antigen penetration for efficient tolerization of CD8+ T cells. IL-10 might be also instrumental for regulatory T-cell differentiation, as demonstrated for Tr1 cells,15,49 and by limiting local activation of hapten-specific CD8+ T cells, may render them more susceptible to CD4+CD25+ T-cell regulation.
This study documents the potent in vivo regulatory effect of CD4+CD25+ T cells on orally induced tolerance of CD8+ T cells mediating antigen-specific tissue inflammation. Successful therapy by oral antigen and CD4+CD25+ cells in Ii°/° mice, who have Th1-biased T-cell responses, supports the potential clinical and therapeutic interest of CD4+CD25+ cells for the control of CD8+ Tc1-mediated diseases.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-03-0727.
Supported by an institutional grant from INSERM and a specific grant from Association pour la Recherche sur le Cancer (no. 4296 to D.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Grégoire Joubert for expert technical assistance with immunohistochemistry and M. Chevalier for hematoxylin/phloxin/safran stainings.

![Figure 6. CD4+ CD25+ cells prevent expansion of hapten-specific CD8+ T cells. Ii°/° mice were either left untreated or were transferred intravenously on day –8 with either naive total CD4+ T cells (10 × 106), CD4+CD25– T cells (9 × 106), or CD4+CD25+ T cells (1 × 106), fed with DNFB on day –7 and skin sensitized on day 0 with DNFB. Purified CD8+ T cells (2 × 105) from spleen and lymph nodes, harvested on day 5 after sensitization, were restimulated in vitro for 3 days with syngeneic mitomycin C–treated spleen cells (5 × 105) either untreated or pulsed with DNBS. T-cell proliferation was determined by [3H]thymidine uptake during the last 8 hours. Results are expressed as Δcpm values (ie, cpm from hapten-derivatized spleen cells – cpm from untreated spleen cells) ± SD of triplicate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-03-0727/6/m_h82135143006.jpeg?Expires=1765951539&Signature=vSGN7QI98cMdO7Qxu~wF7LD-bdwkXYoy20-SBcu4pe20vui2gbozmTBtM~g3Qm5VmGMuirPyGZPCGaZFbWI0cMZvp1lsxKpYkhyxkhI~wY~7hotWK7C~bqlvJNfUFwOPL8iU7tQuMNq2L-bHDdvVXN93xeWT76YxPkGddS2NNAreSS8aGA~iIHjbyR-glxkYY-jPJXAGEzbzJCLCzypRR5Z~2nOh-8mgHL9r4qOfFi1T~11NIMnpBlGZsGgOwRpM7bISrO-iefGPu7NtrCgQt4NST14Mt4YnV0vqHCJ50xo~8N5~1S~F8JurtHixVyK6neY0UzdzW6sbdrSI7ChtTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Innate CD4+ CD25+ cells inhibit hapten-specific CD8+ expansion in vitro. CD8+ T cells (105) were isolated from day-5 DNFB-sensitized C57BL/6 mice and stimulated with hapten-pulsed and mitomycin C–treated BM-DCs (2 × 104) (▦). Graded numbers of CD4+CD25+ T cells, purified from spleen and lymph nodes of either naive (□) or orally tolerant C57BL/6 mice (▨), were added to cultures. Proliferation was determined after 3 days by [3H]thymidine uptake (A) and γ-IFN production was titrated by ELISA in 48 hours cell free supernatants (B). Results are expressed as mean values ± SD and are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-03-0727/6/m_h82135143007.jpeg?Expires=1765951539&Signature=MZmHJpdCJKlsmNKOaZGbq4vVsPYuBiwUX22v3FdMKGHxxII4jR9GadLAzhdEq17qAgW5d712zK4IWP3hdNNzktBibPHkJNdOP4sdffqY7Nwb1PsCvtFGU7bBHAJ1CkS11VFed52bARljKe1C1aUH77KPalZj7ERzLnuIeQ4yKFs9cF1a426qPZZ9CjOY6MGxaVms1xWeOHnz-IBXcZ4v-Y1ItWUR5mDaLWG63hH2P8EMXpDtGqypybqpnWn0ao4DCqopM2cqfJnyuk85ilqO27MYxeTeUIca-NvbNJOjmowEXZw8IEjKu5g9tJvDJgPjPC~ZoQrKp2eiCs7RTaA3zg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal