Abstract
Distinct human dendritic cell (DC) subsets differentially control immunity. Thus, insights into their in vivo functions are important to understand the launching and modulation of immune responses. We show that nonobese diabetic/LtSz-scid/scid (NOD/SCID) mice engrafted with human CD34+ hematopoietic progenitors develop human myeloid and plasmacytoid DCs. The skin displays immature DCs expressing Langerin, while other tissues display interstitial DCs. Myeloid DCs from these mice induce proliferation of allogeneic CD4 T cells in vitro, and bone marrow human cells containing plasmacytoid DCs release interferon-α (IFN-α) upon influenza virus exposure. Injection of influenza virus into reconstituted mice triggers IFN-α release and maturation of mDCs. Thus, these mice may provide a model to study the pathophysiology of human DC subsets.
Introduction
Dendritic cells (DCs) induce and regulate immune responses.1,2 The discovery of in vitro culture systems yielding mouse3 and human4-6 DCs accelerated studies on their biology and led us to understand their complexity. Indeed, DCs have different functions at different stages of maturation. While immature (nonactivated) DCs that capture self-antigens (eg, apoptotic cells) induce tolerance,7,8 mature antigen-loaded DCs induce antigen-specific immunity. Furthermore, several DC subsets exist that have different functions.1 In humans, 2 major DC pathways have been described. A myeloid pathway includes Langerhans cells (LCs), found in stratified epithelia such as skin, and interstitial DCs (intDCs), found in all other tissues. While both subsets produce interleukin-12 (IL-12), only intDCs make IL-10 and induce B-cell proliferation and differentiation.9 The other pathway includes the plasmacytoid DCs (pDCs),10 which secrete large amounts of type I interferons within a few hours following viral encounter.11,12 Depending on the maturation signal that they receive, pDCs modulate T-cell differentiation into either interferon-γ (IFN-γ) or IL-4–producing CD4 T cells13 or into regulatory CD8 T cells.14 Thus, distinct human DC subsets differentially control lymphocytes in vitro.
The in vitro generation of human DCs prompted their use in immunotherapy, particularly for the treatment of cancer. Early trials in humans have shown the safety of tumor-associated antigens (TAA)–loaded DCs as well as some clinical and immune responses (reviewed in Banchereau et al15 ). We also are beginning to unravel the role of DCs in pathogenesis of human diseases, for example, thymic stroma lymphopoietin (TSLP)–triggered mDCs may play a key role in the initiation of allergic inflammation,16 while IFN-α–triggered mDCs appear as a major factor driving the autoimmune reaction in systemic lupus erythematosus.17 Thus, DCs can be considered both as vectors and targets for immunomodulation.
Understanding the specific functions of DC subsets and their interplay in vivo will be critical to understand the launching and modulation of immune responses. Hence, the need for preclinical models of the human immune system. Indeed, conclusions from studies in mice cannot be directly extrapolated to humans because of biologic differences as exemplified by the CD1 antigen presentation system that is different between species.18 SCID mice reconstituted with human cells represent interesting candidates for human disease models19-21 and have been used, for example, in the evaluation of tumor metastasis,22 mechanisms of progression,23 as well as antitumor therapies (reviewed in Bankert et al24 ). However, many difficulties have been encountered. For example, grafting of peripheral blood mononuclear cells (PBMCs) leads to rather limited immune reconstitution that may not allow potent priming of T cells. The grafting of human hematopoietic progenitor cells (HPCs)25,26 improved reconstitution, particularly when fetal tissues coengrafted with human thymus27 or lymph node were used.28 Yet, the complexity of the system limits its general applicability. The introduction of the nonobese diabetic/LtSz-scid/scid (NOD/SCID) mice29 improved engraftment of human cells.30,31 However, residual natural killer (NK) cell activity could interfere with the efficiency of engraftment,32 leading to development of mice with deletion of β2-macroglobulin33,34 or γ chain genes.35
Many of the difficulties encountered in these models could be due to insufficient reconstitution of human DCs, a parameter that has not been extensively studied. Given the key role that DCs play in T-cell homeostasis,36 DC reconstitution might facilitate human T-lymphocyte reconstitution. Indeed, recent studies with ex vivo–generated monocyte-derived DCs suggest their capacity to support CD4 T-cell differentiation as well as humoral responses in vivo.37,38 However, these adoptive transfer models do not permit the evaluation of the interplay between DC subsets. Here, we show that NOD/SCID mice engrafted with human CD34+ HPC develop all subsets of human DCs. Furthermore, we demonstrate that these human DCs are functional both in vitro and in vivo.
Materials and methods
Isolation and culture of CD34+ cells from human umbilical cord blood, fetal liver, and mobilized peripheral blood
Umbilical cord blood (UCB) was obtained after informed consent under an institutional approved institutional review board protocol at Parkland Memorial Hospital from uncomplicated births. Briefly, mononuclear cells were isolated by Ficoll (Pharmacia, Uppsala, Sweden) gradient separation and were enriched for CD34+ cells by positive immunomagnetic isolation according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). Fetal livers (FLs) (at 18-22 weeks gestation) were obtained from Advanced Bioscience Resources (Alameda, CA). To obtain CD34+ cells, liver samples were disrupted in RPMI supplemented with 10% fetal bovine serum (FBS), 50 U/mL penicillin, 50 mg/mL streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, 1 mg/mL collagenase/dispase, and 0.5 U/mL Dnase I. Following disruption, cells were filtered through a 70-μm mesh, and CD34+ cells were isolated as described above. This protocol yielded more than 90% UCB and FL CD34+ cells. CD34+ cells were subsequently stained with mouse anti–human CD34 (clone 581) monoclonal antibodies conjugated to allophycocyanin (APC) or similarly labeled isotype controls (Becton Dickinson, San Jose, CA) and analyzed by flow cytometry for CD34 expression. Cells were immediately frozen following isolation and stored at –80°C until use. Healthy adult volunteers received recombinant granulocyte colony-stimulating factor (G-CSF) (Neupogen; Amgen, Thousand Oaks, CA) 10 μg/kg/d subcutaneously for 5 days, for peripheral blood stem cell mobilization, and then underwent leukapheresis for 2 consecutive days to collect mobilized CD34+HPC (IRB no. 097-053). The cells were processed using the Isolex stem cell concentration system (Baxter, Irvine, CA) to obtain an enriched population of CD34+ HPCs (purity > 90%), which were then cryopreserved until use.
Transplantation
Human UCB, FL, or mobilized peripheral blood (MPB) CD34+ cells (2-3 × 105 UCB CD34+, 0.5-1.5 × 106 FL CD34+, or 3-6 × 106 MPB CD34+ cells per animal except for experiment no. 7 in Table 1, where mice received 1.5 × 106 MPB CD34+ cells) were infused intravenously into separate experimental cohorts of sublethally irradiated (300 centigrays by 137Cs γ-irradiation) NOD/SCID mice as we have previously described.26,39 Tissues were harvested 8-16 weeks after transplantation and analyzed for the presence of indicated cell lineages.
Frequency of engraftment
Experiment . | Age of mice (weeks) . | Source of CD34+ HPCs . | Time reconstituted after transplantation (weeks) . | Reconstitution as human CD45+ cells in bone marrow . | Skin* . | Lung* . | Spleen* . | Liver* . |
|---|---|---|---|---|---|---|---|---|
| 1 | 17 | MPB/CB/FL | 8 | 5/13 | 2/2 | 2/2 | 4/13 | 2/2 |
| 2 | 8-12 | MPB | 14 | 4/4 | 0/4 | 4/4 | 4/4 | 4/4 |
| 3 | 8-12 | CB/FL | 14 | 6/6 | 2/4 | 3/4 | 4/4 | 4/4 |
| 4 | 21 | FL | 16 | 7/7 | 1/1 | 1/1 | 6/6 | ND |
| 5 | 13 | MPB | 15-16 | 4/7 | ND | ND | ND | ND |
| 6 | 12 | CB | 19 | 4/4 | ND | ND | 4/4 | ND |
| 7 | 8 | MPB† | 11 | 6/6 | ND | ND | 6/6 | ND |
Experiment . | Age of mice (weeks) . | Source of CD34+ HPCs . | Time reconstituted after transplantation (weeks) . | Reconstitution as human CD45+ cells in bone marrow . | Skin* . | Lung* . | Spleen* . | Liver* . |
|---|---|---|---|---|---|---|---|---|
| 1 | 17 | MPB/CB/FL | 8 | 5/13 | 2/2 | 2/2 | 4/13 | 2/2 |
| 2 | 8-12 | MPB | 14 | 4/4 | 0/4 | 4/4 | 4/4 | 4/4 |
| 3 | 8-12 | CB/FL | 14 | 6/6 | 2/4 | 3/4 | 4/4 | 4/4 |
| 4 | 21 | FL | 16 | 7/7 | 1/1 | 1/1 | 6/6 | ND |
| 5 | 13 | MPB | 15-16 | 4/7 | ND | ND | ND | ND |
| 6 | 12 | CB | 19 | 4/4 | ND | ND | 4/4 | ND |
| 7 | 8 | MPB† | 11 | 6/6 | ND | ND | 6/6 | ND |
MPB indicates mobilized peripheral blood HPCs (G-CSF); CB, cord blood HPCs; FL, fetal liver HPCs; and ND, not done.
Number of mice displaying human cells/number of tested mice.
1.5 × 106 HPCs/injection/mouse.
Live influenza virus (strain A/PR/8/34, Charles River Laboratories) was resuspended in normal saline at a concentration of 100 μg protein/1 mL. Mice reconstituted with FL CD34+ cells were injected intravenously with 200 μL viral preparation (corresponding to 3600 viral units, as measured by hemagglutinin titer, per mouse). Tissues were harvested 16 hours after administration of the virus. Human recombinant interferon-alpha (IFN-α, Roferon, Schering-Plough, NJ) was obtained from the hospital pharmacy. Mice were injected intravenously with either diluent (100 μL per mouse) or 1 × 106 U of IFN-α (100 μL per mouse corresponding to 4 μg protein/mouse).
Flow cytometry analysis
Total bone marrow cells, blood, and spleen suspensions as well as human cells enriched from bone marrow by depletion of mouse CD45+ cells were stained for 4-color flow cytometric analysis. To determine frequency of human DC subsets, cell preparations were stained using a DC kit (Becton Dickinson) based on a cocktail of fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies against lineage markers CD3, CD14, CD16, CD19, CD20, CD56; CD123-PE, anti–HLA-DR-PerCP, and CD11c-APC. To determine activation/maturation of myeloid DCs enriched human cells in bone marrow, suspensions were stained using the following monoclonal antibodies: CD11c-APC and CD80-PE (BDIS, San Jose, CA), CD86-PE (BD Pharmingen, San Diego, CA), and CD40-PE, CD83-PE, and CD54-PE (Immunotech, Marseille, France).
Immunofluorescence
Harvested tissue was frozen in optimal cutting temperature compound by Tissue Tek (OCT; Allegiance, McGaw, IL). Frozen tissues were cryosectioned and placed on Superfrost Plus Slides (Fisher Scientific, Pittsburgh, PA) and stored at –80°C until staining. Sections were fixed with cold acetone (Sigma-Aldrich, St Louis, MO) and screened with FITC-conjugated CD45 or HLA-DR. In the tissues that were positive, double and triple stainings were performed with the following markers: CD19 FITC (BD, clone SJ25C1), CD45 pure (BD, clone 201), IgD FITC (Southern Biotech, Birmingham, AL), HLA-DR Biotin (BD, clone L243F), CD123 Biotin (BD, clone 7G3), MPO FITC (DAKO, Carpinteria, CA, clone MPO7), CD11b PE (clone Bear 1; Immunotech, Westbrook, ME), Langerin (DCGM4, Immunotech), CD11c pure (clone 3.9; Novacastra UK, distributed by Vector Labs, Burlingame, CA). Donkey anti-mouse IgG conjugated with Texas Red (Jackson ImmunoResearch, West Grove, PA) was added as a secondary antibody. All biotinylated antibodies were conjugated with either streptavidin-Cy5 (Caltag, Burlingame, CA) or streptavidin-Texas Red (Cortex Biochem, San Leandro, CA). Slides were cover-slipped with fluromount G (Southern Biotech) mounting medium containing either Vectashield with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (Vector Labs) or 7-AAD (Sigma-Aldrich) to stain the nuclei.
Enrichment of human cells
Human cells were enriched from the bone marrow of engrafted NOD/SCID mice by depletion of mouse CD45+ cells with a magnetic-activated cell sorter (MACS; Miltenyi Biotec GmbH, Germany) using anti–mouse CD45 Ab-conjugated beads. After depletion of B cells with anti–CD19 Ab, mDCs were positively selected with BDCA-1 beads and BDCA-3 beads with MACS. The population of CD11c+HLA-DR+ cells after positive selection was 27%-46%. Alternatively, mDCs were directly sorted by flow cytometry from the bone marrow of engrafted NOD/SCID mice as lin–HLA-DR+CD11c+CD123– cells. The population of CD11c+ DCs in human cells purified from the bone marrow of engrafted NOD/SCID mice was from 19% to 24%.
TaqMan real-time RT-PCR assay
Reverse transcriptase–polymerase chain reaction (RT-PCR) was carried out using the ABI PRISM 7700 sequence detector (Applied Biosystems, Foster City, CA). Primers, MX1-F: CAGCACCTGATGGCCTATCA, MX1-R: TGGAGCATGAAGAACTGGATGA and FAM-labeled probe (CAGGAGGCCAGCAAGCGCATCT) specific to human MX1 were designed with Primer Express software (Applied Biosystems, Weiterstadt, Germany), and ribosomal 18S PDAR was used as an internal control (Applied Biosystems). Reactions were set up in duplicate using the One Step RT-PCR kit according to manufacturer's recommended protocol with 50 μg total RNA. Cycling conditions were as follows: 48°C for 30 minutes; 95 °C for 10 minutes; 40 cycles of 95°C for 15 seconds; then 60°C for 1 minute. Relative MX1 expression was calculated using the Comparative CT method according to the ABI's protocol.
Functional assays
T-cell proliferation assay. Allogeneic CD4+ T cells were positively selected from a buffy coat of a healthy donor by using MACS. Autologous CD4+ T cells were positively selected from G-CSF–mobilized peripheral blood mononuclear cells. CD4+ T cells (1 × 105/well) were plated in U-bottomed 96-well plates with purified mDCs at graded cell numbers. The proliferation assay was carried out in RPMI 1640 medium supplemented with heat-inactivated 10% human AB serum. After 5 days, tritiated thymidine (1 μCi [0.037 MBq]/well) was added. When autologous T cells were used, human DCs isolated from the bone marrow were pulsed with tetanus toxoid (4 LFU/mL). These cultures were pulsed with thymidine after 6 days. The plates were harvested 16 hours later, and incorporated radioactivity was measured using Wallac scintillation counter.
Quantification of cytokine production. Human cells enriched from bone marrow were cultured overnight (16 hours) in cRPMI 10% fetal calf serum (FCS) with live influenza virus (A/PR/8/34; Charles River Laboratories) in 96 microwells plates. After 16 hours, the plates were spun, and the supernatants were collected and frozen at –20°C. All the collected supernatants and sera from mice injected or not with influenza virus were analyzed using a human interferon alpha kit (Biosource, Camarillo, CA) and developed with tetramethyl-benzidine as substrate according to manufacturer's recommendations.
Results
NOD/SCID mice engrafted with human CD34± HPCs develop human Langerhans cells and interstitial DCs
NOD/SCID mice were irradiated and received transplants of cord blood, fetal liver, or G-CSF–mobilized blood human CD34+ hematopoietic progenitor cells (HPCs). Engrafted mice, as determined by PCR analysis of β-globin expression in blood samples, were used 8-16 weeks after engraftment. Since one of the characteristic features of DCs is their ability to colonize virtually all tissues, we first looked for the presence of human cells as determined by expression of the human pan-leukocyte marker CD45. Human CD45+ cells could be found, albeit at variable numbers, in skin, lung, liver, spleen, heart, and, in some experiments, gut, kidney, and pancreas (Table 1, Figure 1A-C and not shown). Staining was specific, as no cells labeling with anti–human CD45 could be detected in NOD/SCID mice that did not receive human CD34+ HPCs (not shown). The presence of human cells was additionally confirmed by staining with antibody recognizing human β2 microglobulin (not shown). In all peripheral tissues colonized by human cells, there were HLA-DR+ cells that displayed dendritic cell morphology. Skin contained variable numbers of HLA-DR+ CD11c+ cells that expressed Langerin, suggesting their differentiation into Langerhans cells (LCs) (3 of 3 tested mice from different cohorts). LCs were immature as demonstrated by localization of Langerin and HLA-DR within intracytoplasmic vesicles (Figure 2A). Cells with LCs phenotype could also be seen in the lung and rarely in the liver (6 of 6 and 3 of 3 tested mice, respectively) (not shown). HLA-DR+ cells could reproducibly be found in the lung, and the HLA-DR staining was located within intracytoplasmic vesicles, suggesting infiltration with immature DCs (Figure 2B). In contrast to the peripheral tissues, spleen, a secondary lymphoid organ, contained rare HLA-DR+CD11c+ (myeloid DCs) or HLA-DR+CD11c–CD123+ (plasmacytoid DCs) cells as determined both by microscopy analysis of spleen sections (9 of 9 tested mice from different cohorts) (Figure 3 and not shown) and flow cytometry analysis of spleen suspensions (0.4% ± 0.7% and 0.2% ± 0.2% of mDCs and pDCs, respectively, n = 13) (Figure 4). Most human CD45+ cells in the spleen stained with anti–IgD antibody, consistent with a B-cell phenotype (Figure 3). Myeloperoxidase was absent, suggesting lack of human macrophage differentiation (data not shown). Thus, NOD/SCID mice engrafted with human CD34+ HPCs (from multiple sources) develop 2 subsets of human tissular DCs, for instance, interstitial DCs and LCs.
Human cells in tissues of NOD/SCID mice engrafted with human CD34+HPC. Immunofluorescence and confocal microscopy analysis of frozen tissue sections from organs of NOD/SCID mice engrafted with human CD34+ HPC. Staining of skin (A), liver (B), and lung (C) sections with anti–human CD45-FITC. Control staining on spleen sections include isotype control and anti–human CD45-FITC staining on tissue from mice that did not receive human cells (not shown). Sections are from tissues harvested from the same one mouse (no. 4 from experiment 1 in Table 1, which received a transplant of 3 × 105 cord blood CD34+ HPCs). CD45 staining was found in tested samples, harvested from different cohorts of engrafted mice, of skin from 5 mice, liver from 10 mice, and lung from 10 mice. Original magnification, × 63.
Human cells in tissues of NOD/SCID mice engrafted with human CD34+HPC. Immunofluorescence and confocal microscopy analysis of frozen tissue sections from organs of NOD/SCID mice engrafted with human CD34+ HPC. Staining of skin (A), liver (B), and lung (C) sections with anti–human CD45-FITC. Control staining on spleen sections include isotype control and anti–human CD45-FITC staining on tissue from mice that did not receive human cells (not shown). Sections are from tissues harvested from the same one mouse (no. 4 from experiment 1 in Table 1, which received a transplant of 3 × 105 cord blood CD34+ HPCs). CD45 staining was found in tested samples, harvested from different cohorts of engrafted mice, of skin from 5 mice, liver from 10 mice, and lung from 10 mice. Original magnification, × 63.
Immature human DCs in tissues of NOD/SCID mice engrafted with human CD34+ HPC. Immunofluorescence and confocal analysis of frozen tissue sections from skin (A; 3 of 3 tested mice) and lung (B; 6 of 6 tested mice) of NOD/SCID mice engrafted with human CD34+ HPC. (A) Staining with anti–human CD11c-FITC (green), anti–human Langerin-TxR (CD207, red) and anti–human HLA-DRCy5 (blue). Single fluorescence and overlay (no. 4 from experiment 1 in Table 1, which received a transplant of 3 × 105 cord blood CD34+ HPCs). Note the colocalization of Langerin and HLA-DR in cytoplasmic compartments consistent with immature DC phenotype in the skin (× 63 magnification with zoom of × 2. (B) Anti–human HLA-DR-FITC and 7AAD nuclei staining in the lung (projection × 40 magnification and section at zoom × 2). Note intracellular localization of anti–HLA-DR staining. HLA-DR staining was either direct with FITC-conjugated mAb or indirect with streptavidin and is representative of tested samples of lung harvested from 10 different engrafted mice. Note the large infiltration of human cells in the lung as well as dendritic morphology of HLA-DR+ cells.
Immature human DCs in tissues of NOD/SCID mice engrafted with human CD34+ HPC. Immunofluorescence and confocal analysis of frozen tissue sections from skin (A; 3 of 3 tested mice) and lung (B; 6 of 6 tested mice) of NOD/SCID mice engrafted with human CD34+ HPC. (A) Staining with anti–human CD11c-FITC (green), anti–human Langerin-TxR (CD207, red) and anti–human HLA-DRCy5 (blue). Single fluorescence and overlay (no. 4 from experiment 1 in Table 1, which received a transplant of 3 × 105 cord blood CD34+ HPCs). Note the colocalization of Langerin and HLA-DR in cytoplasmic compartments consistent with immature DC phenotype in the skin (× 63 magnification with zoom of × 2. (B) Anti–human HLA-DR-FITC and 7AAD nuclei staining in the lung (projection × 40 magnification and section at zoom × 2). Note intracellular localization of anti–HLA-DR staining. HLA-DR staining was either direct with FITC-conjugated mAb or indirect with streptavidin and is representative of tested samples of lung harvested from 10 different engrafted mice. Note the large infiltration of human cells in the lung as well as dendritic morphology of HLA-DR+ cells.
Spleen contains predominantly human B cells and only rare human DCs. Immunofluorescence and confocal analysis of frozen tissue sections of spleen harvested from different cohorts of mice (A, mouse that received a transplant of 6 × 105 fetal liver CD34+ HPCs; and B, mouse that received a transplant of 5 × 106 adult peripheral blood CD34+ HPCs. (A) Triple staining with anti–human CD11c-FITC (green), anti–human CD45-TxR (red) and anti–human HLA-DR-Cy5 (blue). Single fluorescence and overlay at × 40 magnification. Note only few cells with the phenotype of mDCs. (B) Double staining with anti–human IgD-FITC (green) and anti–human CD45 TxR (red). Single fluorescence and overlay at × 63 magnification.
Spleen contains predominantly human B cells and only rare human DCs. Immunofluorescence and confocal analysis of frozen tissue sections of spleen harvested from different cohorts of mice (A, mouse that received a transplant of 6 × 105 fetal liver CD34+ HPCs; and B, mouse that received a transplant of 5 × 106 adult peripheral blood CD34+ HPCs. (A) Triple staining with anti–human CD11c-FITC (green), anti–human CD45-TxR (red) and anti–human HLA-DR-Cy5 (blue). Single fluorescence and overlay at × 40 magnification. Note only few cells with the phenotype of mDCs. (B) Double staining with anti–human IgD-FITC (green) and anti–human CD45 TxR (red). Single fluorescence and overlay at × 63 magnification.
Plasmacytoid and myeloid DCs in the bone marrow of NOD/SCID mice engrafted with human CD34+ HPC. (A) Frequency of pDCs and mDCs in the total bone marrow (n = 18) as determined by the frequency of lineage negative, HLA-DR+ cells with reciprocal expression of CD123 and CD11c, respectively. (B) Flow cytometry plots of mDCs and pDCs analysis. (C) Low migration of human DCs into spleen is confirmed by the flow cytometry analysis of single cell suspensions (n = 13, all subsets and tissue localization per each of the 13 mice that received transplants of CD34+ HPCs from various sources).
Plasmacytoid and myeloid DCs in the bone marrow of NOD/SCID mice engrafted with human CD34+ HPC. (A) Frequency of pDCs and mDCs in the total bone marrow (n = 18) as determined by the frequency of lineage negative, HLA-DR+ cells with reciprocal expression of CD123 and CD11c, respectively. (B) Flow cytometry plots of mDCs and pDCs analysis. (C) Low migration of human DCs into spleen is confirmed by the flow cytometry analysis of single cell suspensions (n = 13, all subsets and tissue localization per each of the 13 mice that received transplants of CD34+ HPCs from various sources).
NOD/SCID mice develop human plasmacytoid and myeloid DCs
Human CD45+ cells represented 20% to 70% of bone marrow cells (Table 2, the average number of bone marrow cells harvested from 19 animals was 43 ± 11 × 106; range, 22-70 × 106 cells). Bone marrow displayed a remarkably high frequency of both myeloid (average, 3.5% ± 3.3%, n = 18) and plasmacytoid (average, 2.7% ± 2.3%, n = 18) human DCs, defined in the gate for lineage negative, HLA-DR+ cells with reciprocal expression of CD11c or CD123. As shown in Figure 4, pDCs could represent up to 8% of human cells. Phenotypically, both pDCs and mDCs resembled the DC subsets found in human blood40 with regard to reciprocal CD11c and CD123 expression as well as HLA-DR expression with pDCs expressing lower levels of surface HLA-DR (Figure 4B). Spleen contained only few cells with the phenotype of myeloid or plasmacytoid DCs (Figure 4C). Blood contained overall low numbers of human cells (data not shown) but both mDCs and pDCs were present, albeit at low frequency (∼ 0.3% and < 0.1%, respectively, n = 13; Figure 4C) together with CD19+ B cells and CD14+ monocytes. Low levels of CD45+ cells staining with anti–CD3 mAb could be seen, and the characterization of these cells will require further analysis (Table 2). Thus, both human plasmacytoid and myeloid DC subsets were clearly identified in the bone marrow and peripheral blood of all NOD/SCID mice engrafted with human CD34+ cells.
Frequency of human cells in reconstituted NOD/SCID mice bone marrow; B cells (CD19+), monocytes (CD14+), and cells labeling with CD3 are represented as a fraction of human CD45+ cells
. | . | Bone marrow . | . | . | . | Spleen . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment . | HPCs source . | % CD45+ . | % CD19* . | % CD14* . | % CD3* . | % CD45+ . | % CD19* . | ||||
| 17 ± 26 | 87 ± 9 | ||||||||||
| 1, n = 5 | MPB, FL | 31 ± 31 | 62 ± 34 | ND | ND | n = 4 | n = 4 | ||||
| 2, n = 4 | MPB | 59 ± 9 | 63 ± 14 | 9 ± 4.6 | 2 ± 0.4 | ND | ND | ||||
| 3, n = 6 | CB/FL | 34 ± 8 | 75 ± 10 | 11 ± 4 | 1.5 ± 0.6 | ND | ND | ||||
| 4, n = 3† | FL | 38 ± 13 | 37 ± 1.7 | 37 ± 2.6 | 2 ± 0.6 | ND | ND | ||||
| 5, n = 4 | MPB | 9 ± 6.5 | 68 ± 6 | 28 ± 5 | 4.5 ± 2 | 2 ± 2 | 69 ± 10 | ||||
| 6, n = 2 | CB | 60 | ND | ND | 2.8 | ND | ND | ||||
. | . | Bone marrow . | . | . | . | Spleen . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment . | HPCs source . | % CD45+ . | % CD19* . | % CD14* . | % CD3* . | % CD45+ . | % CD19* . | ||||
| 17 ± 26 | 87 ± 9 | ||||||||||
| 1, n = 5 | MPB, FL | 31 ± 31 | 62 ± 34 | ND | ND | n = 4 | n = 4 | ||||
| 2, n = 4 | MPB | 59 ± 9 | 63 ± 14 | 9 ± 4.6 | 2 ± 0.4 | ND | ND | ||||
| 3, n = 6 | CB/FL | 34 ± 8 | 75 ± 10 | 11 ± 4 | 1.5 ± 0.6 | ND | ND | ||||
| 4, n = 3† | FL | 38 ± 13 | 37 ± 1.7 | 37 ± 2.6 | 2 ± 0.6 | ND | ND | ||||
| 5, n = 4 | MPB | 9 ± 6.5 | 68 ± 6 | 28 ± 5 | 4.5 ± 2 | 2 ± 2 | 69 ± 10 | ||||
| 6, n = 2 | CB | 60 | ND | ND | 2.8 | ND | ND | ||||
Average ± standard deviation.
MPB indicates mobilized peripheral blood HPCs (G-CSF); CB, cord blood HPCs; FL, fetal liver HPCs; and ND, not done.
Percentage positive in the gate for CD45+ cells.
Only the data from mice that were reconstituted but did not receive influenza virus are given.
Human DCs generated in NOD/SCID mice are functional in vitro
We next determined whether the human DCs colonizing mouse tissues are functional in vitro upon isolation. To this end, human cells were enriched from bone marrow by depletion of mouse cells using anti–murine CD45 mAb microbeads. The enriched human CD45+ cells were 75% and 95% pure in 2 independent experiments. Human CD11c+ mDCs were further enriched either by depletion of B cells and positive selection using Miltenyi DC beads or by sorting based on lin–HLA-DR+CD11c+CD123–phenotype. As one of the characteristic features of DCs is their capacity to initiate immune responses, purified mDCs were used as stimulators in mixed lymphocyte reaction (MLR) with human allogeneic CD4+ T cells. As shown in Figure 5A, human mDCs isolated from NOD/SCID bone marrow were able to induce vigorous allogeneic T-cell proliferation, thus confirming their DC function. We have also analyzed the capacity of human mDCs isolated from the bone marrow of engrafted mice to present antigens to autologous CD4 T cells. To this end, mDCs were isolated, pulsed with tetanus toxoid (TT) (4 LFU/mL), and cultured with autologous CD4 T cells for 6 days. As shown in Figure 5B, significant thymidine incorporation was observed only in the wells where mDCs were pulsed with TT, suggesting that (1) human mDCs can present recall antigens to autologous T cells, and (2) cross-presentation of murine antigens is very low if at all.
Human plasmacytoid and myeloid DCs isolated from bone marrow of mice that received transplants are functional in vitro. (A) Myeloid DCs are enriched from the bone marrow of engrafted NOD/SCID mice by depletion of mouse CD45+ cells and subsequent positive selection of human mDCs using BDCA-1– and BDCA-3–coated magnetic beads as described in “Material and methods.” Enriched human DCs are irradiated and plated at graded doses (ordinate axis) with allogeneic human CD4 T cells (1 × 105/well). Proliferation is determined at day 5 of culture by thymidine incorporation (vertical axis). Data are representative of 2 independent experiments from 2 different cohorts of mice engrafted with CD34+ HPCs from different sources (cord blood or G-CSF–mobilized peripheral blood). (B) mDCs are isolated, pulsed with TT (4 LFU/mL), and plated at graded doses (ordinate axis) with autologous human CD4 T cells (1 × 105/well). Proliferation is determined at day 6 of culture by thymidine incorporation (vertical axis). Data are representative of 2 independent experiments from a cohort of mice engrafted with low numbers of CD34+ HPCs (1.5 × 106 CD34+ cells per animal) from G-CSF–mobilized peripheral blood. (C) Human cells are enriched from the bone marrow of engrafted NOD/SCID mice by depletion of mouse CD45+ cells and cultured at graded doses (ordinate axis) overnight (16 hours) with live influenza virus at low viral concentration (50 viral units based on hemagglutinin titer). Supernatants are tested for IFN-α by enzyme-linked immunosorbent assay (ELISA). Results from 2 independent experiments using mice from 2 different cohorts are shown. In experiment no. 2, 2 mice were tested. Error bars of triplicates.
Human plasmacytoid and myeloid DCs isolated from bone marrow of mice that received transplants are functional in vitro. (A) Myeloid DCs are enriched from the bone marrow of engrafted NOD/SCID mice by depletion of mouse CD45+ cells and subsequent positive selection of human mDCs using BDCA-1– and BDCA-3–coated magnetic beads as described in “Material and methods.” Enriched human DCs are irradiated and plated at graded doses (ordinate axis) with allogeneic human CD4 T cells (1 × 105/well). Proliferation is determined at day 5 of culture by thymidine incorporation (vertical axis). Data are representative of 2 independent experiments from 2 different cohorts of mice engrafted with CD34+ HPCs from different sources (cord blood or G-CSF–mobilized peripheral blood). (B) mDCs are isolated, pulsed with TT (4 LFU/mL), and plated at graded doses (ordinate axis) with autologous human CD4 T cells (1 × 105/well). Proliferation is determined at day 6 of culture by thymidine incorporation (vertical axis). Data are representative of 2 independent experiments from a cohort of mice engrafted with low numbers of CD34+ HPCs (1.5 × 106 CD34+ cells per animal) from G-CSF–mobilized peripheral blood. (C) Human cells are enriched from the bone marrow of engrafted NOD/SCID mice by depletion of mouse CD45+ cells and cultured at graded doses (ordinate axis) overnight (16 hours) with live influenza virus at low viral concentration (50 viral units based on hemagglutinin titer). Supernatants are tested for IFN-α by enzyme-linked immunosorbent assay (ELISA). Results from 2 independent experiments using mice from 2 different cohorts are shown. In experiment no. 2, 2 mice were tested. Error bars of triplicates.
The main property of pDCs is their unique capacity to promptly release large amounts of IFN-α upon viral triggering. Therefore, to monitor the function of human pDCs, human cells purified from bone marrow were incubated with live influenza virus overnight (∼ 16 hours) to assess production of human IFN-α (Figure 5B). Our in vitro studies show that under these conditions only pDCs, but neither human B cells isolated from blood nor ex vivo–generated mDCs, can release IFN-α (data not shown). Human cells from 3 mice of 2 independent cohorts promptly released human IFN-α, thus suggesting the presence of functional human pDCs.
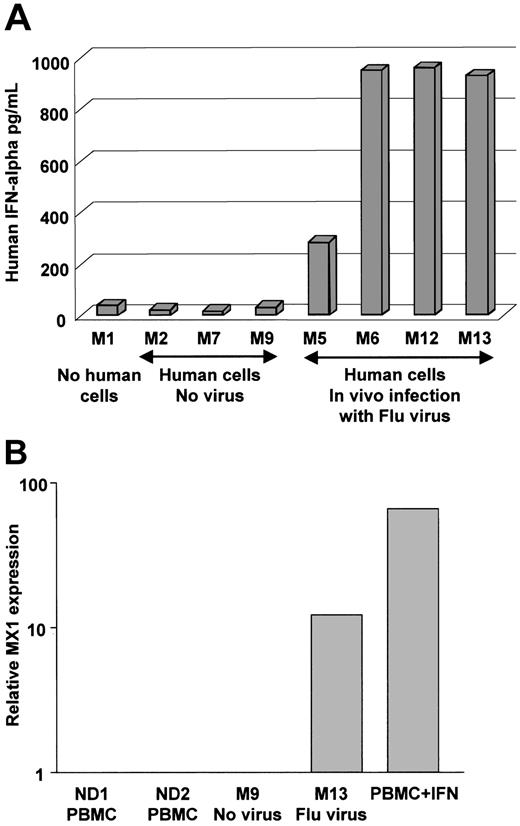
Human cells in NOD/SCID mice release IFN-α in vivo
We next determined whether human DCs would be functional in vivo. To this end, reconstituted and control mice were challenged with live influenza virus (at low virus dose where each mouse was injected intravenously with 3600 viral infectious units as measured by hemagglutinin titer). The response of human cells was assessed 16 hours later. The 4 reconstituted animals that were challenged with the influenza virus displayed considerable levels of human IFN-α (300-900 pg/mL) in their serum (Figure 6A). To further establish the function of human IFN-α in mice exposed to influenza virus, we have measured, using real-time PCR, the expression IFN-α–inducible MX1 gene41 in human cells enriched from NOD/SCID bone marrow. As shown in Figure 6B, human cells enriched from the bone marrow of mouse exposed to virus express high levels of human MX1 message compared to cells enriched from control mouse engrafted with CD34+ HPCs but not exposed to virus. Thus, human cells differentiated in NOD/SCID, possibly pDCs, can promptly release human IFN-α upon viral exposure in vivo, and thereafter, human cells respond to the released IFN-α.
Injection of influenza virus into NOD/SCID mice reconstituted with human DCs triggers IFN-α release into the blood. (A) NOD/SCID mice were engrafted (except for mouse no. 1, which served as control) with human CD34+ HPC and 10 weeks later injected intravenously with either PBS (vehicle control) or 3600 viral units (based on hemagglutinin titer) per mouse. Mice were killed 16 hours after injection. Human IFN-α concentration in the serum was measured using ELISA with human cytokine-specific antibodies that do not cross-react with mouse IFN-α. P = .02 in paired t test. (B) Human cells enriched from bone marrow were analyzed by real-time RT-PCR for the levels of MX1 message expression. Relative RNA expression (vertical axis, log scale) was compared to MX1 expression in PBMCs from healthy controls. As a positive control we have used human PBMCs cultured overnight with IFN-α.
Injection of influenza virus into NOD/SCID mice reconstituted with human DCs triggers IFN-α release into the blood. (A) NOD/SCID mice were engrafted (except for mouse no. 1, which served as control) with human CD34+ HPC and 10 weeks later injected intravenously with either PBS (vehicle control) or 3600 viral units (based on hemagglutinin titer) per mouse. Mice were killed 16 hours after injection. Human IFN-α concentration in the serum was measured using ELISA with human cytokine-specific antibodies that do not cross-react with mouse IFN-α. P = .02 in paired t test. (B) Human cells enriched from bone marrow were analyzed by real-time RT-PCR for the levels of MX1 message expression. Relative RNA expression (vertical axis, log scale) was compared to MX1 expression in PBMCs from healthy controls. As a positive control we have used human PBMCs cultured overnight with IFN-α.
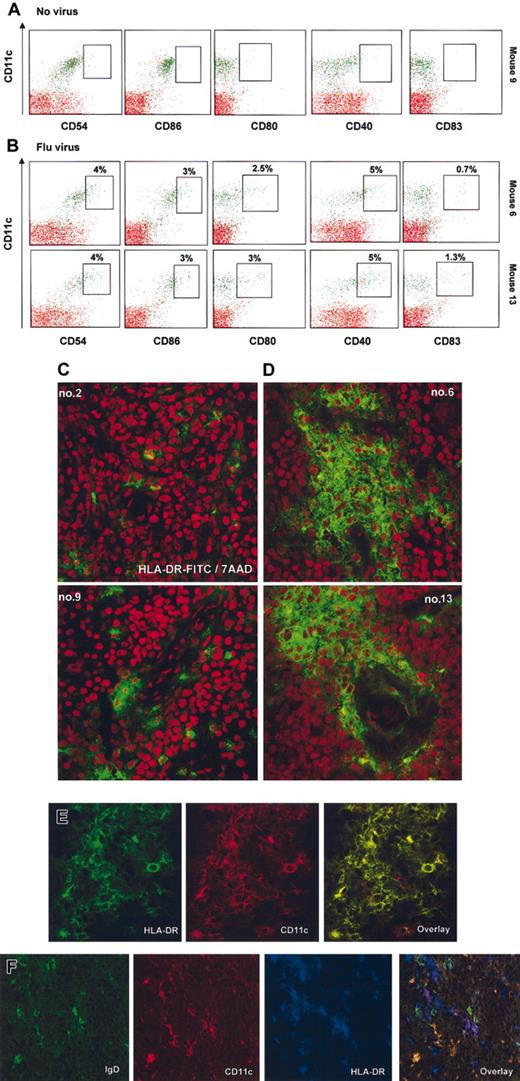
Human mDCs in NOD/SCID mice can mature in vivo
To monitor the in vivo function of human mDCs, we have determined their capacity to undergo maturation in response to environmental stimuli such as exposure of mice to influenza virus. As shown in Figure 7, less than 1% of mDCs from (control/untreated) mice express the maturation antigens CD40, CD80, and CD83 (Figure 7A) as well as HLA-DR (not shown). The CD11c+ mDCs from virus-infected animals coexpressed HLA-DR and CD40, CD80, and CD83 (∼ 4% of mature CD11c+ mDCs in the fraction of human cells enriched from bone marrow) (Figure 7B). In addition, mice exposed to virus displayed lower numbers of human cells, including DCs, in the bone marrow (Table 3). Conversely, sections of spleens harvested from these mice showed multiple areas with aggregates of HLA-DR+ cells (Figure 7C-D). Further analysis showed that these cells coexpressed CD11c but not IgD, thus suggesting infiltration with human mDCs (Figure 7E-F). In a control experiment, mice were injected with human recombinant IFN-α (Roferon; 1 × 106 U/mice corresponding to 4 μg/mice), and 16 hours later serum concentration of IFN-α reached the level of 200-400 pg/mL (data not shown). These mice also displayed activation of mDCs in the bone marrow (not shown). The pDCs phenotype, as determined by HLA-DR, CD11c, and CD123 expression, was not affected. Taken together, these data indicate that the human mDCs within reconstituted NOD/SCID mice are functional in vivo and undergo maturation in response to viral infection.
Injection of influenza virus into NOD/SCID mice reconstituted with human DCs triggers activation and migration of CD11c+ mDCs. (A-B) Bone marrows of mice no. 9, no. 6, and no. 13 in Figure 5 were depleted of murine cells as described in “Material and methods,” and their cell surface phenotype was analyzed by flow cytometry. Surface expression of the DC activation/maturation markers CD54 (ICAM-1), costimulatory molecules such as CD86, CD80, and CD40, as well as CD83 were determined (boxes indicate frequency of CD11c+ cells expressing indicated marker in the total fraction of human cells enriched from the bone marrow, < 1% of expression in mice that did not receive virus). (C-D) Spleen section of mice engrafted with human cells that received either vehicle control (C, left panels) or flu virus (D, right panels). Red illustrates structure of the tissue by nuclei labeling with 7AAD. Human cells are labeled with anti–human HLA-DR-FITC. Spleens of mice infected with influenza virus display aggregation and infiltration of human cells (areas are representative of the whole section). (E-F) Human cellular aggregates in the spleen of NOD/SCID mice infected with virus are composed predominantly of HLA-DR+CD11c+ mDCs, and only rare double staining of CD11c+ with IgD can be seen. (E) Double staining with anti-human HLA-DR-FITC (green), anti–human CD11c-PE (red), and overlay at × 63 magnification (no. 13 from Figure 6). (F) Triple staining with anti–human IgD-FITC (green), anti–human CD11c-PE (red), anti–human HLA-DR-Cy5 (blue), and overlay at × 63 magnification (no. 6 from Figure 6). Note comparatively high levels of expression on DCs (purple) as opposed to IgD+ B cells and strong staining with IgD. Original magnification, × 40.
Injection of influenza virus into NOD/SCID mice reconstituted with human DCs triggers activation and migration of CD11c+ mDCs. (A-B) Bone marrows of mice no. 9, no. 6, and no. 13 in Figure 5 were depleted of murine cells as described in “Material and methods,” and their cell surface phenotype was analyzed by flow cytometry. Surface expression of the DC activation/maturation markers CD54 (ICAM-1), costimulatory molecules such as CD86, CD80, and CD40, as well as CD83 were determined (boxes indicate frequency of CD11c+ cells expressing indicated marker in the total fraction of human cells enriched from the bone marrow, < 1% of expression in mice that did not receive virus). (C-D) Spleen section of mice engrafted with human cells that received either vehicle control (C, left panels) or flu virus (D, right panels). Red illustrates structure of the tissue by nuclei labeling with 7AAD. Human cells are labeled with anti–human HLA-DR-FITC. Spleens of mice infected with influenza virus display aggregation and infiltration of human cells (areas are representative of the whole section). (E-F) Human cellular aggregates in the spleen of NOD/SCID mice infected with virus are composed predominantly of HLA-DR+CD11c+ mDCs, and only rare double staining of CD11c+ with IgD can be seen. (E) Double staining with anti-human HLA-DR-FITC (green), anti–human CD11c-PE (red), and overlay at × 63 magnification (no. 13 from Figure 6). (F) Triple staining with anti–human IgD-FITC (green), anti–human CD11c-PE (red), anti–human HLA-DR-Cy5 (blue), and overlay at × 63 magnification (no. 6 from Figure 6). Note comparatively high levels of expression on DCs (purple) as opposed to IgD+ B cells and strong staining with IgD. Original magnification, × 40.
Frequency of human cells in the bone marrow of SCID mice reconstituted with human CD34+HPCs and challenged or not in vivo with live flu virus
Subsets of human cells . | No virus, n = 3 . | Flu virus, n = 4 . |
|---|---|---|
| CD45 | 52.4 ± 17.6 | 21.6 ± 12.3 (P < .05) |
| CD19 | 19.2 ± 5.8 | 10.2 ± 6 (P = .1) |
| CD14 | 19.5 ± 6.8 | 5.6 ± 3 (P < .05) |
| CD4 | 22 ± 6.5 | 7.9 ± 3.9 (P < .05) |
| CD11c+DC | 12.3 ± 5.8 | 4.4 ± 2.9 (P = .06) |
| CD123+DC | 5.6 ± 3.1 | 2.6 ± 1.7 (P = .1) |
Subsets of human cells . | No virus, n = 3 . | Flu virus, n = 4 . |
|---|---|---|
| CD45 | 52.4 ± 17.6 | 21.6 ± 12.3 (P < .05) |
| CD19 | 19.2 ± 5.8 | 10.2 ± 6 (P = .1) |
| CD14 | 19.5 ± 6.8 | 5.6 ± 3 (P < .05) |
| CD4 | 22 ± 6.5 | 7.9 ± 3.9 (P < .05) |
| CD11c+DC | 12.3 ± 5.8 | 4.4 ± 2.9 (P = .06) |
| CD123+DC | 5.6 ± 3.1 | 2.6 ± 1.7 (P = .1) |
Experiment 4 in Table 1. B cells (CD19+), monocytes (CD14+), and CD4+ cells (DCs) were identified as a fraction of human CD45+ cells. CD11c+ mDCs and CD123+ pDCs were identified as a fraction of total bone marrow. Average ± standard deviation.
Discussion
Here we show that NOD/SCID mice provide an environment permissive for differentiation of functional human myeloid and plasmacytoid DC subsets. Within 8 weeks of transplantation of human CD34+ HPCs, human DCs could be detected in virtually all peripheral tissues. Thus, DCs that express Langerin can be found in the skin. These DCs are immature as indicated by the presence of intracellular major histocompatibility complex (MHC) class II compartments. These characteristics correspond to LCs in the human skin. Immature human DCs also can be detected in the lungs. The presence of human DCs in the lung is of particular importance, as it may permit the analysis of how respiratory pathogens will affect their DC function. Spleen contained only few human DCs. However, as these mice live under sterile conditions, microenvironmental/microbial signals that regulate DC homeostasis and trafficking into secondary lymphoid organs may be lacking. The preliminary data showing accumulation of HLA-DR/CD11c+ cells in the spleen of mice injected with influenza virus are in line with this hypothesis.
Our results indicate that bone marrow may represent a major reservoir of human DCs, in agreement with an earlier study.42 Similar to human blood, the frequency of DC subsets in the blood of engrafted mice is very low. This may provide a model to study the in vivo mobilization and trafficking of DC subsets. Indeed, we have previously shown that FLT 3 ligand and G-CSF differentially mobilize human DC subsets in humans.40 Thus, mice with human DCs may allow us to unravel the mechanisms of such differential mobilization. However, before such studies can be undertaken a detailed analysis of human DCs and murine microenvironment is required to determine potential artifacts. The very exciting finding is the detection of human plasmacytoid DCs in these mice. While the studies of Spits et al suggest that mouse stromal cells can participate in the differentiation of human pDCs in vitro,43 we were surprised by the high density of human pDCs in bone marrow. It could reflect a low rate of release into the periphery in the steady state or that bone marrow may indeed be a major reservoir for these cells, a property that will need further examination in human bone marrow. Indeed, DC subsets in human bone marrow have not been extensively studied. Earlier reports indicated the presence of allostimulatory cells lacking lineage markers within the bone marrow mononuclear cell population.44 The increased numbers of CD4+ cells with dendritic morphology in donor bone marrow have been associated with the increased risk of leukemia relapse in HLA-matched recipients.45 Furthermore, relatively little is known about the localization of human pDCs in vivo in steady state. They have been described to be present in high numbers in the reactive (for example, inflamed tonsils) or neoplastic lymphoid tissue.46,47 More recently they have been found in human thymus.48,49 However, under steady state pDCs are rare in periphery, outside lymphoid organs. Yet, they can be found in the diseased tissue, for example, at the sites of allergic reactions,50 infiltrate skin lesions in lupus,51 as well as in psoriasis and in contact dermatitis.52 Our model of human DCs in NOD/SCID mice may prove excellent to study pDCs trafficking in vivo.
Thus, human DCs can differentiate in NOD/SCID mice engrafted with human HPCs and can be detected not only in the bone marrow as described earlier42 but also in the peripheral tissues, albeit at variable levels. The variability of DCs differentiation may be related to the frequency of DC progenitors among CD34+ HPCs. The absence of DCs in peripheral tissues described in earlier study42 may be related to the use of mononuclear cell fractions rather than to purified CD34+ HPCs. Human DCs that have differentiated in NOD/SCID mice retain their principal functional properties. However, further analysis is needed to determine whether murine environment alters human DC migration from peripheral tissues to secondary lymphoid organs and in vivo functional maturation.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-02-0384.
Supported by grants from Dana Foundation (J.B.), Defense Advanced Research Projects Agency, and Tietze Foundation (J.B., K.P.), National Institutes of Health (RO1 CA78846 [J.B.] and AI39416 [J.V.G.]).
A.K.P. and J.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Authors from the Baylor Institute for Immunology Research wish to thank Olivier Reygrobellet for technical help; Bi-Jue Chang, Susan Hicks, and Dr Joseph Fay for taking care of enrollment and G-CSF mobilization of healthy volunteers; Susan Burkeholder, Jennifer Finholt, and Fabienne Kerneis for HPC isolation and freezing; Drs. Bo Wu and Isabelle Ding for discussion and help in the experiments; and Kathy Brooks for continuous help.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal