Abstract
Acquired thrombotic thrombocytopenic purpura (TTP) has been linked to severe deficiency of ADAMTS-13 activity caused by autoantibodies inhibitory to ADAMTS-13. We report data on a patient with confirmed TTP who had severely reduced ADAMTS-13 activity but showed no ADAMTS-13 inhibition in a widely used fluid phase activity assay. With a newly developed enzyme-linked immunosorbent assay, using immobilized recombinant ADAMTS-13, we found high titers of IgM and IgG antibodies that bound to ADAMTS-13, but did not neutralize protease activity. These autoantibodies probably influenced the half-life of ADAMTS-13 or its binding to the endothelial cell surface, thereby compromising ADAMTS-13 activity in vivo. Given that ADAMTS-13 may interact physiologically with various receptors or ligands, the occurrence, distribution, and the epitope mapping of nonneutralizing antibodies will be an important area for future research.
Introduction
von Willebrand factor (VWF) is proteolytically degraded by a specific VWF-cleaving metalloprotease,1,2 recently termed ADAMTS-13.3 Reduced activity of ADAMTS-13 results in the persistence of unusually large VWF (ULVWF)4-6 that can induce platelet aggregation7 and the formation of platelet- and VWF-rich thrombi under high shear stress conditions.8 Thrombotic thrombocytopenic purpura (TTP), a devastating disease characterized by microangiopathic hemolytic anemia and thrombocytopenia,9 is characterized by severe (< 5%) reduced ADAMTS-13 activity.10 The hereditary form of TTP has been related to specific gene mutations in the ADAMTS13 locus resulting in severe ADAMTS-13 deficiency.11 Acquired TTP has been attributed to autoantibodies that inhibit ADAMTS-13 activity.12-14 Analytically, acquired TTP can be discriminated from hereditary TTP by the detection of inhibitory antibodies using assays measuring ADAMTS-13 proteolytic activity in vitro.12,13 In these assays, the high shear stress found in arterial capillaries is mimicked by the presence of urea that leads to ADAMTS-13 activity under nonphysiologic conditions.1 Absence of inhibitory antibodies is suggestive of hereditary TTP, whereas the presence of inhibitory antibodies implies TTP mediated by autoimmune antibodies.
The gene encoding human ADAMTS13 was recently cloned3,15 and ADAMTS-13 was shown to be the enzyme responsible for the proteolytic degradation of VWF in plasma.16,17 Data suggest that in vivo ADAMTS-13 is active while bound to the endothelial cell surface.18 Other mechanisms, apart from direct protease activity inhibition by autoantibodies, could therefore play a part in causing TTP. For instance, ADAMTS-13 activity might be inhibited by autoantibodies that prevent enzyme binding to endothelial cell surfaces or antibodies that block interactions with cellular or plasmatic modulators, which are necessary for full enzymatic activity in vivo.19 Another possibility is that nonneutralizing antibodies reduce the absolute amount of circulating ADAMTS-13 in the plasma by antibody-mediated clearance.
Study design
For the enzyme-linked immunosorbent assay (ELISA)–based anti–ADAMTS-13 antibody detection in plasma, recombinant ADAMTS-13 was C-terminal His-tagged by the addition of 6 histidine codons to the cDNA and stably expressed in HEK 293 cells (human embryonic kidney fibroblasts; CRL-1573, American Type Culture Collection, Manassas, VA). Protein purification was done by immobilized metal chelate affinity chromatography. His-tagged ADAMTS-13 (2 μg, 2-fold excess) was immobilized on the surface of ELISA (Nunc Maxi Sorb; Nalge Nunc International, Rochester, NY) plate by an anti–His-tag antibody (Qiagen, Hilden, Germany). The patient's plasma was diluted in the range from 1:20 up to 1:1200 in phosphate-buffered saline (PBS)/2% wt/vol bovine serum albumin (BSA) and added to the immobilized protease. After several washing steps (PBS/0.1% vol/vol Tween), bound anti–ADAMTS-13 IgG antibodies were detected by phosphatase-labeled secondary antibodies specifically binding the constant region of human IgG (Sigma-Aldrich, Steinheim, Germany). In a separate experiment, antibodies binding to the human μ chain (Sigma-Aldrich) were used to detect anti–ADAMTS-13 IgM antibodies. Both detection antibodies were specific for their immunoglobulin classes and no cross-reaction was observed. Normal human plasma (NHP) was used as control. The standard deviation (SD) was calculated from several lots of NHP. The cut-off point above which we considered the dilution to be positive was the value obtained for the NHP in the particular experiment plus 2 times the SD calculated from the several NHP lots.
Results and discussion
We report data on a 70-year-old woman who was admitted to the hospital after suffering progressive neurologic derangement for several days. The symptoms increased, and the patient became confused and somnolent. The patient gave informed consent for blood sampling, and the study was performed according to the guidelines defined in the Declaration of Helsinki. Laboratory analysis showed severe thrombocytopenia (9 G/L) and Coombs-negative hemolysis (red blood cell count, 2.6 × 10–9/L; hemoglobin, 7.5 g/dL; reticulocyte count, 181 × 10–6/L; lactate dehydrogenase [LDH], 2410 U/mL; bilirubin, 2.6 mg/dL; haptoglobin, < 12 mg/dL) with red cell fragmentation (47 schistocytes/1000 red cells). The patient was fever free but had arterial hypertension. Her renal function was normal (creatinine, 1.07 mg/dL).
This constellation suggested acquired TTP. Plasma exchange therapy (40 mL/kg body weight daily), supplemented with prednisone (1 mg/kg body weight daily) and packed red cells, was started immediately. Platelet concentrates were avoided. After the third plasma exchange her platelet count increased to 60 × 10–6/L, her LDH normalized, and the neurologic disturbance ceased. Although the patient responded to plasma exchange therapy she experienced at least 7 relapses within the following 2 months. Additional treatment consisted of corticosteroids and protein A immunoadsorption (which resulted in a short-term success); finally a splenectomy was performed. The patient died 64 days after the initial diagnosis during another fulminant relapse of TTP.
Examination of the patient's plasma on admission showed a high VWF:Ag level (3.2 U/mL). Neither ULVWF polymers nor high-molecular-weight VWF multimers were detectable in a sodium dodecyl sulfate–agarose analysis (data not shown). Using a quantitative immunoblot assay1 and a collagen-binding ELISA after ADAMTS-13–induced digestion of VWF,20 ADAMTS-13 activity was found to be severely reduced (< 0.03 U/mL). Samples of the patient's plasma and purified IgG and IgG-depleted fractions from the plasma were repeatedly screened for ADAMTS-13–inhibiting antibodies but no inhibitory antibodies could be detected, which was surprising because the patient's clinical presentation strongly supported a diagnosis of acquired TTP.
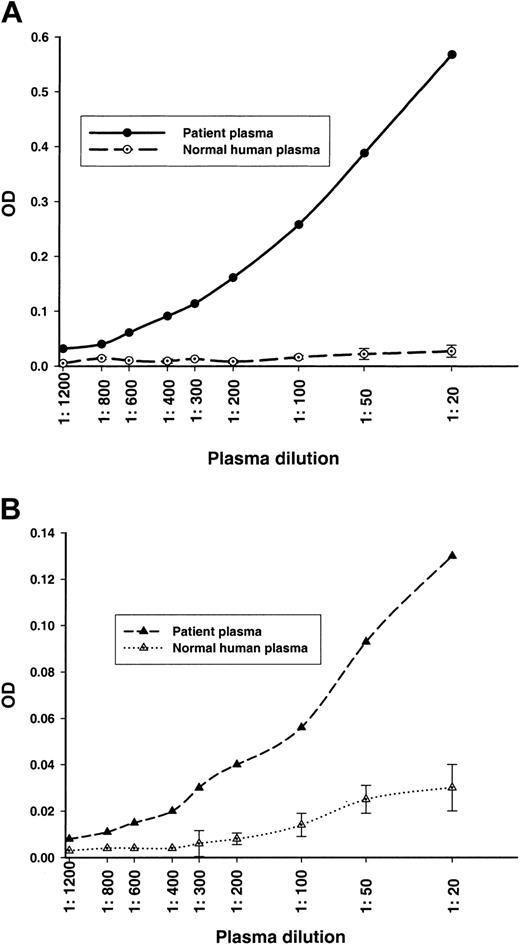
We next analyzed the patient's plasma for anti–ADAMTS-13 antibodies that do not neutralize ADAMTS-13 proteolytic activity, but still bind to ADAMTS-13. We used 2 newly developed ELISAs and found high titers of anti–ADAMTS-13 IgG antibodies (≥ 1:600; Figure 1A) and of anti–ADAMTS-13 IgM antibodies (≥ 1:400; Figure 1B) in the patient's plasma, but not in the control plasma.
Anti–ADAMTS-13 antibodies detected in an ADAMTS-13 antigen-specific ELISA system. NHP was used as a control. The error bars of the NHP indicate the 2 times added SD calculated from several plasma lots. Plasma dilutions ranging from 1:20 to 1:1200 were analyzed. (A) Anti–ADAMTS-13 IgG titer. (B) Anti–ADAMTS-13 IgM titer.
Anti–ADAMTS-13 antibodies detected in an ADAMTS-13 antigen-specific ELISA system. NHP was used as a control. The error bars of the NHP indicate the 2 times added SD calculated from several plasma lots. Plasma dilutions ranging from 1:20 to 1:1200 were analyzed. (A) Anti–ADAMTS-13 IgG titer. (B) Anti–ADAMTS-13 IgM titer.
Our data most likely indicate an early stage of TTP during which plasma VWF levels are increased, but ULVWF and high-molecular-weight VWF are undetectable because they are consumed and deposited during platelet aggregation.21 In accordance with the current pathophysiologic hypothesis,19 we found a severe reduction of ADAMTS-13 proteolytic activity, but we could not detect inhibiting antibodies. Thus, we have shown that IgM and IgG antibodies are present in plasma from a TTP patient and that these antibodies react with ADAMTS-13, but do not inhibit its activity. A possible explanation is that the antibodies bind to regions of the protein that are not necessary for exerting protease activity under the conditions of the in vitro assay. In vivo they could, however, lead to increased clearance of the protease-antibody complexes or interfere with protease binding to the endothelial cell surface, where cleavage of ULVWF probably occurs, or both. On the other hand, we cannot rule out the possibility of the presence of an inhibitor, just sufficient to neutralize enzyme activity in vivo but below the assay's detection limit in vitro.
In summary, the lack of ADAMTS-13 activity in acquired TTP is frequently attributed to the presence of an autoantibody inhibiting ADAMTS-13–mediated cleavage of ULVWF.12-14 We have been able to show the presence of anti–ADAMTS-13 antibodies (IgM and IgG) that do not neutralize ADAMTS-13 activity in standard assays, but most likely impair ADAMTS-13 activity by mechanisms different from merely blocking substrate-cleaving activity. The ELISA-based assay system we used is fast and very simple and may have a much greater sensitivity for detecting anti–ADAMTS-13 antibodies than the more complex standard in vitro assay.
The discovery of the nonneutralizing antibodies could have a considerable impact on our understanding of the pathophysiology of TTP in general and in particular might be important for the diagnosis of hereditary TTP. In addition, our findings support clinical decisions to start plasma exchange therapy without delay in patients with clinically suspected TTP.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-05-1616.
Seven authors (F.S., B.T., B.P., G.M., M.D., F.D., and M.R.) declare a financial interest in Baxter BioScience.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Elise Langdon-Neuner for editing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal