Abstract
A severe lack of von Willebrand factor–cleaving protease (VWF-CP) activity can cause thrombotic thrombocytopenic purpura (TTP). This protease was recently identified as a member of the ADAMTS family, ADAMTS-13. It consists of a preproregion, a metalloprotease domain, a disintegrin-like domain, a thrombospondin type-1 motif (Tsp1), a cysteine-rich domain, a spacer domain, additional Tsp1 repeats, and CUB domains. To explore the structural and functional relationships of ADAMTS-13, we prepared here 13 sequential COOH-terminal truncated mutants and a single-point mutant (ArgGlyAsp [RGD] to ArgGlyGlu [RGE] in the cysteine-rich domain) and compared the activity of each mutant with that of the wild-type protein. The results revealed that the truncation of the cysteine-rich/spacer domains caused a remarkable reduction in VWF-CP activity. We also prepared immunoglobulin G (IgG) fractions containing inhibitory autoantibodies against ADAMTS-13 from plasma from 3 patients with acquired TTP, and we performed mapping of their epitopes using the aforementioned mutants. The major epitopes of these antibodies were found to reside within the cysteine-rich/spacer domains. These results suggest that the ADAMTS-13 cysteine-rich/spacer domains are essential for VWF-CP activity.
Introduction
Von Willebrand factor (VWF) functions as a molecular glue through its anchoring of platelets at sites of injured vessel walls under high shear stress.1 Mature VWF contains 2050–amino acid residues, has a molecular weight of approximately 250 kDa, and is released from endothelial cells as an “unusually large” multimer (UL-VWFM) with a molecular weight of approximately 30 000 kDa.2-5 In healthy individuals, UL-VWFM is converted rapidly in the circulation to smaller forms, a series of multimers with molecular weights ranging from approximately 500 to 20 000 kDa.6 It is known that the larger multimers have more potent biologic activity.7,8 Under shear stress conditions in the circulation, VWF becomes more susceptible to proteolysis,9-12 resulting in the formation of small but consistent proportions of 176-kDa and 140-kDa fragments derived from the approximately 250-kDa VWF subunit.13 On the basis of these findings, the existence of a specific VWF-cleaving protease (VWF-CP) has been proposed.
UL-VWFM has been found in plasma from patients with thrombotic thrombocytopenic purpura (TTP).14 TTP is characterized by thrombocytopenia, microangiopathic hemolytic anemia, renal failure, neurologic dysfunction, and fever.15 In 1996, a metalloprotease that cleaves a peptide bond between amino acid residues Tyr842 and Met843 within the VWF A2 domain was partially purified.16,17 It was also reported that a deficiency in VWF-CP activity was associated with TTP.18 On the basis of these findings, it was proposed that the UL-VWFM produced as a result of the deficiency in VWF-CP activity promotes microvascular thrombus formation. Congenital or acquired deficiency of VWF-CP activity can cause TTP. Congenital TTP with neonatal onset and frequent relapses is often diagnosed as Upshaw-Schulman syndrome,19 and symptoms of acquired TTP develop idiopathically in association with infection, pregnancy, or autoimmune disease. In addition, most adults with TTP have autoantibodies that inhibit VWF-CP activity.20-22
In 2001, VWF-CP was purified and its partial NH2-terminal amino acid sequence was determined.23-25 On the basis of this sequence, the full-length cDNA for VWF-CP was cloned.25,26 These findings revealed that VWF-CP is a new member, designated ADAMTS-13, of the ADAMTS family of metalloproteases,24-26 named for adisintegrin-like and metalloprotease with thrombospondin type I motif. Positional cloning of a gene responsible for congenital TTP also identified the ADAMTS13 gene.27
The human ADAMTS13 gene spans approximately 37 kb on chromosome 9q34 and contains 29 exons. mRNA encoding ADAMTS-13 spans approximately 5 kb. Studies by Northern blot,25-28 polymerase chain reaction (PCR) amplification,26-29 and living-donor–related liver transplantation30 indicated that ADAMTS-13 is produced primarily in the liver and then released into the circulation. Also, it is known that ADAMTS-13 rapidly cleaves UL-VWFM on the endothelial cell surface under flowing conditions.31
ADAMTS-13 comprises 1427 amino acid residues and consists of a preproregion, a reprolysin-like metalloprotease domain, a disintegrin-like domain, a thrombospondin type-1 motif (Tsp1), a cysteine-rich domain, a spacer domain, 7 additional Tsp1 repeats, and 2 CUB domains.25-28 The cysteinerich domain contains the ArgGlyAsp (RGD) sequence, which is widely used for the recognition of integrin.32
Recently, we reported 5 mutations in the ADAMTS13 gene found in 2 families with Upshaw-Schulman syndrome. The effects of these mutations were confirmed by recombinant expression analysis of these ADAMTS-13 mutants. Two mutants, Arg268Pro and Cys508Tyr, when expressed in cultured HeLa cells, had diminished activity caused by secretion disturbances, and 2 other mutants, Gln449Xaa and Pro475Ser, had very low VWF-CP activity despite normal secretion, whereas Gln448Glu showed no difference from the wild-type ADAMTS-13 in VWF-CP activity.33
Several alternatively spliced and/or COOH-terminal truncated ADAMTS-13 isoforms may exist in vivo.23,25-27 Although a considerable amount of data have been accumulated on the properties of ADAMTS-13, little is known about the structural and functional relationship of ADAMTS-13.
In this study, we prepared 13 sequential COOH-terminal truncated molecules and a single point mutant (ArgGlyAsp [RGD] to ArgGlyGlu [RGE] in the cysteine-rich domain) and compared the activity of each mutant protein with that of wild-type recombinant ADAMTS-13. Furthermore, we prepared immunoglobulin G (IgG) fractions containing inhibitory autoantibodies against ADAMTS-13 from the plasma of 3 patients with acquired TTP and performed epitope mapping using the above mutants. These results suggest that the ADAMTS-13 cysteine-rich/spacer domains are essential for expression of VWF-CP activity.
Materials and methods
Construction of expression plasmids of ADAMTS-13 mutants
Transient expression of ADAMTS-13 mutants
Each of the mutant expression plasmids was transfected into HeLa cells by using the polyamine transfection reagent TransIT-LT1 (Mirus, Madison, WI) according to the manufacturer's instructions. Briefly, HeLa cells were cultured in Dulbecco modified Eagle medium (DMEM; Sigma, St Louis, MO) supplemented with 10% fetal bovine serum. Two micrograms of each expression plasmid was transfected into 40% to 70% confluent cells in a 35-mm well. After a 6-hour incubation, the medium was replaced with 2 mL ASF104 serum-free medium (Ajinomoto, Tokyo, Japan), and the cells were incubated for 72 hours. The medium was then collected, and the cells were washed with phosphate-buffered saline (PBS) and lysed with 2 mL sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris (tris(hydroxymethyl) aminomethane)–HCl, 1% SDS, 1% 2-mercaptoethanol, 0.01% bromophenol blue, 5% glycerol, pH 6.8).
Western blot analyses of the expression of ADAMTS-13 mutants
The collected culture media and cell lysates were subjected to 10% SDS–polyacrylamide gel electrophoresis (PAGE) under nonreducing and reducing conditions, respectively, and transferred to an Immobilon polyvinylidene-diflouride membrane (Millipore, Bedford, MA). After blocking with 30% Block Ace (Dainippon Pharmaceutical, Osaka, Japan) in TBST (50 mM Tris-HCl, 100 mM NaCl, and 0.05% Tween 20, pH 7.9) for 1 hour, the membrane was incubated with anti-FLAG M2 monoclonal antibody (MoAb) (Sigma) for 1 hour. After washing, the membrane was incubated with alkaline phosphatase–labeled goat antimouse IgG + M (American Qualex, San Clemente, CA) for 1 hour. The membrane was thoroughly washed with TBST and TBS (50 mM Tris-HCl and 100 mM NaCl, pH 7.9), and labeled bands were visualized with 5-bromo-4-chloro-3-indolylphosphate/4-nitroblue tetrazolium chloride (BCIP/NBT; KPL, Gaithersburg, MD).
Assay of VWF-CP activity
VWF was purified from human pooled plasma cryoprecipitate as previously described.25 VWF-CP activity was detected by using previously reported methods16,17 with some modifications, as reported previously.25 Briefly, test samples were preincubated with 10 mM BaCl2 for 5 minutes, and then purified VWF (approximately 200 μg/mL) was mixed with the samples, and the mixtures were dialyzed overnight with the use of a circular dialysis membrane (Millipore) against 5 mM Tris-HCl, pH 8.0, containing 1.5 M urea at 37°C. Subsequently, the reaction mixtures were subjected to SDS-PAGE in a 5% gel under reducing or nonreducing conditions. The gel was stained with Coomassie Brilliant Blue R-250.
Analysis of VWF multimeric pattern
This assay was performed as reported previously.19 Briefly, 8 μL concentrated culture medium obtained as described in “Transient expression of ADAMTS-13 mutants” was added to 92 μL purified VWF (1 μg) dissolved in reaction buffer (1.5 M urea, 5 mM Tris-HCl, pH 8.0, 10 mM BaCl2, 1 mM phenylmethylsulfonyl fluoride, and 0.05% NaN3) and incubated at 37°C for 24 hours. After that, 10 μL 100 mM EDTA (ethylenediaminetetraacetic acid, pH 8.0) was added to terminate the reaction. A portion of each reaction mixture was analyzed by SDS–agarose gel electrophoresis, and the multimeric state of VWF was visualized by chemiluminescent Western blot analysis, using anti-VWF antibody.
Purification of inhibitory IgG against ADAMTS-13
IgGs were purified from plasma samples isolated from 3 patients with acquired TTP with the use of a HiTrap protein G HP column (Amersham Biosciences AB, Uppsala, Sweden), according to the manufacturer's instructions. Blood samples were collected from all donors after obtaining informed consent. The inhibitory titers against ADAMTS-13 activity of the 3 plasma samples were 55, 34, and 5.2 Bethesda U/mL. The inhibitor against VWF-CP was measured according to the method by Furlan et al21 with some modifications.19 One Bethesda unit of the inhibitor was defined as the amount that reduced ADAMTS-13 activity to 50% compared with an inhibitor negative control. IgG from healthy individuals was used as a control.
Inhibition of VWF-CP activity by inhibitory IgG
Conditioned medium from cells expressing full-length wild-type ADAMTS-13 was preincubated with 10 mM BaCl2 for 5 minutes, and then incubated with an equal volume of purified inhibitory IgG or healthy IgG as negative control for 30 minutes at 37°C. Residual VWF-CP activity of the mixture was performed as described earlier.
Epitope mapping of the inhibitory IgG against ADAMTS-13
Mapping was performed on the basis of Western blot analyses under nonreducing conditions. In practice, 4 membranes containing concentrated (approximately 2- to 20-fold, respectively) culture media of HeLa cells expressing various COOH-terminal truncated ADAMTS-13 mutants were prepared, and each membrane was incubated for 1 hour with anti-FLAG M2 MoAb or 1 of the 3 plasma IgG samples purified as described in “Inhibition of VWF-CP activity by inhibitory IgG.” After washing, the membranes were incubated with alkaline phosphatase–labeled goat antihuman IgG (Rockland, Gilbertsville, PA) for 1 hour. The membranes were washed thoroughly with TBST and TBS, and bound antibody was visualized with BCIP/NBT. Next, to determine the epitope region more precisely, we performed Western blot analysis under nonreducing conditions by using the competitive inhibition method. Briefly, membranes containing the wild-type and the Trp688Xaa mutant proteins were reacted with purified autoantibodies in the absence or presence of a large excess of the concentrated (approximately 10-fold) culture media of HeLa cells expressing full-length wild-type, Trp688Xaa, or Gln449Xaa ADAMTS-13 proteins.
Results
Expression of ADAMTS-13 mutants
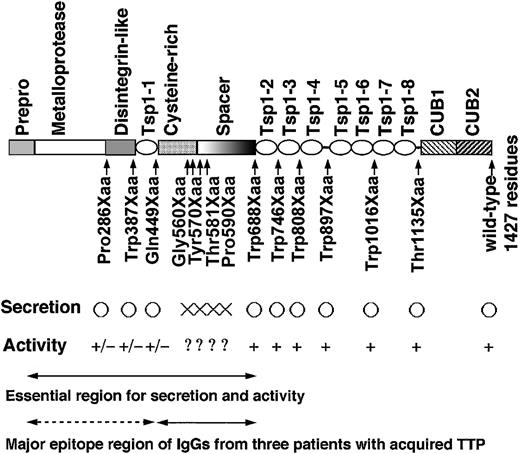
To identify essential domains for VWF-CP activity within ADAMTS-13, expression vectors were prepared for 13 sequential COOH-terminally truncated molecules and a single-point mutant (Asp500Glu, ie, RGD to RGE in the cysteine-rich domain), in addition to the full-length wild-type ADAMTS-13 (Figure 1). The FLAG-tag sequence (DYKDDDDK) was added to the COOH-termini of the wild-type and the mutant proteins to assist in immunochemical detection. These plasmids were expressed transiently in HeLa cells. The secretion of each mutant into the culture medium was assessed by Western blot analysis using anti-FLAG M2 antibody (Figure 2A). The immunoreactive bands of truncated mutants ranged from approximately 30 kDa to 200 kDa, corresponding to their expected molecular weights. For the Gly560Xaa, Tyr570Xaa, Thr581Xaa, and Pro590Xaa mutants, however, immunoreactive bands appeared only in the cell lysates and not in the culture media (Figure 2A-B). Shorter truncated mutants, Gln449Xaa, Trp387Xaa, and Pro285Xaa, were secreted into the culture media, indicating that insertion of a stop codon somewhere between the cysteine-rich domain and the spacer domain inhibited normal secretion into the culture medium.
Schematic representation of the domain organization of ADAMTS-13 and construction of mutants. Truncation positions of a series of 13 domain-deleted mutants and the position of a single point mutation (Asp500Glu★) changing a potential cell adhesion motif, RGD, to RGE, are shown.
Schematic representation of the domain organization of ADAMTS-13 and construction of mutants. Truncation positions of a series of 13 domain-deleted mutants and the position of a single point mutation (Asp500Glu★) changing a potential cell adhesion motif, RGD, to RGE, are shown.
Expression of ADAMTS-13 mutants. HeLa cells were transfected with plasmids encoding FLAG-tagged wild-type or mutant ADAMTS-13. The culture media and cell lysates were analyzed by Western blotting with an anti-FLAG M2 MoAb. (NC) Untransfected culture medium and cell lysate were used as negative controls. (A) Each culture medium sample was subjected to 10% SDS-PAGE under nonreducing conditions. (B) Each cell lysate sample was subjected to 10% SDS-PAGE under reducing conditions.
Expression of ADAMTS-13 mutants. HeLa cells were transfected with plasmids encoding FLAG-tagged wild-type or mutant ADAMTS-13. The culture media and cell lysates were analyzed by Western blotting with an anti-FLAG M2 MoAb. (NC) Untransfected culture medium and cell lysate were used as negative controls. (A) Each culture medium sample was subjected to 10% SDS-PAGE under nonreducing conditions. (B) Each cell lysate sample was subjected to 10% SDS-PAGE under reducing conditions.
A quantitative assay is required for investigating the relationship between the structure and function in detail. However, expression levels of each mutant and the wild type of ADAMTS-13 were too low to precisely determine the protein concentrations. Furthermore, a variety of molecular forms among all constructs made standardization for a quantitative evaluation difficult. Therefore, the subsequent functional assays were qualitative in nature.
VWF-CP activity of ADAMTS-13 mutants
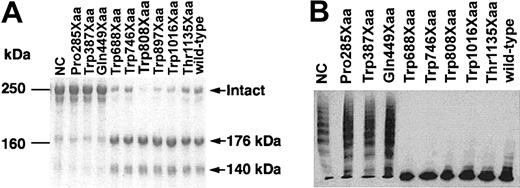
VWF-CP cleaves the Tyr842-Met843 bond within VWF, giving rise to 2 fragments with molecular weights of 140 kDa and 176 kDa. VWF-CP activity in the culture medium was measured qualitatively by SDS-PAGE analysis after incubation of VWF with the culture medium. Culture media expressing the Trp688Xaa, Trp746Xaa, Trp808Xaa, Trp897Xaa, Trp1016Xaa, and Thr1135Xaa mutants, as well as the wild-type ADAMTS-13, showed 2 cleaved fragments after incubation with VWF (Figure 3A). The results of NH2-terminal amino acid sequence analyses of the 176-kDa bands showed that each of these mutant proteins cleaved the Tyr842-Met843 peptide bond within the VWF A2 domain (data not shown). In contrast, it appeared that culture media expressing the Pro285Xaa, Trp387Xaa, and Gln449Xaa mutants, all missing the region from the cysteine-rich domain to the COOH-terminus, showed very low or no activity (Figure 3A), although they were all secreted normally into the culture media (Figure 2A).
VWF-CP activity of ADAMTS-13 mutants. Culture medium of HeLa cells expressing each mutant was incubated with purified VWF. (A) An aliquot of each reaction mixture was subjected to 5% SDS-PAGE under reducing conditions. The gel was stained with Coomassie Brilliant Blue R-250. (B) An aliquot of each reaction mixture was subjected to SDS–agarose gel electrophoresis. The multimeric patterns of VWF were visualized by Western blot analysis with an anti-VWF antibody.
VWF-CP activity of ADAMTS-13 mutants. Culture medium of HeLa cells expressing each mutant was incubated with purified VWF. (A) An aliquot of each reaction mixture was subjected to 5% SDS-PAGE under reducing conditions. The gel was stained with Coomassie Brilliant Blue R-250. (B) An aliquot of each reaction mixture was subjected to SDS–agarose gel electrophoresis. The multimeric patterns of VWF were visualized by Western blot analysis with an anti-VWF antibody.
Next, the enzyme activities of the wild-type and the mutants were measured by assaying degradation of the VWF multimer. Purified VWF was incubated with the culture medium, and the multimeric state of VWF was determined by Western blot analysis after SDS–agarose electrophoresis (Figure 3B). After the incubation of VWF with the medium of untransfected cells, VWF ladders extended into the high molecular weight area, indicating the absence of VWF-CP activity in the medium (Figure 3B, NC). In contrast, the wild-type culture medium eliminated the high molecular weight bands in the VWF ladder. The deletion mutants devoid of the cysteine-rich and spacer domains had dramatically reduced (Gln449Xaa) or almost negative (Pro285Xaa and Trp387Xaa) levels of this VWF-CP activity. The Trp688Xaa, Trp746Xaa, Trp808Xaa, Trp1016Xaa, and Thr1135Xaa mutants, which contain the cysteine-rich and spacer domains, however, retained their VWF-CP activity (Figure 3B).
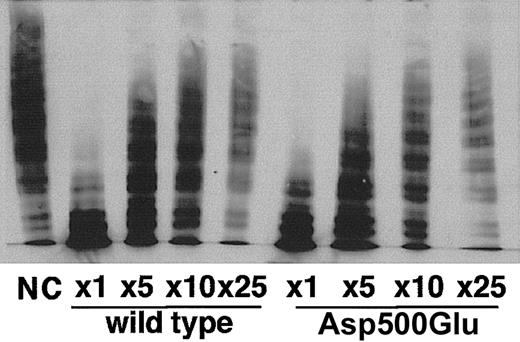
The RGD sequence is widely used by proteins for the recognition of integrin, and ADAMTS-13 contains this sequence within the cysteine-rich domain. Therefore, we examined whether the RGD sequence contributes to the enzymatic activity. The VWF-CP activity of the Asp500Glu mutant, in which the RGD sequence was changed to RGE, was measured using the VWF multimer assay (Figure 4). Culture medium from cells expressing the Asp500Glu mutant showed VWF-CP activity equivalent to that of the wild type, indicating that the RGD sequence in ADAMTS-13 is not necessary for VWF multimer degradation under these assay conditions.
Semiquantitative VWF multimeric state analyses. VWF-CP activity of the wild-type and Asp500Glu mutant proteins was compared. The numbers represent the dilution ratio.
Semiquantitative VWF multimeric state analyses. VWF-CP activity of the wild-type and Asp500Glu mutant proteins was compared. The numbers represent the dilution ratio.
Inhibition of VWF-CP activity by 3 patients' IgGs
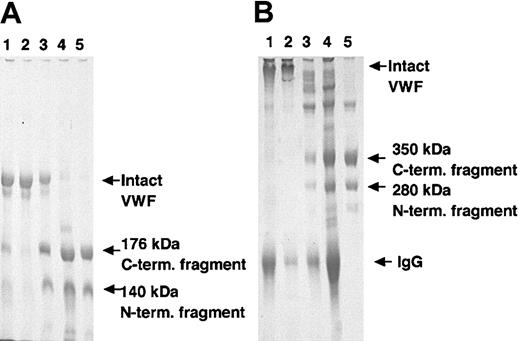
Three IgG fractions were separately purified from 3 patients with acquired (idiopathic) TTP. The inhibitory potency of these IgGs was assessed as described in “Materials and methods.” After overnight incubation of these mixtures with purified VWF, the reaction mixtures were subjected to SDS-PAGE. Cleavage of the Tyr842-Met843 bond within the VWF A2 domain gives rise to 2 fragments with molecular weights of 140 kDa and 176 kDa under reducing conditions, and of approximately 280 kDa and 350 kDa under nonreducing conditions (Figure 5, lanes 4-5). These VWF fragments decreased or disappeared completely in the presence of each patient IgG (Figure 5, lanes 1-3). It was confirmed that these 3 IgG fractions inhibited VWF-CP activity in an in vitro assay system.
Inhibitory activity of VWF-CP activity of rADAMTS-13 by autoantibodies purified from plasma of patients with acquired TTP. Three IgG fractions were separately purified from 3 patients with acquired (idiopathic) TTP. Preincubated conditioned medium of recombinant wild-type ADAMTS-13 with 10 mM BaCl2 was mixed with an equal volume of purified patient A-C IgGs, (lanes 1-3), healthy IgG (lane 4), or buffer (lane 5), respectively. Then these mixtures were incubated with an equal volume of purified VWF followed by overnight dialysis against 5 mM Tris-HCl, pH 8.0, containing 1.5 M urea at 37°C. An aliquot of each reaction mixture was subjected to 5% SDS-PAGE. The gel was stained with Coomassie Brilliant Blue R-250. (A) Under reducing conditions; (B) under nonreducing conditions.
Inhibitory activity of VWF-CP activity of rADAMTS-13 by autoantibodies purified from plasma of patients with acquired TTP. Three IgG fractions were separately purified from 3 patients with acquired (idiopathic) TTP. Preincubated conditioned medium of recombinant wild-type ADAMTS-13 with 10 mM BaCl2 was mixed with an equal volume of purified patient A-C IgGs, (lanes 1-3), healthy IgG (lane 4), or buffer (lane 5), respectively. Then these mixtures were incubated with an equal volume of purified VWF followed by overnight dialysis against 5 mM Tris-HCl, pH 8.0, containing 1.5 M urea at 37°C. An aliquot of each reaction mixture was subjected to 5% SDS-PAGE. The gel was stained with Coomassie Brilliant Blue R-250. (A) Under reducing conditions; (B) under nonreducing conditions.
Epitope mapping of patient IgG fractions
It is of interest to know the epitopes that these inhibitory IgGs recognize. To characterize such autoantibodies, we performed epitope mapping experiments on the basis of Western blot analyses under nonreducing conditions using 9 deletion mutants truncated from the C-terminus and one missense mutant, Asp500Glu. The 3 purified IgGs were used as the primary antibodies for Western blot analysis. As shown in Figure 6, these IgG fractions preferentially bound to the Trp688Xaa, Trp746Xaa, Trp808Xaa, Trp897Xaa, Trp1016Xaa, Thr1135Xaa, and Asp500Glu mutants as well as to the wild-type protein. The Pro285Xaa, Trp387Xaa, and Gln449Xaa mutants, however, did not react with the autoantibodies at all. Therefore, the major epitopes of the autoantibodies appear to exist somewhere within the cysteine-rich domain to the COOH-terminus.
Epitope mapping of anti-ADAMTS-13 autoantibodies in patients with acquired TTP. The culture media of various COOH-terminally truncated mutants were blotted onto polyvinylidene diflouride membranes. The membranes were reacted with anti-FLAG M2 MoAb as a positive control (A) or anti–ADAMTS-13 inhibitory autoantibodies from 3 patients with acquired TTP (B-D).
Epitope mapping of anti-ADAMTS-13 autoantibodies in patients with acquired TTP. The culture media of various COOH-terminally truncated mutants were blotted onto polyvinylidene diflouride membranes. The membranes were reacted with anti-FLAG M2 MoAb as a positive control (A) or anti–ADAMTS-13 inhibitory autoantibodies from 3 patients with acquired TTP (B-D).
Subsequently, we performed competitive Western blot analysis to map the epitopes more precisely. Culture media expressing the wild-type and the Trp688Xaa mutant were mixed and subjected to SDS-PAGE for Western blotting under nonreducing conditions. Again, IgG fractions from the 3 patients reacted with the wild-type and the Trp688Xaa mutant, resulting in immunoreactive bands with molecular masses of approximately 85 kDa (Trp688Xaa) and 210 kDa (wild type), respectively (Figure 7A). Next, the wild-type and the Trp688Xaa mutant proteins on membranes were reacted with purified autoantibodies in the presence of the concentrated culture media of cells expressing Gln449Xaa, Trp688Xaa, or wild-type ADAMTS-13 as competitors for the autoantibodies. The immunopositive bands shown in Figure 7A disappeared completely in the presence of Trp688Xaa or wild-type ADAMTS-13, whereas the addition of Gln449Xaa-containing culture medium caused no effect (Figure 7B). From these results, it is demonstrated that these 3 IgG fractions mainly recognize the region between Trp688Xaa and Gln449Xaa, ie, the cysteine-rich/spacer domains.
Precise epitope mapping of IgG fractions from patients with acquired TTP by competitive immunoblot analysis. Two culture medium samples, one from cells transfected with the full-length wild-type plasmid and the other from cells transfected with the Trp688Xaa plasmid, were premixed, subjected to SDS-PAGE, and blotted onto polyvinylidene diflouride membranes. (A) Each blot was reacted with healthy IgG, anti-FLAG M2 MoAb, or IgGs prepared from 3 patients. (B) Each blot was reacted with IgGs prepared from 3 patients in the presence of 10-fold concentrated cultured media of cells transfected with either the Gln449Xaa, the Trp688Xaa, or the full-length wild-type plasmid.
Precise epitope mapping of IgG fractions from patients with acquired TTP by competitive immunoblot analysis. Two culture medium samples, one from cells transfected with the full-length wild-type plasmid and the other from cells transfected with the Trp688Xaa plasmid, were premixed, subjected to SDS-PAGE, and blotted onto polyvinylidene diflouride membranes. (A) Each blot was reacted with healthy IgG, anti-FLAG M2 MoAb, or IgGs prepared from 3 patients. (B) Each blot was reacted with IgGs prepared from 3 patients in the presence of 10-fold concentrated cultured media of cells transfected with either the Gln449Xaa, the Trp688Xaa, or the full-length wild-type plasmid.
Discussion
We summarized all the results obtained in this study in Figure 8. We prepared 13 sequential COOH-terminally truncated molecules and a single point mutant, Asp500Glu, (RGD to RGE in the cysteine-rich domain), and compared the activity of each mutant with that of wild-type recombinant ADAMTS-13 (Figure 1). Deletions located between the cysteine-rich and spacer domains, such as Gly560Xaa, Tyr570Xaa, Thr581Xaa, and Pro590Xaa, abolished secretion into the conditioned medium (Figure 2). These regions might strongly affect the folding and stability of the protein structure. However, truncation mutants lacking the cysteine-rich/spacer domains, including Gln449Xaa, Trp387Xaa, and Pro285Xaa, exhibited dramatically reduced or almost negative VWF-CP activity (Figure 3). These results were similar to those demonstrated by Zheng et al.36
Schematic representation of the functionally important region of ADAMTS-13. ○ indicates normal secretion; ×, no secretion. +, VWF-CP activity is positive;?, no test; +/–, very low or no activity.
Schematic representation of the functionally important region of ADAMTS-13. ○ indicates normal secretion; ×, no secretion. +, VWF-CP activity is positive;?, no test; +/–, very low or no activity.
On the other hand, Schneppenheim et al37 demonstrated that patients with TTP, with truncation in ADAMTS-13 downstream of the cysteine-rich/spacer domain, such as Arg910Xaa and Arg1034Xaa, suffered from severe deficiency of VWF-CP activity, in that ADAMTS-13 mutants were truncated within the C-terminal Tsp1 motif repeats. However, it is speculated that these truncated forms may not have the ability to form disulfide bonds because a stop codon is inserted within an intradomain of a Tsp1 motif. Therefore, these mutants appear to be very unstable, resulting in the abnormality of secretion. In contrast, the truncated forms we constructed, such as Trp897Xaa, Trp1016Xaa, and Thr1135Xaa, were secreted normally and retained VWF-CP activity, because a stop codon is inserted between domains.
Moreover, we prepared an active site mutated protein, Glu225Ala, which replaced Glu to Ala within the zinc-binding motif (HEXXHXXGXXHD) of metalloprotease domain, to confirm that this domain is responsible for VWF cleavage. As expected, mutation of a critical residue in the active center within the metalloprotease domain, Glu225Ala, caused a loss in activity (data not shown). From these results, we conclude that protease domain to cysteine-rich/spacer domains are indispensable regions for expressing VWF-CP activity under the assay conditions described here.
Binding sites for sulfated glycosaminoglycans are known to exist within the spacer domain of ADAMTS-1 (METH-1).38 According to a previous report on ADAMTS-4 (Aggrecanase-1), binding sites for sulfated glycosaminoglycans attached to the aggrecan core protein are located within the ADAMTS-4 cysteine-rich/spacer domains.39 Furthermore, analyses of chimeras encoding domains of Xenopus ADAMs 10 and 13 suggested that ADAM-13 metalloprotease might use its cysteine-rich domain to improve its specificity or activity in cleaving a substrate(s).40 In addition, the ADAMTS-13 cysteine-rich domain contains one RGD motif, a potential cell adhesion sequence, although the sequence is not required for the expression of VWF-CP activity in the assay conditions as described in “Materials and methods” (Figure 4). On the basis of these findings, it seems possible that the ADAMTS-13 cysteine-rich/spacer domains function as a cell surface receptor binding site and/or substrate recognition exosite.
Results on Western blot analyses demonstrated that the major epitope of IgGs separated from plasma of 3 patients with acquired TTP possessing inhibitory activity against VWF-CP (Figure 5) was located within cysteine-rich/spacer domains (Figures 6, 7). Therefore, it seems reasonable that these IgGs bind to cysteine-rich/spacer domains, resulting in the inhibition of VWF-CP activity. From these results, it is likely that the ADAMTS-13 cysteine-rich/spacer domains are involved in VWF recognition.
It was also speculated that anchorage of ADAMTS-13 on the endothelial cell surface via additional COOH-terminal Tsp1 repeats is necessary for expression of VWF-CP activity in vivo.31 By considering that finding together with our present results, ADAMTS-13 on the endothelial cell surface may interact with VWF through the cysteine-rich/spacer domains and cleave it via the metalloprotease domain in vivo.
The present study clearly demonstrates that ADAMTS-13 cysteine-rich/spacer domains are functionally essential for VWF cleavage; however, it is impossible to deny the theoretical possibility that the results in the present study could be explained by an indirect effect on folding as a result of the deletion of the cysteine-rich/spacer domains. Furthermore, on the epitope mapping experiment using polyclonal autoantibodies, possibilities of a complex conformational epitope involving other N-terminal segments or of contamination of a trace amount of antibodies against other domain(s), for example metalloprotease domain, cannot be excluded. In the future, these possibilities might be examined by the analysis of a complementary set of N-terminal deletions and/or by an experiment using specific antibody against cysteine-rich/spacer domains. Moreover, further studies may reveal that the critical segment is restricted to just the small remaining stretch of cysteine-rich domain or within just the spacer domain, and that one or the other segment is not required.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-03-0908.
Supported in part by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M.M., K.K., T.M.) and the Ministry of Health, Labor and Welfare of Japan for Blood Coagulation Abnormalities (H14-02) (T.M., Y.F.).
K.S. and M.M. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Emeritus Sadaaki Iwanaga (Kyushu University) for his critical reading of the manuscript, as well as Drs Shouichi Higashi (Yokohama City University), Jun Mizuguchi, Shintaro Kamei, and Tsutomu Hamuro (The Chemo-Sero-Therapeutic Research Institute) for many helpful suggestions. We also thank Noriko Mimura and Masaki Hirashima (The Chemo-Sero-Therapeutic Research Institute) for their technical support.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal