Abstract
Hematopoietic stem cell transplantation from unrelated donors is an effective treatment for myeloid malignancies, but its use is usually restricted to young patients without comorbidities. The development of reduced-intensity preparative regimens has allowed the extension of this form of treatment to older and medically infirm patients. We assessed the outcomes of patients older than 54 years who received unrelated donor transplants for the treatment of myeloid malignancies in our institution. There were 29 patients (median age, 59 years) with advanced acute myeloid leukemia (n = 13), myelodysplastic syndrome (n = 7), and chronic myeloid leukemia (n = 9) included. With a median follow-up of 27 months, the probability of overall and event-free survival, and nonrelapse mortality at one year were 44%, 37%, and 55%, respectively. Grades II to IV acute graft-versus-host disease (GVHD) occurred in 41% of patients and chronic GVHD developed in 63% of patients surviving more than 100 days. Of the 11 survivors, 9 were interviewed and reported good quality of life after transplantation using the Functional Assessment of Cancer Therapy–Bone Marrow Transplant Scale (FACT-BMT) questionnaire, with high scores in all dimensions. Unrelated donor transplantation is a treatment option for older patients with myeloid malignancies. The results in this cohort of patients are comparable with those reported in younger patients with similarly advanced disease.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) represents the treatment of choice for selected patients with hematologic malignancies and nonmalignant diseases.1 One factor limiting the full application of allogeneic HSCT is the availability of a human leukocyte antigen (HLA)–compatible donor. HLA-matched sibling donors are available to less than 30% of patients in Europe and North America, and an extended family search usually identifies a suitable mismatched relative in less than 5% of cases.1
The median age of onset of chronic myeloid leukemia (CML) is in the sixth decade of life, while the peak incidence of acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) is in the seventh decade. The median age of patients receiving induction chemotherapy for AML/MDS in our institution in 2001 was 60 years. Unfortunately, less than 20% of the AML patients older than 60 years survive in complete remission 2 years after induction chemotherapy.2-4 Allogeneic transplantation offers the possibility of prolonged remission to a significant fraction of patients with high-risk or advanced leukemia. The number of potential family donors decreases naturally with the aging of the family. In a cohort of patients with AML/MDS receiving chemotherapy in our institution, the median age was 66 years (range, 50-81 years) and the median number of live siblings was 1.5 (range, 0-11). If one is to extend allogeneic transplantation to the majority of patients with myeloid leukemias, alternative donors will have to be used, most commonly provided by a matched unrelated donor. The number of unrelated HSCTs reported to the International Bone Marrow Transplant Registry (IBMTR) is increasing, and currently approximately 25% of hematopoietic transplants are from unrelated donors.5
Older patients have a higher risk of graft-versus-host disease (GVHD), nonrelapse mortality (NRM), and a lower disease-free survival (DFS) than younger subjects.1,6,7 Unrelated donor transplantations are associated with an increased risk of acute GVHD and treatmentrelated mortality. Because of the adverse association between age and outcome after bone marrow transplantation (BMT), many centers limit HSCT with unrelated donors to patients younger than 55 years. Recent developments have allowed the extension of HSCT to older patients through better supportive care and the use of reduced-intensity or nonablative conditioning regimens.4,8,9 Here we review our experience using unrelated donor HSCT for the treatment of AML, MDS, or CML in patients older than 54 years at the University of Texas M. D.Anderson Cancer Center.
Patients, materials, and methods
Eligibility criteria
Patients treated prospectively in protocols conducted in our institution from July 1997 to June 2001 who were older than 54 years with the diagnosis of AML, MDS, or CML were reviewed for this analysis. Transplantation candidates were required to have a good performance status score (Zubrod ≤ 2), no uncontrolled concomitant medical illness or infection, left ventricular ejection fraction more than 40%, creatinine level less than 152.5 μM (2.0 mg/dL), and bilirubin level less than 51.3 μM (3.0 mg/dL). All patients provided written informed consent. The treatment protocols were reviewed and approved by the University of Texas M. D. Anderson Cancer Center institutional review board. Unrelated donors were identified, consented, and harvested through the National Marrow Donor Program (NMDP) according to established procedures.
Human leukocyte antigen typing and compatibility
HLA typing for class I antigens was performed using serologic or intermediate resolution molecular techniques. Low-resolution molecular typing using hybridization techniques of amplified sampled DNA with sequence-specific oligonucleotide probes, followed by high-resolution molecular typing using polymerase chain reaction in the sampled DNA with sequence-specific primers was performed for class II alleles (HLA-DRB1, HLA-DQB1) in all patients.
Conditioning regimen
Patients were treated in consecutive protocols conducted in our institution. We initially investigated the use of fludarabine and 180 mg/m2 melphalan. Subsequently, in an attempt to further minimize regimen-related toxicity and compare the effectiveness of a reduced dose of the alkylating agent, a protocol was written randomizing subjects to 2 different doses of melphalan (180 vs 140 mg/m2) with fludarabine. Most recently, with the availability of an intravenous formulation of busulfan, we incorporated this drug in addition to fludarabine as the preparative regimen. Eligibility criteria for these consecutives studies were similar. From December 1998 to the present all recipients of matched unrelated donor transplants received antithymocyte globulin (ATG) in the preparative regimen, in order to potentiate engraftment and reduce the incidence of GVHD.
There were 23 patients who received a conditioning regimen containing 25 mg/m2 fludarabine per day intravenously for 4 days in combination with 70 mg/m2 melphalan per day intravenously for 2 days (n = 7) or 90 mg/m2 per day intravenously for 2 days (n = 16). There were 5 patients who received 30 mg/m2 fludarabine per day intravenously for 4 days and 0.8 mg/kg busulfan every 6 hours intravenously for 8 to 14 doses. One patient received 30 mg/m2 fludarabine per day intravenously for 4 days, 500 mg/m2 cytarabine per day intravenously for 4 days, and 140 mg/m2 melphalan intravenously for 1 day. Equine ATG (60 mg/kg in divided doses) was part of the conditioning regimen in 18 cases.
Graft-versus-host disease prophylaxis
All patients received GVHD prophylaxis with tacrolimus in combination with 5 mg/m2 methotrexate on days 1, 3, 6, and 11.10 Tacrolimus was administered daily from day –2 as a continuous intravenous infusion adjusted to maintain whole blood trough blood levels at 5 to 15 mg/mL. Tacrolimus was to be continued for 6 to 8 months after transplantation.
Supportive care
Patients were managed in either conventional or laminar airflow rooms. Infection prophylaxis during the peritransplantation period included trimethoprim-sulfamethoxazole for Pneumocystis carinii prophylaxis, fluconazole for antifungal prophylaxis, acyclovir for antiviral prophylaxis, and penicillin with an oral quinolone for antibacterial prophylaxis. Patients underwent twice weekly cytomegalovirus (CMV) surveillance tests for CMV antigenemia, with ganciclovir therapy instituted for positive assays. All patients received 5 μg/kg filgrastim subcutaneously daily beginning on day 7 after transplantation until an absolute neutrophil count (ANC) of 1.5 × 109/L (1500/μL) or higher for 3 consecutive days was achieved. All blood products were filtered and irradiated prior to transfusion.
Engraftment, toxicity, and definition of remission
Day 0 was the stem cell infusion day. Neutrophil count recovery was defined as the first of 3 consecutive days that the absolute neutrophil count exceeded 0.5 × 109/L. Platelet engraftment was defined as the first of 7 consecutive days that the platelet count exceeded 20 × 109/L without transfusion support. Hematopoietic chimerism was evaluated on bone marrow cells by restriction fragment length polymorphisms (RFLPs) at the AY-29 or YNH24 loci,11 by fluorescent in-situ hybridization studies in sex-mismatched cases for Y chromosome or analysis of DNA microsatellite polymorphisms by polymerase chain reaction (PCR) with D6S264, D3S1282, D18S62, and D3S1300 fluorescence-labeled primers then analyzed using GeneScan software (Applied Biosystems, Foster City, CA). Toxicity was graded according to Bearman et al,12 and GVHD was graded according to consensus criteria.13 Patients who survived 100 days or longer were evaluable for chronic GVHD assessment. Complete remission (CR) and partial responses (PRs) were determined by standard disease-specific criteria as defined by the International Bone Marrow Transplant Registry (IBMTR). Relapse was defined by standard morphologic criteria, conventional cytogenetic analysis, or both. Cytogenetic relapse was documented by the presence and persistence in at least 2 consecutive tests of the cytogenetic abnormality that characterized the disease (Philadelphia chromosome for CML and patient-specific cytogenetic abnormalities for AML and MDS). PCR-based studies were not used to define relapse.
Study end points and statistical analysis
Data were collected by comprehensive chart review. Major outcomes of interest were toxicity, survival, nonrelapse mortality (NRM), event-free survival (EFS), engraftment, disease response and relapse, and incidence/severity of GVHD. Overall survival (OS) was measured from transplantation until death from any cause. EFS was determined from transplantation until relapse or death from any cause. Patients with cytogenetic relapse were scored as treatment failure for the purpose of EFS analysis, regardless of their subsequent response to salvage therapy. Patients alive at the time of the analysis were censored at the last follow-up date. NRM was defined as mortality due to any cause other than disease progression within one year of transplantation. Actuarial curves of OS, EFS, and NRM were estimated according to the Kaplan and Meier method,14 and the significance of differences between the curves was estimated by the log-rank test.15 The variables included in the analysis were patient and donor sex, recipient's age, pretransplantation CMV status of donor and recipient, HLA matching, duration of first complete remission, time intervals between diagnosis and BMT, disease status at transplantation, use of ATG in the conditioning regimen, and CD34+ cell doses. Numeric variables were analyzed as categories considering their value above the median of the series as indicated in the results. For the analysis of risk factors influencing engraftment and development of GVHD, univariate analysis of variance was performed with various categoric variables by the chi-square or Fisher exact tests and with continuous variables by the unpaired student t test.
Quality of life (QOL) assessment
A cross-sectional evaluation of QOL was performed using a self-administrated questionnaire sent by mail. Participation was voluntary. The questionnaire was based on the Functional Assessment of Cancer Therapy-Bone Marrow Transplant Scale (FACT-BMT).16 Included in the questionnaire were 2 other sections about the work status of the patient17 before and after transplantation, and assessment of chronic GVHD–related symptoms (such as dry eyes, dry skin, joint stiffness, or frequent infections). The FACT-BMT is a 46-item inventory of questions dealing with physical, functional, social/family, and emotional well-being issues, as well as satisfaction with the physician/patient relationship. It includes a BMT subscale with 12 items specifically designed to address BMT-specific problems. Higher scores in FACT reflect better QOL in the reported dimension. FACT item no. 33 (“I am content with the quality of my life right now”) was considered to give an overall impression of QOL.17
Patient and donor characteristics
Included in this analysis were 29 consecutive patients who fulfilled the selection criteria. Characteristics of patients, diagnosis, and disease status at transplantation are displayed in Table 1. The median age at transplantation was 59 years (range, 55-69 years). Of the patients, 18 were male and 11 were female. The study population included 13 patients (44%) with AML, 9 patients (31%) with CML, and 7 patients (25%) with MDS.
Patient characteristics and transplantation outcomes
. | . | . | Duration of first CR, mo . | Time from diagnosis to transplantation, mo . | Disease status before transplantation . | . | . | Recipient/donor CMV status . | . | Transplantation outcomes . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient UPN . | Age, y . | Diagnosis . | . | . | . | Preparative regimen . | ATG . | . | Chimerism . | Disease status . | Survival, d . | Cause of death . | ||
| 1 | 59 | MDS/RAEB | 13 | 21 | 1st relapse, untreated | FM180 | No | R/R | Donor | CR | 1540 | NA | ||
| 2 | 61 | MDS/RAEB | 0 | 4 | Untreated | FM180 | No | N/N | ND | Dead | 38 | Acute GVHD | ||
| 3 | 62 | CML | 0 | 109 | 2nd AP, untreated | FM140* | Yes | N/N | Donor | CR | 756 | NA | ||
| 4 | 59 | AML | 6.5 | 28 | 1st relapse, untreated | FM180 | No | R/R | Donor | CR | 1642 | NA | ||
| 5 | 59 | CML | 0 | 29 | Myeloid blast crisis | FM180 | No | R/R | NE | Dead | 18 | Multiorgan failure | ||
| 6 | 60 | AML | 12.5 | 25 | 3rd relapse, untreated | FM180 | No | N/R | Donor | Dead | 1181 | Fungal pneumonia | ||
| 7 | 62 | AML | 16 | 19 | 1st relapse, refractory | FM180 | No | N/R | Donor | Dead | 92 | Acute GVHD | ||
| 8 | 58 | AML | 8 | 15 | 1st relapse, refractory | FM180 | No | R/R | Donor | Dead | 42 | Recurrence of disease | ||
| 9 | 57 | CML | 0 | 50 | 2nd AP, untreated | FM180 | No | N/R | ND | Dead | 35 | Sepsis, pulmonary hemorrhage, renal failure | ||
| 10 | 56 | CML | 2.5 | 5 | Myeloid blast crisis | FM180 | No | N/R | ND | Dead | 38 | Acute GVHD | ||
| 11 | 56 | AML | 13.5 | 19 | 1st relapse, refractory | FM180 | No | R/N | Donor | Dead | 72 | Acute GVHD | ||
| 12 | 58 | CML | 0 | 156 | 3rd chronic phase | FM140 | Yes | N/R | NE | Dead | 10 | Cardiac failure, subarachnoid hemorrhage | ||
| 13 | 59 | CML | 0 | 31 | 2nd AP, untreated | FB | Yes | N/N | Donor | CR† | 361 | NA | ||
| 14 | 57 | AML | 2 | 11 | 2nd CR | FM180 | No | R/N | Donor | CR | 1128 | NA | ||
| 15 | 55 | CML | 0 | 16 | AP, untreated | FM180 | Yes | N/R | Donor | CR | 1016 | NA | ||
| 16 | 64 | MDS/RAEB | 0 | 9 | Primary induction failure | FM180 | Yes | N/R | Donor | CR | 872 | NA | ||
| 17 | 62 | AML | 1 | 20 | 1st relapse, refractory | FM140 | No | N/R | Donor | Dead | 92 | Acute GVHD | ||
| 18 | 64 | AML | 12 | 20 | 2nd relapse, refractory | FB | Yes | N/N | Donor | Dead | 56 | Acute GVHD, liver failure | ||
| 19 | 61 | MDS | 7 | 17 | 2nd CR | FM140 | Yes | N/N | Donor | CR | 910 | NA | ||
| 20 | 56 | MDS/RAEB | 0 | 7 | Primary induction failure | FM140 | Yes | N/N | NE | Dead | 8 | Intracerebral hemorrhage | ||
| 21 | 69 | AML | 12 | 21 | 2nd CR | FB | Yes | N/R | Donor | Dead | 217 | Chronic GVHD, intracerebral hemorrhage | ||
| 22 | 59 | CML | 0 | 18 | Chronic phase | FM180 | Yes | N/R | Donor | CR | 495 | NA | ||
| 23 | 55 | AML | 6 | 12 | 2nd CR | FM180 | Yes | R/N | Donor | CR | 505 | NA | ||
| 24 | 64 | AML | 10 | 21 | 1st relapse, refractory | FB | Yes | R/R | Donor | CR | 104 | NA | ||
| 25 | 56 | CML | 2.5 | 15 | 3rd chronic phase | FM180 | Yes | N/R | Donor | CR | 336 | NA | ||
| 26 | 63 | MDS/RAEBt | 0 | 12 | Primary induction failure | FM140 | Yes | N/N | Donor | Dead | 116 | Recurrence of disease | ||
| 27 | 55 | MDS/RAEB | 0 | 9 | Primary induction failure | FM140 | Yes | N/N | Donor | Dead | 293 | Chronic GVHD, Aspergillus pneumonia | ||
| 28 | 68 | AML | 2.5 | 8 | 2nd relapse, untreated | FB | Yes | R/R | Mixed | Dead | 58 | Persistent disease | ||
| 29 | 55 | AML | 9 | 15 | 1st relapse, refractory | FM140 | Yes | R/N | NE | Dead | 17 | Multiorgan failure, pneumonia | ||
. | . | . | Duration of first CR, mo . | Time from diagnosis to transplantation, mo . | Disease status before transplantation . | . | . | Recipient/donor CMV status . | . | Transplantation outcomes . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient UPN . | Age, y . | Diagnosis . | . | . | . | Preparative regimen . | ATG . | . | Chimerism . | Disease status . | Survival, d . | Cause of death . | ||
| 1 | 59 | MDS/RAEB | 13 | 21 | 1st relapse, untreated | FM180 | No | R/R | Donor | CR | 1540 | NA | ||
| 2 | 61 | MDS/RAEB | 0 | 4 | Untreated | FM180 | No | N/N | ND | Dead | 38 | Acute GVHD | ||
| 3 | 62 | CML | 0 | 109 | 2nd AP, untreated | FM140* | Yes | N/N | Donor | CR | 756 | NA | ||
| 4 | 59 | AML | 6.5 | 28 | 1st relapse, untreated | FM180 | No | R/R | Donor | CR | 1642 | NA | ||
| 5 | 59 | CML | 0 | 29 | Myeloid blast crisis | FM180 | No | R/R | NE | Dead | 18 | Multiorgan failure | ||
| 6 | 60 | AML | 12.5 | 25 | 3rd relapse, untreated | FM180 | No | N/R | Donor | Dead | 1181 | Fungal pneumonia | ||
| 7 | 62 | AML | 16 | 19 | 1st relapse, refractory | FM180 | No | N/R | Donor | Dead | 92 | Acute GVHD | ||
| 8 | 58 | AML | 8 | 15 | 1st relapse, refractory | FM180 | No | R/R | Donor | Dead | 42 | Recurrence of disease | ||
| 9 | 57 | CML | 0 | 50 | 2nd AP, untreated | FM180 | No | N/R | ND | Dead | 35 | Sepsis, pulmonary hemorrhage, renal failure | ||
| 10 | 56 | CML | 2.5 | 5 | Myeloid blast crisis | FM180 | No | N/R | ND | Dead | 38 | Acute GVHD | ||
| 11 | 56 | AML | 13.5 | 19 | 1st relapse, refractory | FM180 | No | R/N | Donor | Dead | 72 | Acute GVHD | ||
| 12 | 58 | CML | 0 | 156 | 3rd chronic phase | FM140 | Yes | N/R | NE | Dead | 10 | Cardiac failure, subarachnoid hemorrhage | ||
| 13 | 59 | CML | 0 | 31 | 2nd AP, untreated | FB | Yes | N/N | Donor | CR† | 361 | NA | ||
| 14 | 57 | AML | 2 | 11 | 2nd CR | FM180 | No | R/N | Donor | CR | 1128 | NA | ||
| 15 | 55 | CML | 0 | 16 | AP, untreated | FM180 | Yes | N/R | Donor | CR | 1016 | NA | ||
| 16 | 64 | MDS/RAEB | 0 | 9 | Primary induction failure | FM180 | Yes | N/R | Donor | CR | 872 | NA | ||
| 17 | 62 | AML | 1 | 20 | 1st relapse, refractory | FM140 | No | N/R | Donor | Dead | 92 | Acute GVHD | ||
| 18 | 64 | AML | 12 | 20 | 2nd relapse, refractory | FB | Yes | N/N | Donor | Dead | 56 | Acute GVHD, liver failure | ||
| 19 | 61 | MDS | 7 | 17 | 2nd CR | FM140 | Yes | N/N | Donor | CR | 910 | NA | ||
| 20 | 56 | MDS/RAEB | 0 | 7 | Primary induction failure | FM140 | Yes | N/N | NE | Dead | 8 | Intracerebral hemorrhage | ||
| 21 | 69 | AML | 12 | 21 | 2nd CR | FB | Yes | N/R | Donor | Dead | 217 | Chronic GVHD, intracerebral hemorrhage | ||
| 22 | 59 | CML | 0 | 18 | Chronic phase | FM180 | Yes | N/R | Donor | CR | 495 | NA | ||
| 23 | 55 | AML | 6 | 12 | 2nd CR | FM180 | Yes | R/N | Donor | CR | 505 | NA | ||
| 24 | 64 | AML | 10 | 21 | 1st relapse, refractory | FB | Yes | R/R | Donor | CR | 104 | NA | ||
| 25 | 56 | CML | 2.5 | 15 | 3rd chronic phase | FM180 | Yes | N/R | Donor | CR | 336 | NA | ||
| 26 | 63 | MDS/RAEBt | 0 | 12 | Primary induction failure | FM140 | Yes | N/N | Donor | Dead | 116 | Recurrence of disease | ||
| 27 | 55 | MDS/RAEB | 0 | 9 | Primary induction failure | FM140 | Yes | N/N | Donor | Dead | 293 | Chronic GVHD, Aspergillus pneumonia | ||
| 28 | 68 | AML | 2.5 | 8 | 2nd relapse, untreated | FB | Yes | R/R | Mixed | Dead | 58 | Persistent disease | ||
| 29 | 55 | AML | 9 | 15 | 1st relapse, refractory | FM140 | Yes | R/N | NE | Dead | 17 | Multiorgan failure, pneumonia | ||
UPN indicates unique patient number; CR, complete remission; ATG, antithymocyte globulin; MDS, myelodysplastic syndrome; RAEB, refractory anemia with excess blasts; FM180, fludarabine and 180 mg/m2 melphalan; R, reactive; NA, not applicable; N, nonreactive; ND, not done; CML, chronic myeloid leukemia; AP, accelerated phase; FM140, fludarabine and 140 mg/m2 melphalan; AML, acute myelogenous leukemia; NE, nonevaluable due to early death; FB, fludarabine and busulfan; and RAEBt, refractory anemia with excess blasts in transformation.
Received Ara-c in the preparative regimen.
Developed cytogenetic relapse on day 215 after transplantation treated with ST1571 (achieved hematologic and cytogenetic complete remission).
All except 2 donor/recipient pairs were HLA-A, HLA-B, and HLADRB1 matched. There were 2 pairs that had one HLA-DRB1 antigen mismatch with their donor, and 4 other pairs had HLA-C and/or HLADQB1 antigen mismatches. Of the transplantations, 13 (45%) involved sex-mismatched donor/recipient pairs. There were 2 major and 4 minor ABO mismatches, while 2 pairs had both major and minor ABO incompatibilities. Both patient and donor were CMV negative in 8 pairs (27%). All but one patient received bone marrow transplants. The median number of CD34+ cells infused was 4.46 × 106/kg recipient body weight (range, 0.37-8.6 × 106/kg), and the median number of total nucleated cells (TNCs) infused was 2.8 × 108/kg recipient body weight (range, 0.51-5.96 × 108/kg).
Results
Of the 13 patients with AML, 7 received transplants for treatment of chemotherapy refractory disease. There were 3 patients who underwent transplantation at second or third complete remission, and 3 patients had untreated relapsed disease. Among the 7 patients with MDS, 6 had intermediate risk according to the International Prognostic Scoring System,18 and 1 had high-risk disease at diagnosis. Of the patients with AML, 4 (57%) had chemotherapy refractory disease at transplantation, while 1 was in CR and 2 were untreated. In the AML/MDS group of patients, 4 subjects (20%) underwent transplantation in CR, and the median duration of the first remission was 9.5 months (range, 1-16 months; n = 15). At the time of transplantation, 11 (55%) of the 20 patients had chemotherapy refractory disease (6 patients in refractory relapse and 5 subjects with primary induction failure), and 5 patients had untreated disease (4 of them in untreated relapse). The CML group included 2 patients in myeloid blast crisis, 4 patients in accelerated phase, 2 patients in third chronic phase, and 1 patient in late first chronic phase.
Engraftment
Neutrophil recovery occurred in 25 patients (86%) at a median time of 13 days (range, 11-25 days), and 21 patients (72%) achieved platelet recovery at a median time of 20 days (range, 11-49 days). A platelet count higher than 50 × 109/L was achieved by 20 patients (69%) and by 16 (89%) of the 18 patients surviving at day 100 after transplantation at a median time of 27.5 days (range, 12-95 days). There were 4 patients who did not engraft. They died within 28 days of transplantation due to toxicity and/or infections. One of them received fludarabine and 180 mg/m2 melphalan as conditioning, while the other 3 received fludarabine and 140 mg/m2 melphalan. There were 6 patients who had sustained neutrophil recovery but failed to achieve platelet transfusion independence. No patient surviving longer than 28 days had graft failure.
Chimerism
Chimerism studies were performed at one month after transplantation in 21 of 25 evaluable patients. Initial full-donor chimerism was observed in 18 (86%) of 21 patients, and 3 patients had mixed chimerism. Of the patients with mixed chimerism, 2 converted to full-donor chimerism spontaneously, while the other patient died on day 58 due to persistent AML.
Graft-versus-host disease
Grades II to IV and III/IV acute GVHD was documented in 12 and 6 patients (41% and 20%, respectively). The median time to acute GVHD onset was 25 days (range, 8-54 days). Of 16 patients surviving for at least 100 days after transplantation, 10 developed chronic GVHD (limited in 2 cases and extensive in 8 patients) with onset at a median of 182 days after transplantation (range, 101-360 days).
Infection, treatment-related toxicity, and mortality
In 24 patients, 58 documented episodes of infection occurred (median, 2; range, 0-6 episodes). These included bacteremia (n = 17), other bacterial infection (n = 21), fungal infection (n = 2), CMV antigenemia (n = 8), CMV pneumonitis (n = 2), and other viral infections (n = 8).
There were 13 deaths during the first 100 days (45%): 6 due to acute GVHD, 2 due to disease relapse/progression, and 5 due to regimen-related toxicity (17% regimen-related mortality). There were 6 patients who developed grades III to IV regimen-related toxicity, including 5 deaths. The organ systems affected were renal (n = 2), lung (n = 2), liver (n = 2), cardiac (n = 2), central nervous system (n = 1), and bladder (n = 1). Overall, 17 patients (59%) have died. Causes of death included recurrent or progressive disease in 3 patients (18% of the deaths), acute GVHD and its complications in 6 patients (36%), chronic GVHD and its complications in 2 patients (11%), infection in 4 patients (23%), hemorrhage in 1 patient (6%), and multiorgan failure in 1 patient (6%).
Disease response
Of 16 patients (81%) with the diagnosis of AML or high-risk MDS, 13 achieved complete remission (CR) after transplantation (4 patients received transplant while in CR).
On transplantation day 17 and day 8, 2 patients died early, due to pneumonia and multiorgan failure, and intracerebral hemorrhage, respectively, and were not evaluable for response.
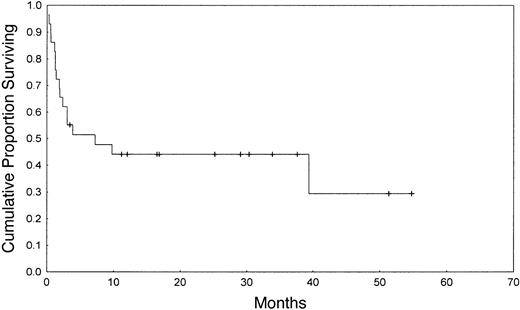
Another patient did not clear bone marrow blasts and died on day 58 with persistent AML. Of the 13 patients who were in CR after transplantation, 2 patients relapsed and died of progressive disease on day 48 and 3 months after transplantation. Another patient relapsed one year after transplantation and was treated with a donor lymphocyte infusion (DLI). He died 40 months after transplantation while in CR, due to fungal pneumonia (Table 1). With follow-up periods of 5 to 51 months, 7 patients remain in CR. The estimated OS and RFS rates at one year were 38% and 34%, respectively.
There were 5 patients with CML (50%) who achieved CR after transplantation. One patient (patient 13) developed cytogenetic relapse 7 months after transplantation and responded to imatinib mesylate treatment. Another patient (patient 22) who achieved CR developed Epstein-Barr virus–related posttransplantation lymphoproliferative disorder, which responded to DLI and Rituximab treatment. Within 100 days of transplantation, 4 patients died of the following: acute GVHD (n = 1), pulmonary hemorrhage (n = 1), multiorgan failure (n = 1), and cardiac arrest (n = 1).
Survival
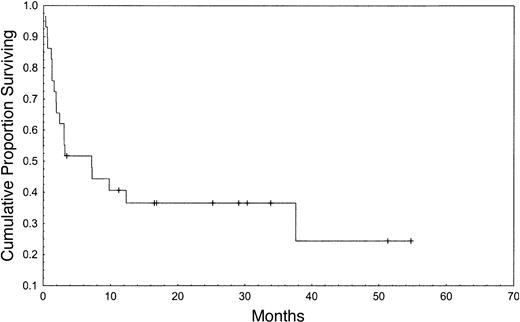
The Kaplan-Meier estimated probability of OS, EFS, and NRM at one year were 44%, 37%, and 55%, respectively (Figures 1, 2). Table 2 summarizes outcomes. In this small group of patients, donor-recipient HLA mismatch was associated with higher NRM (P = .01) and a trend toward lower OS and EFS at one year (P = .09 and .07, respectively) by univariate analysis (Table 3). Patients older than 59 years had similar outcomes to patients younger than 60 years. Subjects receiving ATG in the conditioning regimen showed nonsignificant trend toward better overall survival (51% versus 33%, P = .28), better EFS (45% versus 25%, P = .25), and a lower risk of NRM (39% versus 52%, P = .24), when compared with individuals who did not receive ATG in the conditioning regimen. Higher CD34+ cell dose was also associated with a higher probability of OS at one year (53% versus 33%, P = .35).
Transplantation outcome by diagnosis
. | AML; n = 13 . | MDS; n = 7 . | CML; n = 9 . |
|---|---|---|---|
| Median age, y (range) | 60 (55-69) | 61 (55-64) | 58 (55-62) |
| Median follow-up of patients alive, d (range) | 817 (104-1642) | 910 (872-1540) | 495 (336-1016) |
| Overall survival at 1 year, %* | 36.9 ± 13.8 | 42.9 ± 18.7 | 55.5 ± 16.5 |
| Relapse-free survival at 1 year, %* | 36.9 ± 13.8 | 42.9 ± 18.7 | 44.4 ± 16.5 |
| Transplant-related mortality at 1 year, %* | 55.2 ± 13.8 | 46.4 ± 20.1 | 44.4 ± 16.5 |
. | AML; n = 13 . | MDS; n = 7 . | CML; n = 9 . |
|---|---|---|---|
| Median age, y (range) | 60 (55-69) | 61 (55-64) | 58 (55-62) |
| Median follow-up of patients alive, d (range) | 817 (104-1642) | 910 (872-1540) | 495 (336-1016) |
| Overall survival at 1 year, %* | 36.9 ± 13.8 | 42.9 ± 18.7 | 55.5 ± 16.5 |
| Relapse-free survival at 1 year, %* | 36.9 ± 13.8 | 42.9 ± 18.7 | 44.4 ± 16.5 |
| Transplant-related mortality at 1 year, %* | 55.2 ± 13.8 | 46.4 ± 20.1 | 44.4 ± 16.5 |
Kaplan-Meier estimate and 95% Cl.
Analysis of prognostic factors (univariate analysis)
. | . | Overall survival at 1 year . | . | Relapse-free survival at 1 year . | . | Transplant-related mortality at 1 year . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. of patients . | % survival . | P . | % survival . | P . | % TRM . | P . | |||
| Patient sex | .86 | .99 | .68 | |||||||
| Male | 18 | 44.4 | 38.9 | 48.4 | ||||||
| Female | 11 | 43.6 | 45.4 | 45.5 | ||||||
| Patient age* | .85 | .94 | .61 | |||||||
| Younger than 60 y | 17 | 47.1 | 41.2 | 48.2 | ||||||
| 60 y or older | 12 | 38.9 | 40.0 | 48.1 | ||||||
| Donor sex | .64 | .61 | .21 | |||||||
| Male | 23 | 43.5 | 39.1 | 52.9 | ||||||
| Female | 6 | 50.0 | 50.0 | 25.0 | ||||||
| Patient CMV status | .65 | .62 | .47 | |||||||
| Reactive | 10 | 50.0 | 50.0 | 33.3 | ||||||
| Nonreactive | 19 | 42.1 | 36.8 | 53.7 | ||||||
| Donor CMV status | .84 | .75 | .71 | |||||||
| Reactive | 17 | 46.3 | 46.3 | 45.2 | ||||||
| Nonreactive | 12 | 41.7 | 33.3 | 51.4 | ||||||
| HLA matching | .09 | .07 | .01 | |||||||
| Matched | 23 | 51.2 | 46.8 | 38.3 | ||||||
| Mismatched | 6 | 16.7 | 16.7 | 83.3 | ||||||
| Time to transplantation* | .83 | .76 | .52 | |||||||
| 18 mo or longer | 15 | 45.7 | 38.1 | 54.3 | ||||||
| Less than 18 mo | 14 | 42.9 | 42.9 | 38.8 | ||||||
| Disease status at transplantation for AML/MDS | ||||||||||
| Sensitive | 4 | 75.0 | 75.0 | 25.0 | ||||||
| Refractory | 11 | 12.1 | .03 | 13.6 | .03 | 79.5 | .07 | |||
| Untreated | 5 | 60.0 | .54 | 60.0 | .30 | 20.0 | .98 | |||
| Use of ATG in conditioning | ||||||||||
| Yes | 17 | 51.3 | .28 | 45.3 | .25 | 38.8 | .24 | |||
| No | 12 | 33.0 | 25.0 | 51.9 | ||||||
| CD34+ cell dose* | .35 | .66 | .53 | |||||||
| 4.46 × 106/kg or more | 15 | 53.3 | 46.6 | 41.8 | ||||||
| Less than 4.46 × 106/kg | 14 | 32.6 | 33.3 | 57.1 | ||||||
. | . | Overall survival at 1 year . | . | Relapse-free survival at 1 year . | . | Transplant-related mortality at 1 year . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. of patients . | % survival . | P . | % survival . | P . | % TRM . | P . | |||
| Patient sex | .86 | .99 | .68 | |||||||
| Male | 18 | 44.4 | 38.9 | 48.4 | ||||||
| Female | 11 | 43.6 | 45.4 | 45.5 | ||||||
| Patient age* | .85 | .94 | .61 | |||||||
| Younger than 60 y | 17 | 47.1 | 41.2 | 48.2 | ||||||
| 60 y or older | 12 | 38.9 | 40.0 | 48.1 | ||||||
| Donor sex | .64 | .61 | .21 | |||||||
| Male | 23 | 43.5 | 39.1 | 52.9 | ||||||
| Female | 6 | 50.0 | 50.0 | 25.0 | ||||||
| Patient CMV status | .65 | .62 | .47 | |||||||
| Reactive | 10 | 50.0 | 50.0 | 33.3 | ||||||
| Nonreactive | 19 | 42.1 | 36.8 | 53.7 | ||||||
| Donor CMV status | .84 | .75 | .71 | |||||||
| Reactive | 17 | 46.3 | 46.3 | 45.2 | ||||||
| Nonreactive | 12 | 41.7 | 33.3 | 51.4 | ||||||
| HLA matching | .09 | .07 | .01 | |||||||
| Matched | 23 | 51.2 | 46.8 | 38.3 | ||||||
| Mismatched | 6 | 16.7 | 16.7 | 83.3 | ||||||
| Time to transplantation* | .83 | .76 | .52 | |||||||
| 18 mo or longer | 15 | 45.7 | 38.1 | 54.3 | ||||||
| Less than 18 mo | 14 | 42.9 | 42.9 | 38.8 | ||||||
| Disease status at transplantation for AML/MDS | ||||||||||
| Sensitive | 4 | 75.0 | 75.0 | 25.0 | ||||||
| Refractory | 11 | 12.1 | .03 | 13.6 | .03 | 79.5 | .07 | |||
| Untreated | 5 | 60.0 | .54 | 60.0 | .30 | 20.0 | .98 | |||
| Use of ATG in conditioning | ||||||||||
| Yes | 17 | 51.3 | .28 | 45.3 | .25 | 38.8 | .24 | |||
| No | 12 | 33.0 | 25.0 | 51.9 | ||||||
| CD34+ cell dose* | .35 | .66 | .53 | |||||||
| 4.46 × 106/kg or more | 15 | 53.3 | 46.6 | 41.8 | ||||||
| Less than 4.46 × 106/kg | 14 | 32.6 | 33.3 | 57.1 | ||||||
TRM indicates transplant-related mortality
Above and below the median.
There was no statistically significant difference in survival after treatment with the different preparative regimens used here (P = .25 for the comparison of fludarabine combined with busulfan, and 140 mg/m2 or 180 mg/m2 melphalan). However, actuarial 2-year survival of patients treated with fludarabine and 180 mg/m2 melphalan was 55%, while it was 14% for patients treated with fludarabine and 140 mg/m2 melphalan (P = .1). Actuarial 1-year survival of subjects treated with fludarabine and busulfan was 30%.
Among patients with AML/MDS, disease responsiveness to chemotherapy prior to transplantation was associated with better OS (75% versus 12%, P = .03) and EFS (75% versus 14%, P = .03) at one year after transplantation, when compared with patients with refractory unresponsive disease, and a trend toward lower one-year NRM (25% versus 79%, P = .07).
Quality of life and working status after transplantation
Of the 11 patients who were alive at the time of analysis, 9 completed and returned the questionnaires. In summary, the scores reported in all dimensions were high, indicating good QOL of the patients who survived. For item no. 33, which gives a global impression of QOL, the mean score was 3.67 (Table 4). Concerning the working status of the patients, 1 patient continued to work full time in a professional post after transplantation and another patient who stopped working after the diagnosis of AML went back to working part time after transplantation. After treatment, 5 patients who worked full time (n = 3) or part time (n = 2) before transplantation retired. The other 2 patients had already retired before the diagnosis of their hematologic diseases.
Quality of life assessment
Variables (minimum, maximum scores) . | Mean scores (standard deviation, range) . |
|---|---|
| Physical well-being (0, 28) | 26.35 (3.10, 18.67-28) |
| Social well-being (0, 28) | 25.66 (1.93, 22.4-28) |
| Relationship with doctor (0, 8) | 7.67 (0.71, 6-8) |
| Emotional well-being (0, 24) | 21.33 (3.77, 13-24) |
| Functional well-being (0, 28) | 25.18 (3.20, 19.6-28) |
| FACT total (0, 116) | 106.19 (9.79, 82.27-116) |
| BMT module (0, 48) | 41.00 (5.44, 28.36-48) |
| FACT-BMT (0, 164) | 147.19 (15.12, 110.63-164) |
Variables (minimum, maximum scores) . | Mean scores (standard deviation, range) . |
|---|---|
| Physical well-being (0, 28) | 26.35 (3.10, 18.67-28) |
| Social well-being (0, 28) | 25.66 (1.93, 22.4-28) |
| Relationship with doctor (0, 8) | 7.67 (0.71, 6-8) |
| Emotional well-being (0, 24) | 21.33 (3.77, 13-24) |
| Functional well-being (0, 28) | 25.18 (3.20, 19.6-28) |
| FACT total (0, 116) | 106.19 (9.79, 82.27-116) |
| BMT module (0, 48) | 41.00 (5.44, 28.36-48) |
| FACT-BMT (0, 164) | 147.19 (15.12, 110.63-164) |
Higher scores indicate better quality of life. Quality of life was assessed using the FACT-BMT.15
Dry skin (8 of 9 patients, 88.9%) was the most common chronic GVHD-related symptom, followed by dry eyes (n = 4, 44.4%), dry mouth (n = 4, 44.4%), and joint stiffness (n = 4, 44.4%). Poor memory was reported by 5 subjects (56.%), while 6 patients noticed significant weight gain of more than 20 lb since transplantation and 1 patient noticed weight loss of more than 20 lb since transplantation. Only one patient reported increased frequency of infections.
Discussion
Reduced-intensity preparative regimens were used here in a series of protocols to provide cytoreduction of the malignancy and sufficient immunosuppression to allow donor cell engraftment, allowing development of immunologic “graft-versus-leukemia” effect. This strategy produces less regimen-related toxicities, allowing extension of allogeneic BMT to older patients.19,20
We studied transplantation outcomes of a group of patients older than 55 years who received matched unrelated donor HSC transplant for advanced stage AML, MDS, and CML. The probability of overall and event-free survival, and nonrelapse mortality at one year were 44%, 37%, and 55%, respectively. The majority of our patients had refractory relapsed disease at transplantation, and it is a reasonable assumption that extensive pretransplantation treatment contributed to the high NRM observed here, and that age per se was not the only contributor to treatment-related mortality. Accordingly, the results among the small number of patients who were in remission at the time of transplantation were significantly better than the results obtained among relapsed patients (lower NRM and better survival). Results were poor in patients with resistant relapse, with only 10% survival beyond one year.
The biologic basis underlying the increased incidence of nonrelapse mortality in older patients is not well understood. In some studies acute GVHD incidence increases with age,21 but it is unlikely that the increase in nonrelapse deaths seen in older patients can be attributed solely to an increased incidence of GVHD. It is hypothesized that the release of inflammatory cytokines triggered by tissue destruction produced by the preparative regimen is involved in the pathogenesis of these complications.22 Recently, it has been shown in a murine model that donor T-cell responses are increased both in vivo and in vitro when stimulated by antigen-presenting cells from older mice, suggesting an important role for aging host antigen-presenting cells in the pathogenesis of GVHD in this context.23 The role of immunosenescence in this setting is also poorly understood. Although thymic function has been shown to persist throughout adult life, thymic output decreases markedly with aging.24
The effect of disease stage on NRM among patients older than 45 years was described by Ringden et al.25 They showed that for patients with early-stage leukemia receiving an HLA-identical sibling allograft, 2-year NRM was similar for patients aged 45 to 49 years and for those older than 50 years (38% and 41%, respectively). For patients with advanced disease, however, 2-year NRM was 94%. These studies involved subjects receiving myeloablative regimens, though, and it is largely unknown if the same assumptions are valid in the context of nonmyeloablative transplants. Our group has reported an NRM at 2 years of 44.7% using a less intensive preparative regimen of fludarabine combined with melphalan. We treated patients with hematologic malignancies, with a median age of 52 years, otherwise not eligible for allogeneic transplantation due to concomitant medical conditions or older age. The majority of patients had refractory leukemia or transformed CML.20 Causes of death for recipients of HLA-identical sibling and matched unrelated donor transplants differed. Relapse was the major cause of death among recipients of matched sibling transplants, while acute and chronic GVHD caused the majority of the deaths among unrelated donor recipients.
The rate of acute and chronic GVHD in our cohort was also comparable with that reported previously for younger patients.7,26,27 An NMDP study including 1423 patients with CML (median age, 35 years) reported an incidence of grade III/IV acute GVHD of 33%, while the incidence of extensive chronic GVHD was 60% (95% confidence interval [CI], 56%-63%) at 2 years.7
It would appear that the response rate, and leukemia-free and overall survival of our small cohort is comparable with results reported in the literature for younger, advanced leukemia patients. The NMDP reported a 10%±5% 5-year probability of survival for patients who underwent transplantation for CML in blast crisis.27 Achievement of a second chronic phase has been associated with a one-year survival rate of 44%.28 Similar associations between advanced stage disease and higher NRM and lower disease-free survival have been established for AML and MDS patients undergoing unrelated donor bone marrow transplantations.29,30 Engraftment and hematologic recovery rates in our group of older patients were similar to that reported in larger series.31
The issue of quality of life after transplantation is especially relevant when discussing matched unrelated donor transplantation for older patients. Within the limits of our small sample size, cross-sectional assessment of QOL using the FACT-BMT questionnaire demonstrated that the survivors achieved an overall suitable quality of life after transplantation, with high scores in all dimensions. These results are comparable with those previously reported for younger patients.16,32,33 Chronic GVHD is a major late complication of allogeneic transplantation, and, accordingly, most symptoms were attributable to it. However, these symptoms did not severely impair the patients' performance status. Most patients interested in returning to work did so.
In this study, even though the small number of patients prevented documentation of independent correlation with survival, donor-recipient HLA matching, use of ATG in the preparative regimen, and higher infused CD34+ cell dose were associated with a trend toward better outcomes, while fully HLA-matched donor-recipient pairs had a statistically significant lower risk of NRM. Use of ATG in the conditioning regimen has been shown to reduce the risk of severe acute GVHD and extensive chronic GVHD.34 The occurrence of grades III to IV acute GVHD may be further minimized by allele level matching for class I and II loci.35 Transplantation of unrelated marrows with a higher cell dose may be associated with faster neutrophil and platelet engraftment, and decreased incidence of severe acute GVHD.36
In conclusion, current definitions of “age limits” for unrelated donor transplantation are yet to be defined. These data indicate that unrelated donor transplantation can be successfully performed in patients older than 55 years using a reduced-intensity preparative regimen. Treatment results in patients with advanced myeloid leukemias appeared comparable with those achieved in younger patients. Chronic GVHD was the major late complication, but quality of life was rated as good by most patients. Further studies are required to determine the relative role of unrelated hematopoietic transplantation as an alternative form of treatment for older patients with myeloid malignancies.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-03-0855.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Safa Karandish and M. Jo Lauppe for their review of laboratory data.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal