Abstract
Several studies have compared bone marrow (BM) and peripheral blood (PB) as stem cell sources in patients receiving allografts, but the cell doses infused have not been considered, especially for BM. Using the ALWP/EBMT registry, we retrospectively studied 881 adult patients with acute myelocytic leukemia (AML), who received a non–T-depleted allogeneic BM (n = 515) or mobilized PB (n = 366) standard transplant, in first remission (CR1), from an HLA-identical sibling, over a 5-year period from January 1994. The BM cell dose ranged from 0.17 to 29 × 108/kg with a median of 2.7 × 108/kg. The PB cell dose ranged from 0.02 to 77 × 108/kg with a median of 9.3 × 108/kg. The median dose for patients receiving BM (2.7 × 108/kg) gave the greatest discrimination. In multivariate analyses, high-dose BM compared to PB was associated with lower transplant-related mortality (RR = 0.61; 95% CI, 0.39-0.98; P = .04), better leukemia-free survival (RR = 0.65; 95% CI, 0.46-0.91; P = .013), and better overall survival (RR = 0.64; 95% CI, 0.44-0.92; P = .016). The present study in patients with AML receiving allografts in first remission indicates a better outcome with BM as compared to PB, when the dose of BM infused is rich.
Introduction
In the past decade, the use of peripheral blood (PB) as a source of hematopoietic stem cells for allogeneic transplantation has increased considerably. A large number of retrospective studies,1-6 several prospective and randomized,7-10 have compared the outcome of patients receiving allografts with bone marrow (BM) versus PB, using a family identical sibling; in all studies PB has resulted in faster engraftment and shorter hospital stay. In most studies, with the notable exception of the EBMT study,9 the incidence and severity of acute graft-versus-host disease (aGVHD) has been similar with PB and BM; PB on the other hand has been associated with more chronic GVHD (cGVHD). Finally, the outcome with PB, in terms of leukemia-free survival (LFS) and overall survival (OS), has been identical to BM and sometimes superior.11 All things considered, for practicality reasons, PB has therefore become a first choice for many transplantation teams. It has been postulated that the benefit brought by PB was at least in part due to the higher cell dose infused to the recipient when compared to BM.12
In the same period, other studies on BM and PB transplantations have drawn attention to the importance of the dose of stem cells infused; a lower transplant-related mortality (TRM) and also in some studies a lower relapse rate after transplantation have been observed in patients undergoing allografting13,14 or autografting15-19 with higher BM cell doses.
In the present retrospective study based on the ALWP/EBMT registry, we compared the outcome of patients with acute myelocytic leukemia (AML) receiving allografts in first remission (CR1) using BM or PB as a source of stem cells, focusing on the dose of stem cells infused. We found a significantly better outcome in patients receiving higher BM cell doses (rich marrow), when compared to the others, that is, patients receiving low-dose BM or high- or low-dose PB.
Patients and methods
Data collection and patient selection
The European Blood and Marrow Transplant (EBMT) Registry is a voluntary working group of more than 500 transplantation centers. Participants are required once a year to report all consecutive transplantations and follow-up. The Acute Leukemia Working Party (ALWP) of the EBMT is in charge of validating and checking submitted data to ensure data quality.
This study included 881 patients with AML, older than 16 years of age, who received a non–T-depleted allogeneic BM (n = 515) or mobilized PB (n = 366) standard transplant, in CR1, from an HLA-identical sibling, over a 5-year period from January 1994 (date of the first allogeneic PB transplantation) to January 2001. The dose of cells infused with the graft was in nucleated cells per kilogram. The study was approved by the EBMT review board.
End points of the study
Hematopoietic recovery. Neutrophil and platelet recoveries were analyzed separately and defined by a neutrophil count equal to or more than 0.5 × 109/L for 3 consecutive days and a platelet count equal to or more than 50 × 109/L for 7 consecutive days with no platelet support, respectively. The median time to recovery was calculated using the product limit method.
Mortality and relapse. Transplant-related mortality (TRM) was defined as nonleukemic deaths. Relapse incidence (RI) was defined on the basis of morphologic evidence of leukemia in BM or other extramedullary sites. To evaluate the probability of relapse, patients dying either from direct toxicity of the procedure or from any other cause not related to leukemia were censored. Leukemia-free survival (LFS) was defined as the time interval from transplantation to the first event (either relapse or death in complete remission).
GVHD. Acute GVHD (aGVHD) was diagnosed and graded at each transplantation center according to Seattle criteria.20 Only patients with grade II or superior were considered as having aGHVD. For chronic GVHD (cGVHD), only patients surviving without relapse for more than 100 days after transplantation with sustained donor engraftment were considered as evaluable; cGVHD was defined according to standard criteria (limited and extensive).
Statistical analyses
All analyses were performed with the SPSS statistical analysis program (SPSS, Chicago, IL). Values reported for quantitative variables were median and range. The following patient or graft characteristics were analyzed for their potential prognostic value on each of the outcomes: patient's and donor's characteristics (age, sex, and sex matching), disease factors (white blood cell count at diagnosis, French-American-British [FAB] classification, interval from diagnosis to CR1, interval from CR1 to transplantation), and transplant-related factors (source of stem cells, nucleated cell dose infused per kilogram, year of transplantation, nature of the conditioning regimen including or not total body irradiation [TBI]). For these prognostic analyses, continuous variables were categorized according to the median value. To compare the distribution between the subgroups of patients we used the χ2 test for categorical variables and the nonparametric Mann-Whitney U test for continuous variables.
Patients were censored at the time of relapse or the last follow-up. Probability of LFS, RI, TRM, and OS were estimated by the product-limit method.21 The significance of differences between curves was estimated by the log-rank test (Mantel-Cox).22 All variables associated with outcome with a P value of less than .1 in univariate analyses and characteristics statistically different (P < .05) between subgroups of patients were included in a multivariate analysis. Because a center effect had been observed in a previous EBMT23 study in patients receiving a BM transplant for AML in CR1, all further multivariate analyses were adjusted on center.
Results
Patient populations in relation to hematopoietic stem cell doses infused and univariate analyses
The outcome was identical when comparing BM and PB transplantations: At 2 years, the TRM with BM and PB was 22% ± 2% and 22% ± 2% (P = .98), the RI 19% ± 2% and 22% ± 3% (P = .61), the LFS 63% ± 2% and 61% ± 3% (P = .72), and the OS 66% ± 2% and 66 ± 3% (P = .82), respectively.
The BM cell dose ranged from 0.17 to 29 × 108/kg recipient weight with a median of 2.7 × 108/kg. The PB cell dose ranged from 2.2 to 20 × 108/kg with a median of 9.3 × 108/kg.
There was no difference for outcome when taking the median dose of PB cells infused as a cutoff within the PB group: The 3-year LFS was 64% ± 6% in patients receiving doses below the median and 59% ± 7% for those receiving doses above the median (P = .76). For patients receiving BM, the median dose (2.7 × 108/kg) gave the greatest discrimination for all end points studied.
We therefore studied 3 populations of patients: those receiving higher doses (above median) of marrow (n = 257), those receiving lower (below median) doses of marrow (n = 258), and those receiving PB whatever the dose (n = 366).
Table 1 gives the distribution of the 3 groups for disease and transplantation characteristics; the only differences concerned the year of transplantation, which was more recent for PB, and more TBI and a trend for fewer female donor–to–male recipient combinations in the group receiving higher doses of BM.
Distribution of patients by source and dose of nucleated cells/kg infused
. | BM . | . | . | P . | . | ||
|---|---|---|---|---|---|---|---|
. | NC less than 2.7 × 108/kg, n = 258 . | NC more than 2.7 × 108/kg, n = 257 . | PB, n = 366 . | BM more than 2.7 vs BM less than 2.7 × 108/kg . | PB vs BM less than 2.7 × 108/kg . | ||
| Year of BMT (range) | 1996 (1994-2000) | 1996 (1994-2000) | 1998 (1994-2001) | .03 | < .0001 | ||
| Age, y (range) | |||||||
| Patients | 36 (16-58) | 37 (16-57) | 37 (16-66) | .25 | .04 | ||
| Donors | 35 (5-65) | 35 (10-59) | 37 (14-67) | .81 | .008 | ||
| Patient sex (%) | |||||||
| Male | 138 (53) | 126 (49) | 189 (52) | ||||
| Female | 120 (46) | 130 (51) | 174 (48) | .33 | .73 | ||
| Donor sex (%) | |||||||
| Male | 128 (51) | 146 (58) | 203 (56) | ||||
| Female | 124 (49) | 104 (42) | 162 (44) | .08 | .24 | ||
| Female donor to male recipient, % | 27 | 18 | 24 | .02 | .43 | ||
| White blood cell count at diagnosis, × 109/L | 11 (0.2-420) | 10.6 (0.7-500) | 13.5 (0.7-710) | .57 | .49 | ||
| FAB classification (%) | |||||||
| M0 | 3 (1) | 1 (0.4) | 3 (1) | ||||
| M1 | 46 (20) | 53 (23) | 52 (16) | ||||
| M2 | 67 (29) | 84 (36) | 111 (34) | ||||
| M3 | 13 (5) | 9 (4) | 11 (3) | .23 | .66 | ||
| M4 | 58 (25) | 46 (20) | 81 (25) | ||||
| M5 | 37 (16) | 29 (12) | 52 (16) | ||||
| M6 | 6 (2) | 10 (4) | 13 (4) | ||||
| M7 | 4 (2) | 1 (0.4) | 4 (1) | ||||
| Cytogenetics, n (%) | n = 125 | n = 115 | n = 126 | — | — | ||
| Good | 21 (17) | 15 (13) | 21 (17) | — | — | ||
| Intermediate | 95 (76) | 93 (81) | 96 (76) | — | — | ||
| Poor | 9 (7) | 7 (6) | 9 (7) | .65 | 1 | ||
| Interval diagnosis-CR1, d (range) | 40 (12-305) | 43 (17-480) | 41 (17-432) | .34 | .96 | ||
| Interval CR1-transplantation, d (range) | 99 (13-369) | 94 (11-2375) | 97 (12-594) | .29 | .37 | ||
| TBI, % | 45 | 57 | 37 | .005 | .06 | ||
| NCs infused, × 108/kg (range) | 2 (0.17-2.7) | 3.68 (2.7-29) | 9.3 (2.2-77) | — | — | ||
| aGVHD grades II-IV, % | 38 | 35 | 36 | .5 | .61 | ||
| aGVHD grades III-IV, % | 13 | 9 | 12 | .18 | .64 | ||
| Follow-up, mo (range) | 33 (1-79) | 35 (1-81) | 16 (1-72) | — | — | ||
. | BM . | . | . | P . | . | ||
|---|---|---|---|---|---|---|---|
. | NC less than 2.7 × 108/kg, n = 258 . | NC more than 2.7 × 108/kg, n = 257 . | PB, n = 366 . | BM more than 2.7 vs BM less than 2.7 × 108/kg . | PB vs BM less than 2.7 × 108/kg . | ||
| Year of BMT (range) | 1996 (1994-2000) | 1996 (1994-2000) | 1998 (1994-2001) | .03 | < .0001 | ||
| Age, y (range) | |||||||
| Patients | 36 (16-58) | 37 (16-57) | 37 (16-66) | .25 | .04 | ||
| Donors | 35 (5-65) | 35 (10-59) | 37 (14-67) | .81 | .008 | ||
| Patient sex (%) | |||||||
| Male | 138 (53) | 126 (49) | 189 (52) | ||||
| Female | 120 (46) | 130 (51) | 174 (48) | .33 | .73 | ||
| Donor sex (%) | |||||||
| Male | 128 (51) | 146 (58) | 203 (56) | ||||
| Female | 124 (49) | 104 (42) | 162 (44) | .08 | .24 | ||
| Female donor to male recipient, % | 27 | 18 | 24 | .02 | .43 | ||
| White blood cell count at diagnosis, × 109/L | 11 (0.2-420) | 10.6 (0.7-500) | 13.5 (0.7-710) | .57 | .49 | ||
| FAB classification (%) | |||||||
| M0 | 3 (1) | 1 (0.4) | 3 (1) | ||||
| M1 | 46 (20) | 53 (23) | 52 (16) | ||||
| M2 | 67 (29) | 84 (36) | 111 (34) | ||||
| M3 | 13 (5) | 9 (4) | 11 (3) | .23 | .66 | ||
| M4 | 58 (25) | 46 (20) | 81 (25) | ||||
| M5 | 37 (16) | 29 (12) | 52 (16) | ||||
| M6 | 6 (2) | 10 (4) | 13 (4) | ||||
| M7 | 4 (2) | 1 (0.4) | 4 (1) | ||||
| Cytogenetics, n (%) | n = 125 | n = 115 | n = 126 | — | — | ||
| Good | 21 (17) | 15 (13) | 21 (17) | — | — | ||
| Intermediate | 95 (76) | 93 (81) | 96 (76) | — | — | ||
| Poor | 9 (7) | 7 (6) | 9 (7) | .65 | 1 | ||
| Interval diagnosis-CR1, d (range) | 40 (12-305) | 43 (17-480) | 41 (17-432) | .34 | .96 | ||
| Interval CR1-transplantation, d (range) | 99 (13-369) | 94 (11-2375) | 97 (12-594) | .29 | .37 | ||
| TBI, % | 45 | 57 | 37 | .005 | .06 | ||
| NCs infused, × 108/kg (range) | 2 (0.17-2.7) | 3.68 (2.7-29) | 9.3 (2.2-77) | — | — | ||
| aGVHD grades II-IV, % | 38 | 35 | 36 | .5 | .61 | ||
| aGVHD grades III-IV, % | 13 | 9 | 12 | .18 | .64 | ||
| Follow-up, mo (range) | 33 (1-79) | 35 (1-81) | 16 (1-72) | — | — | ||
NC indicates nucleated cells; —, not applicable.
The distribution was even for patient age and sex, white blood cell count at diagnosis, FAB classification, and pretransplantation intervals.
Information on cytogenetics was available for 366 patients. In keeping with our previous work,24 57 patients were in the good-risk category (t(15;17), t(8;21) and inv16), 25 in the poor-risk category (abnormality 5 or 7, 11q–), and 284 in the intermediate-risk category. The number of missing values was too high to include cytogenetics in the multivariate analysis. However, the distribution by cytogenetics over the 3 groups of patients was even.
Recovery of polymorphonuclear cells (PMNs) to 500/mm3 with high-dose BM, low-dose BM, and PB occurred at days 18 (range, days 10-39), 19 (range, days 10-53), and 14 (range, days 10-42), respectively. The differences were statistically significant between rich and poor BM (P = .009) and PB and poor BM or rich BM (P < 10–4 for both). Recovery of platelets to 50 000/mm3 with high-dose BM, low-dose BM, and PB occurred at days 24 (range, days 12-385), 27 (range, days 12-263), and 17 (range, days 7-343), respectively. The differences were statistically significant between rich and poor BM (P = .02), PB and poor BM (P = .0002) or rich BM (P < 10–4).
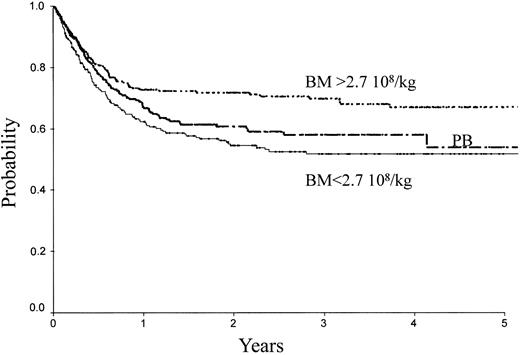
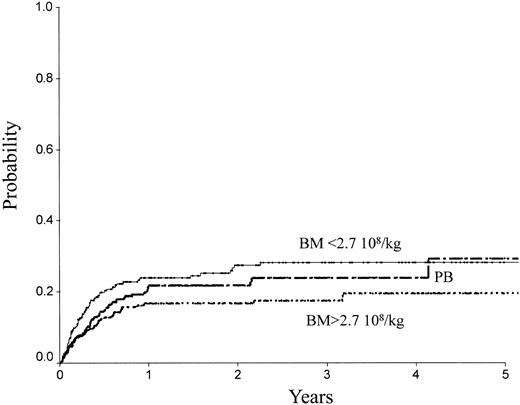
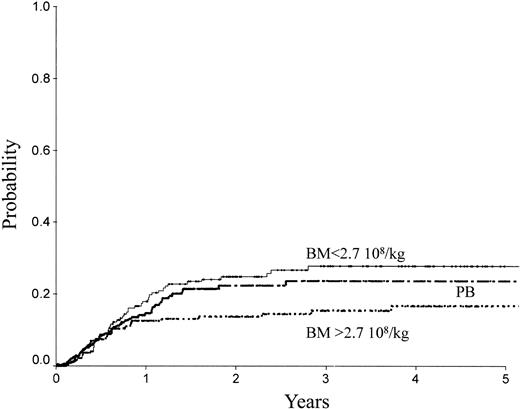
Table 2 indicates the outcome at 2 years in these 3 groups of patients. The LFS was 72% ± 3% with the high BM dose versus 54% ± 3% with the low BM dose (P = .0007) and 61% ± 3% with PB (P = .04; Figure 1). The TRM (Figure 2) and the RI (Figure 3) were lower with the high BM dose compared to the low BM dose (TRM, 17% ± 2% versus 27% ± 3%, P = .02; RI, 14% ± 2% versus 25% ± 3%, P = .02). The incidence of aGVHD was similar in the 3 groups. Patients receiving PB had the highest incidence of cGVHD evaluated at 1 year (50% ± 4%) over low-dose BM (43% ± 5%, P = .002) and high-dose BM (40% ± 5%; P = .0006).
Outcome of patients by source and dose of nucleated cells/kg infused
. | BM, %* . | . | . | P . | . | ||
|---|---|---|---|---|---|---|---|
. | NC less than 2.7 × 108/kg . | NC more than 2.7 × 108/kg . | PB, %* . | PB vs BM less than 2.7 × 108/kg . | PB vs BM more than 2.7 × 108/kg . | ||
| 2-y outcome | |||||||
| LFS | 54 ± 3 | 72 ± 3 | 61 ± 3 | .17 | .04 | ||
| RI | 25 ± 3 | 14 ± 2 | 22 ± 3 | .23 | .17 | ||
| TRM | 27 ± 3 | 17 ± 2 | 22 ± 2 | .23 | .17 | ||
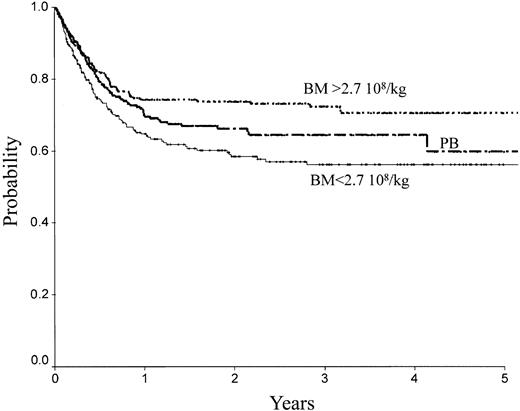
| OS | 58 ± 3 | 74 ± 3 | 66 ± 3 | .08 | .14 | ||
| 1-y cGVHD | 43 ± 5 | 40 ± 5 | 50 ± 4 | .002 | .0006 | ||
. | BM, %* . | . | . | P . | . | ||
|---|---|---|---|---|---|---|---|
. | NC less than 2.7 × 108/kg . | NC more than 2.7 × 108/kg . | PB, %* . | PB vs BM less than 2.7 × 108/kg . | PB vs BM more than 2.7 × 108/kg . | ||
| 2-y outcome | |||||||
| LFS | 54 ± 3 | 72 ± 3 | 61 ± 3 | .17 | .04 | ||
| RI | 25 ± 3 | 14 ± 2 | 22 ± 3 | .23 | .17 | ||
| TRM | 27 ± 3 | 17 ± 2 | 22 ± 2 | .23 | .17 | ||
| OS | 58 ± 3 | 74 ± 3 | 66 ± 3 | .08 | .14 | ||
| 1-y cGVHD | 43 ± 5 | 40 ± 5 | 50 ± 4 | .002 | .0006 | ||
Values given as mean ± SD.
LFS of patients receiving transplants with high-dose BM, low-dose BM, or PB.
TRM of patients receiving transplants with high-dose BM, low-dose BM, or PB.
RI of patients receiving transplants with high-dose BM, low-dose BM, or PB.
Table 3 lists prognostic factors other than cell dose influencing outcome. Not surprisingly, a younger age for the patient and for the donor, a short interval from diagnosis to CR1 (rapid remitters), and a short interval from CR1 to transplantation were all significantly associated with a lower TRM. A female donor–to–male recipient combination resulted in a higher TRM. aGVHD (score II and over) resulted in higher TRM, lower RI, and lower LFS and OS. Of particular interest was the confirmation of the existence of a center effect affecting TRM, LFS, and OS, but not RI, as we previously reported.23
Prognostic factors other than cell dose influencing outcome, P (log-rank test)
. | LFS . | RI . | TRM . | OS . |
|---|---|---|---|---|
| Patient sex | .91 | .89 | .78 | .53 |
| Donor sex | .86 | .1 | .09 | .68 |
| Female to male | .54 | .1 | .02† | .15 |
| FAB M5 versus other | .88 | .83 | .98 | .7 |
| TBI | .72 | .79 | .48 | .57 |
| Patient age | .06 | .86 | .007† | .02† |
| Donor age | .009† | .28 | .01† | .012† |
| Year of transplantation (before 1997 versus after 1997) | .26 | .16 | .79 | .92 |
| Interval diagnosis-CR1, d | .08 | .88 | .03† | .02† |
| Interval CR1-transplantation, d | .16 | .52 | .02† | .14 |
| White blood cell count at diagnosis | .94 | .82 | .77 | .51 |
| Center* | .02† | .22 | .03 | .01† |
. | LFS . | RI . | TRM . | OS . |
|---|---|---|---|---|
| Patient sex | .91 | .89 | .78 | .53 |
| Donor sex | .86 | .1 | .09 | .68 |
| Female to male | .54 | .1 | .02† | .15 |
| FAB M5 versus other | .88 | .83 | .98 | .7 |
| TBI | .72 | .79 | .48 | .57 |
| Patient age | .06 | .86 | .007† | .02† |
| Donor age | .009† | .28 | .01† | .012† |
| Year of transplantation (before 1997 versus after 1997) | .26 | .16 | .79 | .92 |
| Interval diagnosis-CR1, d | .08 | .88 | .03† | .02† |
| Interval CR1-transplantation, d | .16 | .52 | .02† | .14 |
| White blood cell count at diagnosis | .94 | .82 | .77 | .51 |
| Center* | .02† | .22 | .03 | .01† |
One class grouping all centers performing fewer than 10 transplantations during the period.
Significant difference.
Table 4 gives the causes of death.
Causes of death
. | BM . | . | . | |
|---|---|---|---|---|
. | NC less than 2.7 × 108/kg, % . | NC more than 2.7 × 108/kg, % . | PB, % . | |
| Failure/rejection | 2 | 0 | 2 | |
| Infection | 21 | 16 | 14 | |
| Interstitial pneumonitis | 2 | 13 | 7 | |
| GVHD | 28 | 21 | 31 | |
| Leukemia | 43 | 45 | 40 | |
| Other | 4 | 5 | 6 | |
. | BM . | . | . | |
|---|---|---|---|---|
. | NC less than 2.7 × 108/kg, % . | NC more than 2.7 × 108/kg, % . | PB, % . | |
| Failure/rejection | 2 | 0 | 2 | |
| Infection | 21 | 16 | 14 | |
| Interstitial pneumonitis | 2 | 13 | 7 | |
| GVHD | 28 | 21 | 31 | |
| Leukemia | 43 | 45 | 40 | |
| Other | 4 | 5 | 6 | |
Multivariate analyses
There was no difference between PB and low-dose BM in terms of TRM, RI, LFS, and OS. In contrast, high-dose BM compared to PB was associated with lower TRM (Figure 2; RR = 0.61; 95% CI, 0.39-0.98; P = .04), better LFS (Figure 1; RR = 0.65; 95% CI, 0.46-0.91; P = .013), and better OS (Figure 4; RR = 0.64; 95% CI, 0.44-0.92; P = .016). No significant association was found for RI (Figure 4). Marrow at low and high dose induced less cGVHD than PB (low-dose BM versus PB, P = .006; high-dose BM versus PB, P = .003).
OS of patients receiving transplants with high-dose BM, low-dose BM, or PB.
None of the other studied factors (patient and donor age and sex, female donor to male recipient, year of transplantation, and use of TBI) influenced any component of the outcome.
Discussion
In the past 5 years several prospective randomized studies have compared BM and PB as alternative sources of stem cells for allogeneic stem cell transplantation using HLA-identical siblings.7-10 Conclusions from these studies have been that PB is associated with faster engraftment, similar or greater incidence of aGVHD, and higher incidence of cGVHD. LFS and OS have been found identical or even better with PB.11 All these studies have combined transplantations for several hematologic malignancies. A more limited number of retrospective studies have suggested poorer results with PB in more risky situations, such as with mismatched related donors,25 unrelated donors,26 or more advanced diseases. Also, it has been recently suggested that results may vary for different diseases, with the observation in transplantations using unrelated donors of similar outcome with BM and PB in AML contrasting with a worse outcome with PB in acute lymphocytic leukemia (ALL).26
The minimum doses of cells to ensure safe engraftment have been established very early with the development of BM and then PB transplantation. For allogeneic stem cell transplantation, it is generally accepted that a BM graft should contain more than 2 × 108 nucleated cells/kg and a PB graft (as initially defined in the setting of autologous PB stem cell transplantation) more than 2 or even better 5 × 106 CD34+ cells/kg. These thresholds have been established from observations on the kinetics of engraftment and, with PB, infusion of doses more than 5 × 106 CD34+ cells/kg has been shown not to further reduce the duration of aplasia. Only recently, however, has attention focused on the possibility that increasing the doses of stem cells above these thresholds might not only reduce the TRM but also and more unexpectedly reduce the relapse/progression rate of the underlying disease. In the field of AML specifically, in the context of autologous BM transplantation with BM purged by mafosfamide, the dose of stem cells infused has been identified as an important prognostic factor for outcome.15-19 Modeling of prognostic groups for LFS generated 3 groups of patients.18 The best one consisted of patients who received a stem cell dose evaluated before purging in granulocyte-macrophage colony-forming units (CFU-GMs)/kg (agar cultures with placenta conditioned medium and no cytokines) over the median (> 5.46 × 104/kg) and an actual residual CFU-GM dose evaluated after mafosfamide purging below the median (< 0.02 × 104/kg); in this group the LFS at 12 years was 70% with a 2% TRM. More recently, with PB autologous transplantation in AML, the EORTCGIMEMA group (AML 10 study) reported on an increase in relapse incidence following autografting with PB, linked to higher doses of CD34+ cells infused27 but in a subsequent paper28 linked this observation to the presence in the graft of large amounts of leukemic CD34+ cells. In patients over 60 years of age, receiving autografts for AML in CR1, high-dose PB and BM infusion were associated with a lower RI than low-dose PB.19 Following allogeneic BM transplantation with an HLA-identical twin or sibling29,30 for various hematologic malignancies, a greater dose of nucleated or progenitor CD34+ cells has been shown to reduce fungal infections and TRM and to result in a better OS. As an example in 212 patients who received a transplant of an unmanipulated graft from an HLA-identical sibling donor,30 5-year survival and 180-day TRM were, respectively, 64% and 19% for patients receiving a CD34+ cell dose of 3 × 106/kg or more and 40% and 37% for the other. EBMT similarly has recently reported on the impact of the dose of BM on the outcome of patients receiving allotransplants for AML with a geno-identical family donor; higher doses of cells infused expressed in nucleated cells/kg (above median value) not only were associated with a lower TRM but also with a reduced RI, with no effect on GVHD.14 In high-risk patients, the Seattle group, studying transplantation with unrelated donors, made similar observations,13 that is, an increase in LFS with higher stem cell doses due mainly to a reduction in TRM but also at least in part to an enhanced graft-versus-leukemia (GVL) effect. To explain the reduced RI in relation to the higher dose of cells infused, 2 mechanisms have been proposed, a stem cell competition effect whereby an expanded normal stem cell pool might have a growth advantage over the minimal residual tumor population, and higher numbers of lymphocytes infused with the richer stem cell graft inducing more GVL, both effects possibly combining.
PB grafts contain 5 to 10 times more nucleated cells and about 10 times more T lymphocytes than BM grafts.12 PB following mobilization with granulocyte colony-stimulating factor (G-CSF) has more T polarized cells (Th2) with antiinflammatory cytokines (hence supposedly the absence of more GVHD, as initially feared),31 and more CD14+ cells. In contrast, mesenchymal cells are present only in BM (approximately 1/104 BM cells/kg) and virtually absent in mobilized PB.32 Richer marrows may contain higher doses of mesenchymal stem cells. Other types of accessory cells also may be differently distributed in the 2 products and there may be so far unforeseen advantages in using BM, or even combining BM and blood. Allografting with PB is associated not only with better kinetics of engraftment but also more rapid immune reconstitution.12 Despite an increase in cGVHD and a questionable increase in aGVHD with PB, which would favor the use of BM, PB tends presently to be preferred by many teams, but the general assumption is that most of its benefit comes from the higher stem cell dose infused. However, in all studies comparing PB to BM the dose of BM infused never has been considered. In addition, in all studies comparing PB to BM, the median number of BM nucleated cells infused was lower than in the present study. In this respect this study may be of importance because it draws attention to the fact that infusion of rich BM during allogeneic stem cell transplantation from an HLA-identical sibling gives a better outcome than PB, whereas low-dose BM and PB are equivalent. Previous studies comparing PB to BM not only have not taken into account the doses for BM, but also have mixed several diseases and disease status. Because the present study is homogeneous in that it concerns only patients with AML in CR1, the present finding may not necessarily apply to other hematologic malignancies or more advanced stages of AML.
Appendix
The following investigators contributed to the ALWP: Manuel Abecasis, Institute Portugues Oncologia, Lisboa, Portugal; Jitka Abráhamová, Thomayer Memorial Teaching Hospital, Prague, Czech Republic; B.V. Afanassiev, SPb State I. Pavlov Medical University, St. Petersburg, Russia; Massimo Aglietta, Istituto per la Ricerca e la Cura del Cancro, Candiolo, Italy; Abdulaziz Alabdulaaly, Riyadh Armed Forces Hospital, Riyadh, Saudi Arabia; Olga Aleinikova, Belorussian Centre for Paediatric, Minsk, Belarus; E. Paolo Alessandrino, Policlinico San Matteo, Pavia, Italy; Salem H. Al Shemmari, Hamid Al-Essa Center, Jabriya, Kuwait; D. Amadori, Morgagni-Pierantoni Hospital, Forli, Italy; Sergio Amadori, University Tor Vergata, St. Eugenio Hospital, Rome, Italy; Toren Amos, Chaim Sheba Medical Center, Tel-Hashomer, Israel; Marino Andolina, Istituto Per. L'Infanzia Burlo Garofolo, Trieste, Italy; Reinhard Andreesen, University Regensburg, Regensburg, Germany; Emanuele Angelucci, Ospedale A. Businco, Cagliari, Italy; Jürgen Anhuf, St. John's Hospital,, Duisburg, Germany; Renate Arnold, CHARIT, Berlin, Germany; Fikret Arpaci, Gülhane Military Medical Academy, Ankara, Turkey; Michel Attal, Hopital de Purpan, Toulouse, France; Wellington Azevedo, Hospital Amaral Carvalho, Sao Paulo, Brazil; Hamdy A. Azim, Oncology Centre, Cairo, Egypt; Michele Baccarani, Hospital San Orsola, Bologna, Italy; Andrea Bacigalupo, Ospedale San Martino, Genova, Italy; Tiziano Barbui, Ospedale Bergamo, Bergamo, Italy; Mario Bargetzi, Center of Oncology/Hematology, Aarau, Switzerland; D.L. Barnard, St James' University Hospital, Leeds, United Kingdom; H.H. Bartsch, Klinik für Tumorbiologie, Freiburg, Germany; André Baruchel, Hôpital St. Louis, Paris, France; Camillo Battista, Divisione di Oncologia e Trapianto di Midollo Osseo Ospedale, Napoli, Italy; Jacques-Olivier Bay, Fédération de Greffe de Moelle et de Thérapie Cellulaire d'Auvergne, Clermont-Ferrand, France; Mahmut Bayik, Marmara University, School of Medicine, Istanbul, Turkey; Ali Bazarbachi, American University of Beirut, Beirut, Lebanon; Y. Beguin, University of Liege, Liege, Belgium; Jose Luis Bello López, Hospital Xeral de Calicia, Santiago de Compostela, Spain; Istvan Benedek, Sectia Clinica de Hematolgoie si Trasnpalnt de Maduva, Targu-Mures, Romania; Fabio Benedetti, Policlinico G.B. Rossi, Verona, Italy; Carmelo Bengala, St. Chirara Hospital, Pisa, Italy; Alain Berrebi, Kaplan Hospital, Rehovot, Israel; J. Besalduch, Hospital Universitari Son Dureta, Palma de Mallorca, Spain; Douwe Biesma, St. Antonius Hospital, Nieuwegein, The Netherlands; Pierre Biron, Centre Leon Berard, Lyon, France; Magnus Björkholm, Karolinska Hospital, Stockholm, Sweden; Didier Blaise, Institut Paoli Calmettes, Marseille, France; Norbert E. Blesing, The Great Western Hospital, Swindon, United Kingdom; Marc Boasson, CHRU, Angers, France; Dragan Bobev, University Hospital “Queen Johanna,” Sofia, Bulgaria; Mario Boccadoro, Ematologia Universitaria, Torino, Italy; Zahit Bolaman, Adnan Menderes University Med. Faculty, Turkey; Marc A. Boogaerts, University Hospital Gasthuisberg, Leuven, Belgium; Dominique Bordessoule, CHRU Limoges, Limoges, France; Alberto Bosi, Ospedale di Careggi, Firenze, Italy; Botelho Sousa Aida, Hospital dos Capuchos, Lisboa, Portugal; Jean Henri Bourhis, Institut Gustave Roussy, Villejuif Cedex, France; George Bourikas, Thrace University Medical School, Alexandroupolis, Greece; David T. Bowen, Ninewells Hospital, Dundee, United Kingdom; Marco Bregni, Istituto Scientifico H.S. Raffaele, Milano, Italy; Greet Bries, Virga Jesseziekenhuis, Hasselt, Belgium; Lorentz Brinch, Rikshospitalet, Oslo, Norway; D. Brittain, West Rand Oncology Centre, Weltevredenpark, South Africa; Dominique Bron, Institut Jules Bordet, Brussels, Belgium; Mats Brune, Sahlgrenska University Hospital, Goeteborg, Sweden; Eduardo O. Bullorsky, Hospital Británico, Buenos Aires, Argentina; Donald Bunjes, Medizinische Klinik und Poliklinik, Ulm, Germany; Stefan Burdach, Martin-Luther-University HalleWittenberg, Halle, Germany; Alan K. Burnett, College of Medicine, Cardiff, United Kingdom; Agnes Buzyn, Hôpital Necker, Paris, France; Dolores Caballero, Hospital Clínico, Salamanca, Spain; Seckin Cagirgan, Ege University Medical School, Bornova-Izmir, Turkey; Jean-Yves Cahn, Hopital Jean Minjoz, Besancon, France; Carlos Osvaldo Canepa, UTMO— Hospital CUCAIBA, La Plata, Argentina; Antonio Cao, Ospedale Regionale Microcitemie, Cagliari, Italy; Angelo Michele Carella, IRCCS, Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy; Fernández Dolores Carrera, Hospital Covadonga, Oviedo, Spain; Anne-Sophie Carret, The Montréal Children's Hospital, Montréal, Canada; Stefano Cascinu, Medical Oncology Division, Parma, Italy; Victorial Castel, Hospital Infantil La Fe, Valencia, Spain; Mark Caswell, Royal Liverpool Children's NHS Trust, Liverpool, United Kingdom; Luigi Cavanna, Ospedale Civile—Piacenza, Piacenza, Italy; Gian Luigi Cetto, Ospedale Civile Maggiore, University Verona, Verona, Italy; Bernard Chapuis, Hôpital Cantonal Universitaire, Geneva, Switzerland; Richard Chasty, North Staffordshire Hospital, Stokeon-Trent, United Kingdom; Yao-Chang Chen, National Taiwan University Hospital, Taipei, Taiwan; Teodoro Chisesi, Ospedale Civile SS. Giovanni e Paolo, Venezia, Italy; Raj Chopra, Christie NHS Trust Hospital, Manchester, United Kingdom; Alicja Chybicka, Oncology, Wroclaw Medical University, Wroclaw, Poland; R.E. Clark, Royal Liverpool University Hospital, Liverpool, United Kingdom; P. Colombat, Hopital Bretonneau, Tours, France; Milica D. Colovic, University Clinical Center, Belgrade, Serbia-Montenegro; Manuel Constenla-Figueiras, Hospital Montecelo, Pontevedra, Spain; Marcela Contreras, NBS—North London, London, United Kingdom; Licinio Contu, P.O. “R. Binaghi,” Cagliari, Italy; Catherine Cordonnier, Hôpital Henri Mondor, Creteil, France; J.J. Cornelissen, Erasmus MC-Daniel den Hoed Cancer Centre, Rotterdam, The Netherlands; Jacqueline Cornish, Bristol Royal Hospital for Children, Bristol, United Kingdom; Paolo Coser, Hospital San Maurizio, Bolzano, Italy; Nascimento Costa, Hospital da Unviersidade de Coimbra, Coimbra, Portugal; Carole Coze, Hopital d'Enfants de la Timone, Marseille, France; C. Craddock, University Hospital Birmingham NHS Trust, Birmingham, United Kingdom; John Crown, St. Vincent's Hospital, Dublin, Ireland; Dominic J. Culligan, Grampian University Hospitals Trust, Aberdeen, United Kingdom; Marco Danova, University of Pavia—IRCCS Policlinico, Pavia, Italy; P.J. Darbyshire, Birmingham Children's Hospital, Birmingham, United Kingdom; John M. Davies, Western General Hospital, Edinburgh, United Kingdom; R. de Bock, AZ Middelheim, Antwerp, Belgium; José Maria de Pablos Gallego, Hospital University Virgen de las Nieves, Granada, Spain; Bernard De Prijck, CHR La Citadelle, Liege, Belgium; Thierry de Revel, Hôpital Percy, Clamart, France; Giulio De Rossi, Ospedale Bambino Gesú, Rome, Italy; Carmino A. De Souza, University Est. de Campinas/TMO/UNICAMP, Campinas, Brazil; Giovanni Deb, Ospedale Bambino Gesu, Rome, Italy; Laurent Degos, Hôpital St. Louis, Paris, France; Hilde Demuynck, Heilig Hartziekenhuis, Roeselare, Belgium; Ioannis Dervenoulas, University of Athens, Athens, Greece; Paolo Di Bartolomeo, Ospedale Civile, Pescara, Italy; Nicola Di Renzo, Centro di Riferimento Oncologico di Basilicad, Rionero in Vulture, Italy; Miguel Angel Diaz, Niño Jesus Children's Hospital, Madrid, Spain; Volker Diehl, University of Cologne, Cologne, Germany; J.L. Diez-Martin, Hospital G.U. Gregorio Maranon, Madrid, Spain; Suleyman Dincer, Azerbajian Central Clinic Hospital, Baki, Azerbaijan, and Ankara Numune Education, Ankara, Turkey; Dini Giorgio, Institute G. Gaslini, Genova, Italy; Anna Dmoszynska, Medical University of Lublin, Lublin, Poland; Gottfried Doelken, Medizinische Universitätsklinik C, Greifswald, Germany; Peter Dreger Peter, AK St. Georg., Hamburg, Germany; Frederico Dulley, University of Sao Paolo, Sao Paulo, Brazil; Jose Easow, Apollo Speciality Hospital, Chennai, India; Wolfram Ebell, Charité-Virchow Klinikum, Humboldt-Univ., Berlin, Germany; Anna Efremidis, St.Savvas Oncology Hospital, Athens, Greece; G. Ehninger, Universitaetsklinikum Dresden, Dresden, Germany; Hermann Eichler, Faculty of Clinical Medicine Mannheim, Mannheim, Germany; Hartmut Eimermacher, Medizinische Klinik II, Hagen, Germany; Arno Enno, Hunter Haematology Unit, Mater Hospital, Hunter, Australia; Luis Errazquin, Hospital Universitario Virgen Macarena, Sevilla, Spain; Jesús Estella Aguado, Hospital Sant Joan de Deu, Barcelona, Spain; Hele Everaus, Tartu University Clinics, Tartu, Estonia; Franca Fagioli, Ospedale Regina Margherita, Torino, Italy; Michele Falda, Azienda Ospedaliera S. Giovanni, Torino, Italy; Renato Fanin, Udine University Hospital, Udine, Italy; Athanasios Fassas, Hospital of Thessaloniki, Thessaloniki, Greece; Anders Fasth, Queen Silvia Children's Hospital, Goeteborg, Sweden; Lawrence B. Faulkner, Oncoematologia-Reparto Interdisciplinare, Firenze, Italy; A.A. Fauser, Klinik für Knochenmarktrans-plantation, Idar-Oberstein, Germany; Leonardo Feldman, Unidad de Transplante de Medula Osea, Buenos Aires, Argentina; W. Feremans, U.L.B.— Hopital Erasme, Brussels, Belgium; Burhan Ferhanoglu, Cerrahpasa Medical School, Istanbul, Turkey; M.N. Fernández, Clinica Puerta de Hierro, Madrid, Spain; José M. Fernández-Ranada, Hospital de la Princesa, Madrid, Spain; Augustin Ferrant, Cliniques Universitaires St. Luc, Brussels, Belgium; Felicetto Ferrara, Cardarelli Hospital, Napoli, Italy; Jürgen Finke, University of Freiburg, Freiburg, Germany; Alain Fischer, Hôpital Necker, Paris, France; J. Fischer, II Med.Klinik, Stadt. Klinikum, Karlsruhe, Germany; Ted Fitzsimons, The Western Infirmary, Glasgow, United Kingdom; Filomena Floristan, Hospital de Cruces, Barakaldo (Bilbao), Spain; J.M.F. Forjaz de Lacerda, Hospital de Santa Maria, Lisboa, Portugal; Franca Fossati-Bellani, Istituto Nazionale per lo Studio e la Cura del Tumori, Milano, Italy; Vinicio Fosser, San Bortolo Hospital, Vicenza, Italy; Ian Franklin, Glasgow Royal Infirmary, Glasgow, United Kingdom; Mathias Freund, Universität Rostock, Rostock, Germany; N. Frickhofen, Dr. Horst-Schmidt-Kliniken, Wiesbaden, Germany; Attilio Gabbas, Ospedale “San Francesco,” Nuoro, Italy; Helmut Gadner, St. Anna Kinderspital, Vienna, Austria; Andrea Gallamini, Az. Ospedaliera S. Croce e Carle, Cuneo, Italy; M.C. Galvin, Pinderfields General Hospital, West Yorkshire, United Kingdom; Joan Garcia López, Hospital Duran i Reynals, Barcelona, Spain; Javier García-Conde, Hospital Clínico Universitario, Valencia, Spain; Tobias Gaska, St. Marienkrankenhaus Siegen, Siegen, Germany; Günther Gastl, University Hospital Innsbruck, Innsbruck, Austria; Gündüz Gedikoglu, Istanbul University, Istanbul, Turkey; Ardeshir Ghavamzadeh, Shariati Hospital, Teheran, Iran; Alessandro Gianni, Istituto Nazionale Tumori, Milano, Italy; Brenda E. Gibson, Royal Hospital for Sick Children, Glasgow, United Kingdom; José L. Gil, Hospital Universitario “Puerta del Mar,” Cádiz, Spain; Maria H. Gilleece, Ysbyty Gwynedd, Bangor, United Kingdom; C. Gisselbrecht, Hôpital St. Louis, Paris, France; Bertram Glass, Universitätsklinikum Göttingen, Gottingen, Germany; Eliane Gluckman, Hopital St. Louis, Paris, France; Jürg Gmür, Klinik Im Park, Zurich, Switzerland; Ulrich Göbel, Klinik für Kinder-Onkologie, Düsseldorf, Germany; John M. Goldman, Hammersmith Hospital at Imperial College, London, United Kingdom; Antony H. Goldstone, University College London Hospital, London, United Kingdom; Jose David González San Miguel, Hospital Insular, Las Palmas de Gran Canaria, Spain; MiguelAngel González-López, Hospital Xeral-Calde, Lugo, Spain; Norbert C. Gorin, Hopital Saint Antoine, Paris, France; Stelios Grafakos, St. Sophia' Children's Hospital, Athens, Greece; Martin Gramatzki, University Erlangen, Erlangen, Germany; Albert Grañena, Institut Universitari Dexeus, Barcelona, Spain, and Institut Catala d'Oncologia, Barcelona, Spain; Nicol Gratecos, Hôpital de l'ARCHET I, Nice, France; Alois Gratwohl, Kantonsspital, Basel, Switzerland; Hildegard T. Greinix, AKH und Universitaetskliniken Wien, Vienna, Austria; Luigi Gugliotta, Unita Operativa Ematologia, Reggio Emilia, Italy; F. Guilhot, Hopital La Miletrie, Poitiers, France; José Eduardo Guimaraes, Hospital Sao Joao, Porto, Portugal; Zafer Gülbas, Osmangazi University, Fac. of Medicine, Eskisehir, Turkey; Ozturk Gulyuz, Gazi University Medical School, Ankara, Turkey; Gunhan Gurman, Ankara University Medical School, Ankara, Turkey; Martín Martín Gutierrez, Hospital Clínico, Zaragoza, Spain; Rainer Haas, Klinik für Hämat, Onkol, Klin. Immun., Düsseldorf, Germany; Rose-Marie Hamladji, Centre Pierre et Marie Curie, Alger, Algeria; M.D. Hamon, Plymouth Hospitals NHS Trust, Plymouth, United Kingdom; Niels Ebbe Hansen, Herlev Hospital, Herlev, Denmark; Nicholas Harhalakis, Evangelismos Hospital, Athens, Greece; J.L. Harousseau, Hotel Dieu, Nantes, France; Reiner Hartenstein, Städt Krankenhaus München Harlaching, München, Germany; Olivier Hartmann, Institut Gustave Roussy, Villejuif, France; Hubert Hausmaninger, LKA Salzburg, Salzburg, Austria; Rauf Haznedar, Gazi Universitesi Tip Fakültesi, Ankara, Turkey; W. Heit, Med.Klinik/Haematologie/Onkologie, Essen, Germany; Andrzej Hellmann, Medical University of Gdansk, Gdansk, Poland; R.P. Herrmann, Royal Perth Hospital, Perth, Australia; Bernd Hertenstein, Medical School of Hannover, Hannover, Germany; U. Hess, Hematol./Oncol. Kantonsspital St.Gallen, St. Gallen, Switzerland; Wolfgang Hinterberger, Donauspital Vienna, Vienna, Austria; Anthony Dick Ho, University of Heidelberg, Heidelberg, Germany; Dieter Hoelzer, Universität Frankfurt, Frankfurt, Germany; J. Holowiecki, University Dept. of Haematology and BMT, Katowice, Poland; Heinz-August Horst, Christian-Albrechts-University, Kiel, Germany; Dieter K. Hossfeld, Universitätsklinikum Eppendorf, Hamburg, Germany; Lothar Huebsch, Ottawa General Hospital, Ottawa, Canada; A.E. Hunter, Leicester Royal Infirmary, Leicester, United Kingdom; Pasquale Iacopino, Azienda Ospedaliera, Reggio Calabria, Italy; Emilio Iannitto, University di Palermo, Div. di Ematologia, Palermo, Italy; Karel Indrák, University Hospital, Olomouc, Czech Republic; A. Iriondo, Hospital Universitario, Santander, Spain; Teodosio Izzi, Spedali Civili—Brescia, Brescia, Italy; G.L. Jackson, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom; Peter Jacobs, Constantiaberg Medi-Clinic, Cape Town, South Africa; Niels Jacobsen, Rigshospitalet, Copenhagen, Denmark; M. Janvier, Centre Rene Huguenin, Saint Cloud, France; Ladislav Jebavy, Charles University Hospital, Hradec Králové, Czech Republic; Heikki Joensuu, Helsinki University Central Hospital, Helsinki, Finland; Schubert Joerg, University of Saarland, Homburg, Germany; F.G.C. Jones, Belfast City Hospital, Belfast, United Kingdom; J.P. Jouet, Hopital Claude Huriez, Lille, France; Miles V. Joyner, Royal Deevon and Exeter Hospital, Exeter, United Kingdom; Gunnar Juliusson, University Hospital, Linköping, Sweden; Herbert Jürgens, Klinik /Poliklinik für Kinderheilkunde, Münster, Germany; Sevgi Kalayoglu-Besisik, Istanbul Tip Fakueltesi, Istanbul, Turkey; Nagy Kalman, Postgraduate Medical School, Miskolc, Hungary; Maria Kalmanti, University Hospital of Heraklion, Heraklion, Crete, Greece; Savas Kansoy, Ege Universitesi Typ Fakultesi, BornovaIzmir, Turkey; Emin Kansu, Macettepe University, Ankara, Turkey; L. Kanz, Medizinische Klinik, Tübingen, Germany; G. Karianakis, Diagnostic & Therapeutic Center ofAthens, Athens, Greece; Yves Kernéis, Centre Hospitalier René Dubos, Pontoise, France; Omari Khalifeh, King Hussein Medical Centre, Amman, Jordan; Viktor Khomenko, Kiev City BMT Center, Kiev, Ukraine; J. Kienast, University of Münster, Münster, Germany; Sally Killick, Royal Bournemouth Hospital, Bournemouth, United Kingdom; H.H. Kirchner, Klinikum Hannover—Siloah, Hannover, Germany; Thomas Klingebiel, University Children's Hospital III, Frankfurt, Germany; Wolfgang Knauf, Klin. Benjamin Franklin, FU Berlin, Berlin, Germany; Michael Koenigsmann, Med. Fakultät d. Otto-von-Guericke-, Magdeburg, Germany; Pirjo Koistinen, Oulu University Central Hospital, Oulu, Finland; Elli Koivunen, Tampere University Hospital, Tampere, Finland; Hans-Jochem Kolb, Med. Klinik III, München, Germany; K. Kolbe, Johannes-Gutenberg-University, Mainz, Germany; Elisabeth Koller, Hanuschkrankenhaus, Vienna, Austria; M. Komarnicki, K. Marcinkowski University, Poznan, Poland; Ewa Koscielniak, Olgahospital Stuttgart, Kinderklinik, Stuttgart, Germany; Tibor Kovacsovics, Universitaire Vaudois, Lausanne, Switzerland; Jerzy R. Kowalczyk, Children's University Hospital, Lublin, Poland; Vladimir Koza, Charles University Hospital, Pilsen, Czech Republic; Tomas Kozak, Charles Univ., Prague, Czech Republic; John Kugler, OHACI, Peoria, IL, USA; K. Kuliczkowski, Medical Academy, Wroclaw, Poland; Stein Kvaloy, The Norwegian Radium Hospital, Oslo, Norway; Boris Labar, University Hospital Centre— Rebro, Zagreb, Croatia; P. Laciura, Unita Operativa Di Oncologia Medica, Cuneo, Italy; Juan Jose Lahuerta Palacios, Hospital University 12 de Octubre, Madrid, Spain; Jan Lakota, National Cancer Institute, Bratislava, Slovakia; Deliliers Giorgio Lambertenghi, Ospedale Maggiore di Milano, Milano, Italy; Andrzej Lange, K. Dluski Hospital, Wroclaw, Poland; Francesco Lanza, St. Anna Hospital, Ferrara, Italy; Ramon Lasa Isasti, Hospital Aranzazu, San Sebastian, Spain; Francesco Lauria, Policlinico Le Scotte, Siena, Italy; Francoise Le Moine, Hopital de la Ville Esch/Alzette, Esch-Alzette, Luxembourg; Veronique Leblond, Pitie-Salpetriere, Paris, France; Giorgio Lelli, Hospital Casa Sollievo Della Sofferenza, San Giovanni Rotondo, Italy; Stig Lenhoff, University Hospital, Lund, Sweden; Lara A. Leon, Hospital del SAS, Cádiz, Spain; Leda Leoncini-Franscini, Laboratory Trapianti e Immunofenotipizzazione, Bellinzona, Switzerland; Giuseppe Leone, Universita Cattolica S. Cuore, Rome, Italy; Pietro Leoni, Ospedale di Torrette, Ancona-Torrette, Italy; Alessandro Levis, S.S. Antonio e Biagio e C. Arrigo, Alessandria, Italy; Serge Leyvraz, Centre Coordonné d'Oncologie, Lausanne, Switzerland; Marina Liberati, University of Perugia, Perugia, Italy; Hartmut Link, Medizinische Klinik I, Kaiserslautern, Germany; Werner Linkesch, Karl-Franzens-UniversityGraz, Graz, Austria; Vincenzo Liso, Universita degli Studi di Bari, Bari, Italy; Igor A. Lisukov, Institute of Clinical Immunology, Novosibirsk, Russia; Tim Littlewood, The Oxford Radcliffe Hospital, Oxford, United Kingdom; Per Ljungman, Huddinge University Hospital, Huddinge, Sweden; Franco Locatelli, IRCCS Policlinico San Matteo, Pavia, Italy; Hajna Losonczy, University of Pécs, Pécs, Hungary; Jean-Pierre Lotz, Hôpital Tenon, Paris, France; Heinz Ludwig, Wilhelminenspital, Vienna, Austria; Jozef Lukac, Pediatric University Teaching Hospital, Bratislava, Slovakia; Dieter Lutz, Elisabethinen-Hospital, Linz, Austria; Perantonio Macchia, University of Pisa, Pisa, Italy; Alejandro Madrigal, The Royal Free Hospital, London, United Kingdom; Angelo Maiolino, Hospital Universitario, Rio de Janeiro, Brazil; Ignazio Majolino, Ospedale S. Camillo-Forlanini, Rome, Italy; Juan Maldonado Eloy-García, Hospital Regional de Malaga, Málaga, Spain; Milomir Malesevic, Military Medical Academy, Belgrade, Serbia-Montenegro; Franco Mandelli, Univ.“La Sapienza,” Rome, Italy; André Marc, CHNDRF, Charleroi, Belgium; Robert Marcus, Addenbrookes Hospital, Box 234, Cambridge, United Kingdom; Bozena Marianska, Institute of Haematology and Blood Transfusion, Warsaw, Poland; Imrich Markuljak, Roosevelt Hospital, Banská Bystrica, Slovakia; J.C.W. Marsh, St. George's Hospital, Medical School, London, United Kingdom; Massimo F. Martelli, University of Perugia, Perugia, Italy; Josep M. Marti Tutusaus, Hospital Mutua de Terrassa, Barcelona, Spain; Sonja Martin, Robert-Bosch-Krankenhaus, Stuttgart, Germany; M. Martin, Hospital Universitario San Carlos, Madrid, Spain; Giovanni Martinelli, European Institute of Oncology, Milano, Italy; A.M. Martínez-Rubio, Hospital Infantil La Paz, Madrid, Spain; Andrea Martoni, Policlinico S. Orsola— Malpighi, Bologna, Italy; Alexei Maschan, Russian's Children's Hospital, Moscow, Russia; Georg Maschmeyer, Univ. Charité der Humboldt Universität, Berlin, Germany; Tamás Masszi, St. László Hospital, Budapest, Hungary; Patrizio Mazza, Hospedale Nord, Taranto, Italy; Shaun McCann, St. James Hospital Trinity College, Dublin, Ireland; Carl Richard Meier, Medizinische Klinik I, Bremen, Germany; Chiara Messina, Clinica di Oncoematologia Pediatrica, Padova, Italy; V. Mettivier, Hematology Unit A. Cardarelli Hospital, Napoli, Italy; Bernd Metzner, Klinikum Oldenburg, Oldenburg, Germany; Mauricette Michallet, Hopital E. Herriot, Lyon, France; M. Michieli, CRO Aviano, Aviano, Italy; J. Michon, Institute Curie, Paris, France; D.W. Milligan, Birmingham Heartlands Hospital, Birmingham, United Kingdom; Jorge H. Milone, ITMO - Instituto de Transplante de Medula Osea, La Plata, Argentina; Giuseppe Milone Giuseppe, Ospedale Ferrarotto, Catania, Italy; Hrvoje Minigo, Clinical Hospital “Merkur,” Zagreb, Croatia; M. Mistrik, University Hospital, Bratislava, Slovakia; A.D. Moicean, Fundeni University Hospital - Bldg. B, Bucharest, Romania; Silvio Monfardini, Centro Oncologia Regionale, Padova, Italy; Emilio Montserrat, Institute of Hematology & Oncology, Barcelona, Spain; José M. Moraleda Jimenez, Hospital Morales Meseguer, Murcia, Spain; Alfonso Morales-Lazaro, Hospital University “Virgen de la Arrixaca,” Murcia, Spain; Sergio Morandi, Midollo Osseo, Medicina II, Cremona, Italy; Enrica Morra, Ospedale di Niguarda Ca' Granda, Milano, Italy; G.J. Mufti, GKT School of Medicine, London, United Kingdom; Maurizio Musso, Ospedale La Maddalena—Dpt. Oncologico, Palermo, Italy; Arnon Nagler, Chaim Sheba Medical Center, Tel-Hashomer, Israel; Giulio Nalli, Sezione di Oncoematologia, Lodi, Italy; Elizabeth Naparstek, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Franco Narni, Uni. Modena, Policlinico, Modena, Italy; Maja Nenadov-Beck, Department of Pediatrics, Lausanne, Switzerland; Andreas Neubauer, Klinik fuer Haematologie, Onkologie, Marburg, Germany; A.C. Newland, The Royal London Hospital, London, United Kingdom; Dietger Niederwieser, Univ. Leipzig, Div. of Internal Med.II, Leipzig, Germany; D. Niethammer, University Hospital, Tübingen, Germany; L.A. Noens, University Hospital Gent, Gent, Belgium; Tapio Nousiainen, Kuopio University Hospital, Kuopio, Finland; Andrei Novik, Military Medical Academy, St. Petersburg, Russia; Nicolas Novitzky, UCT Medical School, Cape Town, South Africa; Domenico Occhini, National Cancer Institute, Sabratha, Libya; Jesus Odriozolas, Hospital Ramon y Cajal, Madrid, Spain; Jesus Maria Ojanguren, Hospital Galdakao, Galdakao, Spain; Anne O'Meara, Our Lady's Hospital for Sick Children, Dublin, Ireland; Haluk Onat, University of Istanbul, Istanbul, Turkey; Kim Orchard, Southampton General Hospital, Southampton, United Kingdom; Juan J. Ortega, Hospital M. Infantil Vall d'Hebron, Barcelona, Spain; Rainhardt Osieka, Medizinische Klinik IV, Aachen, Germany; G.J. Ossenkoppele, Free University Hospital Amsterdam, Amsterdam, The Netherlands; Ben Othman, Centre Nat. de Greffe de Moelle Osseuse, Tunis, Tunisia; Ercument Ovali, Karadeniz Technical University, Trabzon, Turkey; Osman I. Ozcebe, Hacettepe University, Ankara, Turkey; Korkut Ozerkan, Maltepe Medical Faculty, Istanbul, Turkey; Ahmet Ozturk, GATA Haydarpasa Egitim Hst, Istanbul, Turkey; Antonis Papatryfonos, Makarios Hospital III, Nicosia, Cyprus; Jane E. Parker, University Hospital, Norwich, United Kingdom; Maria Pastore, Hospital Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy; Franco Patrone, University of Genova, Genova, Italy; Nigel Patton, Canterbury Health Laboratory, Christchurch, New Zealand; Dusan Pejin, Clinic of Hematiology, Novi Sad, SerbiaMontenegro; María Jesús Peñarrubia, Hospital Rio Hortega, Valladolid, Spain; Encarnacion Pérez Equiza, Hospital de Navarra, Pamplona, Spain; Christian Peschel, Klinkum Rechts der Isar, München, Germany; Andrea Pession, Pediatriche Mediche e Chirurgiche, Bologna, Italy; Anna Pigaditou, Athens Medical Center, Athens, Greece; Bernard Pignon, Hôpital Robert Debre, Reims, France; Ulla Pihkala, University of Helsinki, Helsinki, Finland; Pedro Pimentel, Inst. Portugues Oncologia, Porto, Portugal; Vincenzo Pitini, Oncology - Pathology - Blood Center, Messina, Italy; Eleonora Podoltseva, Clinical Center for Advanced Medical, St. Petersburg, Russia; Enrico Maria Pogliani, University di Milano-Bicocca, Monza, Italy; Anna Poros Anna, National Medical Centre, Budapest, Hungary; Fulvio Porta, Universitá degli Studi di Brescia, Brescia, Italy; Michael Potter, Royal Free Hospital and School of Med., London, United Kingdom; Ray Powles, Royal Marsden Hospital, Sutton, United Kingdom; Grant H. Prentice, The London Clinic, London, United Kingdom; Joze Pretnar, University Med. Center, Ljubljana, Slovenia; Vadim Ptushkin, N.N. Blokhin Cancer Research Center, Moscow, Russia; Giovanni Quarta, Perrino Hospital, Brindisi, Italy; J. Reiffers, Hôpital Haut-leveque, Pessac, France; Alfred Reiter, University Childrens' Hospital, Giessen, Germany; Kari Remes, Turku University Central Hospital, Turku, Finland; Sigrun Reykdal, Landspitali University Hospital, Reykjavik, Iceland; Josep Maria Ribera Santasusana, Hospital Universitari, Barcelona, Spain; Jose Rifón, Clínica Universitaria de Navarra, Pamplona, Spain; Bernard Rio, Hotel Dieu, Paris, France; Vittorio Rizzoli, Univ. of Parma, Parma, Italy; Tadeusz Robak, Medical University of Lodz, Lodz, Poland; Anibal Jorge Robinson, Navy Hospital `Pedromallo,' Buenos Aires, Argentina; Francesco Rodeghiero, S. Bortolo Hospital, Vicenza, Italy; Juan M. Rodríguez Fernández, Hospital `Virgen del Rocio,' Sevilla, Spain; Yannis Rombos, University of Athens, Athens, Greece; Kenneth R. Romeril, Wellington Regional Oncology Unit, Wellington, New Zealand; Agathe Rosenmayr, Austrian Bone Marrow Donor Registry, Vienna, Austria; Jean Francois Rossi, University Hospital, Montpellier, France; Giovanni Rosti, Oncology-Hematology Department, Ravenna, Italy; Bruno Rotoli, University of Napoli, Napoli, Italy; Jacob M. Rowe, Rambam Medical Center, Haifa, Israel; N.H. Russell, Nottingham City Hospital, Nottingham, United Kingdom; Tapani Ruutu, Helsinki University Central Hospital, Helsinki, Finland; O. Ryzhak, Kiev Regional Oncologic Hospital, Kiev, Ukraine; Piotr Rzepecki, Military Medical Academy, Warsaw, Poland; Giuseppe Saglio, Ospedale San Luigi Orbassano, Torino, Italy; Hans Salwender, Allgemeines Krankenhaus Altona, Hamburg, Germany; Hellmut Samonigg, Karl-Franzens-UniversityGraz, Graz, Austria; Armando Santoro, Sezione Trapianto Midollo Osseo, Milano, Italy; Miguel A. Sanz, Hospital Universitario La Fe, Valencia, Spain; H.G. Sayer, Klinik f. Innere Medizin II, Jena, Germany; Alberto Scanni, Ospedale Fatebenefratelli e Oftalmico, Milano, Italy; M.R. Schaafsma, Medisch Spectrum Twente, Enschede, The Netherlands; U.W. Schaefer, University Hospital, Essen, Germany; Urs Schanz, University Hospital, Zurich, Switzerland; A. Schattenberg, University Medical Center St. Radboud, Nijmegen, The Netherlands; S.A.M. Schey, Guy's Hospital, London, United Kingdom; G. Schlimok, II Medizinische Klinik, Augsburg, Germany; Hans-Joachim Schmoll, Universität, Hämatologie/Onkologie, Halle, Germany; R. Schots, University Hospital VUB, Brussels, Belgium; Harry Schouten, University Hospital Maastricht, Maastricht, The Netherlands; Anthony P. Schwarer, Alfred Hospital, BMT Programme, Melbourne, Australia; R. Schwerdtfeger, Deutsche Klinik für Diagnostik, Wiesbaden, Germany; Rosanna Scimè, Ospedale V. Cervello, Palermo, Italy; Erik Segel, University Department of Hematology, Aarhus, Denmark; Reinhard Seger, University Children's Hospital, Zurich, Switzerland; D. Selleslag, A.Z. Sint-Jan, Brugge, Belgium; Margit Serban, University of Medicine and Pharmacy, Timisoara, Romania; Sameh Shamaa, Mansoura University Hospital, Mansoura, Egypt; Peter J. Shaw, Oncology Unit, Sydney, Australia; Wolfgang Siegert, Medizinische Klinik und Poliklinik II, Berlin, Germany; Salvatore Siena, Ospedale Niguarda Ca'Granda, Milano, Italy; Jorge Sierra, Hospital Santa Creu i Sant Pau, Barcelona, Spain; Bengt Simonsson, University Hospital, Uppsala, Sweden; Charles R.J. Singer, Royal United Hospital, Bath Avon, United Kingdom; Girolamo Sirchia, Ospedale Policlinico, Milano, Italy; Aleksander B. Skotnicki, Jagiellonian University, Krakow, Poland; Shimon Slavin, Hadassah University Hospital, Jerusalem, Israel; John Snowden, Royal Hallamshire Hospital, Sheffield, United Kingdom; Jean Jacques Sotto, Hopital A. Michallon, Grenoble, France; Atila Tanyeli, BMT Unit, Cukurova University, Balcali, Turkey; Lucilla Tedeschi, Oncology Department, Milano, Italy; U. Tidefelt, Orebro University Hospital, Orebro, Sweden; Jean-Daniel Tissot, Centre Régional de Transfusion Sanguine, Lausanne, Switzerland; Andreas Tobler, University Hospital Bern, Bern, Switzerland; José F. Tomas, Fundación Jimenez Díaz, Madrid, Spain; J.P. Torres, Hospital Juan Canalejo, La Coruña, Spain; Gomez A. Torres, Córdoba Hospital, Córdoba, Spain; Jean-Louis Touraine, Hôpital E. Herriot, Lyon, France; Marek Trneny, Charles University Hospital, Prague, Czech Republic; Cornelio Uderzo, Ospedale San Gerardo, Monza, Italy; Emel Unal, School of Medicine, Ankara, Turkey; Ali Unal, Erciyes University, Kayseri, Turkey; Levent Undar, Akdeniz University School of Medicine, Antalya, Turkey; Christian Urban, University Children's Hospital, Graz, Austria; H. Van den Berg, Academisch Ziekenhuis bij deUniversiteit, Amsterdam, The Netherlands; Kooy M. van Marwijk, Isala Kliniecken Zwolle, Zwolle, The Netherlands; E. Vellenga, University Hospital Groningen, Groningen, The Netherlands; Marco Venturini, Medical Oncology 1, Genova, Italy; Leo F. Verdonck, University Medical Centre, Utrecht, The Netherlands; Paul Veys, Great Ormond Street Hospital, London, United Kingdom; Jordi Vilardell, Catalan Health Service, Barcelona, Spain; Orazio Vinante, Civic Hospital, Noale, Italy; G. Visani, Pesaro Hospital, Pesaro, Italy; Antonin Vitek, Institute of Hematology and Blood Transfusion, Prague, Czech Republic; Pilar Vivancos, Centro Medico Teknon, Barcelona, Spain; Ettore Volpe, Ematologia'Giovanni di Guglielmo', Avellino, Italy; Ajay Vora, Sheffield Children's Hospital, Sheffield, United Kingdom; Jiri Vorlicek, University Hospital Brno, Brno, Czech Republic; Marcus Vowels, Sydney Children's Hospital, Randwick, Australia; Dragana Vujic, Mother and Child Health Institute, Belgrade, Serbia-Montenegro; Jacek Wachowiak, K. Marcinkowski University of Med. Sciences, Poznañ, Poland; T. Wagner, Medizinische Universität zu Lübeck, Lübeck, Germany; Anders Wahlin, Umea University Hospital, Umea, Sweden; Jan Walewski, Curie-Memorial Inst., Warsaw, Poland; Hannes Wandt, Klinikum Nürnberg, Nürnberg, Germany; Florian Weissinger, Medizinische Poliklinik der Universität, Wuerzburg, Germany; P.W. Wijermans, Leyenburg Hospital, The Hague, The Netherlands; Wieslaw Wiktor-Jedrzejczak, The Medical University of Warsaw, Warsaw, Poland; Andrew M. Will, Royal Manchester Children's Hospital, Pendlebury, United Kingdom; Roelof Willemze, Leiden University Hospital, Leiden, The Netherlands; Ewald Woell, University Hospital Innsbruck, Innsbruck, Austria; Bernhard Wörmann, Städtisches Klinikum Braunschweig, Braunschweig, Germany; Isaac Yaniv, Schneider Children's Medical Center, Petach-Tikva, Israel; M. Akif Yesilipek, Akdeniz University Medical School, Antalya, Turkey; Ugur Yilmaz, Dokuz Eylül Universitesi, Izmir, Turkey; Agnes Yong, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; Pierre Zachée, AZ Stuivenberg, Antwerp, Belgium; Alberto Zambelli, Fondazione S. Maugeri, Pavia, Italy; Axel R. Zander, University Hospital Eppendorf, Hamburg, Germany; Felix Zintl, University of Jena, Jena, Germany; and Nicholas C. Zoumbos, Patras University Medical School, Patras, Greece.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2003-03-0665.
Supported in part by EBMT funds and Association Claude Bernard.
A complete list of EBMT members contributing to the ALWP appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal