Abstract
Human herpesvirus 6 (HHV-6) is a potentially immunosuppressive agent that has been suggested to act as a cofactor in the progression of HIV disease. Exposure of human macrophages to HHV-6A or HHV-6B profoundly impaired their ability to produce interleukin 12 (IL-12) upon stimulation with interferon-γ (IFN-γ) and lipopolysaccharide (LPS). By contrast, the production of tumor necrosis factor–α (TNF-α); regulated on activation, normal T-cell expressed and secreted (RANTES); and macrophage inflammatory protein 1β (MIP-1β) was not negatively affected. To exclude the involvement of IL-12–suppressive cytokines, such as IL-10 and TNF-α, the viral stocks were fractionated by ultra-centrifugation. The bulk of the suppressive activity was recovered within the virion-rich pelleted fraction that was virtually devoid of such cytokines. IL-12 suppression was independent of viral replication, and the effect was not abrogated upon ultraviolet-light inactivation of the viral inoculum. The mechanism of HHV-6–mediated IL-12 suppression was investigated by RNase protection assays, which demonstrated unaltered levels of IL-12 p35 mRNA and only a modest reduction in p40 mRNA, which was insufficient to account for the near-complete loss of both extracellular and intracellular IL-12 protein. Moreover, both the IFN-γ and the LPS signaling pathways were intact in HHV-6–treated cells. These data suggest that HHV-6 can dramatically affect the generation of effective cellular immune responses, providing a novel potential mechanism of HHV-6–mediated immunosuppression.
Introduction
Human herpesvirus 6 (HHV-6) is a member of the betaherpetovirinae subfamily,1 along with HHV-7 and human cytomegalovirus (hCMV, or HHV-5). HHV-6 isolates can be divided into 2 major subgroups, A and B, that exhibit significant genetic, immunologic, and biologic divergence, albeit insufficient for classification as different viral species. Subgroup B is extremely diffused in the human population and is usually acquired within the first 2 years of life. Primary infection with this subgroup has been linked to exanthema subitum, a benign childhood disease.2 Subgroup A is apparently less prevalent, with reported identifications being predominantly from severely immunosuppressed patients,3-5 although an association with febrile episodes in early infancy has been documented in Central Africa.6 Like other herpesviruses, HHV-6 is able to induce latent infection and may persist almost indefinitely in the host. In immunocompromised patients, HHV-6 reactivation or reinfection may cause severe opportunistic diseases, including encephalitis, pneumonitis, hepatitis, and retinitis, as well as bone marrow graft failure.7 Moreover, in patients who have received transplants, HHV-6 infection has been fingered as a cofactor in hCMV disease.8
Multiple lines of clinical and experimental evidence suggest that HHV-6, besides causing opportunistic infections, may be an immunosuppressive agent in its own right. In vitro, HHV-6 shows a predominant tropism for CD4+ T lymphocytes, exerting marked cytopathic effects on them.9 In severe combined immunodeficiency mice engrafted with a human thymic implant (SCID–hu thy/liv), both HHV-6A and HHV-6B cause a rapid destruction of the graft, with dramatic thymocyte depletion affecting all major intrathymic cell populations.10 Consistent with these experimental observations, disseminated coinfection with HHV-6A and HHV-6B was etiologically linked with thymic atrophy and progressive immunodeficiency in a child who showed no evidence of human immunodeficiency virus 1 (HIV-1) infection.11 In patients who received stem cell transplants, there was a correlation between HHV-6 DNAemia and a lack of hCMV-specific lymphoproliferative responses, suggesting a direct immunosuppressive role of HHV-6 in this setting.12 Moreover, HHV-6 may act as a cofactor that can accelerate the course of HIV infection (reviewed in Lusso and Gallo9 ), as suggested by several observations: the ability of HHV-6 to preferentially target and destroy CD4+ T lymphocytes; the enhanced cytopathic effects seen upon in vitro coinfection with HIV; the ability of HHV-6A to induce de novo CD4 expression in CD8+ T lymphocytes and other cytotoxic effector cells, thus broadening the spectrum of HIV-susceptible cells; the accelerated progression toward full-blown acquired immunodeficiency syndrome (AIDS) in pig-tailed macaques coinfected with HHV-6A and simian immunodeficiency virus (SIV); and, finally, the frequent detection of active HHV-6 infection in patients with progressive HIV disease.9 Thus, there is growing evidence that HHV-6 may directly and profoundly affect the function of the immune system, although the mechanisms by which this occurs remain largely unknown.
We recently demonstrated that HHV-6 uses CD46, also designated membrane cofactor protein (MCP), as a cellular receptor.13 CD46 is a type-1 glycoprotein expressed on the surface membrane of virtually all nucleated human cells.14 The classical role of CD46 is to protect autologous cells from complement attack by acting as a cofactor to plasma protease factor 1 in the inactivation of C3b and C4b, thereby blocking the formation of C3 convertases. For this reason, CD46 is classified into a family of proteins designated regulators of complement activation (RCAs). In recent years, however, CD46 has been shown to serve as a cellular receptor for several human pathogens, including measles virus (MV), Neisseria gonorrhoeae and meningitidis, and some serotypes of group-A Streptococci.15-17 Besides its role as a complement regulator, CD46 appears to provide a liaison between innate and adaptive immunity via regulation of interleukin 12 (IL-12) production, as documented in the MV system.18 IL-12, a cytokine that is produced mainly by antigen-presenting cells such as mononuclear phagocytic cells and dendritic cells upon stimulation with microbial products, represents a pivotal element in the polarization of immune responses toward a T-helper 1 (Th1) cytokine–secretion pattern.19 Th1 cells, characterized by the selective production of interferon-γ (IFN-γ) and IL-2, play a critical role in the generation of cytotoxic T lymphocytes (CTLs) and effective immune responses against intracellular microorganisms, including viruses.20 Thus, suppression or dysregulation of IL-12 may constitute a critical survival strategy for viruses. For example, MV was shown to dramatically down-modulate IL-12 secretion, and this effect was associated with ligation of CD46 on the surface of human monocytes.18 Clinically, measles is characterized by profound abnormalities in virtually all compartments of the immune system, resulting in a dramatic and prolonged immunosuppression that can be explained, at least in part, by the ability of MV to suppress IL-12 production.21 In fact, the in vitro data have been confirmed with the analysis of IL-12 production in peripheral blood monocytes from patients with measles.22
In light of the observation that HHV-6 also uses CD46 as a receptor and is potentially associated with immunosuppression in vivo, we investigated whether HHV-6 can affect the production of IL-12 by human mononuclear phagocytic cells.
Materials and methods
Human macrophage (Mϕ) cultures
Peripheral blood mononuclear cells (PBMCs) were isolated from buffycoat preparations from healthy donors by means of Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. Monocytes were isolated by plating PBMCs in 12-well plates (Nalge Nunc, Roskilde, Denmark) at a cell density of 6 × 106/mL for 1 hour. Nonadherent cells were then removed by repeated washes, and adherent cells were cultured in Dulbecco minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) (Bio-Whittaker Europe, Verviers, Belgium). All reagents were tested for endotoxin contamination by means of the Limulus Amebocyte Lysate, Pyrogen Plus detection kit (Bio-Whittaker Europe) with a sensitivity of 0.125 endotoxin units (EUs) per milliliter.
HHV-6 infection and IL-12 stimulation protocol
The viral strains used in this study were GS,3 subgroup A, and PL-1, subgroup B.10 Viral stocks were generated by expansion in primary cord blood mononuclear cells (CBMCs) previously activated for 3 days with phytohemagglutinin (PHA) (Sigma, Saint Louis, MO) and then by the collection of cell-free culture supernatants at day 7 or when the majority of the cells showed morphologic signs of infection. Infectious supernatants were confirmed for endotoxin negativity and stored at –80°C until use. Adherent Mϕs cultured for 4 to 5 days in vitro were infected for 17 hours with such supernatants at the approximate multiplicity of infection (MOI) of 1 (0.5 mL virus stock containing 106 tissue-culture infectious doses per milliliter for approximately 500 000 cells), after which the cells were washed, kept for an additional 24 hours in culture, and then stimulated with 300 U/mL IFN-γ (Cabru, Milan, Italy) for 17 hours and with 1 μg/mL lipopolysaccharide (LPS) (Sigma) for an additional 24 hours. Cell-free culture supernatants were harvested for the measurement of cytokines and chemokines. For the infection of PBMCs, cells were stimulated with 5 μg/mL PHA for 3 days in RPMI–10% FCS and then exposed to infectious supernatants at the approximate MOI of 1. After 2 hours at 37°C, the cells were diluted with fresh RPMI-10% FCS and cultured at 1 × 106/mL.
Cytokine and chemokine assays
The levels of cytokines and chemokines (IL-12 p70; tumor necrosis factor–α [TNF-α]; IL-10; regulated on activation, normal T-cell expressed and secreted [RANTES]; and macrophage inflammatory protein–1β [MIP-1β]) released into the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) following the manufacturer's instructions. According to the manufacturer's specifications, the IL-12 p70 ELISA used shows no cross-reactivity with or interference by recombinant human IL-12 p40 (rhIL-12) p40 or rhIL-12 p35 monomers or homodimers. ELISA results were calculated as means from multiple donors ± standard deviation (SD). P values were calculated by means of a paired, 2-tailed t test.
Ultracentrifugation and ultraviolet (UV)–light inactivation of viral stocks
HHV-6A infectious supernatants were spun over a 20% sucrose cushion at 36 000g for 1 hour with the use of a type 50 titanium (Ti) rotor in a Beckman L-80 Ultracentrifuge (Beckman Instruments, Palo Alto, CA). The ultraspin pellet was resuspended in DMEM–10% FCS. For UV-light inactivation, HHV-6A infectious supernatants were directly exposed to UV light for 5 minutes at a distance of 20 cm from the source, and kept on ice until use.
Quantitation of HHV-6 DNA by real-time quantitative polymerase chain reaction (PCR)
HHV-6 replication was assessed by quantitative PCR for HHV-6 DNA, based on the TaqMan technology with the use of an ABI PRISM 7700 sequence detector (Applied Biosystems, Foster City, CA), as described.23
Fluorocytometric analyses
Fluorocytometric analysis was used to determine the levels of expression of intracellular IL-12 in Mϕs, as well as of surface CD14 and IFN-γ receptor in Mϕs and promonocytic (U937) cells. The primary antibodies used were unconjugated murine monoclonal antibodies (mAbs) against CD14 (Becton Dickinson, San Jose, CA), IFN-γ receptor α-chain (γR99; a kind gift from Dr Francesco Novelli, Department of Clinical and Biological Sciences, University of Turin, Italy), or IFN-γ receptor β-chain (clone MMHGR-2; PBL Biomedical Laboratories, Piscataway, NJ); or phycoerythrin (PE)–labeled anti–IL-12 p40/p70 mAb (BD BioSciences, Erembodegem, Belgium), which detects both the p70 heterodimer and the p40 monomer of IL-12, but not the p35 monomer. A PE-labeled irrelevant isotype–matched mAb (BD BioSciences) was used as a control. For surface staining, the cells were washed with cold phosphate-buffered saline (PBS) supplemented with 2% human serum and then incubated for 30 minutes on ice with the primary antibodies. After 2 washes with PBS, the cells were incubated for 30 minutes with PE-conjugated goat-antimouse immunoglobulin G (IgG) antiserum (Sigma). The cells were further washed with PBS and analyzed. To examine intracellular levels of IL-12, Mϕs, pretreated or not with HHV-6, were stimulated for 17 hours with IFN-γ followed by 4 hours with LPS; subsequently, they were treated for 4 hours with 10 μg/mL Brefeldin A, after which they were fixed with 4% paraformaldehyde (Sigma) for 15 minutes at 4°C. The cells were harvested by scraping and washed 2 times with fresh PBS containing 3% human serum (Bio-Whittaker Europe) and once with buffer containing 0.1% wt/vol saponin, 0.05% sodium azide in PBS (SAP buffer) (Sigma). Then, 3 × 105 Mϕs per test were incubated for 20 minutes at room temperature in SAP buffer and stained for 45 minutes in the dark with the mAbs. The samples were washed twice with SAP buffer and once with PBS and finally resuspended in PBS for the analysis. Fluorocytometric analysis was performed by means of a FacScan analyzer (Becton Dickinson). Appropriate gating was used to exclude dead cells; at least 10 000 events were acquired for each sample.
RNase protection assay (RPA)
Total RNA was extracted by means of guanidinium thiocyanate followed by phenol-chloroform extraction. RPA was carried out by means of the PharMingen Multiprobe system with a custom probe set according to the manufacturer's instructions (PharMingen, San Diego, CA). The data were analyzed by means of the KODAK 1D Image Analysis Software (Eastman Kodak, Rochester, NY) and calculated as the ratio between the mean intensity obtained for the gene of interest and the mean intensities of ribosomal protein L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The ratio generated by stimulation with IFN-γ plus LPS was set as 100%, while values from unstimulated and HHV-6–pretreated cells were calculated as a percentage of that value.
Western blot
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (8%) under reducing conditions was performed with 25 μg total protein for each sample. The gel was then electroblotted onto Hybond-P membrane (Amersham Pharmacia Biotech Italia, Milan, Italy). After being blocked with PBS plus 0.1% Tween 20 containing 10% milk overnight at 4°C, the membrane was incubated with signal transduction and activator of transcription 1 (STAT-1)–specific and phospho–STAT-1 (Try701)–specific polyclonal antibodies (New England Biolabs, Beverly, MA) in 5% milk in PBS-Tween. The membrane was then washed repeatedly with PBS-Tween before incubation with goat-antirabbit antiserum conjugated to horseradish peroxidase (HRP) used at a dilution of 1:1000 (DAKO, Glostrup, Denmark). After 3 washes with PBS-Tween, the membrane was subjected to an enhanced chemiluminescence detection procedure (Pierce, Rockford, IL).
Results
HHV-6A and HHV-6B inhibit the production of IL-12 by human macrophages
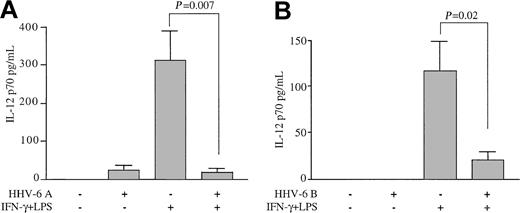
To evaluate the effects of HHV-6 on the production of IL-12, human Mϕs derived from the peripheral blood of healthy adult blood donors were exposed to HHV-6A, strain GS, prior to stimulation with IFN-γ and LPS with the use of a classic protocol for the induction of IL-12 production. Figure 1A shows that unstimulated Mϕs failed to produce significant levels of IL-12 p70, as detected in the culture supernatants by ELISA. A slight increase was observed in Mϕs pre-exposed to HHV-6, although the difference was not statistically significant. As expected, sequential treatment with IFN-γ and LPS induced the release of high levels of the IL-12 p70 heterodimer into the culture supernatants. However, pretreatment with HHV-6 had a dramatic suppressive effect, reducing the level of IL-12 production by more than 90% in Mϕ cultures derived from multiple healthy blood donors (P = .007 for the comparison between stimulated cultures exposed to HHV-6 and controls; n = 6).
Inhibition of IL-12 p70 production from primary human Mϕs by HHV-6. Human peripheral blood–derived Mϕs were pre-exposed to HHV-6 and then stimulated or not with IFN-γ plus LPS. Control Mϕs were not pre-exposed to HHV-6. Culture supernatants were analyzed for IL-12 p70 levels by ELISA 24 hours after LPS addition. (A) Mean results (± SD) of Mϕs from 6 donors pretreated with HHV-6 subgroup A (strain GS). (B) Mean results (± SD) of Mϕs from 4 donors pretreated with HHV-6 subgroup B (strain PL-1).
Inhibition of IL-12 p70 production from primary human Mϕs by HHV-6. Human peripheral blood–derived Mϕs were pre-exposed to HHV-6 and then stimulated or not with IFN-γ plus LPS. Control Mϕs were not pre-exposed to HHV-6. Culture supernatants were analyzed for IL-12 p70 levels by ELISA 24 hours after LPS addition. (A) Mean results (± SD) of Mϕs from 6 donors pretreated with HHV-6 subgroup A (strain GS). (B) Mean results (± SD) of Mϕs from 4 donors pretreated with HHV-6 subgroup B (strain PL-1).
To elucidate whether the suppression of IL-12 was specific for the HHV-6A subgroup, which is frequently associated with immunosuppression, similar experiments were conducted with a strain belonging to subgroup B (PL-1). As illustrated in Figure 1B, a dramatic suppression of IL-12 was also observed with this strain (P = .02 for the comparison between stimulated cultures exposed to HHV-6 and controls; n = 4). These results demonstrate that HHV-6, irrespective of the viral subgroup, profoundly affects the production of IL-12 by human Mϕs.
Lack of suppression of other cytokines and chemokines by HHV-6
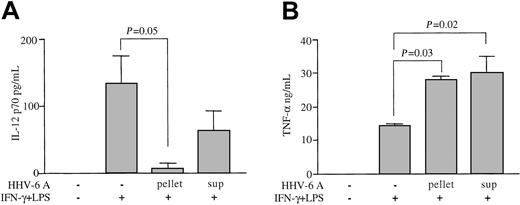
To investigate the extent of functional impairment caused by HHV-6 in human Mϕs, the levels of other soluble factors produced by IFN-γ/LPS-stimulated Mϕs, namely, TNF-α, RANTES, and MIP-1β, were analyzed by ELISA. The production of TNF-α, RANTES, and MIP-1β was not inhibited in Mϕs pretreated with HHV-6A and then stimulated with IFN-γ/LPS (Figure 2). In fact, HHV-6 induced a significant increase in TNF-α release upon IFN-γ/LPS stimulation (P = .05), whereas the levels of RANTES and MIP-1β were only moderately enhanced (P > .1). In unstimulated Mϕs, HHV-6 pretreatment caused a slight enhancement of both TNF-α and MIP-1β (P > .1), while the low-level basal production of RANTES was not modified. Moreover, HHV-6 did not induce any detectable production of IFN-γ from unstimulated Mϕs and did not significantly alter the levels of IFN-γ in stimulated Mϕs, as compared with controls (data not shown). These data demonstrate that the suppression of IL-12 by HHV-6 is a specific effect that does not result from a generalized functional impairment of Mϕs.
Failure of HHV-6 to inhibit the production of TNF-α, RANTES, and MIP-1β from activated Mϕs. Culture supernatants of Mϕs pre-exposed to HHV-6A, either unstimulated or stimulated with IFN-γ and LPS, were tested by ELISA. Mean results (± SD) from 4 donors are presented. (A) TNF-α production. (B) RANTES production. (C) MIP-1β production.
Failure of HHV-6 to inhibit the production of TNF-α, RANTES, and MIP-1β from activated Mϕs. Culture supernatants of Mϕs pre-exposed to HHV-6A, either unstimulated or stimulated with IFN-γ and LPS, were tested by ELISA. Mean results (± SD) from 4 donors are presented. (A) TNF-α production. (B) RANTES production. (C) MIP-1β production.
HHV-6 virions, not soluble factors, are responsible for IL-12 suppression
Since the HHV-6 stocks used for infection were derived from culture supernatants of in vitro–infected human CBMCs, they contained multiple soluble factors of cellular origin, besides specific HHV-6 proteins, which could be responsible for the observed IL-12 suppression. For example, cytokines such as TNF-α, IL-4, IL-10, IL-13, and tumor growth factor–β (TGF-β), which are likely to be present in infectious supernatants, have been reported to inhibit the production of IL-12.24-27 To rule out the possibility that the IL-12–suppressive activity could be due to these or other, yet-undefined soluble factors, the HHV-6A stock was subjected to ultracentrifugation to separate structurally intact virions from soluble, nonpelletable material. To verify the efficacy of the ultracentrifugation procedure, both the pellet and the ultraspin supernatants were tested by ELISA for the presence of 3 cytokines, namely, IL-4, TNF-α, and IL-10, as well as for HHV-6 infectivity. Low to undetectable levels of the 3 cytokines were measured in the ultraspin supernatant (IL-4, not detected; IL-10, 88 pg/mL; TNF-α, 92 pg/mL), and none of them reached sufficient concentrations to cause IL-12 suppression; as expected, the pellet was devoid of detectable cytokines. Conversely, viral infectivity was associated exclusively with the pelleted material (data not shown).
The results shown in Figure 3A demonstrate that the bulk of the IL-12–suppressive activity was recovered within the virion-rich ultraspin pellet. Although a moderate reduction of IL-12 release was also seen with virion-free ultraspin supernatants, the difference was not statistically significant (P = .32; n = 3). The latter observation may be interpreted either as the effect of shed viral envelope proteins due to virion disruption during the ultracentrifugation procedure, or as the combined effect of multiple IL-12–suppressive cytokines present in the infectious supernatants. Again, IL-12 inhibition was selective, as neither the virion-rich pellet nor the soluble fraction inhibited TNF-α production upon IFN-γ/LPS stimulation (Figure 3B). In fact, both the pelleted HHV-6 virions and the ultraspin supernatant caused a significant enhancement in TNF-α production.
Inhibition of IL-12 primarily by HHV-6 virions and not by soluble factors. HHV-6A infectious supernatants were ultracentrifuged; both the ultraspin supernatant (sup) and the pellet were tested for their ability to inhibit IL-12 p70 production upon IFN-γ and LPS stimulation (A). TNF-α levels were also tested in parallel (B). Mean results (± SD) from 3 donors are presented.
Inhibition of IL-12 primarily by HHV-6 virions and not by soluble factors. HHV-6A infectious supernatants were ultracentrifuged; both the ultraspin supernatant (sup) and the pellet were tested for their ability to inhibit IL-12 p70 production upon IFN-γ and LPS stimulation (A). TNF-α levels were also tested in parallel (B). Mean results (± SD) from 3 donors are presented.
Productive HHV-6 infection is not required for IL-12 suppression
Previous studies have demonstrated that HHV-6 cannot effi-ciently establish productive infection in cells of the mononuclear phagocytic system, even though such cells have been suggested to represent a reservoir for viral persistence in vivo.28 To investigate whether productive HHV-6 infection is required for IL-12 suppression in human Mϕs, the level of virus replication was analyzed by means of a quantitative real-time PCR measuring the kinetics of accumulation of viral genome equivalents at various time points after infection. As shown in Figure 4A, no significant accumulation of HHV-6 genome equivalents was observed in Mϕ cultures at day 6 after infection, whereas the number of viral copies was dramatically increased in parallel control cultures of activated PBMCs infected with the same viral stock. Furthermore, in Mϕs treated with HHV-6, we found no detectable expression of 2 viral immediate-early genes (U16/17 and U89/90) or of 1 late gene (U60/61), as measured by RT-PCR,29 nor did we find intracellular expression of the viral phosphoprotein pp41, as assessed by flow cytometry (data not shown), supporting the concept that Mϕs, at least in vitro, do not sustain productive HHV-6 infection.
Productive HHV-6 infection and IL-12 suppression. Productive HHV-6 infection is not required for IL-12 suppression. (A) PBMCs and Mϕs were infected with HHV-6A. At 6 days later, the cells were harvested and analyzed for viral DNA by means of a quantitative real-time PCR assay. β-actin DNA was measured in parallel to normalize for cell number. The data shown are from one representative donor and are expressed as HHV-6 genome equivalents per cell. (B) PBMCs were incubated with HHV-6A supernatant that was previously inactivated or not by UV-light treatment. Cells were immediately harvested for the initial time point (0) and then after 4 and 6 days. (C) Mϕs were incubated for 17 hours with HHV-6A and treated or not with UV light, after which the infectious supernatants were replaced with fresh media. At 24 hours later, the cells were stimulated with IFN-γ and LPS. Supernatants were harvested and tested for IL-12 p70 by ELISA. Mean results (± standard error [SE]) from 2 donors are presented.
Productive HHV-6 infection and IL-12 suppression. Productive HHV-6 infection is not required for IL-12 suppression. (A) PBMCs and Mϕs were infected with HHV-6A. At 6 days later, the cells were harvested and analyzed for viral DNA by means of a quantitative real-time PCR assay. β-actin DNA was measured in parallel to normalize for cell number. The data shown are from one representative donor and are expressed as HHV-6 genome equivalents per cell. (B) PBMCs were incubated with HHV-6A supernatant that was previously inactivated or not by UV-light treatment. Cells were immediately harvested for the initial time point (0) and then after 4 and 6 days. (C) Mϕs were incubated for 17 hours with HHV-6A and treated or not with UV light, after which the infectious supernatants were replaced with fresh media. At 24 hours later, the cells were stimulated with IFN-γ and LPS. Supernatants were harvested and tested for IL-12 p70 by ELISA. Mean results (± standard error [SE]) from 2 donors are presented.
To further prove that productive HHV-6 infection is not required for the observed IL-12 blockade, Mϕs were exposed to UV light–inactivated virus, which is unable to replicate. In control cultures, UV light–inactivated HHV-6 failed to induce productive infection in activated PBMCs, while infection with live virus resulted in a marked increase in the number of viral genome equivalents (Figure 4B). Nevertheless, UV light–treated HHV-6 maintained the ability to suppress IL-12 production with an efficiency that was similar to that of untreated virus (Figure 4C). The levels of TNF-α, which could contribute to the IL-12–suppressive activity, were similar in Mϕ cultures pre-exposed to either live or UV-treated HHV-6 (data not shown). Taken together, these results conclusively demonstrate that HHV-6 is able to suppress the production of IL-12 without establishing a productive viral life cycle.
Intact IFN-γ/LPS signaling in HHV-6–treated human macrophages
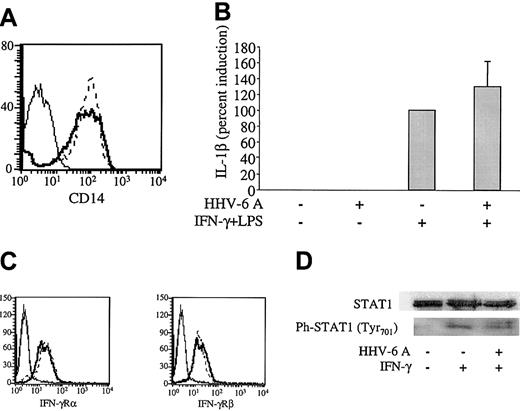
To investigate the mechanisms whereby HHV-6 affects the production of IL-12 by human Mϕs, we evaluated the IFN-γ– and LPS-elicited signaling pathways in human Mϕs treated with HHV-6. First, we investigated by flow cytometry the level of surface expression of CD14, an LPS receptor molecule that, upon association with Toll-like receptor 4 (TLR-4), turns on signaling pathways resulting in nuclear factor κB (NFκB) activation and induction of LPS-responsive genes such as TNF-α and IL-1β. Figure 5A shows that the levels of CD14 expression were not reduced in Mϕs 48 hours after treatment with HHV-6A. Consistent with an intact TLR-4 signaling pathway, TNF-α production by Mϕs stimulated with IFN-γ/LPS was not inhibited by pretreatment with HHV-6A (Figure 2A). Furthermore, by RPA, we measured the levels of IL-1β messenger ribonucleic acid (mRNA), another downstream marker of LPS-elicited signaling. Not only was IL-1β mRNA not reduced in HHV-6–pretreated Mϕs, but a moderate, albeit not significant, increase was observed (Figure 5B). The lack of down-modulation of CD14 and the production of TNF-α protein and IL-1β mRNA by HHV-6–pretreated Mϕs clearly demonstrate that HHV-6 pretreatment does not interfere with the LPS/CD14/TLR-4 signaling pathway.
Intact IFN-γ/LPS signaling pathways in HHV-6–pretreated Mϕs. (A) Mϕs were incubated for 17 hours with HHV-6A (bold line) or mock infected (dashed line) and 48 hours later analyzed for CD14 levels by flow cytometry. Thin line indicates negative control. (B) Mϕs were pretreated or not with HHV-6A and then stimulated with IFN-γ and LPS as described in “Materials and methods.” After 3 hours of stimulation, Mϕs were harvested and analyzed for IL-1β mRNA levels by RPA. The data (mean from 6 donors ± SD) are shown as induction percentages, with the positive control set as 100%. (C) U937 cells were treated for 17 hours with HHV-6 GS (solid line) or mock infected (dashed line) and then analyzed for IFN-γRα and IFN-γRβ levels by flow cytometry. (D) Mϕs were isolated and treated with HHV-6A or left uninfected. After 17 hours, the cells were treated with IFN-γ for 30 minutes, after which they were harvested and analyzed for STAT-1 and phosphorylated STAT-1 levels.
Intact IFN-γ/LPS signaling pathways in HHV-6–pretreated Mϕs. (A) Mϕs were incubated for 17 hours with HHV-6A (bold line) or mock infected (dashed line) and 48 hours later analyzed for CD14 levels by flow cytometry. Thin line indicates negative control. (B) Mϕs were pretreated or not with HHV-6A and then stimulated with IFN-γ and LPS as described in “Materials and methods.” After 3 hours of stimulation, Mϕs were harvested and analyzed for IL-1β mRNA levels by RPA. The data (mean from 6 donors ± SD) are shown as induction percentages, with the positive control set as 100%. (C) U937 cells were treated for 17 hours with HHV-6 GS (solid line) or mock infected (dashed line) and then analyzed for IFN-γRα and IFN-γRβ levels by flow cytometry. (D) Mϕs were isolated and treated with HHV-6A or left uninfected. After 17 hours, the cells were treated with IFN-γ for 30 minutes, after which they were harvested and analyzed for STAT-1 and phosphorylated STAT-1 levels.
The stimulation procedure used to obtain maximal IL-12 production from human Mϕs involved a priming step with IFN-γ for 17 hours, before addition of LPS, to enhance TLR-4 expression, as well as to activate the IL-12 p40 promoter.30,31 As shown in Figure 5C, the levels of IFN-γ receptor chains α and β in U937 promonocytic cells were not affected by HHV-6. Similar data, despite markedly lower basal levels of expression, were obtained with primary Mϕs (data not shown). Next, we investigated whether the IFN-γ signaling pathway was altered by HHV-6. Such a pathway involves the Janus family kinase (JAK)/STAT cascade, with specific recruitment and phosphorylation of STAT-1 upon IFN-γ receptor binding.32 To investigate whether the JAK/STAT pathway was intact in HHV-6–pretreated human Mϕs, STAT-1 phosphorylation was analyzed by Western blotting. Mϕs were treated with HHV-6A or left uninfected for 17 hours. IFN-γ was added for 30 minutes, after which the cells were lysed and analyzed. As shown in Figure 5D, the STAT-1 protein was expressed and correctly phosphorylated in IFN-γ–treated Mϕs in both the presence and the absence of HHV-6A. Taken together, these data suggest that HHV-6–pretreated Mϕs are able to correctly signal through the IFN-γ–elicited pathway and that IL-12 suppression is not due to interruption of IFN-γ–induced priming of the IL-12 p40 promoter.
HHV-6 blocks IL-12 production at the posttranscriptional level
To further dissect the mechanism of HHV-6–mediated IL-12 suppression, we investigated if the effect occurs at the transcriptional or posttranscriptional level. The biologically active form of IL-12 is a heterodimer consisting of 2 subunits, the α-chain (p35) and the β-chain (p40), covalently linked by a disulfide bridge.20 Of note, the β-chain is also a component of the IL-23 heterodimer.33 The 2 chains are encoded by different genes, and the transcription of each subunit is independently regulated.34 In systems where IL-12 regulation has been molecularly defined, as in the case of TNF-α or IL-10 treatment, its production was shown to be modulated at the transcriptional level.24,25 To investigate the level of blockade in IL-12 p70 production in HHV-6–treated Mϕs, we used a specific RPA to measure IL-12 p40 and p35 chain mRNA. The mean results from a panel of different donors are shown in Figure 6A, while data summarizing IL-12 protein and mRNA expression in a representative donor are shown in Figure 6B-C. Contrary to previous observations with TNF-α and IL-10, which can reduce IL-12 p40 mRNA levels by 90% or more,24,25 IL-12 p40 mRNA was only moderately diminished in Mϕs pretreated with HHV-6 GS (less than 35% reduction at 3 hours after stimulation), while IL-12 p35 mRNA was even slightly up-regulated, suggesting that HHV-6–induced inhibition of IL-12 p70 occurs predominantly at the posttranscriptional level.
Posttranscriptional nature of IL-12 suppression by HHV-6. IL-12 suppression by HHV-6 occurs predominantly at the posttranscriptional level. (A) RPA analysis of IL-12 p40 (□), IL-12 p35 (▦), TNF-α (▪), and IL-1β (▧) mRNA in HHV-6–pretreated Mϕs. Mϕs were exposed to HHV-6 as described in “Materials and methods.” Cells were stimulated for 17 hours with IFN-γ and then with LPS and harvested at either 3 hours (mean ± SD of 6 donors) or 6 hours (mean ± SD of 4 donors) after LPS addition. The data are shown as induction percentages relative to the positive control (dotted line), which was set as 100%. Data obtained 24 hours after stimulation are not shown, since at this time point IL-12 mRNA levels returned to the baseline level seen in unstimulated cells. (B) Lack of intracellular accumulation of IL-12 p40/p70 protein in Mϕs after HHV-6 pretreatment. Mϕs from a representative donor were isolated and exposed to HHV-6A for 17 hours, after which the cells were stimulated for 17 hours with IFN-γ and an additional 8 hours with LPS. The cells were fixed/permeabilized and stained with a specific anti–IL-12 p40/p70 antibody. Intracellular IL-12 levels were analyzed by fluorocytometry; the monocyte gate was selected with the use of the physical parameters (forward scatter [FSC] and side scatter [SSC]) and CD14 positivity. (C) Mϕs from the same donor as in panel B were harvested after 8 hours of LPS stimulation for RPA analysis of IL-12 p35 and IL-12 p40 mRNA expression. Dotted line indicates positive control level (100%).
Posttranscriptional nature of IL-12 suppression by HHV-6. IL-12 suppression by HHV-6 occurs predominantly at the posttranscriptional level. (A) RPA analysis of IL-12 p40 (□), IL-12 p35 (▦), TNF-α (▪), and IL-1β (▧) mRNA in HHV-6–pretreated Mϕs. Mϕs were exposed to HHV-6 as described in “Materials and methods.” Cells were stimulated for 17 hours with IFN-γ and then with LPS and harvested at either 3 hours (mean ± SD of 6 donors) or 6 hours (mean ± SD of 4 donors) after LPS addition. The data are shown as induction percentages relative to the positive control (dotted line), which was set as 100%. Data obtained 24 hours after stimulation are not shown, since at this time point IL-12 mRNA levels returned to the baseline level seen in unstimulated cells. (B) Lack of intracellular accumulation of IL-12 p40/p70 protein in Mϕs after HHV-6 pretreatment. Mϕs from a representative donor were isolated and exposed to HHV-6A for 17 hours, after which the cells were stimulated for 17 hours with IFN-γ and an additional 8 hours with LPS. The cells were fixed/permeabilized and stained with a specific anti–IL-12 p40/p70 antibody. Intracellular IL-12 levels were analyzed by fluorocytometry; the monocyte gate was selected with the use of the physical parameters (forward scatter [FSC] and side scatter [SSC]) and CD14 positivity. (C) Mϕs from the same donor as in panel B were harvested after 8 hours of LPS stimulation for RPA analysis of IL-12 p35 and IL-12 p40 mRNA expression. Dotted line indicates positive control level (100%).
Recent data demonstrated that IL-12 p70 can persist intracellularly for a prolonged time in an immature form, and that its maturation and subsequent secretion are dependent upon glycosylation.35 To rule out the possibility that HHV-6 pretreatment of human Mϕs could have resulted in the intracellular trapping of IL-12 p70 owing to maturation inhibition, IL-12 levels were examined by means of intracellular staining 8 hours after LPS stimulation. Since the antibody used recognizes both the p70 heterodimer and the p40 monomer, the presence of isolated p35 monomer was not assessed. RPAs for IL-12 p40 and p35 expression were performed in parallel. As illustrated in Figure 6B, no intracellular accumulation of IL-12 p40/p70 was seen in the absence of stimulation regardless of HHV-6 pretreatment. Upon stimulation, a distinct cell population positive for IL-12 p40/p70 was detected; however, pretreatment with HHV-6 completely prevented the intracellular expression of IL-12 p40/p70. The lack of a detectable p40 chain signal indirectly suggests that HHV-6 treatment may also have suppressed the production of IL-23. By contrast, RPA analysis documented the presence of mRNA for both p40 and p35 despite a slight reduction (less than 25%) in p40 levels (Figure 6C). Taken together, these data strongly suggest that the near-complete IL-12 suppression seen at the protein level (both intracellular and extracellular) cannot be ascribed to a lack of mRNA transcription and is not due to defective protein maturation resulting in intracellular accumulation.
Discussion
Several lines of evidence suggest that HHV-6, a predominantly T-lymphotropic herpesvirus, may act as an immunosuppressive agent, although the clinical observations are still limited and little is known about the potential mechanisms of HHV-6–mediated immunosuppression. In this report, we demonstrate one possible mechanism of HHV-6–induced immune dysregulation by showing that both HHV-6A and HHV-6B dramatically inhibit the production of IL-12 p70 from in vitro–activated macrophages. Interestingly, we found that this effect is not dependent upon productive viral infection; is not primarily mediated by the induction of IL-12–suppressive cytokines, such as IL-4, IL-10, and TNF-α; and does not involve blockade of the IFN-γ/LPS signaling pathways. Strikingly, other markers of Mϕ activation, such as the production of TNF-α, RANTES, and MIP-1β, were not negatively regulated by HHV-6, suggesting that IL-12 suppression is a specific effect not resulting from a generalized abatement of Mϕ function.
We investigated the potential mechanism of HHV-6–mediated IL-12 suppression in Mϕs, showing that the action of HHV-6 occurs predominantly at the posttranscriptional level. Indeed, the dramatic down-modulation of IL-12 p70 seen at the protein level cannot be explained by a reduction in mRNA levels since IL-12 p35 mRNA was not down-modulated and IL-12 p40 mRNA was only moderately reduced. Similar results were recently reported by microarray analysis of a productively infected T-cell line, which demonstrated no significant effect of HHV-6 on IL-12 mRNA expression.36 These observations contrast with the results obtained with the use of IL-12–suppressive cytokines such as TNF-α and IL-10,24,25 further proving that the effect of HHV-6 is not primarily dependent upon the induction of such cytokines. For example, TNF-α, which is induced by HHV-6, is known to inhibit IL-12 p70 via transcriptional down-modulation of IL-12 p40. Thus, it is possible that the moderate decrease in IL-12 p40 mRNA observed in our system was due to up-regulation of endogenous TNF-α production; however, this slight decrease in IL-12 p40 mRNA cannot account for the near-complete loss of IL-12 protein, suggesting that the relative effect of TNF-α was marginal. Down-modulation of IL-12 p70 production has also been observed with the use of other CD46 ligands such as C3b and measles virus (MV),18 strongly suggesting that this effect is induced by the downstream action of CD46-mediated signaling or, alternatively, by uncoupling of CD46-mediated signaling from other activatory signals, resulting in the induction of a state of selective anergy. There is increasing evidence that CD46 is an active signaling membrane receptor. In T cells, CD46 cross-linking by antibodies induces phosphorylation of both p120CBL and linkers for activation of T cells (LATs), 2 adaptor proteins involved with T-cell receptor (TCR)–mediated signaling.37 In this model, CD46/CD3 costimulation strongly induces T-cell proliferation. In epithelial cells, binding of type IV Neisseria pili to CD46 induces intracellular Ca++ mobilization.38 Work done on Mϕs demonstrated that CD46 ligation results in the recruitment of the protein tyrosine phosphatase SHP-1 and increased production of nitric oxide.39,40 These examples illustrate the complexity of CD46-mediated signaling, which is further complicated by the diversity in CD46 isoformspecific tyrosine phosphorylation.41 Recent results obtained in mice transgenic for different human CD46 isoforms showed that CD46 plays a role in the regulation of T-cell responses, acting as a costimulatory signal or setting a threshold for T-cell activation.42 Elucidating the exact nature of the signals induced by CD46 binding on human monocytes requires further investigation. Particular interest in this area is raised by the fact that CD46 seems to provide a link between innate and adaptive immunity, adding more grounds for the concept that these 2 domains of the immune system do not act as independent compartments, but rather continuously interact in a complex network that involves both cellular and soluble components.
Clinically, the observed down-modulation of IL-12 by HHV-6 is consistent with the concept that HHV-6 may be capable of directly causing immunosuppression by inhibiting Th1-polarized immune responses. In apparent contrast with this concept, Mayne et al,36 using microarray technology, recently documented a preferential expression of Th1-type cytokines in T cells productively infected with HHV-6 in vitro. However, their analysis was conducted on an immortalized T-cell line of neoplastic origin (SupT1), in the absence of antigen-presenting cells and physiologic stimuli; thus, the putative Th1 bias needs to be verified with primary T lymphocytes. To provide additional grounds for our in vitro observations, it will be important to verify them in vivo, by testing the ability of Mϕs isolated from patients with primary HHV-6 infection for their ability to produce IL-12 p70 upon stimulation. Furthermore, as IL-12 plays a critical role in the development of effective antiviral responses, the immunosuppressive function described here is consistent with the notion that HHV-6 may act as a cofactor in the course of HIV-1 infection by accelerating the progression to full-blown AIDS.9 Interestingly, PBMCs from patients infected with HIV-1 were shown to produce lower levels of IL-12 p70 upon in vitro stimulation with Staphylococcus, compared with uninfected controls.43 The status of HHV-6 infection was not determined for these patients, and the possibility that HHV-6 reactivation and/or reinfection may be involved in the reduction of IL-12 production in HIV-1–infected individuals should be investigated.
Down-modulation of IL-12 may represent a common strategy of evasion and subversion of the host immune system by viruses. The long parallel evolution of viral pathogens with their human hosts has resulted in the development of an elaborate repertoire of viral mechanisms to counteract both specific and native host immune responses. For herpesviruses, diverse strategies of immune evasion have been documented, including the capture and reprogramming of a variety of host genes, such as those encoding cytokines/chemokines or cytokine/chemokine receptors, the modulation of major histocompatibility complex (MHC) class I expression, as well as the induction of a reduced susceptibility of the host cells to apoptosis.44 The observation that HHV-6 is able to specifically down-modulate the production of IL-12 from human Mϕs represents a novel mechanism for herpesvirus evasion of host immune control, in addition to its well-documented ability to directly infect and kill CD4+ T cells.9 However, an important caveat that should be considered relates to the ongoing controversy as to the importance of IL-12 in the generation of protective antiviral immune responses. Indeed, individuals with genetic defects in either IL-12 or IL-12 receptor genes have not so far been reported to suffer from recurrent viral infections,45 although spontaneous fulminant varicella was diagnosed in one child with autosomal recessive IL-12 β-chain deficiency.46 This may indicate that IL-12–independent mechanisms of IFN-γ induction usually compensate for such defects in vivo. Nevertheless, the adjuvant effect exerted by IL-12 on an anti–herpes simplex virus-2 vaccine,47 as well as the therapeutic benefit of IL-12 against different viral infections in mice (ie, CMV, vesicular stomatitis virus, lymphocytic choriomeninigtis virus),48-50 suggests that IL-12 suppression may be a strategy that facilitates virus persistence in vivo. Further investigation of the effect of HHV-6 on the interactions between T cells and antigen-presenting cells will permit a full evaluation of the potential immunosuppressive action of this herpesvirus.
In conclusion, we have shown that HHV-6, like MV, is able to profoundly down-modulate the production of IL-12 from human Mϕs, providing a novel mechanism for HHV-6–mediated immunosuppression. Although this effect is probably mediated by the direct engagement of the CD46 receptor, which is shared by HHV-6 and MV, this hypothesis could not be directly verified owing to the lack of appropriate reagents. Indeed, anti-CD46 antibodies that prevent HHV-6 binding cannot be used because engagement of CD46 is sufficient per se to induce IL-12 p70 inhibition.18 Conversely, the use of antibodies directed to HHV-6 envelope glycoproteins is problematic because none of the available reagents, regardless of their neutralizing effect in infectivity assays, have the ability to block the interaction with CD46 (F.S. et al, unpublished results, 2002). Understanding the fine molecular mechanisms of IL-12 suppression by HHV-6 could be useful in devising novel therapeutic approaches for specifically counteracting the immunosuppressive effects of both HHV-6 and MV. Moreover, the ability of these viruses to selectively block the production of IL-12 and, thereby, the generation of effective Th1-polarized immune responses could be exploited for the development of innovative strategies for reducing such responses in the treatment of autoimmune disorders or in the prevention of graft rejection in patients receiving transplants.
Supported in part by the Istituto Superiore di Sanità (ISS) AIDS Program, Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-10-3152.
We thank Dr Edward Berger, Dr Giovanni Fagà, and Dr Chiara Bovolenta for critical suggestions; Dr Francesco Novelli for the kind gift of mAb γR99; and Richard Koch for assistance in the establishment of the RPA protocol.


![Figure 4. Productive HHV-6 infection and IL-12 suppression. Productive HHV-6 infection is not required for IL-12 suppression. (A) PBMCs and Mϕs were infected with HHV-6A. At 6 days later, the cells were harvested and analyzed for viral DNA by means of a quantitative real-time PCR assay. β-actin DNA was measured in parallel to normalize for cell number. The data shown are from one representative donor and are expressed as HHV-6 genome equivalents per cell. (B) PBMCs were incubated with HHV-6A supernatant that was previously inactivated or not by UV-light treatment. Cells were immediately harvested for the initial time point (0) and then after 4 and 6 days. (C) Mϕs were incubated for 17 hours with HHV-6A and treated or not with UV light, after which the infectious supernatants were replaced with fresh media. At 24 hours later, the cells were stimulated with IFN-γ and LPS. Supernatants were harvested and tested for IL-12 p70 by ELISA. Mean results (± standard error [SE]) from 2 donors are presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2002-10-3152/6/m_h82035085004.jpeg?Expires=1767728581&Signature=yAt4s-3c7344Vr-Cidh7fSbb7zUlesU734up5Vzup1sutQj~nYeWx4foqnsKtYywD2o1WwhWxGibgPs4pBHU3y-YsCdhZlca2rCWVOa8CJdO1fhcbtsRo6s9gY7wvZD54GVATiJTapusa2e-RkcmvVCiR~zZdhypYAa8nVwi8KKDFLNI8PU47R6OMP2TG0D6uZUZT69KV4FirO7VJpUDm5292hvcPIp9N7bHMJ5c2Tx45mLq9jckfxbZyr2y9jRmmVoqPN1C-47E-yViPoCFrv3E-EWsKbNnvOHps76pccFmKlriqWAfKxMqqGBQwDT80fyvpmq17xC3YJFS3lnC5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Posttranscriptional nature of IL-12 suppression by HHV-6. IL-12 suppression by HHV-6 occurs predominantly at the posttranscriptional level. (A) RPA analysis of IL-12 p40 (□), IL-12 p35 (▦), TNF-α (▪), and IL-1β (▧) mRNA in HHV-6–pretreated Mϕs. Mϕs were exposed to HHV-6 as described in “Materials and methods.” Cells were stimulated for 17 hours with IFN-γ and then with LPS and harvested at either 3 hours (mean ± SD of 6 donors) or 6 hours (mean ± SD of 4 donors) after LPS addition. The data are shown as induction percentages relative to the positive control (dotted line), which was set as 100%. Data obtained 24 hours after stimulation are not shown, since at this time point IL-12 mRNA levels returned to the baseline level seen in unstimulated cells. (B) Lack of intracellular accumulation of IL-12 p40/p70 protein in Mϕs after HHV-6 pretreatment. Mϕs from a representative donor were isolated and exposed to HHV-6A for 17 hours, after which the cells were stimulated for 17 hours with IFN-γ and an additional 8 hours with LPS. The cells were fixed/permeabilized and stained with a specific anti–IL-12 p40/p70 antibody. Intracellular IL-12 levels were analyzed by fluorocytometry; the monocyte gate was selected with the use of the physical parameters (forward scatter [FSC] and side scatter [SSC]) and CD14 positivity. (C) Mϕs from the same donor as in panel B were harvested after 8 hours of LPS stimulation for RPA analysis of IL-12 p35 and IL-12 p40 mRNA expression. Dotted line indicates positive control level (100%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2002-10-3152/6/m_h82035085006.jpeg?Expires=1767728581&Signature=1ZM8uczfoHt3kIC7ks9ptjLNIAjUpzzRkopkCAQ7VZxFDGpdn9TEI4qowX-JHraBUc7A4LzUJQo-vrLtZGe2Xq9TA5Gz1MgZdLpu7m-2eoVAJDcyo0aftIFlcoV3FB~vlOEWTixYrIpqvNEcCVF4HBColIO6jPk3zzbEslGq4jkjES-YL02vW5OLVXgXoVYk~E6CNGCSRu-EuHrYmVnw8tkhiT3VnRAnDnEXl0OKd9PCGv9GzjpDLiR7LHTO2FGObTkQJXPQPzYKn8S56Rk9wu-bPqMEWY6PE0n1iQddeT7N-VqyTjYPsgvlcFAiWXRYCn6Yj3Eusem7BwnHqaq6eA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal