Abstract
We have investigated the density of the collagen receptors glycoprotein VI (GPVI) and α2β1 on human platelets and their relationship to polymorphisms within the GPVI gene. GPVI levels varied 1.5-fold and showed a weak correlation (r = 0.35) with the levels of α2β1, which varied 3-fold. GPVI genotype had a significant effect on receptor levels with carriers of the proline 219 allele (approximately 22% of the population) having 10% lower GPVI levels than the more common serine homozygotes. GPVI and α2β1 levels were found to be significantly decreased on platelets from patients with myeloproliferative disorders (MPDs). In both the MPD and the control group, GPVI levels were found not to affect platelet function under high shear in whole blood. Similarly murine platelets that express up to 5-fold lower levels of GPVI showed no significant difference than controls in thrombus formation on a high-density collagen-coated surface. However platelets lacking the GPVI/Fc receptor γ-chain (FcR γ-chain) complex or a functional FcR γ-chain (immunoreceptor tyrosine-based activation motif [ITAM] point mutant) exhibited severely abrogated thrombus formation at 800 s–1 and 1500 s–1. These results demonstrate that GPVI levels are tightly controlled and play a critical role in thrombus formation on collagen; nevertheless, a range of receptor densities can support platelet function under high shear.
Introduction
Platelet adhesion and activation in response to blood vessel damage are both crucial for normal hemostasis and are also thought to be primary events in arterial thrombosis. The interaction of platelet receptors with subendothelial components such as collagen is central to this response. There are at least 2 receptors that mediate the direct platelet-collagen interaction, the integrin α2β1 and glycoprotein VI (GPVI), although there are likely to be others.1 The traditional 2-site, 2-state model2 describes collagen initially binding to the integrin α2β1 followed by binding and activation of a second receptor now known to be GPVI. This model was revised in the light of evidence that α2β1 exists in multiple affinity states3 with collagen initially binding to either α2β1 or GPVI followed by rapid binding to the other receptor. In an alternative version of this model4 the initial interaction between platelets and collagen is proposed to be via GPVI, which generates signals that in turn switch the α2β1 integrin into its high-affinity binding conformation.
Platelet glycoprotein polymorphisms have been intensively investigated as genetic risk factors for thrombotic disease in the past few years. Of those reported within the integrin α2 subunit gene, the proximal 5′-regulatory region as well as those in exons 7 and 8 combine to give a range of receptor densities at the platelet surface.5,6 The highest of these, allele 1 (807T), has been shown to be associated with increased risk for myocardial infarction among younger individuals,7 although results in other groups of patients are contradictory.8,9 The density of α2β1 integrin on platelets is reported to vary between 3- to 4-fold, as measured by flow cytometry or radiolabeling.10 This correlates with a variation in platelet adhesiveness on type I and type III collagen of 20-fold and 5-fold, respectively.10 The 3 cases of human deficiency of α2β1 in platelets that have been described are associated with reduced collagen responses and excessive bleeding, although other complications may have contributed to this phenotype.11-13 In contrast, mice lacking either the α2- or the β1-subunit on their platelets exhibit normal tail bleeding times but exhibit a small delay in onset of platelet aggregation.4,14,15 The effect on adhesion to collagen is dependent on the experimental conditions, with normal adhesion observed in whole blood4,15 but a dramatic loss observed under washed conditions.14 The GPVI signaling complex requires the presence of the Fc receptor γ-chain (FcR γ-chain) at the platelet surface, and signaling occurs via a similar pathway to that of immune receptors.16 FcR γ-chain–deficient platelets do not express GPVI at their surface17 and do not undergo aggregation to collagen.4,16,18 The small number of human cases of GPVI deficiency show similar traits to the FcR γ-chain–deficient mice, notably failure of platelets to aggregate and undergo thrombus formation on collagen-coated surfaces under flow.19-22
Recent reports of polymorphisms within the human GPVI gene suggest a possible link with myocardial infarction.23,24 The GPVI protein has 339 amino acids and consists of 2 immunoglobulin (Ig)–like domains at the amino terminus, responsible for ligand binding, on a highly glycosylated stalk with a short cytoplasmic tail. There have been 9 polymorphisms observed within the GPVI gene coding sequence,23 6 within the extracellular region and 3 in the cytoplasmic tail of the molecule, with further polymorphisms upstream of the translation start site. Of these, 5 led to amino acid substitutions, 3 in the highly glycosylated stalk and 2 in the cytoplasmic domain. The gene has 2 main haplotypes, with Caucasoid gene frequencies of 0.81 and 0.13, which can be distinguished by an amino acid substitution of serine 219 by proline (Ser219Pro) and at the DNA level as 13254T>C. The pivotal role of GPVI in platelet-collagen interaction and its polymorphic nature raise the question as to whether these differences have an impact on the receptor at a functional level. This study has addressed this by measurement of GPVI levels in 102 healthy donors and the relationship to the Ser219Pro polymorphism. The influence of a 5-fold variation in GPVI levels in a mouse model of thrombus formation under high shear has also been investigated.
Materials and methods
Donors were recruited from members of staff within the Department of Pharmacology and from donors attending the National Blood Services plateletpheresis program in Oxford, United Kingdom. The study was approved by the Oxfordshire clinical research ethics committee. All donors gave informed consent and answered negatively to routine deferral questions such as ingestion of aspirin. Blood samples were taken prior to apheresis from subjects who had not donated within the previous 4 weeks and analyzed within 4 hours. Blood was collected into Vacutainer tubes (Becton Dickinson, Oxford, United Kingdom) containing either tripotassium EDTA (ethylenediaminetetraacetic acid) or buffered sodium citrate (0.105 M). A full blood count was performed on the EDTA sample with mean platelet volume (MPV) measured in the citrated sample. Blood samples were also collected from consenting patients diagnosed with essential thrombocythemia (ET) or polycythemia vera (PRV) attending the Haematology Clinic at the John Radcliffe Hospital, Oxford, United Kingdom.
Measurement of platelet surface glycoprotein levels
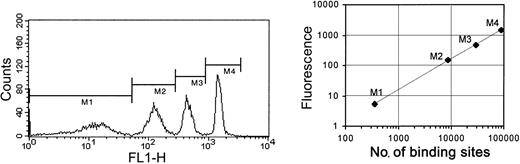
Surface expression of GPVI, the α2 subunit of the α2β1 integrin (CD49b), GPIb (CD42b), and GPIV (CD36) were measured in platelet-rich plasma by quantitative flow cytometry using a Platelet Calibrator kit (Biocytex, Marseille, France) according to the manufacturer's instructions. Kits from a single batch were used throughout the study. This is an indirect immunofluorescent method using 4 calibration beads coated with known concentrations of mouse IgG (360, 8500, 29 000, and 90 000 molecules for the batch used) and a polyclonal anti–mouse IgG–fluorescein isothiocyanate (FITC) as a staining reagent. This allows conversion of the mean fluorescence intensity (MFI) of stained platelets into the number of glycoprotein molecules per cell. All the antibodies used were mouse monoclonal antibodies (mAbs) as follows: 204-11 (anti-GPVI),25 anti-CD49b, anti-CD42b (both from BD Pharmingen, Oxford, United Kingdom), and anti-CD36 (Immunotech, Marseille Cedex, France). Antibodies were used at a saturating concentration (10 μg/mL) as determined in preliminary experiments. A calibration curve was performed simultaneously with each set of samples, and receptor numbers were calculated from this after subtraction of the corresponding negative isotypic control measurement (Figure 1). Samples were analyzed on a Facscalibur flow cytometer (Becton Dickinson, San Jose, CA) by collecting 10 000 events and analyzing the data using CellQuest software (Becton Dickinson, San Jose, CA).
Quantitative flow cytometry of platelet glycoproteins. Platelet surface glycoproteins were measured in platelet-rich plasma (PRP) using specific mouse monoclonal antibodies by flow cytometry. Platelets were stained with a glycoprotein-specific antibody (10 μg/mL) for 15 minutes and then an FITC-labeled antimouse antibody was added and incubated for a further 15 minutes in the dark. Simultaneously, calibration beads were incubated with the antimouse FITC secondary for 15 minutes. Diluent was added to all tubes, which were analyzed by flow cytometry. The calibration curve was obtained by plotting the geometric mean fluorescence intensity (GMFI) for each peak of the histogram (M1,M2,M3, and M4 in the left panel) against the known number of antibody binding sites for that peak (right panel). The value of the GMFI for the isotype control is always subtracted from the GMFI of the specific antibody binding before the number of specific sites is calculated.
Quantitative flow cytometry of platelet glycoproteins. Platelet surface glycoproteins were measured in platelet-rich plasma (PRP) using specific mouse monoclonal antibodies by flow cytometry. Platelets were stained with a glycoprotein-specific antibody (10 μg/mL) for 15 minutes and then an FITC-labeled antimouse antibody was added and incubated for a further 15 minutes in the dark. Simultaneously, calibration beads were incubated with the antimouse FITC secondary for 15 minutes. Diluent was added to all tubes, which were analyzed by flow cytometry. The calibration curve was obtained by plotting the geometric mean fluorescence intensity (GMFI) for each peak of the histogram (M1,M2,M3, and M4 in the left panel) against the known number of antibody binding sites for that peak (right panel). The value of the GMFI for the isotype control is always subtracted from the GMFI of the specific antibody binding before the number of specific sites is calculated.
Generation of RBL-2H3 expressing GPVI
RBL-2H3 cells were grown in Dulbecco modified Eagle medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (FBS) under 5% CO2/95% air in a humidified incubator. Cells were kept at exponential phase of growth. Adherent cells were detached by incubating with trypsin-EDTA for 5 minutes at 37°C before washing. The cDNA for human GPVI was subcloned into HindIII/XbaI sites of pRc plasmid. The RBL-2H3 cells were stably transfected with this construct as follows: 1 × 107 RBL-2H3 cells were washed once with serum-free medium and once with cytomix buffer (8.9 mg/mL KCl, 0.02 mg/mL CaCl2, 1.7 mg/mL K2HPO4, 1.4 mg/mL KH2PO4, 6 mg/mL HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.8 mg/mL EGTA [ethylene glycol tetraacetic acid], 5 mM MgCl2, pH 7.6), which was supplemented on the day of experiment with 0.37 mg/mL glutathione. Cells were resuspended in 400 mL cytomix buffer and placed into an electroporation cuvette containing 40 mg plasmid DNA. Cells were electroporated, added to 15 mL completed medium, and incubated for 48 hours, before selection by 500 mg/mL Geneticin. Surface expression of GPVI was confirmed by flow cytometry using GPVI-specific snake venom convulxin and anticonvulxin antibody. GPVI receptor levels were also measured with (mAb) 204-11 using the quantitative flow cytometry already described.
Convulxin staining
RBL-2H3 cells were resuspended in Tyrode-HEPES buffer containing 1 mg/mL bovine serum albumin (BSA). All incubations were performed for 30 minutes unless otherwise indicated. For GPVI detection, RBL-2H3 cells were incubated with 10 μg/mL convulxin, washed, and incubated with 0.4 μg/mL anticonvulxin antibody, washed again, and finally incubated with FITC-conjugated anti–rabbit IgG secondary antibody diluted 1:500 (Sigma, Poole, Dorset, United Kingdom). Stained cells were analyzed immediately using a FACScalibur flow cytometer. Data were recorded and analyzed using CellQuest software. Convulxin and anticonvulxin antibody were generous gifts from Drs Mireille Leduc and Cassian Bon (Institut Pasteur, Paris, France).
Platelet function analyser (PFA-100)
The PFA-100 (Dade, Miami, FL) was used according to the manufacturer's instructions. The 2 cartridges, 1 with collagen and epinephrine (CEPI) and 1 with collagen and adenosine diphosphate (CADP), were used for each donor, and closure time (CT) was measured within 4 hours of blood being drawn into buffered sodium citrate (0.105 M) Vacutainer tubes. A single batch of cartridges was purchased at the beginning of this study to ensure uniformity throughout the program.
Genotyping
DNA was prepared from peripheral blood samples by standard techniques. Donors were genotyped for 2 polymorphisms in the GPVI gene, 13254T>C (Ser219Pro) and –154C>T. GPVI 13254T>C was determined by polymerase chain reaction (PCR) amplification of a 279-bp region of exon 5 using primers 5′-ACATCCACAACAGTCCAGTG (forward) and 5′-ATCGAGAAGTCTAGGCAGAG (reverse) followed by MspI (New England Biolabs [NEB], Beverly, MA) digestion of the product. This yields constant fragments of 112 bp and 47 bp in addition to 120 bp in the presence of the T allele and 95 bp and 25 bp in the presence of the C allele. The –154C>T status was determined by NaeI (NEB) digestion of the 199-bp PCR product using primers 5′-CAGGGAGTTTATGGGAGCAC (forward) and 5′-AGAATGCTCTCCTCCCTTCC (reverse). Fragments of 133 bp and 68 bp are evident in the presence of the C allele. Donors were typed for the 3 major α2 alleles.26 Allele 1 (807T/1648G/2531C), allele 2 (807C/1648G/2531C), and allele 3 (807C/1648A/2531C) were detected by BglII/AseI (NEB) digestion of the 1332-bp product of amplification of intron G and most of exon 8 using the following primers: 5′-GATTTAACTTTCCCGACTGCCTTC (forward) and 5′-CTCTCTAGATTGTCATGGTTGCATTGATCAATCAC (reverse). Allele 1 yielded fragments of 536 bp, 439 bp, and 343 bp; allele 2, 879 bp, 268 bp, and 171 bp; and allele 3, 879 bp and 439 bp.
von Willebrand factor antigen (VWF:Ag) levels
Plasma levels of VWF:Ag were measured using a fully automated immunoturbidometric assay (Sta-Liatest VWF kit; Diagnostic Stago & Roche Diagnostics, Lewes, United Kingdom) on an MDA-180 analyser (Biomerieux, Basingstoke, Hampshire, United Kingdom). The assay was performed according to the manufacturer's instructions and calibrated using the Eighth British Reference Plasma (98/734; NIBSC, South Mimms, Herts, United Kingdom).
Genetically modified mice
Breeding pairs of C57Bl/6 mice deficient in the FcR γ-chain (FcR γ(–/–)), FcR γ(+/–) mice, FcR γ-chain transgenic (FcRγ-Tg), and FcR γ-chain immunoreceptor tyrosine-based activation motif (ITAM) mutant (with phenylalanine residues replacing the 2 tyrosine in the ITAM) (ITAM PM) mice were kindly supplied by Dr Takashi Saito, Chiba University Graduate School, Japan. FcR γ-Tg mice express a transgene for FcR γ-chain in an FcR γ-chain–null background and were crossed with FcR γ(–/–) to reduce the level of expression of the GPVI–FcR γ-chain complex to approximately 20% of that of controls.27 The level of GPVI in the FcR γ-chain ITAM mutant was approximately 40% of controls. The generation of these mice will be described (T.S., manuscript in preparation).
Flow adhesion assay
Mouse blood was taken into sodium heparin (10 U/mL) by cardiac puncture and used within 30 minutes of collection. The blood was perfused for 2 minutes through a glass microslide, 1 × 0.1 mm inner diameter (Camlab, Cambridge, United Kingdom), that had been coated with 1 mg/mL type I collagen from equine tendon (Horm; Nycomed, Munich, Germany) before blocking with 2% BSA in phosphate-buffered saline. Shear rates of 800 s–1 and 1500 s–1 with corresponding flow rates of 0.08 mL/min and 0.15 mL/min were generated by a syringe pump (Harvard Apparatus, Southnatick, MA). After 2 minutes of perfusion with whole blood, modified Tyrode-HEPES buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2, pH 7.3) was perfused for 8 minutes at the same shear rate as the blood. Platelet thrombi that had formed on the surface of the collagen were visualized with an inverted stage videomicroscope system (DM IRB; Leica, Milton Keynes, United Kingdom) and surface coverage was analyzed using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). Subsequently adherent platelets were lysed in ice-cold nonidet P-40 (NP-40) lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane], 300 mM NaCl, 2 mM EGTA, 2 mM EDTA, 2% [vol/vol] NP-40, 1 mM phenylmethylsulphonyl fluoride, 2 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1 μg/mL pepstatin A, pH 7.3) for protein estimation, RC DC protein assay (Bio-Rad, Hemel Hempsted, United Kingdom), and immunoblotting for actin (antiactin antibody; Sigma).
Statistical analysis
Results were expressed as the mean ± SD. Differences between groups were detected with an unpaired Student t test as well as by simple linear regression and correlation using Graphpad Prism (Graphpad Software, San Diego, CA) software. Further multiple regression analysis was performed using the SPSS (Chicago, IL) program. r is the correlation coefficient and R2 is square of the correlation coefficient, also called the coefficient of determination. P values are reported except when P values are less than .001, and differences were considered significant when P values were less than .05.
Results
Quantitation of GPVI and other glycoproteins on platelets
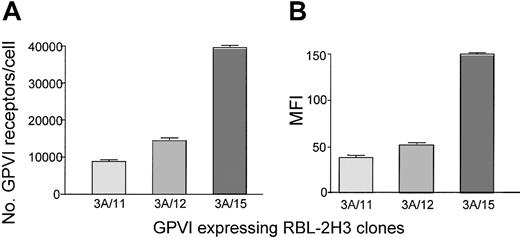
To measure the level of GPVI and several other glycoprotein receptors, we used a quantitative flow cytometry method28 that has previously been used to measure the levels of Par-1,29 GpIb,30 and GpIIb/IIIa31 on the platelet surface. For GPVI we used a recently characterized mouse monoclonal antibody, mAb 204-11.25 The reproducibility of the method was established by measuring platelet GPVI and α2β1 levels from both a female and a male donor at regular intervals over periods of 6 months (n = 20) and 3 months (n = 8), respectively. There was no specific fluctuation of glycoprotein levels over time with an overall coefficient of variation of approximately 10%. In addition, the level of GPVI was not altered by prior activation of protease-activated receptors or in the presence of the inhibitor PP2, which inhibits Src family kinases, and prostacyclin, which elevates cyclic adenosine monophosphate (not shown). These studies were performed in washed platelets because of the high level of plasma binding of PP2. Measurement of GPVI levels on cell lines that had been transfected with the receptor32 confirmed the ability of this method to detect differences in receptor expression (Figure 2). Differing levels of GPVI expression found in 3 clones of transfected RBL-2H3 cells were analyzed using mAb 204-11 by quantitative flow cytometry and compared with the MFI of convulxin staining. The fold shift in GPVI expression of the clones is similar between the 2 methods with mAb 204-11 showing a 2.7-fold and 4.5-fold increase in GPVI levels of clone 3A/15 compared with clones 3A/11 and 3A/12, respectively. Convulxin staining of the same clones shows a 2.9-fold and 4-fold increase in MFI of clone 3A/15 compared with clones 3A/11 and 3A/12, respectively.
Comparison of GPVI levels expressed on different clones of transfected RBL-2H3 cells. Surface expression of GPVI was analyzed on 3 different clones of RBL-2H3 cells transfected with human GPVI. (A) GPVI levels were measured on transfected RBL-2H3 cells using mAb 204-11 by quantitative flow cytometry. Cells were stained with mAb 204-11 (10 μg/mL) for 15 minutes, and then an FITC-labeled antimouse antibody was added and incubated for a further 15 minutes in the dark. Simultaneously, calibration beads were incubated with the antimouse FITC secondary for 15 minutes. Diluent was added to all tubes, which were then analyzed by flow cytometry. A calibration curve was constructed and the number of GPVI receptors per cell was determined. (B) GPVI-transfected RBL-2H3 cells were stained with convulxin (10 μg/mL) for 30 minutes, washed, and incubated with a rabbit IgG anticonvulxin antibody for 30 minutes. After further washing, the cells were incubated with anti–rabbit IgG FITC for 30 minutes, then diluted and analyzed by flow cytometry. All results are expressed ± SEM, n = 4.
Comparison of GPVI levels expressed on different clones of transfected RBL-2H3 cells. Surface expression of GPVI was analyzed on 3 different clones of RBL-2H3 cells transfected with human GPVI. (A) GPVI levels were measured on transfected RBL-2H3 cells using mAb 204-11 by quantitative flow cytometry. Cells were stained with mAb 204-11 (10 μg/mL) for 15 minutes, and then an FITC-labeled antimouse antibody was added and incubated for a further 15 minutes in the dark. Simultaneously, calibration beads were incubated with the antimouse FITC secondary for 15 minutes. Diluent was added to all tubes, which were then analyzed by flow cytometry. A calibration curve was constructed and the number of GPVI receptors per cell was determined. (B) GPVI-transfected RBL-2H3 cells were stained with convulxin (10 μg/mL) for 30 minutes, washed, and incubated with a rabbit IgG anticonvulxin antibody for 30 minutes. After further washing, the cells were incubated with anti–rabbit IgG FITC for 30 minutes, then diluted and analyzed by flow cytometry. All results are expressed ± SEM, n = 4.
GPVI levels on healthy human platelets are less variable than those of the α2β1 integrin
The level of GPVI, α2 integrin subunit, GPIb, and CD36 were measured in 102 healthy donors. The mean age of the donors was 41 ± 10 years, with 65% males and 13% smokers. The platelet count was 278 ± 62 (× 109/L) and the mean platelet volume was 9.1 ± 0.78 fL. All other parameters in the hematology screen fell within normal limits for each donor. Analysis of platelets from these donors gave a mean GPVI receptor density of 3730 ± 453 per platelet. There was a 1.5-fold variation in the level of GPVI between individuals and a coefficient of variation of 12.2%. The narrow variation in expression of GPVI was confirmed in 62 of these donors using a second GPVI antibody, HY101, that was kindly donated by Dr Mark Kahn (data not shown). This is in contrast to the 3-fold variation in levels of α2β1 determined in the same donors using an antibody to the α2 subunit. The level of expression was estimated as 1731 ± 432 sites per platelet with a coefficient of variation of 24.9%. These levels and degree of variation agree favorably with previously published values of α2 subunit expression determined by a radiolabeling assay10 or by flow cytometry using a direct immunofluorescence procedure.33 For example, Kunicki et al10 estimated the levels of the α2 subunit to be in the order of 968 to 2874 molecules per cell. GPIb levels are also comparable with those determined by direct immunofluorescence33 with 19 510 ± 2206 sites per platelet and a narrow range of variability of 1.5-fold as previously published.34
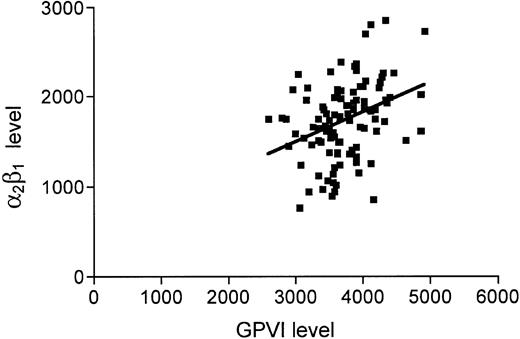
There has been speculation that levels of α2β1 and GPVI on platelets may in some way be coordinated. Although our data (Figure 3) do show a significant relationship between the levels of the 2 receptors (P < .001), the r value of 0.35 and R2 = 0.12 indicate that this is not a strong association. Intriguingly there also appears to be a weak but statistically significant relationship between GPVI receptor levels and GPIb levels (P = .005, R2 = 0.076, r = 0.28). We performed regression analysis of the covariates on GPVI by entering the independent variables in linear regression in a stepwise fashion, that is, most significant entered into the equation first, with GPVI as the dependent variable. This gave a model with high significance (P < .001 and R2 = 0.239), with α2β1 level being the most significant predictor of GPVI level followed by GPIb level, smoking, and finally VWF level.
Levels of GPVI and α2β1 on human platelets. Levels of GPVI and α2β1 were measured by flow cytometry as described in “Materials and methods.” A weak but statistically significant relationship was observed between GPVI (abscissa) and α2β1 (ordinate) levels: R2 = 0.122 and P < .001.
Levels of GPVI and α2β1 on human platelets. Levels of GPVI and α2β1 were measured by flow cytometry as described in “Materials and methods.” A weak but statistically significant relationship was observed between GPVI (abscissa) and α2β1 (ordinate) levels: R2 = 0.122 and P < .001.
Effect of genetic variation in the GPVI gene
To investigate whether polymorphisms in the GPVI gene affect platelet GPVI levels, donors were genotyped for 13254T>C dimorphism, which codes for a Ser to Pro substitution at position 219 of the protein. The polymorphic variation in the coding region falls into 2 main haplotypes that can be broadly defined by 13254T>C (Ser219Pro). Our results show 77% of donors to be homozygous for Ser219, 22% to be heterozygous, and only 1 individual homozygous for the Pro219 allele. This broadly agrees with previously published data.23 Genotype distributions for both polymorphisms did not differ from Hardy-Weinberg equilibrium. It is interesting to note that genotype does have an effect on GPVI levels with a significantly greater number of sites (3814 ± 439 sites, P < .001) present in the Ser/Ser219 donors compared with 3437 ± 353 sites in Ser219Pro heterozygotes. The single Pro/Pro219 donor had one of the lowest levels of GPVI with 2598 sites but increased numbers of this genotype are needed to verify this observation. The relationship between a polymorphism in the promoter region of the GPVI gene –154C>T and the level of expression of GPVI was also investigated. The following distributions were found: 27% of donors, –154TT; 52%, –154C>T; and 21%, –154CC. There was no association with GPVI levels.
Effect of genetic variation in the α2 gene
Donors were genotyped for the 3 α2 alleles (A1, A2, A3) to detect possible co-ordination of genetic control between α2β1 and GPVI. The frequencies were in broad agreement with published data (data not shown).26 There were more α2 binding sites observed in A1A1 donors, 2110 ± 419, and reduced numbers, 1207 ± 195, in A2A2 donors. We also observed that homozygosity for the A1 allele is associated with significantly lower GPVI levels. Stepwise linear regression analysis was performed without prior adjustment for covariates to test which of the measured parameters influence GPVI levels. Inclusion of genotypes and covariates produces a final model with GPVI Ser219Pro genotype being the most significant predictor of GPVI levels followed by α2β1 level, homozygosity for α2 A1, GPIb level, and VWF level, (P < .001 and R2 = 0.446 for the model).
Platelet function analysis
The PFA-100 measures platelet plug formation on a collagen-coated aperture under high shear stress (5000 s–1). There are 2 cartridge types, 1 coated with collagen and ADP (CADP) and 1 with collagen and epinephrine (CEPI). The PFA-100 is becoming a recognized substitute for bleeding time measurements in the diagnosis of von Willebrand disease and congenital or drug-induced platelet disorders.35-37 We observed a mean closure time (CT) with CADP and CEPI cartridges of 92 ± 40 seconds and 139 ± 68 seconds, respectively. As reported previously,38 the level of von Willebrand factor has the most significant effect on closure time (Table 1). GPVI levels had a small but significant effect on both CADP (P = .005, R2 = 0.076) and CEPI (P = .013, R2 = 0.060) cartridges, as did MPV. The effect of GPVI and MPV was no longer significant when stepwise linear regression of covariates was performed, with VWF levels remaining the only significant indicator of closure time with both cartridges. Neither of the 2 GPVI polymorphisms, nor any of the α2 haplotypes, showed any significant association with closure time and similarly there was no effect evident of α2β1, GPIb, or CD36 levels.
Relationship between PFA-100 closure time and study parameters
. | Collagen + ADP* . | Collagen + epinephrine†closure time (± 2 SD) 139 s (± 68) n = 102 . |
|---|---|---|
| GPVI levels | ||
| P | .0052‡ | .0131‡ |
| r | -0.28 | -0.24 |
| R2 | 0.076 | 0.060 |
| α2 integrin levels | ||
| P | .5939 | .5442 |
| r | -0.53 | -0.06 |
| R2 | 0.003 | 0.004 |
| GPIb levels | ||
| P | .4654 | .1644 |
| r | -0.073 | -0.14 |
| R2 | 0.005 | 0.019 |
| CD36 levels | ||
| P | .8526 | .3541 |
| r | -0.019 | -0.092 |
| R2 | 0.0003 | 0.009 |
| von Willebrand factor | ||
| P | <.0001‡ | <.0001‡ |
| r | -0.497 | -0.388 |
| R2 | 0.246 | 0.1512 |
| Mean platelet volume | ||
| P | .0192‡ | .0047‡ |
| r | -0.232 | -0.278 |
| R2 | 0.054 | 0.077 |
. | Collagen + ADP* . | Collagen + epinephrine†closure time (± 2 SD) 139 s (± 68) n = 102 . |
|---|---|---|
| GPVI levels | ||
| P | .0052‡ | .0131‡ |
| r | -0.28 | -0.24 |
| R2 | 0.076 | 0.060 |
| α2 integrin levels | ||
| P | .5939 | .5442 |
| r | -0.53 | -0.06 |
| R2 | 0.003 | 0.004 |
| GPIb levels | ||
| P | .4654 | .1644 |
| r | -0.073 | -0.14 |
| R2 | 0.005 | 0.019 |
| CD36 levels | ||
| P | .8526 | .3541 |
| r | -0.019 | -0.092 |
| R2 | 0.0003 | 0.009 |
| von Willebrand factor | ||
| P | <.0001‡ | <.0001‡ |
| r | -0.497 | -0.388 |
| R2 | 0.246 | 0.1512 |
| Mean platelet volume | ||
| P | .0192‡ | .0047‡ |
| r | -0.232 | -0.278 |
| R2 | 0.054 | 0.077 |
Closure time was measured in citrated whole blood within 4 hours of venepuncture using 2 cartridges, 1 with collagen and epinephrine and 1 with collagen and ADP. PFA-100 closure time was inversely correlated to VWF antigen levels, and a weak relationship was also indicated with MPV and GPVI levels.
Mean closure time of 92 ± 40 seconds (mean ± 2 SD); n = 102.
Mean closure time of 139 ± 68 seconds (mean ± 2 SD); n = 102.
Statistically significant at P < .05.
Glycoprotein levels in platelets from patients with myeloproliferative disorders
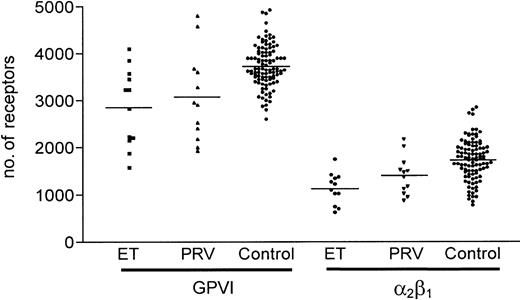
Platelets in these diseases have previously been described to have altered levels of glycoprotein receptors such as GPIb, GPIV (CD36), and αIIb β3.39 We therefore measured the levels of several glycoproteins including GPVI in blood samples from 12 patients with ET and 12 patients with PRV. Surface glycoprotein levels in these patients were widely distributed (Figure 4). Mean levels of GPVI per platelet were 2853 ± 830 for ET and 3063 ± 956 for PRV, with α2β1 levels of 1133 ± 335 and 1402 ± 408, respectively. These levels are significantly lower in both patient groups compared with healthy donors: ET group—GPVI, P = .004 and α2β1, P < .001; and the PRV group—GPVI, P = .035 and α2β1, P = .014. It was noteworthy that not all patients with unusually low GPVI levels exhibited low α2β1 integrin levels. As previously reported in myeloproliferative disorders, platelet surface CD36 levels are significantly increased in both patient groups, with mean values of 11 870 ± 2571 for ET (P < .001) and 12 030 ± 2469 for PRV (P < .001) compared with 8795 ± 1990 for the control group, while GPIb levels are largely unaffected. Results for CADP closure time were 85 ± 25 seconds in the ET group and 104 ± 48 seconds in the PRV group, which are not significantly different from healthy donors despite their altered levels of platelet surface glycoproteins. CEPI closure times were prolonged 251 ± 67 seconds for ET (P < .001) and 243 ± 61 seconds for PRV (P < .001), which might be due to aspirin prophylaxis. There was no significant difference in MPV, although the mean platelet count was higher (373 ± 110) in the ET group compared with the healthy donor group (278 ± 62).
Comparison between levels of GPVI and α2β1 in myeloproliferative disorder and healthy donors. Levels of GPVI and α2β1 were measured by flow cytometry in platelet-rich plasma from 12 patients with essential thrombocythemia (ET) and 12 with polycythemia vera (PRV).
Comparison between levels of GPVI and α2β1 in myeloproliferative disorder and healthy donors. Levels of GPVI and α2β1 were measured by flow cytometry in platelet-rich plasma from 12 patients with essential thrombocythemia (ET) and 12 with polycythemia vera (PRV).
Platelet adhesion and thrombus formation is dependent on GPVI signaling
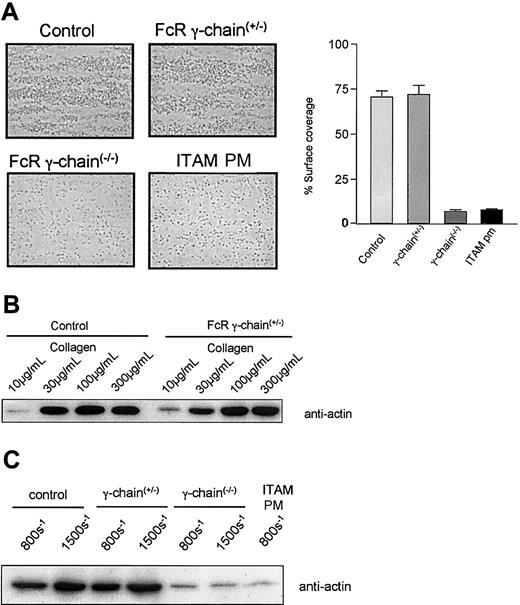
To investigate the effect of a wider variation in GPVI levels on platelet function under shear conditions we used blood from genetically modified mice with varying amounts of GPVI on their platelets. Blood was flowed over a high-density collagen-coated surface at 800 s–1 or 1500 s–1 and thrombus formation was visualized by microscopy. Subsequently, adherent platelets were collected for protein estimation and immunoblotting for actin. Blood from control mice exhibited robust thrombus formation at 800 s–1, which equates to 11.6 ± 1.6 μg protein deposited on the collagen-coated surface during 2 minutes (Figure 5A). Thrombus formation in FcR γ-chain heterozygote blood, which expresses 50% of control levels of GPVI,27 was not significantly different than the control with protein levels of 9.8 ± 0.9 μg deposited during 2 minutes. Quantitation of percent of surface coverage of the collagen-coated microslide gave results that are consistent with the protein assay, with control platelets giving 70 ± 4% coverage compared with 72 ± 5% for the heterozygotes. It is important to note that, unlike the protein assay, this measurement does not take into consideration the 3-dimensional structure of the thrombus. A concentration-response effect on thrombus formation was evident when control and FcR γ-chain heterozygote blood was flowed over lower density collagen-coated surfaces at 800 s–1 (Figure 5B), with protein estimation demonstrating a less than 10% difference between the 2 at each concentration. Blood from a FcRγ-Tg/knockout mouse, which expressed 20% of the control level of GPVI, was analyzed in triplicate and found to have no significant differences in thrombus size (data not shown). FcR γ-chain–deficient mice, with no GPVI, exhibit abrogated thrombus formation, although adhesion of single platelets was consistently observed (Figure 5A). This resulted in a protein content below the lowest value of the standard curve, although there was 7 ± 1% surface coverage of the collagen-coated microslide by single FcR γ-chain–deficient platelets. Immunoblotting for actin demonstrated recovery of a small amount of protein, which was considerably lower than that in controls (Figure 5C). At higher shear, 1500 s–1, there was a greater deposition of platelets in both control and FcR γ-chain heterozygote mice but not in the FcR γ-chain–deficient mice (Figure 5C). FcR γ-chain–deficient platelets that express an FcR γ-chain ITAM mutant transgene at a level that is approximately 40% of the wild type demonstrated profoundly decreased thrombus formation (Figure 5A,C) presenting a similar picture to FcR γ-chain(–/–) platelets with surface coverage of 8 ± 1%. Platelets from these mice also show a complete loss of aggregation and tyrosine phosphorylation in response to collagen (data not shown).
Thrombus formation in blood from mice genetically modified to express different levels of GPVI. (A) Blood from control mice, FcR γ-chain(+/–) mice with a 50% reduction in GPVI levels, FcR γ-chain(–/–) mice deficient in GPVI, and FcR γ-chain(–/–) mice that express an FcR γ-chain ITAM (ITAM PM) mutant transgene at a level that is approximately 40% of the wild type was flowed over a collagen-coated surface at 800 s–1 Thrombus formation was abolished in the FcR γ-chain(–/–) mice and in blood from the ITAM PM mice, although there was some adherence of single platelets. Quantitation of percent of surface coverage of the collagen-coated microslide using Image-Pro plus software demonstrates control platelets giving 70 ± 4% coverage compared with 72 ± 5% for the heterozygotes, 7 ± 1% for FcR γ-chain–deficient platelets, and 8 ± 1% ITAM PM platelets. These results are presented as ± SEM and are representative of 3 to 8 experiments. Original magnification, × 630. (B) Blood from control mice and FcR γ-chain(+/–) mice, with a 50% reduction in GPVI levels, was flowed over differing concentrations of collagen-coated surface as indicated. Platelets adherent to the collagen-coated surface were lysed in 1% NP-40 lysis buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was probed with an anti-Actin antibody. These results are representative of 3 experiments. Thrombus formation varied by less than 10% as measured by protein estimation of adherent platelets between control and FcR γ-chain(+/–) mice. (C) Blood from genetically modified mice as indicated was flowed over a collagen-coated surface at 800 s–1 and 1500 s–1. Platelets adherent to the collagen-coated surface were lysed in 1% NP-40 lysis buffer and subjected to SDS-PAGE and transferred to PVDF membrane. The membrane was probed with an anti-Actin antibody. These results are representative of 3 to 8 experiments.
Thrombus formation in blood from mice genetically modified to express different levels of GPVI. (A) Blood from control mice, FcR γ-chain(+/–) mice with a 50% reduction in GPVI levels, FcR γ-chain(–/–) mice deficient in GPVI, and FcR γ-chain(–/–) mice that express an FcR γ-chain ITAM (ITAM PM) mutant transgene at a level that is approximately 40% of the wild type was flowed over a collagen-coated surface at 800 s–1 Thrombus formation was abolished in the FcR γ-chain(–/–) mice and in blood from the ITAM PM mice, although there was some adherence of single platelets. Quantitation of percent of surface coverage of the collagen-coated microslide using Image-Pro plus software demonstrates control platelets giving 70 ± 4% coverage compared with 72 ± 5% for the heterozygotes, 7 ± 1% for FcR γ-chain–deficient platelets, and 8 ± 1% ITAM PM platelets. These results are presented as ± SEM and are representative of 3 to 8 experiments. Original magnification, × 630. (B) Blood from control mice and FcR γ-chain(+/–) mice, with a 50% reduction in GPVI levels, was flowed over differing concentrations of collagen-coated surface as indicated. Platelets adherent to the collagen-coated surface were lysed in 1% NP-40 lysis buffer and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was probed with an anti-Actin antibody. These results are representative of 3 experiments. Thrombus formation varied by less than 10% as measured by protein estimation of adherent platelets between control and FcR γ-chain(+/–) mice. (C) Blood from genetically modified mice as indicated was flowed over a collagen-coated surface at 800 s–1 and 1500 s–1. Platelets adherent to the collagen-coated surface were lysed in 1% NP-40 lysis buffer and subjected to SDS-PAGE and transferred to PVDF membrane. The membrane was probed with an anti-Actin antibody. These results are representative of 3 to 8 experiments.
Discussion
The purpose of this study was to gain a better understanding of the distribution of collagen receptor levels on platelets from healthy donors while determining both the functional effect and the influence of genetic polymorphisms. It has already been established that levels of one of the collagen receptors, the integrin α2β1, varies between 3- and 4-fold on platelets, dependent on genotype, and some studies suggest that individuals that have the α2 A1A1 (807TT) genotype may be at increased risk of myocardial infarction. We have examined levels of the major activatory collagen receptor, GPVI, in 102 healthy donors and found the level of the glycoprotein to be tightly controlled varying by only 1.5-fold. This was in contrast to levels of the α2 integrin, which varied by 3-fold in the same set of donors. We have also demonstrated that the level of GPVI is maintained over time in healthy donors. This does not preclude the presence of low or high levels of GPVI in a small number of donors or in other ethnic groups as might become apparent in a large-scale study.
Despite the modest variation in GPVI levels we were able to detect a lower receptor density in donors carrying the GPVI Pro219 allele who account for about 22% of the population. This is noteworthy given a recent report of increased risk of myocardial infarction in individuals homozygous for the Pro219 allele.23 The only donor with this genotype in our study had GPVI levels within the bottom 5% of results for the normal population. While it is not possible to draw firm conclusions from a single donor, the association with lower levels holds with carriers of one Pro219 allele. Polymorphisms in the promoter region of the α2β1 gene have been shown to be responsible for alteration in transcription of the integrin. In contrast the –154C>T polymorphism in the GPVI gene does not appear to have an effect on GPVI level. Indeed several other polymorphisms in this region were recently investigated and also found to have no effect on transcription.40 Since the known genetic variation in the coding region falls into 2 haplotypes defined by Ser219Pro,23 and promoter variation appears not to influence GPVI level, it is likely that most of the genetically determined interindividual variation in GPVI levels is accounted for by Pro219 alone or in combination with one of the other amino acid changes on the same haplotype.
Recent work has suggested that there may be co-ordination of receptor density between GPVI and α2 β1,41 whereas another study was unable to confirm these findings.42 The concept, while attractive, is not strongly supported by our results. We find a weak correlation between GPVI and α2β1 levels, although α2β1 was less important than the GPVI genotype in determining GPVI levels in a stepwise linear regression model. This regression model also indicates that GPIb levels have an effect on GPVI levels. This may suggest a general link between density of receptors that, from our analysis, does not appear to be provided by platelet volume, which varies by 9% among our donors. Further, the level of CD36 does not exhibit the same relationship. The link between increased α2β1 integrin levels and risk of myocardial infarction or cerebrovascular disease is intriguing, as the receptor seems to play an accessory role to GPVI in collagen adhesion and signaling. This effect is not necessarily platelet mediated as α2β1 is highly expressed on many cell types, including endothelial cells where it has been implicated in cell migration, attachment, and spreading as well as collagen matrix remodeling and angiogenesis.43-45 Intriguingly, a recent retrospective study of factors influencing the development of coronary artery disease after heart transplantation demonstrates that donor α2 807TT genotype had a greater impact in relation to risk than recipient genotype.46
The results of our functional testing show little evidence of collagen receptor density affecting PFA-100 closure time. The weak correlation between GPVI levels and closure time disappeared once stepwise linear regression was performed to remove the influence of covariates. Levels of VWF have a major impact on closure time in the PFA-100, and the subtle effects of receptor levels may be masked in healthy donors. Consistent with this is the observation that patients with type I von Willebrand disease are more at risk of bleeding if they carry the 807CC genotype associated with low levels of α2 β1.47 This may suggest a more important role for the integrin in situations where the major collagen pathway via VWF and GPVI is compromised. The initial platelet response to exposed collagen at high shear is the formation and breakage of tethers via GPIb binding to VWF collagen slowing the platelet and allowing it to further interact specifically via GPVI and α2 β14,48 and indirectly via αIIbβ3.4
Any effect of GPVI in pathogenesis would have to be mediated by platelets or terminally differentiated megakaryocytes, as expression of the receptor is restricted to this lineage.49,50 Given the central role of GPVI in collagen signaling in platelets, the potential impact of a similar variation to that of α2β1 could be much greater. We examined platelets from genetically modified mice that exhibit a range of GPVI densities but unchanged levels of α2 β127 to study this effect. We found that platelets from FcR γ-chain–deficient mice, with no GPVI, are unable to form a thrombus on a collagen-coated surface at shear rates comparable with those found in arteries, although some single platelets form stable adhesions. FcR γ-chain(+/–) platelets, with a 50% reduction in GPVI levels, were not significantly different from controls with respect to thrombus formation. A similar result was also found for murine platelets that have a 5-fold reduction in GPVI relative to controls. It is of interest to note that thrombus formation in blood from FcR γ-chain–deficient platelets that express an FcR γ-chain ITAM mutant transgene (in which the 2 phosphorylated tyrosines have been converted to phenylalanines) is reduced to a similar level as that of FcR γ-chain(–/–) despite having 40% of control GPVI levels. This demonstrates that signaling via GPVI is crucial to the collagen response and emphasizes that the receptor alone is unable to support adhesion under high shear conditions.51
The observation that GPVI levels can be depressed by up to 50% in platelets from patients with a myeloproliferative disorder emphasizes the importance of murine platelets with reduced levels of the glycoprotein as a model system. As in the murine system, a 50% reduction in GPVI levels on human platelets does not affect thrombus formation under high shear, albeit with the caveat that the cartridges used in the PFA-100 are coated with either ADP or epinephrine in addition to collagen. These results suggest that collagen signaling via GPVI to α2β1 and αIIbβ is efficient at low receptor density levels. Evidence from heterozygotic forms of Bernard-Soulier and Glanzmann thrombasthenia indicate that patients whose platelets possess half the normal amounts of GPIb or αIIb β3 also have normal hemostasis.52,53 Similarly, reducing levels of GPIb by 50% using a monoclonal antibody does not affect platelet adhesion under flow conditions.54 It is possible that low receptor density may become more influential in the presence of other exacerbating factors such as decreased VWF levels or other coagulation defects.
In conclusion, our study shows that the level of GPVI on platelets of healthy individuals is tightly regulated, varying by no more than 1.5-fold, compared with the 3-fold variation in the level of α2β1. It is interesting to speculate as to why GPVI levels vary so little when it appears that a wide range of densities in both murine and human models can support robust platelet function as seen in in vitro systems. It may be, for example, that this is not the case in all vascular beds or that a high density of GPVI is essential for normal hemostasis in vivo. Alternatively, the apparent overcapacity of GPVI may have given an evolutionary advantage in helping to cope with severe vascular bleeds. In this context, it is noteworthy that evidence from other platelet glycoprotein receptors such as GPIb or αIIbβ3 indicates that there is overcapacity of receptor expression. The tight regulation of GPVI is consistent with its pivotal role in platelet activation in vivo and emphasizes the fact that GPVI remains one of the most promising pharmacologic targets for antiplatelet therapy, especially given its restricted distribution.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2003-01-0231.
Supported by the British Heart Foundation (S.P.W., D.B., Y.S., G.J.); National Blood Service, University of Oxford, Howard Hughes Medical Institute (D.R.).
One of the authors, P.H., has declared a financial interest in a company whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Note added in proof. Since the submission of the manuscript, another study has shown the low frequency GPVI allele characterized by Pro/Pro219 to be associated with reduced expression of the receptor.55
S.P.W. holds a British Heart Foundation chair.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal