Abstract
Interferon-γ receptor-1 (IFNγR1) deficiency is a rare inherited immunodeficiency. We performed a nonmyeloablative allogeneic stem cell transplantation on a boy with complete IFNγR1 deficiency and refractory disseminated Myco-bacterium avium infection. Despite the patient's profound immune defect, early donor stem cell engraftment was low. Full donor engraftment was accomplished only following multiple donor lymphocyte infusions. Detection of IFNγR1 expression on peripheral blood monocytes and neutrophils corresponded with establishment of stable, complete donor hematopoietic chimerism. However, expression of, and signaling through IFNγR1 disappeared shortly thereafter. Disseminated Mycobacterium avium infection persisted and the patient died. Coculture of Myco-bacterium avium with normal myeloid cells resulted in an IFNγ signaling defect similar to that observed in vivo. Active disseminated Mycobacterium avium infection may significantly compromise normal immune reconstitution following allogeneic stem cell transplantation. Patients with IFNγR1 deficiency should receive transplants before developing refractory mycobacterial infections. (Blood. 2003;102:2692-2694)
Introduction
Interferon-γ receptor-1 deficiency (IFNγR1) is a rare immunodeficiency in which patients are susceptible to a broad range of pathogenic and nonpathogenic mycobacteria, Salmonellae, Listeria monocytogenes, histoplasma, and severe viral infections.1-4 Patients with autosomal recessive IFNγR1 deficiency typically have a complete lack of interferon-γ responsiveness and usually die of disseminated mycobacterial infections in childhood.1
Allogeneic stem cell transplantation (SCT) has the potential of restoring normal immune function by providing normal interferon-γ-responsive myeloid cells.5 The potential for allogeneic stem cell transplantation to facilitate clearance of an established mycobacterial infection has not previously been tested. Therefore, we performed a nonmyeloablative SCT on a chronically ill boy with autosomal recessive IFNγR1 deficiency and refractory disseminated Mycobacterium avium infection. Following engraftment, the donor cells acquired a defect in IFNγR1 surface expression and signaling. In vitro studies suggest a role for M avium in the signaling defect.
Study design
With parental informed consent, the child was enrolled as a compassionate exemption to National Institutes of Health protocol 98-I-0104 (nonmyeloa blative stem cell transplantation for chronic granulomatous disease). Using high-resolution typing, the patient's mother was found to be identical at the HLA-A, -B, -DRB1, -DQB1, and -DRB loci. Although heterozygous for the IFNγR1 mutation, maternal peripheral blood mononuclear cells demonstrated normal in vitro IFNγR1 expression and signaling.6 Nonmyeloablative conditioning consisted of intravenous cyclophosphamide (60 mg/kg on days -7 and -6), fludarabine (25 mg/m2 on days -5 to -1), and equine antithymocyte globulin (40 mg/kg on days -5 to -2). Following conditioning, a granulocyte colony-stimulating factor (G-CSF)-mobilized, T-cell-depleted (Isolex; Nexell Therapeutics, Irvine, CA) peripheral blood stem cell graft consisting of 52.2 × 106 CD34+ cells/kg and 5.4 × 104 CD3+ cells/kg (recipient body weight) was infused. Donor chimerism was determined by quantitative polymerase chain reaction (PCR) amplification of informative microsatellites using DNA isolated from CD14+ and CD15+ cells that were purified using immunomagnetic beads. Cyclosporine (target trough levels of 200-350 μg/L) was administered until day 100 as prophylaxis for graft-versus-host disease (GVHD) and graft rejection. Donor lymphocyte infusions (DLIs) were performed on days +30 (2 × 106 CD3+ cells/kg), +60 (1 × 107 CD3+ cells/kg), and +100 (5.3 × 108 CD3+ cells/kg) to augment donor stem cell engraftment. Cells for the day +30 and day +60 DLIs were obtained from a dedicated lymphocyte apheresis of the donor. Cells for the day +100 DLI were obtained following G-CSF mobilization of the donor and contained 20.4 × 106 CD34+ cells/kg. Antimycobacterial therapy consisting of amikacin, azithromycin, ethambutol, levofloxacin, and rifabutin was continued throughout the SCT. Lineage-specific chimerism analysis was performed by microsatellite analysis on CD14+ monocytes, CD15+ neutrophils, and CD3+ T cells.7
M avium coculture (5 × 105 organisms/mL) was performed on normal peripheral blood mononuclear cells (1 × 106 cells/mL) in RPMI supplemented with 10% human type AB serum for 6 days. The M avium isolate used in these experiments was derived from the patient. Flow cytometric analysis was performed using anti-IFNγR1 (Genzyme, Cambridge, MA) and antiphosphorylated STAT1 (signal transducers and activators of transcription 1; New England Biolabs, Beverly, MA) as previously described.8
Results and discussion
The patient was a chronically ill-appearing 5-year-old boy with marked hepatosplenomegaly, renal insufficiency, and severe restrictive and reversible obstructive airway disease. All organ pathology was felt to be a consequence of a disseminated M avium infection originally diagnosed at 8 months of age. Blood, sputum, and urine cultures grew M avium (colonies too numerous to quantify) despite aggressive antimicrobial therapy. The patient's genetic defect has been previously described.6 In vitro analysis of peripheral blood demonstrated complete unresponsiveness to interferon-γ and absence of IFNγR1 expression by flow cytometry.
The conditioning regimen was tolerated without significant complications. The patient's absolute neutrophil count was below 0.5 × 109/L (500/μL) for 6 days and the platelet count nadir was 74 × 109/L (74 000/μL). Full donor stem cell engraftment was accomplished following the DLI given on day 100 of the transplantation (Figure 1). Coincident with engraftment was development of grade 3 GVHD involving the skin and intestine that resolved with corticosteroid and cyclosporine therapy. Despite resolution of GVHD, the patient had continued debilitating generalized inflammation characterized by cachexia, metabolic acidosis, azotemia, bronchoconstriction, splenomegaly, and chylous ascites. High-level mycobacterial bacteremia persisted. In addition, multifocal calcification developed in the spleen, atria, and cerebral vessels. Prednisolone at doses that ranged from 0.5 to 3 mg/kg was essential to maintain clinical stability. Approximately 218 days following SCT, we first observed decline of surface IFNγR1 expression (Figure 1). This was progressive and became complete (Figure 2A). Concurrent with loss of surface expression of IFNγR1 was loss of normal phosphorylation of STAT1 in response to interferon-γ stimulation in engrafted cells but not in cells collected directly from the donor (Figure 2A-D). The patient died 12 months after SCT of acute polymicrobial sepsis. An autopsy was not permitted.
Engraftment of CD14+ donor monocytes (▪), CD15+donor neutrophils (•), and percent cells positive for surface expression of interferon-γ receptor-1 on donor CD14+monocytes (▴). Graft rejection was prevented by infusion of escalating doses of donor lymphocytes. Following full donor stem cell engraftment, surface expression of the interferon-γ receptor progressively diminished. Downward pointing arrows indicate microsatellite chimerism data prior to transplantation (accompanied by the donor pattern) day 134 and day 296 following the transplantation.
Engraftment of CD14+ donor monocytes (▪), CD15+donor neutrophils (•), and percent cells positive for surface expression of interferon-γ receptor-1 on donor CD14+monocytes (▴). Graft rejection was prevented by infusion of escalating doses of donor lymphocytes. Following full donor stem cell engraftment, surface expression of the interferon-γ receptor progressively diminished. Downward pointing arrows indicate microsatellite chimerism data prior to transplantation (accompanied by the donor pattern) day 134 and day 296 following the transplantation.
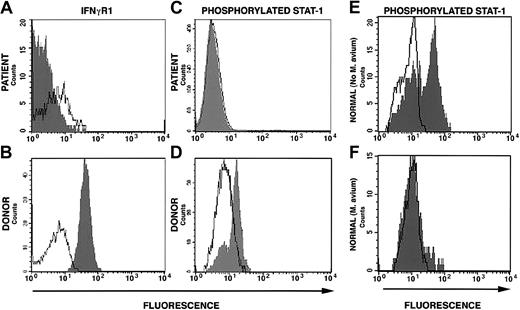
Surface interferon-γ receptor-1 expression and STAT1 phosphorylation. (A) Down-regulation of surface expression of IFNγR1 on mononuclear cells from the patient 322 days after allogeneic stem cell transplantation (160 days after complete donor stem cell engraftment). (B) Down-regulation of STAT1 phosphorylation following stimulation with IFNγ of cells from patient at the same time as in panel A. (C) Normal IFNγR1 expression on donor mononuclear cells and (D) normal STAT1 phosphorylation following IFNγ stimulation of donor cells. (E) STAT1 phosphorylation in response to IFNγ stimulation in normal cells cultured in the absence of M avium and (F) in the presence of M avium.
Surface interferon-γ receptor-1 expression and STAT1 phosphorylation. (A) Down-regulation of surface expression of IFNγR1 on mononuclear cells from the patient 322 days after allogeneic stem cell transplantation (160 days after complete donor stem cell engraftment). (B) Down-regulation of STAT1 phosphorylation following stimulation with IFNγ of cells from patient at the same time as in panel A. (C) Normal IFNγR1 expression on donor mononuclear cells and (D) normal STAT1 phosphorylation following IFNγ stimulation of donor cells. (E) STAT1 phosphorylation in response to IFNγ stimulation in normal cells cultured in the absence of M avium and (F) in the presence of M avium.
This is the first reported case of allogeneic SCT of a patient with primary immunodeficiency who is actively infected with M avium. Using a nonmyeloablative bone marrow conditioning regimen and delayed DLI, complete donor stem cell engraftment was achieved with minimal conditioning-related toxicity. Prolonged corticosteroid therapy was mandated not by refractory GVHD but by a profound systemic inflammatory state that may have been part of the appropriate donor cell response against disseminated M avium. The acquired defect in IFNγR1 surface expression and signaling through STAT1 by the previously normal donor cells was likely caused by, and contributed to, the persistent M avium infection in this patient. Coculture of mononuclear cells from 2 healthy volunteers with a concentration of M avium similar to what had been found in our patient's blood simulated the in vivo defect in STAT1 phosphorylation (Figure 2E-F). This observation is consistent with the findings of Hussain et al9 who demonstrated downregulation of STAT1 phosphorylation and interferon-γ-inducible genes following interferon-γ stimulation in murine macrophages infected with M avium. That we can replicate the in vivo finding of defective IFNγ receptor signaling in vitro argues against the possibility that the patient developed blocking antibodies to IFNγR1 following SCT. Furthermore, formation of such antibodies would be highly unlikely given the prolonged use of immunosuppressive medications.
This case demonstrated 2 important points. First, transplantation of normal functioning hematopoietic stem cells into a M avium-infected host resulted in a sustained, steroid responsive inflammatory response. In the early days following stem cell engraftment, the inflammatory response was indistinguishable from classic GVHD. What role the M avium infection played in initiating the GVHD is unknown; however, conventional GVHD treatment (corticosteroids and cyclosporine) resulted in resolution of typical manifestations. The subsequent symptomatology characterized by high fever, multifocal calcifications, nephritis, and chylous ascites are atypical for GVHD. Second, reconstitution of the interferon-γ-responsive pathways responsible for host defense was impaired by the ongoing high-grade M avium infection. Our experience suggests that restoration of normal immune function with allogeneic SCT in patients with IFNγR1 deficiency may not be possible in the setting of disseminated M avium infection. This case has important implications for other SCT candidates with mycobacterial infections as a consequence of primary or secondary (chemotherapy-induced) immunodeficiency. Further studies are needed to characterize the mechanism by which M avium affects interferon-γ signaling in human cells.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2003-04-1268.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal