Abstract
Recent studies in mice have shown that although interleukin 15 (IL-15) plays an important role in regulating homeostasis of memory CD8+ T cells, it has no apparent function in controlling homeostatic proliferation of naive T cells. We here assessed the influence of IL-15 on antigen-independent expansion and differentiation of human CD8+ T cells. Both naive and primed human T cells divided in response to IL-15. In this process, naive CD8+ T cells successively down-regulated CD45RA and CD28 but maintained CD27 expression. Concomitant with these phenotypic changes, naive cells acquired the ability to produce interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α), expressed perforin and granzyme B, and acquired cytotoxic properties. Primed CD8+ T cells, from both noncytotoxic (CD45RA-CD27+) and cytotoxic (CD45RA+CD27-) subsets, responded to IL-15 and yielded ample numbers of cytokine-secreting and cytotoxic effector cells. In summary, all human CD8+ T-cell subsets had the ability to respond to IL-15, which suggests a generic influence of this cytokine on CD8+ T-cell homeostasis in man. (Blood. 2003;102:2541-2546)
Introduction
The ability of the specific immune system to respond to a variety of pathogens requires the continuous presence of a pool of T cells expressing a diverse array of T-cell receptors. T cells are produced in the thymus and subsequently transported to the periphery. Studies in humans have shown that in the absence of antigenic stimulation, so-called naive T cells have a very slow turnover rate and may be preserved for years.1 In contrast, T cells that have been in contact with antigen (primed or memory T cells) show a significantly higher cell division rate.1 Homeostasis of the peripheral T-cell pool not only depends on the production of new T cells by the thymus but also on the maintenance of both naive and primed T cells through the action of cytokines that not only induce proliferation but also promote cell survival.2
The cytokines interleukin 7 (IL-7) and IL-15, both binding to the common γ-chain-containing cytokine receptors, have been implicated in regulating T-cell homeostasis in mice. IL-7 appears to be the key cytokine involved in the maintenance of mouse naive CD8+ T-cell numbers.3-5 On the basis of several complementary observations, it has been implied that IL-15, a 14- to 15-kDa cytokine belonging to the 4 α-helix-bundle cytokine family, may exert its prime function on memory CD8+ T cells.5-11 First, mice deficient in either IL-15 or IL-15 receptor (R) α show a marker reduction in CD8+ T-cell numbers in the lymph nodes and in the frequency of primed (CD44bright/CD62Llow) T cells.2,11,12 On the other hand, an increase in CD44high CD8+ memory T cells is found in IL-15 transgenic mice,13 and T cells with a memory phenotype divide after IL-15 injection.6 Although these observations strongly associate IL-15 with the maintenance of memory CD8+ T cells, it should be noted that IL-15Rα-/- mice also have a marked reduction in numbers of single-positive CD8+ T cells in the thymus,13 suggesting effects of IL-15 on non-antigen-primed T cells.
Recently, Geginat et al14 have shown that within the human CD4+ compartment, only effector-memory CD4+ T cells were able to divide upon IL-15 stimulation, whereas Kanegane and Tosato15 have reported that both primed (CD45R0+) and naive (CD45RA+) CD8+ T cells proliferate in response to IL-15. The latter observation, however may be complicated by the fact that CD45RA positivity is not a reliable marker for naive CD8+ T cells since it has now been established that CD45RA+CD27- T cells are in fact primed T cells.16-18 We here evaluated the role of IL-15 in the homeostasis of human naive CD8+ T cells. We found that naive CD45RA+CD27+ T cells, obtained from either adult or cord blood, proliferated in response to IL-15 stimulation and during this process changed their phenotype and acquired effector functions.
Materials and methods
Reagents and mAbs
The monoclonal antibodies (mAbs) peridinin chlorophyll A protein (PerCP)-conjugated CD3, allophycocyanin (APC)-conjugated CD3, PerCP-conjugated CD8, PerCP-conjugated CD4, phycoerythrin (PE)-conjugated CD16, PE-conjugated CD27, PE-conjugated CD28, PE-conjugated CD62L, biotin-conjugated CD122, biotin-conjugated CD132, anti-CCR7, biotinconjugated rat antimouse immunoglobulin M (IgM), APC-conjugated streptavidin, PE-conjugated anti-interferon γ (IFN-γ), PE-conjugated anti-tumor necrosis factor α (TNF-α), and PE-conjugated gamma-1 were purchased from Becton Dickinson (San Jose, CA). Biotin-conjugated polyclonal anti-IL-15Rα was obtained from R&D Systems (Europe). Isotype controls consisted of mouse IgG1, rat IgG2b, and goat IgG (kind gifts from Dr Ramon Arens). APC-conjugated CD8, PE-conjugated CD45RA, and APC-conjugated CD56 mAbs were obtained from Immunotech Coulter (Miami, FL). PE-conjugated antiperforin mAb was obtained from Holzel Diagnostika (Koln, Germany). PE-conjugated anti-granzyme B mAb was purchased from Sanquin (Amsterdam, the Netherlands). Microbeads coated with human CD8 (CD8 microbeads) were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany).
Cell preparation
Human peripheral blood mononuclear cells (PBMCs) from buffy coats of healthy donors were isolated by Ficoll-Isopaque density gradient centrifugation (Nycomed; Pharma, Oslo, Norway). Umbilical cord blood mononuclear cells (UCBMCs) were used to obtain naive T cells. The current study was approved by the local medical ethics committee of the Academic Medical Center.
CD8+ purification and sorting
CD8+ T cells were purified either from total PBMCs or UCBMCs by positive selection using the MACS system. Briefly, PBMCs were stained with CD8 microbeads for 15 minutes at 8°C. After washing, cells were resuspended in incubation buffer and enrichment was performed with the VarioMACS magnet according to manufacturer's instructions. The sample purity was assessed by fluorescence-activated cell sorter (FACS) with APC-conjugated CD8 and PerCP-conjugated CD3 mAbs (purity, >95% CD3+ CD8+). For subset purification, purified peripheral blood CD8+ T cells were stained with PE-conjugated CD45RA and fluorescein isothiocyanate (FITC)-conjugated CD27 mAbs and sorted into CD45RA+CD27+, CD45RA+CD27-, and CD45RA-CD27+ populations on a FACS sorter (Moflow; Dako-Cytomation, Carpinteria, CA). The purity assessed by FACS was higher than 95%.
CFSE labeling
UCBMCs or purified CD8+ T cell subsets were pelleted and resuspended in phosphate-buffered saline (PBS) at a final concentration of 5 to 10 × 106 cells/mL. Next, cells were labeled with 2.5 μM (final concentration) of 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Europe BV, Leiden, the Netherlands) in PBS for 10 minutes at 37°C. Cells were washed and subsequently resuspended in Iscoves modified Dulbecco medium (IMDM) supplemented with L-glutamine, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Biowhittaker, Verviers, Belgium) containing 10% human pool serum (HPS; Biowhittaker), streptamycin (100 ng/mL; Gibco BRL, Paisley, Scotland), penicillin (10 U/mL; Yamanouchi, Pharma, Leiderdorp, the Netherlands), and 3.57 × 10-4 percent (vol/vol) β-mercapto ethanol culture medium (Merck, West Point, PA).
Cell culture
CFSE-labeled UCBMCs, or purified CD8+ T-cell subsets, were cultured for 7 days at 37°C in 5% CO2 atmosphere, in culture medium in the presence or absence of IL-15 (R&D Systems). The precursor frequency (percentage of cells in the initial population that undergone one or more divisions after culture) was calculated as follows: [∑n≥1(Pn/2n)]/[∑n≥0(Pn/2n)], where n is the division number that cells have gone through and Pn is the number of cells in division n.19
FACS analysis
CFSE-labeled cells stained with PE-, PerCP-, or APC-labeled mAbs were analyzed on a FACSCalibur (Becton Dickinson). The cytokine-producing capacity was assessed after phorbol myristate acetate (PMA; 2 ng/mL) and ionomycin (1 μM/mL) stimulation in the presence of brefeldin A (1 μM; all from Sigma Chemical, St Louis, MO) for 4 hours. Cells were fixed in 2% paraformaldehyde, permeabilized with PBS containing 0.5% (wt/vol) bovine serum albumin (BSA), and 0.5% saponin followed by staining with PE-labeled anti-IFN-γ, anti-TNF-α, and a control mAb (gamma-1). Intracellular detection of granzyme B and perforin was performed as described previously.16
Redirected cytotoxicity assay
The cytotoxic activity of (un)stimulated CD8+ T cells was determined in a 4-hour 51Cr release assay using Fc receptor (FcR)γ-bearing P815 cells as targets. Targets cells were labeled with 200 μCi (7.4 MBq) of 51Cr (Amersham, Arlington Heights, IL) in 100 μL of fetal calf serum (FCS) for 1 hour at 37°C. After 3 washes, target cells were plated in 96-well round-bottom plates at 5000 cells per well. CD8+ T cells were mixed with target cells at effector-to-target ratios of 20:1, 10:1, 5:1, and 1:1, in the presence or absence of 5 μg/mL anti-CD3 mAbs (clone CD3.4/1; Sanquin, Amsterdam, the Netherlands). After 4 hours of incubation, supernatants were harvested and counted in a gamma counter. Specific lysis was calculated according to the following formula: % specific release = [(experimental release - spontaneous release)/(maximum release - spontaneous release)] × 100.
Results
Human naive CD8+ T cells can respond to IL-15
A previous study suggested that both naive and memory human CD8+ T cells are able to respond to IL-15 in a T-cell receptor (TCR)-independent way.15 However, in that study these 2 populations were separated based on the expression of 2 different isoforms of leukocyte common antigen (CD45) and therefore did not take into account the notion that primed CD8+ T cells with abundant effector functions can express CD45RA.16-19
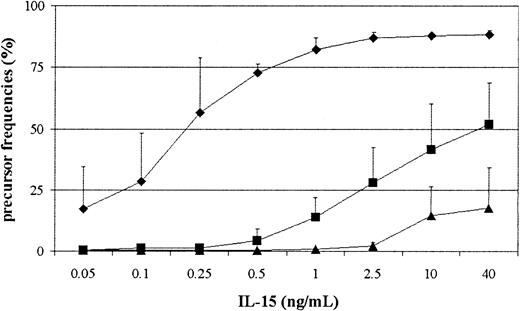
To assess whether truly naive CD8+ T cells were indeed able to respond to IL-15, we used UCBMCs as a source of human naive lymphocytes. In parallel we measured the response of naive CD4+ T and natural killer (NK) cells. UCBMCs were labeled with CFSE and stimulated with IL-15 for 7 days with graded concentrations of IL-15. As shown in Figure 1, umbilical cord NK cells responded to low doses of IL-15 and most NK cells divided at a concentration of 1 ng/mL. Naive CD8+ T cells needed higher concentrations of IL-15 to proliferate and at 40 ng/mL around 50% of the cells within this subset responded. Still, also at lower concentrations (1-2.5 ng/mL), 20% of the cells underwent at least one or more rounds of division. Finally, we also observed that naive CD4+ T cells proliferated, although the amount of cells entering cell cycle was much lower in comparison with the CD8+ T-cell response. The differences among the different lineages were the same when PBMCs from adults were used (data not shown), which indicated that the ability to proliferate in response to IL-15 is independent from prior antigenic challenge.
Naive CD8+, CD4+T cells, and NK cells require different amounts of IL-15 to expand. UCBMCs were labeled with CFSE and cultured in the presence of variable concentrations of IL-15 for 7 days, followed by FACS analysis. NK cells (♦) were gated on CD3-CD16+CD56+, CD4+ T cells (▴) on CD3+CD4+, and CD8+ T cells (▪) on CD3+CD8+. The percentage of cells in the initial population that had undergone one or more rounds of division after IL-15 stimulation was calculated as described in He et al.19 Data depicted are the mean ± SD from 3 experiments performed.
Naive CD8+, CD4+T cells, and NK cells require different amounts of IL-15 to expand. UCBMCs were labeled with CFSE and cultured in the presence of variable concentrations of IL-15 for 7 days, followed by FACS analysis. NK cells (♦) were gated on CD3-CD16+CD56+, CD4+ T cells (▴) on CD3+CD4+, and CD8+ T cells (▪) on CD3+CD8+. The percentage of cells in the initial population that had undergone one or more rounds of division after IL-15 stimulation was calculated as described in He et al.19 Data depicted are the mean ± SD from 3 experiments performed.
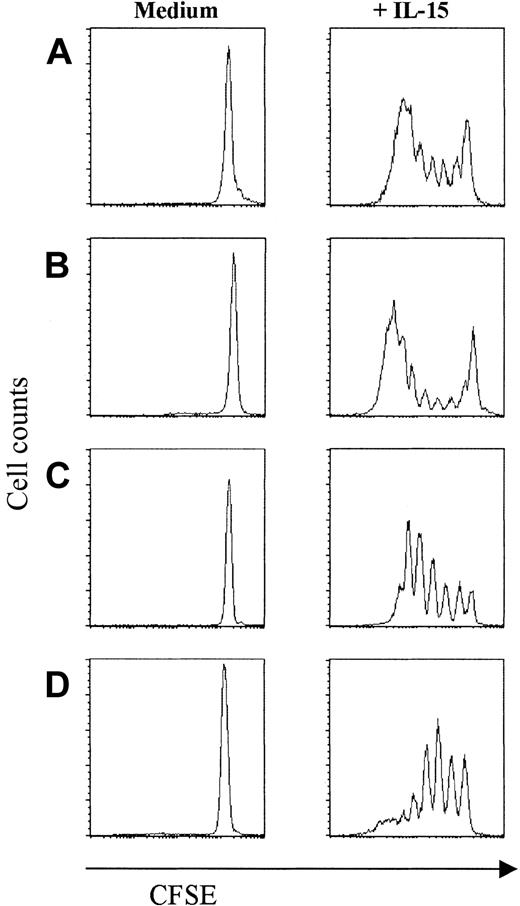
Lodolce et al2 have shown that in vivo responses of CD8+ T cells to IL-15 depend on the expression of IL-15Rα on hematopoietic cells but not necessarily on IL-15Rα expressed on CD8+ T cells suggesting an interplay between different cell types for the bystander proliferation. As we found that umbilical cord NK cells are by far the most IL-15-sensitive cell type, we questioned whether the IL-15-induced proliferation of naive CD8+ T cells could be indirectly influenced by NK cell-derived cytokines. To this end, CD8+ T cells were purified from UCBMCs using CD8+ magnetic beads. Additionally, CD8+ T cells from adult blood were sorted in naive (CD27brightCD45RAbright) and 2 different primed populations (ie, cytotoxic, CD27-CD45RAbright; and noncytotoxic, CD27+CD45RAdull/neg) T cells, to assess possible differences in IL-15 responses between these subsets. Purified naive CD8+ T cells from umbilical cord responded strongly to IL-15 (precursor frequency = 41.5%; Figure 2A). Also in all subsets obtained from adult blood we detected proliferation (Figure 2B-D), although the percentage of cells responding to IL-15 was highest in the CD27-CD45RAbright subset (precursor frequency = 55%; Figure 2C). Thus, naive human CD8+ T cells expanded directly to IL-15 stimulation in an antigen-independent way.
Purified naive and cytotoxic- and noncytotoxic-primed CD8+ T cells proliferate upon IL-15 stimulation. Sorted populations were labeled with CFSE and stimulated for 7 days with IL-15 (10 ng/mL), followed by FACS analysis. (A) UCB naive CD8+ T cells. (B) Peripheral naive CD8+ T cells. (C) Cytotoxic CD8+ T cells (CD27-CD45RAbright). (D) Noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg). Data are one representative experiment of 4 performed.
Purified naive and cytotoxic- and noncytotoxic-primed CD8+ T cells proliferate upon IL-15 stimulation. Sorted populations were labeled with CFSE and stimulated for 7 days with IL-15 (10 ng/mL), followed by FACS analysis. (A) UCB naive CD8+ T cells. (B) Peripheral naive CD8+ T cells. (C) Cytotoxic CD8+ T cells (CD27-CD45RAbright). (D) Noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg). Data are one representative experiment of 4 performed.
Changes in cell surface phenotype in IL-15-stimulated CD8+ subsets
Recently Liu et al20 have concluded on the basis of gene expression profiles that IL-15 mimics TCR stimulation in CD8+ memory T cells. Particularly, both stimuli gave similar responses with respect to proliferation and effector functions. Therefore, having found that all CD8+ T-cell subsets were able to respond to IL-15, we examined whether IL-15-induced activation would induce cellular differentiation that can be characterized by changes at the cell surface.
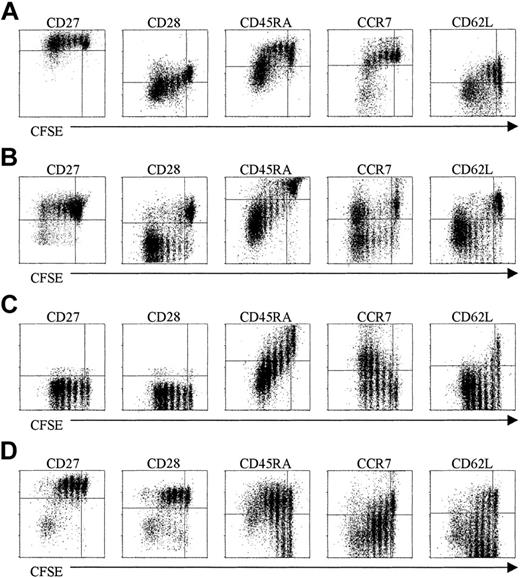
The sorted naive CD8+ T-cell population was CD27bright CD45RAbrightCD28+CCR7+CD62L+ (data not shown). After stimulation, there was a successive down-modulation of CD45RA but CD27 remained largely unchanged (Figure 3A-B). Expression of CD28, CCR7, and CD62L was down-regulated in cells that underwent more than 3 to 4 rounds of divisions (Figure 3A). These latter data suggest that after contact with IL-15 for long periods the naive population might alter its recirculation ability.
Phenotypic properties of purified subsets after IL-15 stimulation. Purified subsets were labeled with CFSE and stimulated with IL-15 (10 ng/mL). After 7 days the cells were stained for the indicated cell surface markers, followed by FACS analysis. (A) UCB naive CD8+ T cells. (B) Peripheral naive CD8+ T cells. (C) Cytotoxic CD8+ T cells (CD27-CD45RAbright). (D) Noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg). Data are one representative experiment of 4 performed.
Phenotypic properties of purified subsets after IL-15 stimulation. Purified subsets were labeled with CFSE and stimulated with IL-15 (10 ng/mL). After 7 days the cells were stained for the indicated cell surface markers, followed by FACS analysis. (A) UCB naive CD8+ T cells. (B) Peripheral naive CD8+ T cells. (C) Cytotoxic CD8+ T cells (CD27-CD45RAbright). (D) Noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg). Data are one representative experiment of 4 performed.
After culturing in the presence of IL-15, the cytotoxic population (CD27-CD45RAbrightCD28-CCR7-CD62L+/-; data not shown) remained CD27- and CD28- indicating that the expression of these markers once lost, is not regained during cytokine-driven proliferation (Figure 3C). There was a down-modulation of CD45RA as in naive population. Surprisingly, the expression of CCR7 was regained in a fraction of the cells but was not accompanied by CD62L re-expression (Figure 3C). Nevertheless the re-expression of CCR7 in cells that had lost this receptor suggest that after contact with IL-15 some cells might reacquire the ability to respond to the CCR7 ligands, secondary lymphoid tissue chemokine (SLC), and macrophage inflammatory protein 3β (MIP-3β).
In the noncytotoxic fraction (CD27+CD45RA-CD28+CCR7+/- CD62L+/-, data not shown), the expression of CD27 and CD28 was maintained. Based on the subset classification proposed by Lanzavecchia and colleagues,21 memory cells after contact with IL-15 shifted to an effector phenotype, as shown by the down-regulation of CCR7 and up-regulation of CD45RA. CD62L expression was down-modulated in the cells that underwent more than 3 to 4 rounds of division (Figure 3D).
IL-15 enhances the expression of IL-2/15Rβ in naive CD8+ T cells
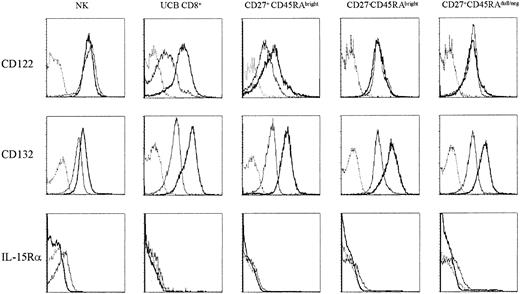
IL-15 binds to a specific trimeric receptor consisting of IL-15Rα, β, and γc chains. It shares with IL-2 two signaling receptor subunits, IL-2/15Rβ (CD122) and IL-2/15Rγ (CD132), using the common JAK3/STAT5 (Janus kinase 3/signal transducers and activators of transcription 5) signaling pathway.22-24 In mice, CD122 expression is higher in memory relatively to naive CD8+ T cells,7 which favors the response of memory cells to IL-15. We examined the expression of IL-15 receptor components by FACS analysis on resting T cells and after IL-15 stimulation. In resting cells the expression of CD122 was lower in the naive population in comparison with cytotoxic and noncytotoxic cells and with NK cells. After stimulation, we detected a pronounced up-regulation of CD122 in the naive population to levels comparable to the ones obtained in primed subsets (Figure 4 top row). CD132 is constitutively expressed on human lymphocytes. However after contact with IL-15 there was an increased expression of this receptor in all subsets (Figure 4 middle row). IL-15Rα was almost undetectable on T-cell subsets (slightly higher in primed subsets in comparison with naive cells), NK cells being the only population where it was clearly detected. After stimulation IL-15Rα was no longer detected in any of the lymphocyte subsets (Figure 4 bottom row).
Up-regulation of IL-2/15Rβ (CD122) on naive CD8+T cells and of IL-2/15 Rγ (CD132) on all subsets after IL-15 stimulation. The purified subsets were labeled with CFSE and stimulated with IL-15 (10 ng/mL) for 7 days. NK cells gated on CD3-CD16+CD56+ (NK), CD8+ T cells purified from UCB (UCB CD8+), peripheral naive CD8+ T cells (CD27+CD45RAbright), cytotoxic CD8+ T cells (CD27-CD45RAbright), and noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg) were stained for CD122 (IL-2/15 Rβ), CD132 (IL-2/15 Rγ), and IL-15Rα followed by FACS analysis. Dashed line indicates isotype control; thin line, resting cells; and bold line, IL-15-stimulated cells. Data are one representative experiment of 3 performed.
Up-regulation of IL-2/15Rβ (CD122) on naive CD8+T cells and of IL-2/15 Rγ (CD132) on all subsets after IL-15 stimulation. The purified subsets were labeled with CFSE and stimulated with IL-15 (10 ng/mL) for 7 days. NK cells gated on CD3-CD16+CD56+ (NK), CD8+ T cells purified from UCB (UCB CD8+), peripheral naive CD8+ T cells (CD27+CD45RAbright), cytotoxic CD8+ T cells (CD27-CD45RAbright), and noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg) were stained for CD122 (IL-2/15 Rβ), CD132 (IL-2/15 Rγ), and IL-15Rα followed by FACS analysis. Dashed line indicates isotype control; thin line, resting cells; and bold line, IL-15-stimulated cells. Data are one representative experiment of 3 performed.
IL-15 induces IFN-γ and TNF-α production in naive CD8+ T cells
A prime feature of the CD8+ T-cell activation pathway is the synthesis of proinflammatory cytokines, such as IFN-γ and TNF-α. In previous studies we have shown that human-primed CD8+ T cells abundantly produce IFN-γ and TNF-α after PMA/ionomycin activation, whereas naive cells synthesize only low amounts of these cytokines.16,18 After a 7-day culture period in the presence of IL-15, the production of IFN-γ and TNF-α was measured intracellulary after PMA/ionomycin activation in stimulated CD8+ T-cell subsets. IL-15 strongly induced TNF-α (producing cells, 30%-53%, n = 4 donors) and IFN-γ production (producing cells, 12%-45%, n = 4 donors) in purified naive CD8+ T cells from umbilical cord blood. Highest production was found in the cells with strongest CFSE dilution (Figure 5A). Furthermore, naive T cells purified from adult blood acquired a strong ability to produce these cytokines (Figure 5B). In contrast to the naive populations, both primed T-cell subsets contained ample cytokine-secreting cells in the undivided fraction that was maintained in the dividing ones (Figures 5C-D).
Functional properties of purified subsets after IL-15-stimulation. The purified subsets were labeled with CFSE and stimulated with IL-15 (10 ng/mL). After 7 days the cells were restimulated with PMA/ionomycine and brefeldin A and stained intracellular for indicated cytokines followed by FACS analysis (gamma-1 [g-1] used as control mAbs). (A) CD8+ T cells purified from UCB. (B) Peripheral naive CD8+ T cells. (C) Cytotoxic CD8+ T cells (CD27-CD45RAbright). (D) Noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg). Data are one representative experiment of 4 performed.
Functional properties of purified subsets after IL-15-stimulation. The purified subsets were labeled with CFSE and stimulated with IL-15 (10 ng/mL). After 7 days the cells were restimulated with PMA/ionomycine and brefeldin A and stained intracellular for indicated cytokines followed by FACS analysis (gamma-1 [g-1] used as control mAbs). (A) CD8+ T cells purified from UCB. (B) Peripheral naive CD8+ T cells. (C) Cytotoxic CD8+ T cells (CD27-CD45RAbright). (D) Noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg). Data are one representative experiment of 4 performed.
IL-15 induces cytotoxic effector function in naive CD8+ T cells
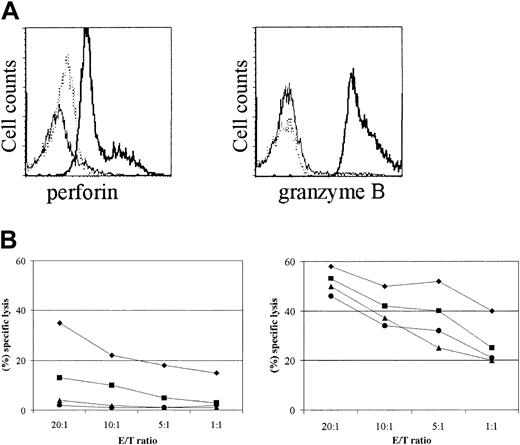
Upon IL-15 stimulation, naive CD8+ T cells acquired a primed phenotype, indicated by changes in their cell surface phenotype and by the ability to produce proinflammatory cytokines. We next assessed whether IL-15 also induced the expression of cytotoxic effector molecules in purified naive cord blood CD8+ T cells. Indeed, strong induction of perforin and granzyme B was found in naive CD8+ T cells upon IL-15 activation (Figure 6A). Moreover, we determined cytotoxic activity against P815 cells in an antibody-dependent cell-mediated cytotoxicity assay. In contrast to unstimulated cells, naive CD8+ T cells, either from umbilical cord or peripheral blood, stimulated with IL-15 exhibited potent cytotoxic activity (Figure 6B). Additionally, the cytotoxic (CD27- CD45RAbright) and noncytotoxic (CD27+CD45RAdull/neg) CD8+ T-cell-primed subsets showed an enhanced cytotoxic potential after IL-15 stimulation (Figure 6B). Hence, IL-15 effectively induces phenotypic and functional changes in naive CD8+ T cells.
Naive CD8+ T cells acquire cytotoxic properties upon IL-15 stimulation. The purified subsets were stimulated with IL-15 (10 ng/mL) for 7 days. (A) Naive CD8+ T cells were stained intracellular for perforin and granzyme B, followed by FACS analysis. Dotted line indicates control mAbs; thin line, resting; and bold line, IL-15-stimulated cells. (B) Purified CD8+ T-cell subsets were tested in a redirected cytotoxicity assay. Targets (P815 cells) were mixed with CD8+ T cells from UCB (•), peripheral naive CD8+ T cells (▴), cytotoxic CD8+ T cells (CD27-CD45RAbright) (♦), and noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg) (•) in the presence of anti-CD3 mAbs, resting (left panel) or IL-15-stimulated (right panel). Resting and IL-15-stimulated purified subsets did not lyse P815 cells in the absence of CD3 mAbs (data not shown). Data are one representative experiment of 3 performed. E/T ratio indicates effector-to-target ratio.
Naive CD8+ T cells acquire cytotoxic properties upon IL-15 stimulation. The purified subsets were stimulated with IL-15 (10 ng/mL) for 7 days. (A) Naive CD8+ T cells were stained intracellular for perforin and granzyme B, followed by FACS analysis. Dotted line indicates control mAbs; thin line, resting; and bold line, IL-15-stimulated cells. (B) Purified CD8+ T-cell subsets were tested in a redirected cytotoxicity assay. Targets (P815 cells) were mixed with CD8+ T cells from UCB (•), peripheral naive CD8+ T cells (▴), cytotoxic CD8+ T cells (CD27-CD45RAbright) (♦), and noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg) (•) in the presence of anti-CD3 mAbs, resting (left panel) or IL-15-stimulated (right panel). Resting and IL-15-stimulated purified subsets did not lyse P815 cells in the absence of CD3 mAbs (data not shown). Data are one representative experiment of 3 performed. E/T ratio indicates effector-to-target ratio.
Discussion
Herein we have demonstrated that naive CD8+ T cells are responsive to IL-15 in an antigen-independent way, with concomitant phenotypic changes and acquisition of functional properties that are difficult to distinguish from antigen-primed T cells. For most experiments we have used a dose of IL-15 (10 ng/mL) that is comparable to or lower than the doses used in other reports.6,14,15,25,26 When UCBMCs or PBMCs were used, NK cells were by far the most IL-15-sensitive cellular subset, responding to very low cytokine concentrations (0.5-1 ng/mL). The huge difference in IL-15 sensitivity between NK and T cells raises 2 important issues. First, as has been elegantly described by Lodolce et al2 using a mouse model, the effect of IL-15 on naive CD8+ T cells in these in vitro cultures could be indirect, via the induction of cytokines and possible other factors by NK cells, rather than direct. However, our studies with purified CD8+ subsets showed that the response of these cells is similar to that of the CD8+ T cells in the unfractionated cultures (compare Figures 1 and 2). Second, an obvious question that can be raised is whether CD8+ T cells in vivo, in competition with NK cells that are apparently much more apt to respond to IL-15, would be in contact with such concentrations that proliferation and phenotypic changes could be induced. We would favor the possibility that the maintenance of CD8+ T cells would involve additional signals, which might regulate the ability of CD8+ T cells to use IL-15.
IL-15 is expressed by a large range of cell types, from bone marrow stroma cells and epithelial cells to monocytes.22,27-29 Importantly, it is highly produced in the gut microenvironment during stress or infection,28 which raises the possibility that CD8+ homeostasis is specifically regulated under conditions of immune activation. Moreover, IL-15 is bioactively expressed at the cell surface by human spleen-derived fibroblasts30 and it can be presented at the cell surface of activated monocytes, thereby activating target cells expressing either intermediate or high-affinity IL-15R in trans.31 These observations therefore suggest that IL-15, associated with cell surface, signals in a cell contact-dependent manner and while recirculating through the secondary lymphoid organs, naive CD8+ T cells may be stimulated through this mechanism.
As shown by others15,32 we observed that NK cells as well as CD8 T cells express the intermediate-affinity IL-15R (β and γ chains), although the expression of the β chain as lower in naive population. IL-15Rα was only clearly detected in NK cells, which may explain the ability of NK cells to respond to low doses of IL-15 (Figure 1). Upon activation IL-15Rα expression was lost from all subsets. It has been shown that IL-15 binds to IL-15Rα with high affinity and is subsequently internalized and recycled.31 The inability to detect IL-15Rα after IL-15 stimulation may therefore be due to receptor occupancy and/or redistribution of IL-15Rα to the intracellular compartment.
It has been shown by different groups that naive CD8+ T cells undergoing homeostatic-driven proliferation showed a phenotypic and functional conversion to memory-type cells.33-35 Importantly, these phenotypic and functional alterations may revert when homeostatic proliferation stops.34 Goldrath et al34 showed that naive CD8+ T cells undergoing homeostatic proliferation up-regulate CD122 (IL-2/15 Rβ), which could place naive T cells under the control of IL-15. Zhang et al6 have shown that the expression of IL-2/15Rβ was higher on murine memory CD8+ T cells, the only subset able to proliferate upon stimulation via IL-15. However our data indicate that the response to IL-15 is not limited by the level of IL-2/15Rβ expression as (1) in the naive population the expression of this receptor is lower in comparison to the effector and memory populations and (2) no gross differences between UBCMCs (containing only naive T cells) and PBMCs (containing various subsets of primed T cells) in the response to IL-15 was evident (Figure 1; data not shown). Rather, all subsets responded to IL-15 and in this process naive cells strongly up-regulate CD122. These findings suggest that the relatively low amount of CD122 on naive human T cells is sufficient to initiate responses to IL-15.
In mice models, it has been shown that IL-7 is the key cytokine in controlling survival and homeostatic proliferation of naive T cells working simultaneously with low levels of TCR signaling from contact with self-major histocompatibility complex (MHC)/peptide ligands,3-5 whereas it has been stressed that IL-15 is important for the generation and maintenance of memory CD8+ T cells.3-11,36 Still, IL-15Rα-/- mice also have a defect in generating single-positive CD8+ T cells in the thymus and a marked CD8+ T-cell lymphopenia is found in these animals, which can unlikely be solely attributed to diminution of effector/memory CD8+ T cells.12 Additionally, the interpretation of the studies on IL-15-induced proliferation of effector-memory cells in vivo are complicated by the fact that IL-15 induced rapid phenotypic changes and as a result it may be difficult to distinguish expanded antigen-primed effector-memory cells from cells that have acquired such a phenotype through the effect of IL-15 or other homeostatic proliferation signals. At very least, our findings do suggest that in humans IL-15 may participate in the maintenance and homeostatic control of human naive CD8+ T-cell pool. In contrast, and in agreement with Geginat et al,14 the effect of IL-15 on naive CD4+ T cells appears to be low with only 10% of the cells responding (10 ng/mL; Figure 1).
In a recent report, Kim et al25 have shown that IL-15 combined with a low dose of IL-12 induces a CD28- phenotype in human cord blood cells but in this report no information was provided on the response of cord blood T cells to IL-15 alone. In agreement with our findings, their study demonstrated that naive T cells become rapidly cytotoxic upon IL-15 stimulation. The observation that IL-15 by it self can induce effector T-cell function is supported by functional and DNA microarray data of Liu et al20 that showed that IL-15 mimics TCR activation in the cellular proliferation, gene expression profile, and induction of cytotoxic activity in memory CD8+ T cells.
The finding that cytokines like IL-15 alter function and phenotype of responding T cells precludes attributing particular changes related to effector or memory T-cell formation directly to antigen-specific responses. Cell cycle progression is believed to relieve epigenetic repression of certain genes allowing their expression37 and was previously seen in relation to signals specifically derived from the CD3/TCR complex. In light of our data and those of Kim et al,25 it would be interesting to investigate whether IL-15-induced proliferation would induce changes in chromatin organization facilitating expression of cytokines and cytotoxic effector molecules in naive cells.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2003-01-0183.
Supported by Foundation for Science and Technology (FCT) Portugal grant no. SFR/BD/2740/200 (N.L.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very thankful to Drs Eric Eldering, Laila Gamadia, and Michiel van Oosterwijk for discussion and comments on the manuscript. We thank Paul Baars and Ester Remmerswaal for all technical support.

![Figure 5. Functional properties of purified subsets after IL-15-stimulation. The purified subsets were labeled with CFSE and stimulated with IL-15 (10 ng/mL). After 7 days the cells were restimulated with PMA/ionomycine and brefeldin A and stained intracellular for indicated cytokines followed by FACS analysis (gamma-1 [g-1] used as control mAbs). (A) CD8+ T cells purified from UCB. (B) Peripheral naive CD8+ T cells. (C) Cytotoxic CD8+ T cells (CD27-CD45RAbright). (D) Noncytotoxic CD8+ T cells (CD27+CD45RAdull/neg). Data are one representative experiment of 4 performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2003-01-0183/6/m_h81934985005.jpeg?Expires=1769095315&Signature=oFJfaGIJzl~AIRW8sGEMEBA3CPvtS-VZU225cJLn8Wt4PBpprHzs62pjqEo7BqgAkHA~uEWsqHEjgzTjnNtgz61iGuc1OnPEoALh~oiKBj5Mig8xSHIGzW3C3B-tl7n9EkggPXdc49HvWnqmYUc5~sWT16nlQ~n3t-dZDNTJ~0jql3xk3HWXoMdjdQGhIJFWrZ9bSHyarB72PHiHGjEvnGxm5kdyEhsqIaa8FyT4OMcLYbbAEjaFj1xr-Oqdp37lwtyzbnUijTTmV2d7zvJAqabPjEW9ADBmBkOY1KjsalwpBQV85cJcMa-2p1DPgVIg-b1d1T7llq7cnRFLQEiTBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal