Abstract
Apoptosis or programmed cell death plays an important role in a wide variety of physiologic processes and is regulated by proteins of the Bcl-2 family consisting of both antiapoptotic and proapoptotic factors. The direct involvement of the Bcl-2 protein family in the process of mast cell apoptosis has not been clarified. In the present work we have used a single-chain antibody (scFv) raised against Bcl-2 derived from a semisynthetic human phage-display antibody library. The addition of TAT sequence, which is responsible for translocation through the membrane, endows the anti-Bcl-2-scFv with the ability to penetrate living cells. Moreover, it specifically neutralizes Bcl-2 intracellularly by binding to the BH1 domain and eradicates its antiapoptotic activity in 2 types of mast cells and in a human breast cancer cell line. (Blood. 2003;102:2506-2512)
Introduction
Apoptosis or programmed cell death plays an important role in a wide variety of physiologic processes. The mechanism of this process is highly conserved throughout all eukaryotic cells.1,2 The Bcl-2 family of proteins are central regulators of apoptotic death consisting of both antiapoptotic (Bcl-2, BclXL, Mcl-1, and A1/Bfl-1) and proapoptotic (BclXS, Bax, Bak, and Bok/Mtd) factors.3,4 The ability of many Bcl-2 family members to form homo- as well as heterodimers through their Bcl-2 homology (BH) domains is important for activation of specific functions such as causing changes in mitochondrial membrane potential and initiating the process of apoptosis, and also for neutralizing these functions in the cells.2,5,6 Most pro-survival factors, which can inhibit apoptosis in response to a wide variety of cytotoxic damages, contain BH1, BH2, and BH3 domains and those most similar to Bcl-2 have all 4 BH domains.2 It is thought that the relative ratio of antiapoptotic versus proapoptotic dimers plays a pivotal role in determining the resistance of a cell to apoptosis.
The biochemical events leading to apoptosis in mast cells, which are the major cellular players in allergy, have recently started to be explored. The number of mast cells in tissues has been found to be constant under normal conditions, which probably reflects an equilibrium among cell proliferation, migration, and death.7 The involvement of the Bcl-2 family proteins in human mast cell survival was indicated by the observation that stem cell factor induced elevation of Bcl-2 and BclXL in these cells.7 Several studies on mast cell leukemia show overexpressed levels of Bcl-2, pointing out the importance of this antiapoptotic protein in mast cell homeostasis.8
Other experiments reveal that upon stimulation via the aggregation of high-affinity immunoglobulin E (IgE) receptors (FcϵRI), mouse mast cells synthesize the antiapoptotic component A1 rather than Bcl-2.9 However, it is still uncertain whether A1 plays a direct role in mediating cell survival in these cells. Later, it was found that only monomeric IgE binding to its mast cell high-affinity receptor (FcϵRI) suppresses the apoptosis induced after growth factor deprivation.10 This induction of mast cell survival was observed without detectable changes in the expression of other members of the Bcl-2 family of proteins. Thus, from all the data mentioned above it remains unclear whether Bcl-2 is indeed involved in promoting mast cell survival.
In the present work we addressed this topic by using a semisynthetic human phage-display antibody library.11 Phage-display libraries of peptides have been used since mid 1980 as a highly effective method for selection of interacting partners.12 The display of antibody fragments as single-chain Fv (scFv) on phage and the subsequent antigen-driven selection provided a new way for the development of human monoclonal antibodies with potential therapeutic applications.13
After selection against recombinant Bcl-2, specific anti-Bcl-2-scFv was isolated and modified by genetic engineering. Moreover, we have found that anti-Bcl-2-scFv linked to the TAT peptide sequence penetrates living cells, binds specifically to Bcl-2 at a region including BH1 domain, and eradicates the antiapoptotic activity of the cellular protein in 2 types of mast cells and in a human breast cancer cell line.
Materials and methods
Cell culture
Rat basophilic leukemia (RBL) and breast cancer (MCF7) cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, antibiotic mix (100 U/mL penicillin, 100 μg/mL streptomycin), 2 mM nonessential amino acids (Biological Industries, Bet Haemek, Israel), and 50 μM β-mercaptoethanol (Fisher Science, Medford, MA). Femoral bone marrow cells derived from wild-type mice (C57BL/6) were cultured in interleukin 3 (IL-3)-containing medium for 3 weeks to generate bone marrow-derived mast cells (BMMCs) as previously described.14 All the cells were grown in a humidified incubator at 37°C with 5% CO2.
Purification of bacterially expressed GST proteins
GST-Bcl-2 (ΔC21) was expressed from pGEX-Bcl-2 (a kind gift from Dr A. Gross, Weizmann Institute, Rehovot, Israel). The expression of the recombinant proteins was induced by 1 mM isopropyl β-D-thiogalactoside (IPTG) for 2 hours at 30°C and their purification was conducted following the manufacturer's instructions with glutathione sepharose 4B beads (Amersham Biosciences, Uppsala, Sweden).
Selection of scFv against Bcl-2 from phage-display library
We employed a human semisynthetic scFv library constructed from a single human framework for VH (DP-47 and JH4) and VL (DPK9 and JK1) joined by a linker, in which diversity was incorporated in CDR3 and CDR2.11 The screening of the phage-display antibody libraries was performed as previously described.15 Briefly, immunotubes (Nunc-Immuno Tubes, Maxi-Sorp, Nunc, Denmark) were coated with 10 μg/mL GST-Bcl-2 in phosphate-buffered saline (PBS) and exposed for 2 hours to 1013 transforming units (tu) of the phage library.
For enrichment of the GST-Bcl-2-bound phage, E coli TG-1 was infected with the eluted phages and rescued by the helper phage. The panning process was repeated 4 times and E coli TG-1 was infected with the final phage preparations and individual ampicillin-resistant colonies (phage clones) were selected for further analysis.
Screening and sequencing of Bcl-2-specific phage clones
Screening for positive anti-Bcl-2 phage clones was first done by enzyme-linked immunosorbent assay (ELISA), as previously described.16 Microtiter plate (Nunc) wells were coated with antigen by adding to each well 100 μL GST-Bcl-2 (10 μg/mL in PBS) using GST as negative control and incubated with 100 μL phage suspension for 90 minutes. After removal of the supernatants, the amount of bound phage was determined using peroxidase-labeled anti-M13 antibodies (Amersham Biosciences) and developed by TMB (3,3′5,5′ tetramethylbenzidine) following the manufacturer's instructions (Sigma Chemical, St Louis, MO). The reaction was monitored in a Spectra Max 340 ELISA reader (Molecular Devices, Sunnyville, CA) at 450 nm with a reference wavelength of 650 nm.
The entire scFv DNA fragment of the selected phage clones with specific anti-Bcl-2 activity was sequenced using the primers LMB-3 (5′-C AGGAAACAGCTATGAC) and Fd-Seq (5′-GAATTTTCTGTATGAGG).
Production and purification of anti-Bcl-2-scFv
The Bcl-2-reactive phage clones obtained from E coli TG-1 bacteria were used to infect E coli HB2151 nonsuppressor bacterial strain to obtain soluble scFv.15 After overnight induction with 1 mM IPTG at 30°C, the antibody fragments derived from the VH3 family were harvested from the supernatant and periplasmic space as described15 and purified on a protein A affinity column (Amersham Biosciences).16 To append the basic domain comprising residues 47 to 57 (YGRKKRRQRRR) of TAT protein enabling membrane translocation17 at the C-terminus of anti-Bcl-2 and control scFv, the encoding sequences of the different scFvs were amplified with the primers LMB3 (5′-CAGGAAACAGCTATGAC-3`) and TAT (5′-AAGGAAAAAATGCGGCCGCTCTTCGTCGCTGTCTCCGCTTCTTCCTGCCATACCGTTTGATTTCCACCTTGGTCCC-3`). The polymerase chain reaction (PCR) products were further cloned into the expression vector pTrcHis2-TOPO (Invitrogen). For the purification of the different single-chain TAT, Ni-beads TALON (Clontech, Palo Alto, CA) were used for incubation at 4°C overnight and the recombinant antibodies were eluted with freshly prepared 1 mM imidazole (Sigma). The antibodies were dialyzed against PBS before biologic experiments, verified by Western blotting with protein A-horseradish peroxidase (HRP) antibody16 (Zymed Laboratories, San Francisco, CA).
Membrane translocation of scFvs-TAT
RBL and MCF7 cells were grown on cover slips coated with collagen in 6-well plates (4 × 105 cells/well) in complete medium for 24 hours at 37°C. The cells were treated for 90 minutes with different scFv-TAT at various concentrations (5, 15, and 20 μg/mL) and fixed with 3.7% formaldehyde in PBS. After permeabilization with 0.8% Triton in PBS containing 0.5% bovine serum albumin (BSA) and blocking with normal horse serum (Dako Diagnostics AG, Glostrup, Denmark), the fixed cells were exposed to protein A-fluorescein isothiocyanate (FITC) (ICN Biomedicals, Aurora, OH) to determine the presence of the intracellular scFv.16 Specimens were examined, analyzed, and photographed by laser scanning confocal microscopy using the Zeiss LSM 410 confocal laser system connected to a Zeiss Axiovert 135M microscope with 40×/1.2 C-Apochromat water immersion lens (Zeiss, Jena, Germany). Green fluorescence of FITC was excited with argon laser (488-nm excitation line with 515-nm-long pass barrier filter).
Immunoprecipitation assays
RBL cells were treated with 20 μg/mL anti-Bcl-2-scFv-TAT or the nonspecific antibody for 90 minutes at 37°C. RBL cells were lysed by the addition of 250 μL cold lysis buffer (0.01 M Tris-HCl, pH 7.4, 1% deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 0.15 M NaCl, and 0.25 μM phenylmethyl-sulfonylfluoride) and 10 μL protease inhibitor cocktail (Sigma). After homogenization, the lysates were incubated overnight at 4°C with anti-Bcl-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and then for an additional 2 hours with 10 mg protein-A agarose beads (Invitrogen). After washing 3 times with cold lysis buffer (1:2), recovered immunocomplexes were solubilized in Laemmli sample buffer containing 0.5% SDS and the presence of the scFv-TAT was visualized by Western blotting using anti-myc antibody (Santa Cruz) (Figure 3A). In order to determine the specificity of the anti-Bcl-2-scFv-TAT, RBL cell lysates were incubated overnight with one of the following polyclonal antibodies: anti-Bcl-2, anti-BclXL, anti-A1, or anti-Mcl-1 (all from Santa Cruz). Samples were then incubated for an additional 2 hours with protein-A agarose beads (Invitrogen) as described before. The specificity of anti-Bcl-2-scFv-TAT was determined by using it in Western blot, followed by protein-A-HRP (Zymed) as secondary antibody (Figure 4). For verifying the protein level of Bcl-2, RBL cells were treated with 30 μL/mL anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for various periods of time (0 to 12 hours). After immunoprecipitation of Bcl-2 from the cell lysates, the level of Bcl-2 protein was determined by Western blotting using anti-Bcl-2 antibody (Santa Cruz) and the interaction between Bax and Bcl-2 was resolved using the same blot with anti-Bax antibody (Pharmingen, San Diego, CA). The presence of scFv-TAT within the Bcl-2-Bax complex was identified by anti-myc antibody (Santa Cruz). Anti-actin (Santa Cruz) was used as a loading control (Figure 8).
The BH1 domain mediates the interaction between anti-Bcl-2-scFv-TAT and Bcl-2. (A) Binding of anti-Bcl-2-scFv-TAT to intracellular Bcl-2. Bcl-2 was immunoprecipitated from RBL cells with anti-Bcl-2 immobilized on protein A-agarose. The binding of anti-Bcl-2-scFv-TAT (lane 1) or nonspecific scFv-TAT (lane 2) to intracellular Bcl-2 was determined by Western blot analysis using antibody against the myc tag on the scFv. (B) The sequence of the Bcl-2-BH domains and their homology to other BH domains in the Bcl-2-family proteins. The box encloses the BH domains, and the Bcl-2 sequences before and after the box complete the fragments for PCR. (C) Bcl-2 fragments BH1, BH2, BH3, and BH4 were labeled with [35S]-methionine in vitro and added to anti-Bcl-2-scFv-TAT immobilized on agarose beads. Retained [35S]-labeled fragments were determined by SDS-PAGE and autoradiography. One representative experiment of 3 is shown.
The BH1 domain mediates the interaction between anti-Bcl-2-scFv-TAT and Bcl-2. (A) Binding of anti-Bcl-2-scFv-TAT to intracellular Bcl-2. Bcl-2 was immunoprecipitated from RBL cells with anti-Bcl-2 immobilized on protein A-agarose. The binding of anti-Bcl-2-scFv-TAT (lane 1) or nonspecific scFv-TAT (lane 2) to intracellular Bcl-2 was determined by Western blot analysis using antibody against the myc tag on the scFv. (B) The sequence of the Bcl-2-BH domains and their homology to other BH domains in the Bcl-2-family proteins. The box encloses the BH domains, and the Bcl-2 sequences before and after the box complete the fragments for PCR. (C) Bcl-2 fragments BH1, BH2, BH3, and BH4 were labeled with [35S]-methionine in vitro and added to anti-Bcl-2-scFv-TAT immobilized on agarose beads. Retained [35S]-labeled fragments were determined by SDS-PAGE and autoradiography. One representative experiment of 3 is shown.
Anti-Bcl-2-scFv-TAT is a Bcl-2-specific antibody. The various antiapoptotic Bcl-2-family proteins were immunoprecipitated (IP) using their specific antibodies. Each immunoprecipitated protein was first identified by Western blotting (WB) with its specific antibody (top row). The same immunoblot was probed with anti-Bcl-2-scFv-TAT and protein A-HRP as secondary antibody (bottom row). One representative experiment of 3 is shown.
Anti-Bcl-2-scFv-TAT is a Bcl-2-specific antibody. The various antiapoptotic Bcl-2-family proteins were immunoprecipitated (IP) using their specific antibodies. Each immunoprecipitated protein was first identified by Western blotting (WB) with its specific antibody (top row). The same immunoblot was probed with anti-Bcl-2-scFv-TAT and protein A-HRP as secondary antibody (bottom row). One representative experiment of 3 is shown.
The effect of anti-Bcl-2-scFv-TAT on the level of Bcl-2 and on its interaction with Bax. The level of Bcl-2 protein was analyzed in RBL cells treated for various periods of time with either anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT. After immunoprecipitation with anti-Bcl-2 antibody, Bcl-2 was identified by Western blotting (top row). The same immunoblot was exposed to anti-Bax antibody in order to determine the interaction between Bcl-2 and Bax (second row). The presence of scFv-TAT was visualized using anti-myc antibody (third row). Anti-actin was used as a loading control (fourth row).
The effect of anti-Bcl-2-scFv-TAT on the level of Bcl-2 and on its interaction with Bax. The level of Bcl-2 protein was analyzed in RBL cells treated for various periods of time with either anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT. After immunoprecipitation with anti-Bcl-2 antibody, Bcl-2 was identified by Western blotting (top row). The same immunoblot was exposed to anti-Bax antibody in order to determine the interaction between Bcl-2 and Bax (second row). The presence of scFv-TAT was visualized using anti-myc antibody (third row). Anti-actin was used as a loading control (fourth row).
In vitro protein interaction assay
Protease-deficient E coli BL21 bacteria expressing recombinant anti-Bcl2-scFv-TAT were lysed in BugBuster buffer (Novagen, Madison, WI) supplemented with bacterial protease inhibitor cocktail (Sigma) at a dilution of 1:100 and clarified by centrifugation before purification of recombinant proteins using TALON affinity resins (Clontech). Bcl-2 and its various BH domains (BH1, BH2, BH3, BH4) were produced and [35S]-methionine labeled in a coupled transcription/translation system using the rabbit reticulocyte lysate (Promega, Madison, WI) with specific primers containing the T7 promoter and ribosomal binding domain, as follows: BH1 sense: 5′-CTAATACGACTCACTATAGG GAAGGAGATATACATATGCTGACGCCCTTCACC; BH1 antisense: 5′-TTACATCCCACCGAACTCAAAGAA; BH2 sense: 5′- CTAATACGACTCACTATAGGGAAGGAGATATACATATGGTGGACAACATCGCC; BH2 antisense: 5′-TTACATCCGCATGCTGGGGCC; BH3 sense: 5′-CTAATACGACTCACTATAGGGAAGGAGATATACATATGGGCGCCGCCGCGGGG; BH3 antisense: 5′- TTACATTTACATCGTCAGGTGCAGCTG; BH4 sense: 5′-CTAA TACGACTCACTATAGGGAAGGAGATATACATATGATGGCGCACGCTGGG; BH4 antisense: 5′-TTACATGGCGCCCACATCTCC. Pull-down assays were performed by mixing equal amounts of recombinant anti-Bcl-2-scFv-TAT immobilized onto protein A-agarose beads (Gibco, Carlsbad, CA) after preincubation for 1 hour at 4°C in 1 mL binding buffer (100 mM KCl, 20 mM Hepes, 1 mM dithiothreitol, 1 mM EDTA, 5% glycerol, 0.1% Nonidet P-40). One to 10 μL [35S]-labeled proteins (Bcl-2 and BH1-4 domains) were added to each preincubation mix and the binding reaction carried out overnight at 4°C. Proteins retained on the agarose beads were washed 4 times in 1 mL PBS/290 mM NaCl, boiled for 7 minutes in sample buffer, and resolved using SDS-polyacrylamide gel electrophoresis (PAGE) (14%). Following electrophoresis, the gels were treated with GelCode staining solution (Pierce, Rockford, IL), dried, and subjected to autoradiography to detect binding between anti-Bcl-2-scFv-TAT and Bcl-2 and its BH domain(s).
Flow cytometry
RBL cells were incubated in the presence of 30 μg/mL anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for various periods of time (0-24 hours). For mitochondrial staining, 1 × 106 cells were removed from the medium and MitoTracker Red CMXRos (Molecular Probes, Eugene, OR) was added to a final concentration of 50 nM for 20 minutes at 37°C. After pelleting at 500g for 3 minutes, cells were resuspended in 1 mL PBS (pH 7.4) and the cells were analyzed with a FACScan cytometer (Becton Dickinson, Mountain View, CA) equipped with an argon ion laser emitting at 488 nm, in FL2 channel.
Cell death assay
BMMCs (1 × 104) were cultured in 96-well round-bottom plates (Nunc) and RBL cells (5 × 103) in 96-well flat-bottom plates (Nunc) in 0.15 mL cultured medium supplemented with either anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT at different concentrations. Cell viability was determined by trypan blue exclusion following 24, 36, 48, and 72 hours in culture.
Apoptosis assay
RBL and MCF7 cells were cultured on cover slips coated with collagen in 6-well plates (4 × 105 cells/well) in 2 mL RPMI containing 10% FCS for 24 hours at 37°C, 5% CO2. After treatement with 30 μg/mL of the different scFv-TAT for 72 hours, the apoptotic cells were determined by using the DeadEnd Fluorometric TUNEL system (Promega) following the manufacturer's instructions.
Results
Characterization of the positive phage clones and the construction of anti-Bcl-2-scFv-TAT
Following 4 rounds of selection using a semisynthetic human phage-display antibody library, several positive clones were isolated. Clones were considered positive only if they did not bind to negative GST control and showed a 450-nm optical density (OD) of at least 3-fold over the background (Figure 1A).
Construction of a novel antibody derived from a positive phage clone against Bcl-2 with the TAT sequence. (A) Monoclonal ELISA: binding and specificity of phage clones to GST-Bcl-2. Isolated individual phage clones, after the fourth round of biopanning, were assayed for binding to either GST-Bcl-2 (▦)or GST (□), by phage monoclonal ELISA as described in “Materials and methods.” Three positive clones (H1, G2, and A9) were identified as showing specific binding to GST-Bcl-2. (B) Molecular structure and sequence of the anti-Bcl-2-scFv-TAT. The light and the heavy variable chains were linked by a flexible linker, which confers the possibility of conformational change. (C) Western blot analysis of the purified scFvs-TAT. After purification, the recombinant antibody anti-Bcl-2-scFv-TAT (lane 1) and nonspecific scFv-TAT (lane 2) were electroporated under denaturating conditions, transferred onto nitrocellulose paper and the 30-kDa bands were visualized using protein A-HRP.
Construction of a novel antibody derived from a positive phage clone against Bcl-2 with the TAT sequence. (A) Monoclonal ELISA: binding and specificity of phage clones to GST-Bcl-2. Isolated individual phage clones, after the fourth round of biopanning, were assayed for binding to either GST-Bcl-2 (▦)or GST (□), by phage monoclonal ELISA as described in “Materials and methods.” Three positive clones (H1, G2, and A9) were identified as showing specific binding to GST-Bcl-2. (B) Molecular structure and sequence of the anti-Bcl-2-scFv-TAT. The light and the heavy variable chains were linked by a flexible linker, which confers the possibility of conformational change. (C) Western blot analysis of the purified scFvs-TAT. After purification, the recombinant antibody anti-Bcl-2-scFv-TAT (lane 1) and nonspecific scFv-TAT (lane 2) were electroporated under denaturating conditions, transferred onto nitrocellulose paper and the 30-kDa bands were visualized using protein A-HRP.
Three positive scFv clones were used to infect nonsupressive E coli HB1251 bacteria to obtain soluble expression of the selected scFv. Only one positive clone (A9) was chosen, after it was assessed against GST-Bcl-2 and native Bcl-2 from different lysates (data not shown). We then added TAT sequence (YGRKKRQRRR),17 which is responsible for translocation through the membrane, to the positive clone (A9) and to the negative clone, which did not show any specificity against either Bcl-2 or GST. After verifiying the sequence (Figure 1B), vectors containing the recombinant antibodies were further transformed into competent E coli bacteria, induced with IPTG and the proteins were purified on affinity columns. This procedure enabled us to obtain large-scale amounts of different scFv-TATs (∼4 mg protein per liter culture). The integrity of the recombinant antibodies was assessed by Western blotting and identified with protein A-HRP, as shown in Figure 1C.
Translocation of anti-Bcl-2-scFv-TAT into cells
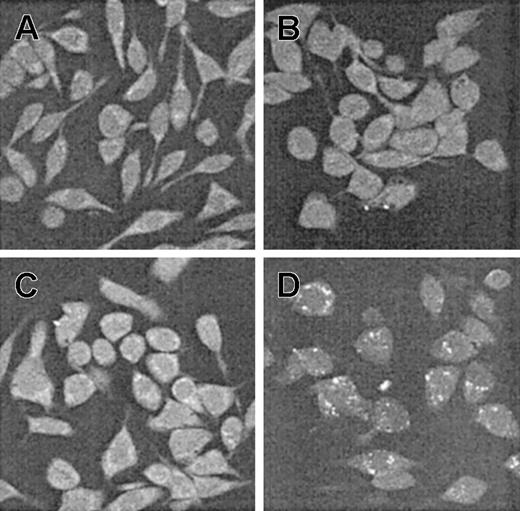
The penetration of the anti-Bcl-2-scFv-TAT into living cells was assessed using immunofluorescence. RBL and MCF7 cells were grown on collagen-coated cover slips and incubated for 90 minutes at 37°C with anti-Bcl-2-scFv-TAT, anti-Bcl-2-scFv (without TAT), and nonspecific scFv-TAT as controls. Figure 2 shows the translocation of anti-Bcl-2-scFv-TAT into RBL cells (Figure 2C-D). Moreover, the internalization follows a dose-dependent curve as observed by the intensity of the recorded signals (Figure 2C-D). The translocation of the control nonspecific scFv-TAT through the cell membrane was also verified (data not shown). In contrast, cells treated with anti-Bcl-2-scFv alone did not display such fluorescent signals (Figure 2B). Similar results were obtained using MCF7 cells (data not shown).
Translocation of scFv-TAT through the cell membrane. RBL cells (untreated, panel A) were exposed for 90 minutes to anti-Bcl-2-scFv-TAT at different concentrations (5 μg/mL, panel C; 15 μg/mL, panel D), and to anti-Bcl-2-scFv (10 μg/mL, panel B). The translocation of anti-Bcl-2-scFv-TAT into the cell cytoplasm was visualized using protein A-FITC, seen in the figure as white dots (C-D). Cells were observed under a fluorescent microscope at ×40 magnification.
Translocation of scFv-TAT through the cell membrane. RBL cells (untreated, panel A) were exposed for 90 minutes to anti-Bcl-2-scFv-TAT at different concentrations (5 μg/mL, panel C; 15 μg/mL, panel D), and to anti-Bcl-2-scFv (10 μg/mL, panel B). The translocation of anti-Bcl-2-scFv-TAT into the cell cytoplasm was visualized using protein A-FITC, seen in the figure as white dots (C-D). Cells were observed under a fluorescent microscope at ×40 magnification.
These results strongly suggest that the gain in fluorescence in the different scFv-TAT-treated cells reflects the membrane-translocating activity of the recombinant antibodies.
The involvement of the BH1 domain of Bcl-2 in the interaction with anti-Bcl-2-scFv-TAT
After testing antigen recognition and TAT-mediated translocation into the cells, we next assayed the in vivo ability of anti-Bcl-2-scFv-TAT to bind intracellular Bcl-2. After incubation of RBL cells with anti-Bcl-2-scFv-TAT or with the control nonspecific scFv-TAT, the proteins were immunoprecipitated with commercial anti-Bcl-2 bound to protein A-agarose. The presence of the anti-Bcl-2-scFv-TAT in the cells was detected by Western blot analysis using anti-myc antibodies15 against the myc epitope on the scFv. Figure 3A demonstrates that only the anti-Bcl-2-scFv-TAT is bound to the native Bcl-2.
A pull-down assay was carried out in order to identify the domain(s) of Bcl2 that is responsible for the interaction with anti-Bcl-2-scFv-TAT. PCR fragments coding for regions including the various BH-Bcl-2 domains were used for the assay. Since the different BH domains were very short, we amplified larger, specific Bcl-2 fragments containing these domains, as shown in the multiple sequence alignment (Figure 3B). Anti-Bcl-2-scFv-TAT was expressed in bacteria, immobilized on protein A-agarose beads, and assayed for its ability to retain the in vitro-translated Bcl-2 and its BH domains labeled with [35S]-methionine. The results clearly show that only the fragment containing the BH1 domain binds to anti-Bcl-2-scFv-TAT (Figure 3C). In contrast, the other BH domains did not bind to anti-Bcl-2-scFv-TAT. The anti-Bcl-2-scFv-TAT also interacted with the full-length of Bcl-2 (data not shown).
The specificity of the anti-Bcl-2-scFv-TAT
The specific binding of anti-Bcl-2-scFv-TAT to Bcl-2 rather than to the other antiapoptotic Bcl-2 family proteins was then determined. For this purpose, the proteins Bcl-2, BclXL, A1, and Mcl-1 were immunoprecipitated from RBL cell lysates using their specific antibodies and then identified by the same antibodies (Figure 4, upper pannel). The specificity of the anti-Bcl-2-scFv-TAT was determined by Western blotting using the same blots (Figure 4, lower pannel). As shown, the band corresponding to the purified Bcl-2 from the cell lysate was specifically identified by anti-Bcl-2-scFv-TAT. As a control, we used GST-Bcl-2, which was detected by anti-Bcl-2-scFv-TAT (data not shown). None of the other antiapoptotic proteins were recognized by anti-Bcl-2-scFv-TAT.
The effect of anti-Bcl-2-scFv-TAT on the mitochondrial membrane potential
For further characterization of the mechanism involved in the inhibition of the Bcl-2 function by anti-Bcl-2-scFv-TAT, changes in the mitochondrial membrane potential (ΔΨm) were monitored following cell treatement with 30 μg/mL of the different scFvs-TAT and at various intervals of time. The analysis was performed by observing the accumulation of the fluorescent dye MitoTracker Red in the cell mitochondria. Figure 5 shows the specific effect of the anti-Bcl-2-scFv-TAT on ΔΨm of the cells, as can be noticed by the decrease in the fluorescence after 12 hours (Figure 5A, right panel) in comparison to the 1-hour treatement (Figure 5A, left panel). In contrast, the nonspecific scFv-TAT did not cause any decrease in the fluorescence as represented at the different points of time (Figure 5B).
The effect of anti-Bcl-2-scFv-TAT on the mitochondrial membrane potential. RBL cells were exposed to 30 μg/mL anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for various periods of time and were then stained with MitoTracker Red, as described in “Materials and methods.” The changes in mitochondrial membrane potential were monitored by FACS. Control represents unstained cells. One representative experiment of 3 is shown. (A) Changes in fluorescence in RBL cells after 1 hour of treatment (left panel) and after 12 hours (right panel). (B) Mean fluorescence intensity monitored as function of the cell time treatment (anti-Bcl-2-scFv-TAT [□]; and nonspecific scFv-TAT [▦]).
The effect of anti-Bcl-2-scFv-TAT on the mitochondrial membrane potential. RBL cells were exposed to 30 μg/mL anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for various periods of time and were then stained with MitoTracker Red, as described in “Materials and methods.” The changes in mitochondrial membrane potential were monitored by FACS. Control represents unstained cells. One representative experiment of 3 is shown. (A) Changes in fluorescence in RBL cells after 1 hour of treatment (left panel) and after 12 hours (right panel). (B) Mean fluorescence intensity monitored as function of the cell time treatment (anti-Bcl-2-scFv-TAT [□]; and nonspecific scFv-TAT [▦]).
The effect of anti-Bcl-2-scFv-TAT on cell survival
BMMCs and RBL cells were incubated for 72 hours and 36 hours, respectively, with anti-Bcl-2-scFv-TAT or with the control nonspecific scFv-TAT, and cell survival was tested by trypan blue exclusion and cell counting (Figure 6).
The effect of anti-Bcl-2-scFv-TAT treatment on cell survival. Primary culture of BMMCs (A) and RBL cells (B) were treated with various concentrations of scFvs-TAT (untreated cells [□], anti-Bcl-2-scFv-TAT [▪], nonspecific scFv-TAT [▦]). The viability of the cells was assessed by trypan blue exclusion (mean ± SE; n = 3).
The effect of anti-Bcl-2-scFv-TAT treatment on cell survival. Primary culture of BMMCs (A) and RBL cells (B) were treated with various concentrations of scFvs-TAT (untreated cells [□], anti-Bcl-2-scFv-TAT [▪], nonspecific scFv-TAT [▦]). The viability of the cells was assessed by trypan blue exclusion (mean ± SE; n = 3).
The viability of the BMMCs decreased with increasing anti-Bcl-2-scFv-TAT concentrations. BMMCs treated with nonspecific scFv-TAT showed a percentage of viability similar to that observed for untreated BMMCs (Figure 6A). The same assay was also carried out in RBL cells. A significant decrease in cell viability was shown only in those cells treated with anti-Bcl-2-scFv-TAT (Figure 6B).
Anti-Bcl-2-scFv-TAT-induced apoptosis
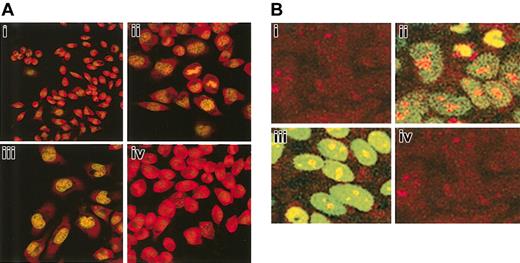
The possible induction of apoptosis by anti-Bcl-2-scFv-TAT was examined in RBL (Figure 7A) and MCF7 (Figure 7B) cells by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) analysis. RBL or MCF7 cells were grown in 6-well plates for 24 hours and exposed to 30 μg/mL anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for 72 hours. Cells were treated with DNaseI as a positive control, which specifically causes DNA fragmentation without affecting the membrane integrity of the cells, thus these cells are not apoptotic (Figure 7Aii,Bii). As seen in Figure 7Aiii and 7Biii, cells treated with anti-Bcl-2-scFv-TAT showed signs of apoptosis, which include DNA fragmentation, nuclear damage, cellular shrinkage, and blebbing. In contrast, the morphology of cells treated with nonspecific scFv-TAT was similar to that of nontreated cells (Figure 7Ai,Bi,Aiv,Biv). These results clearly indicate that the new recombinant antibody is effective in eliminating the target protein, thus ablating the pro-survival activity of Bcl-2.
Induction of apoptosis in RBL and MCF7 cells after treatment with anti-Bcl-2-scFv-TAT. Cells were exposed to anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for 72 hours. Apoptosis was determined by TUNEL assay and the cells were observed under a fluorescent microscope at × 40 magnification. Untreated RBL (Ai) and MCF7 (Bi) cells. RBL (Aii) and MCF7 (Bii) cells exposed to DNaseI as positive control. The cells show DNA fragmentation and fluorescent labeling. RBL (Aiii) and MCF7 (Biii) cells treated with anti-Bcl-2-scFv-TAT. The nuclei of the cells show fragmentation and fluorescent staining. RBL (Aiv) and MCF7 (Biv) cells treated with nonspecific scFv-TAT. The cells show morphologic similarity to untreated cells.
Induction of apoptosis in RBL and MCF7 cells after treatment with anti-Bcl-2-scFv-TAT. Cells were exposed to anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for 72 hours. Apoptosis was determined by TUNEL assay and the cells were observed under a fluorescent microscope at × 40 magnification. Untreated RBL (Ai) and MCF7 (Bi) cells. RBL (Aii) and MCF7 (Bii) cells exposed to DNaseI as positive control. The cells show DNA fragmentation and fluorescent labeling. RBL (Aiii) and MCF7 (Biii) cells treated with anti-Bcl-2-scFv-TAT. The nuclei of the cells show fragmentation and fluorescent staining. RBL (Aiv) and MCF7 (Biv) cells treated with nonspecific scFv-TAT. The cells show morphologic similarity to untreated cells.
Anti-Bcl-2-scFv-TAT affects the level of Bcl-2 and its interaction with Bax
The observations that anti-Bcl-2-scFv-TAT directly binds to Bcl-2 and induces cell apoptosis via its effect on the mitochondrial membrane potential were further analyzed by determining the protein level of Bcl-2 in RBL cells treated for various periods of time with either the specific or the nonspecific antibody (Figure 8). In contrast with cells exposed to nonspecific scFv-TAT, which show a constant level of Bcl-2 throughout 12 hours, the level of Bcl-2 significantly decreased in cells treated with anti-Bcl-2-scFv-TAT (Figure 8, first row). Moreover, the interaction of Bcl-2 with the Bax protein was increased with time only in the cells treated with the specific antibody (Figure 8, second row). The presence of scFv-TAT was identified only in the cells treated with anti-Bcl-2-scFv-TAT (Figure 8, third row). Anti-actin was used as a loading control (Figure 8, fourth row).
Discussion
In this study, we have described a novel approach that allows the efficient delivery of single-chain antibodies into several types of living cells and thus modulates the protein-protein interaction of the antiapoptotic Bcl-2 protein.
The developments in antibody engineering and recombinant DNA technology have made it possible to generate recombinant antibodies with a high degree of specificity and affinity for any antigens by employing phage-display technology and constructing very large repertoires of antibodies that are displayed on the surface of filamentous phage.18,19
The use of single-chain antibodies enables the use of smaller molecules than regular antibodies, which are simpler to introduce into cells. Two mRNA degradation strategies, RNAi and antisense RNA, are now available for the study of the function of specific genes. However, these 2 approaches are useful especially against short-lived proteins and therefore are not 100% effective for other molecules. The advantage of using single chains is their ability to adopt 3-dimensional conformations and to homodimerize as whole antibodies, in this way mimicking the immune system. This feature makes single-chain antibodies attractive not only for research, but also for gene therapy. One approach is loading the cells with dominant-negative proteins, so the native protein is in competition with its dominant-negative form and the cell is under stress. When using single-chain antibodies, the cell is able to continue its cycle until a definite point since the recombinant antibody neutralizes the native protein successively and noncompetitively. The use of proteins has a further advantage that in the future, genetic engineering could be employed to intracellularly express the antibody, thus allowing homing to specific cellular compartments, without the need for conjugation of peptides to oligonucleotides or specific drugs.
As we show in this study, the single-chain Fv selected from the phage-display library was raised specifically against Bcl-2 and its intracellular uptake was totally dependent on the basic residues of TAT signal sequence. Several studies have recently shown that TAT protein is able to induce apoptosis in several types of cells.20 However, we used only the fragment of TAT that is responsible for protein translocation and the control scFv conjugated to TAT did not increase cell death as compared with untreated cells. These results also support the notion that the antibody not only bound intracellularly to Bcl-2, but also inhibited the activity of this antiapoptotic protein. It thus seems that TAT transport of a single chain can deliver a biologically active antibody into cells.
The mechanism of translocation of the TAT basic peptide could be analogous to the model proposed for the Antennapedia homeodomain peptide.21 A tight ionic interaction between the basic groups of the peptide side chains and the negative charges of the phospholipid heads could induce a local invagination of the plasma membrane. The local reorganization of the phospholipid bilayer would then lead to the formation of inverted micelles with the protein enclosed in the hydrophilic cavity and ultimately to the cytoplasmic release of the scFv-TAT.22
Since a nuclear localization sequence is present in TAT peptide, we expected to find the recombinant antibody also in the nucleus. However, the high affinity of anti-Bcl-2-scFv-TAT for its cellular target leads to the binding of the antibody fragment to Bcl-2 and its localization in cytosol alone.
Bcl-2 is a cytoplasmic protein anchored mainly in the outside mitochondrial membrane and in the outside membrane of the nucleus. Confocal microscopy showed cytoplasmic distribution of the recombinant antibody and absence of nuclear staining. Thus, we attribute the biologic activity to the scFv that was carried inside the cell by TAT. Moreover, our results from the coimmunoprecipitation showed the binding of the specific single chain to native Bcl-2, indicating the high affinity and specificity of the novel anti-Bcl-2-scFv-TAT, whereas the control antibody was detected inside the cells, however not bound to Bcl-2.
Previous mutagenesis and deletion analyses of Bcl-2 family members have concentrated primarily on the regions of homology: BH1, BH2, and BH3. These domains have been shown to be involved in mediating dimerization between the various family members.23,24 Using domain mapping studies, we demonstrated that the amino acids 136-155, containing the Bcl-2-BH1 domain, are responsible for binding of the anti-Bcl-2-scFv-TAT. Both BH1 and BH2 domains are required for the inhibition of apoptosis since heterodimerization of Bcl-2 with Bax through these 2 domains leads to apoptosis.23 Consistent with the results of the in vitro interaction, our results demonstrate the direct and specific binding of anti-Bcl-2-scFv-TAT to Bcl-2, but not other antiapoptotic proteins such as BclXL, A1 or Mcl-1.
To investigate possible intracellular mechanisms for the observed increase in cell apoptosis, we examined the effect of anti-Bcl-2-scFv-TAT on the mitochondrial membrane potential ΔΨm. Only cells exposed to the specific scFv-TAT exhibited substantial mitochondrial depolarization as evidenced by loss of MitoTracker Red staining as a function of time. These results suggest that mitochondrial dysfunction plays a pivotal role in anti-Bcl-2-scFv-TAT-induced apoptosis and is caused by modulation of Bcl-2 activity in mitochondria. To address the molecular basis for these observations, we studied the expression of the pro and antiapoptotic proteins Bcl-2 and Bax in RBL cells exposed to the different scFvs-TAT. Bcl-2 protein expression showed a linear decrease over a period of 12 hours in RBL cells treated with anti-Bcl-2-scFv-TAT, in contrast with Bcl-2 amounts, which did not change noticeably in cells exposed to nonspecific scFv-TAT. The fact that our antibody modulated Bcl-2 and induced apoptosis prompted us to investigate the binding of proapoptotic protein Bax to Bcl-2. We clearly showed a constant increase in Bax-binding to Bcl-2 only in anti-Bcl-2-scFv-TAT-treated RBL cells.
Furthermore, we demonstrated that the binding of anti-Bcl-2-scFv-TAT with Bcl-2 induced the binding of Bax to this protein complex, leading to changes in the potential membrane of the mitochondria.
Our data implicate that the association of the Bcl-2-BH1 domain with anti-Bcl-2-scFv-TAT regulates the ability of Bcl-2 to serve as an antiapoptotic protein. Since the sequence used in this study is larger than the BH1 domain alone and unique to Bcl-2, we suggest that the activity mechanism of anti-Bcl-2-scFv-TAT might increase the affinity of Bax to Bcl-2. Such an interaction may change the relative ratio of the pro- and antiapoptotic factors in the cells.
The biologic effect of scFvs-TAT was studied in the basophilic cell line from rat leukemia, in mouse primary culture of BMMCs, and in the human breast cancer MCF7 cells. Only cells treated with the specific anti-Bcl-2 recombinant antibody displayed morphologic changes characteristic of apoptotic cell death (ie, chromatin condensation, margination, cellular shrinkage, and blebbing), with no such changes observed in untreated cells or cells treated with nonspecific scFv-TAT.
In addition, the use of Bcl-2-overexpressing breast cancer MCF7 cells25 underlines the efficiency of anti-Bcl-2-scFv-TAT in neutralizing Bcl-2, therefore leading to cell apoptosis.
Moreover, the results of the proliferation assay emphasize the importance of Bcl-2 in mast cell survival. The effect of the anti-Bcl-2-scFv-TAT was greater in transformed mast cells (RBL cells) than in the primary culture of BMMCs. This observation indicates that IL-3, the growth factor added to the medium, may antagonize the effect of anti-Bcl-2-scFv-TAT by up-regulating Bcl-2 level in the cells.
The mechanisms by which Bcl-2 regulates apoptosis in mast cells are poorly understood. In a recent study, a significant decrease in the survival of gastric mucosal mast cells derived from Bcl-2-/- mice was observed, whereas in Bax-/- mice an increase in the survival of these cells was seen.26 Thus, Bcl-2 probably also plays an important role in the survival in vivo of mucosal mast cells, which share many features with BMMCs. The normal number of skin mast cells observed in that study could be explained by different survival factors secreted in the skin environment. Altogether, the results from the study by Maurer et al26 and our study suggest that Bcl-2 plays an important role in mast cell survival under certain circumstances.
Our results support the claim that Bcl-2 has a major role in mast cell survival.7 It seems that anti-Bcl-2-scFv-TAT triggers apoptosis through a mechanism that involves direct targeting of Bcl-2 on the mitochondria. Further studies are needed to demonstrate whether inhibition of Bcl-2 also affects mast cell survival in vivo.
Inhibiting the function of intracellular proteins is an important goal for anyone studying the role of a specific protein in biochemical pathways and for those who try to develop specific therapeutic substances. The in vitro biologic activity of anti-Bcl-2-scFv-TAT should be widely applicable to the study of diverse biologic questions and makes it attractive for clinical applications.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2002-12-3921.
Supported by Israeli Ministry of Science grant no. 00-2-1456 (E.R., H.N., and A.N).
C.C.-S. and H.N. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Miri Shmueli for technical assistance and Dr Gillian Kay for figures and manuscript preparation. We are grateful to Dr Hanoch Cassuto for very helpful discussions and constructive criticism throughout the project. We also thank Dr Sagi Tshori for comments and members of Razin's laboratory for support.

![Figure 3. The BH1 domain mediates the interaction between anti-Bcl-2-scFv-TAT and Bcl-2. (A) Binding of anti-Bcl-2-scFv-TAT to intracellular Bcl-2. Bcl-2 was immunoprecipitated from RBL cells with anti-Bcl-2 immobilized on protein A-agarose. The binding of anti-Bcl-2-scFv-TAT (lane 1) or nonspecific scFv-TAT (lane 2) to intracellular Bcl-2 was determined by Western blot analysis using antibody against the myc tag on the scFv. (B) The sequence of the Bcl-2-BH domains and their homology to other BH domains in the Bcl-2-family proteins. The box encloses the BH domains, and the Bcl-2 sequences before and after the box complete the fragments for PCR. (C) Bcl-2 fragments BH1, BH2, BH3, and BH4 were labeled with [35S]-methionine in vitro and added to anti-Bcl-2-scFv-TAT immobilized on agarose beads. Retained [35S]-labeled fragments were determined by SDS-PAGE and autoradiography. One representative experiment of 3 is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2002-12-3921/6/m_h81935002003.jpeg?Expires=1767750832&Signature=MmSe7Koc5qfztTMRZd9P6q59UIaYOSbWUKpELhURIBF3nE8keA5SWax-arp0dldbOo50YgFDOMkHDFxDxKVOOd0veOqhHV2P~Ojpr7SlEuWHnxtLLsXH2IU-1f4H3rexa96dmFOY1F6PZReWhhvdoZOIUn4P~dYTRkwszuWuir2LpjKiEug~d16OyGv6bubOfvBE~WgCF0wB4MgfuSn8PIaNqAl92uDjTnJTp28M8UR1TifJWhr2CLPu4Ul0iJC3q2pZ4kgz5ifRpSvL4mwNfXje0LtJ1YEdh720D6fwro7qKJuUppLhWKbioMqOVq4n8di-Ka8wsvxWs4UUo19uyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 5. The effect of anti-Bcl-2-scFv-TAT on the mitochondrial membrane potential. RBL cells were exposed to 30 μg/mL anti-Bcl-2-scFv-TAT or nonspecific scFv-TAT for various periods of time and were then stained with MitoTracker Red, as described in “Materials and methods.” The changes in mitochondrial membrane potential were monitored by FACS. Control represents unstained cells. One representative experiment of 3 is shown. (A) Changes in fluorescence in RBL cells after 1 hour of treatment (left panel) and after 12 hours (right panel). (B) Mean fluorescence intensity monitored as function of the cell time treatment (anti-Bcl-2-scFv-TAT [□]; and nonspecific scFv-TAT [▦]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2002-12-3921/6/m_h81935002005.jpeg?Expires=1767750833&Signature=prlpviFtDFSn5XZlmS3SjNWoIPCyPi8NKna1jPX0r~~lsSaCccMSiI~Wl1c4W-cvHvVJ87Ay0prcuTYIN2-twzf4Nno0w32HAh~XlkGlLQMUw804~uPHciaBZwOi0CqWidzc4rMHChiWnRRZGe7gco7us8IOM~7lQJKMTZhjDa~bDEhoBbwn71un76KCUERU0ToH1g7UoCKJSSCLSogXjaIwLFtu4eoMWGUGgSYG313xQs~Rbczvg~XDC7rrH1o17gWNqjTBIOgjsHzxtxeAkQzk3o0RjSFVHBgNR0mDKmRsgqOfqDBKZlaY0y628YVDYMJPVXekWILCtnA7lKk8qA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. The effect of anti-Bcl-2-scFv-TAT treatment on cell survival. Primary culture of BMMCs (A) and RBL cells (B) were treated with various concentrations of scFvs-TAT (untreated cells [□], anti-Bcl-2-scFv-TAT [▪], nonspecific scFv-TAT [▦]). The viability of the cells was assessed by trypan blue exclusion (mean ± SE; n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2002-12-3921/6/m_h81935002006.jpeg?Expires=1767750833&Signature=AinX6cpJMIKojaqcNjy1fPwRUBwjd2OvM3ZnqxyMS5TYNGJIoFdFZZlNcmOGd3eSzTjZZbNaLh~8zQ9Lg-pO0IdpUZ6RuzbDj6egzWF~8jCj5JC7HWXHY8U5y4hLgx4Ju8q1gqwhSIniVAg2cwUVE-Y7Je-B1neUZ-2GkiAcJr9PvdoPiQJ-W5odgz-L3wFw9386vYZV9xW5VCeGPQkc1col9j-CCAeRa-AwLjBl5Q5rLzbUSqv1RFKoy8eNKdkXpD37GH7eYlv67eRbwYtO1n--SLGfcvrWFywxaZ-ykc~d2r2RfmNZED-HYjxBIxM3DiRGCRMQsp8zkB5U0IV7AQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal