Abstract
Angiopoietin-1 is implicated in the maturation and remodeling of the vascular network during embryo development and in adult life. Through its tyrosine kinase receptor Tie-2 it stimulates endothelial cells to migrate and change shape. Here we show that angiopoietin-1 elicits chemokinesis of endothelial cells by a phosphoinositide 3-OH kinase/son of sevenless-dependent modulation of Rac1 and RhoA. The resulting temporal events are associated with cytoskeletal rearrangements and occur in discrete zones of the cell. Endothelial cells carrying dominant-negative mutants of RhoA and Rac1 or treated with LY294002, an inhibitor of phosphoinositide 3-OH kinase, dramatically decrease their chemokinetic velocity. Taken together, these results further expand our understanding of angiopoietin-1-mediated endothelial cell motility during vascular network assembly and angiogenesis. (Blood. 2003;102:2482-2490)
Introduction
Angiogenesis is a crucial event in embryonic development and plays a critical role in many pathologic processes.1 Sprouting of new blood vessels from pre-existing ones is a multistep process that requires digestion of extracellular matrix, and migration and proliferation of endothelial cells (ECs).1 Finally, cells change their shape and fold up to form capillaries that are surrounded by pericytes, necessary for the cells' stabilization.2 A cascade of molecules whose biologic activities are partially overlapped drives this process. Among them, vascular endothelial growth factor (VEGF) and angiopoietin (Ang) families have restricted activities on ECs. Through VEGF receptor-1 and -2, VEGFs primarily regulate migration, proliferation, and survival of ECs. By analysis of genetic and pathologic models, Ang-1, a ligand of Tie-2 receptor, is instead supposed to stabilize EC networks, presumably by stimulating interactions between ECs and pericytes.3-5 In vitro experiments show that Ang-1 causes ECs to form capillary networks6 and sprouts from preassembled spheroids.7 Ang-1 induces EC chemotaxis8,9 and chemokinesis,10,11 which require the activation of the downstream effector phosphoinositide 3-OH kinase (PI 3-kinase) and the phosphorylation of focal adhesion kinase and paxillin.10,12 Such morphogenetic events are dependent on cell motility and shape rearrangement, which imply dramatic changes in cytoskeletal dynamics; this suggests that Ang-1 could be directly implicated in the regulation of the motility machinery in migrating cells.
Rho guanosine triphosphatases (Rho GTPases) are major regulators of cell polarization and motility. In fibroblasts, Cdc42 mediates filipodia extension, Rac1 lamellipodia formation and membrane ruffling, and RhoA stress fiber formation.13 Besides Rac1, RhoA is involved in ruffling as well.14 Nevertheless, considering that GTPase activities have antagonist effects in the regulation of different steps of cell motility15 and discrete sub-cellular localization,16-18 the above model seems to be more complex. Recent evidence suggests that Rho GTPases participate to the EC signaling pathways triggered by angiogenic inducers19-21 and, more in general, regulate biologic responses requiring changes in EC shape.22-25
Here we analyze the spatiotemporal modulation and localization of Rac1 and RhoA during EC locomotion induced by Ang-1 and we investigate the motility of ECs carrying RhoA and Rac1 dominant-negative molecules. Furthermore we studied the signaling pathway from Tie-2 receptor to these enzymes through PI 3-kinase activity.
Materials and methods
Reagents
Recombinant Ang-1 was produced in a baculovirus expression system as described and was able to phosphorylate Tie-2 in ECs.11 Glutathione-S-transferase (GST)-Pak 1B binding domain (GST-PBD), GST-rhotekin binding domain (GST-RBD), and GST-Wiskott-Aldrich syndrome protein (WASP) binding domain (GST-WBD) fusion proteins were purified as described.26 GST-PBD, GST-RBD, and GST-WBD bind the bound form of Rac1 and Cdc42, of RhoA, and of Cdc42, respectively.27 N17Rac1 and N19RhoA cDNAs28,29 were subcloned into the BamHI/EcoRI site of Pinco retroviral vector30 and expressed under the control of the 2 long-terminal repeats. Green fluorescent protein (GFP) cDNA was under the control of cytomegalovirus promoter. cDNA of RacQ61L (Rac1QL), RhoQ63L (RhoAQL), and Cdc42Q61L (Cdc42QL)31,32 was subcloned into the HindIII/NotI site of Pinco. GST-RBD and GST-PBD cDNAs were subcloned into pGEX2TK and pGEX3X, respectively, and expressed in Escherichia coli BL21 cells. Bacteria were sonicated in buffer A (50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 7.5, 100 mM NaCl, 1% Triton X-100, 2 mM dithiotreitol, and 5% glycerol) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 50 μg/mL leupeptin, 10 μg/mL aprotinin, and 5 μg/mL pepstatin). Lysates were purified by affinity-chromatography with glutathione-coupled Sepharose 4B beads (Amersham-Biotech-Pharmacia, Milan, Italy). For pull-down assays, beads were washed in washing buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0,5% Triton X-100, 5 mM MgCl2, and 1 mM dithiotreitol) and stored at -80°C with 10% glycerol. For immunolocalization assay, GST-PBD and GST-WBD were eluted with buffer B (50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 2 mM dithiotreitol, 5% glycerol, and 20 mM reduced glutathione) and GST-RBD in buffer B with 50 mM NaCl, and stored at -80°C.
pEGFP-C1-Sos-1 Dbl homology domain (DH), in which the residues Glu357 and Leu358 were replaced by alanine and have dominant-negative activity on endogenous son of sevenless 1 (Sos-1),33,34 was used to transfect porcine aortic ECs (PAE) constitutively expressing human Tie-2 (E.A. et al, unpublished data, January 2001) by lipofectamine procedure (Invitrogen, Paisley, United Kingdom).
Cell culture
Human vein ECs were cultured and used as previously described.35 ECs were infected by 1 to 3 rounds of infection as described.36 In some experiments, ECs were coinfected with N17Rac1, N19RhoA, and RhoAQL. The infection efficiency was evaluated by fluorescence-activated cell sorting (FACS) analysis of GFP expression (FACS Advantage SE; Becton Dickinson, San Jose, CA) (Figure S1 on the Blood website; see the Supplemental Figure link at the top of the online article). The expression of transgene molecules was evaluated by Western blot analysis and always gave an increase of proteins recognized by specific antibody (Ab) anti-Rac1 (Becton Dickinson) or anti-RhoA (Santa Cruz Biotechnology, Santa Cruz, CA) compared with ECs infected with vector alone (not shown). COS7 cells (American Type Culture Collection, Teddington, United Kingdom) were transfected with Rac1QL, RhoAQL, and Cdc42QL by calcium-phosphate technique (Invitrogen). In some experiments ECs were transfected with pEGFP-C3 Rac1 or pEGFP-C3 RhoA37 by lipofectamine (Invitrogen).
Motility assays
ECs carrying N17Rac1, N19RhoA, or vector were suspended at concentrations of 1.7 × 106 cells/mL of M199 with 20% fetal calf serum (FCS; Invitrogen), and 20 μL of this suspension was spotted on dishes previously coated in gelatin 1% (Sigma-Aldrich, Milano, Italy) and dried under sterile air. When cells were spread, the medium was added to cover the dish until cells formed confluent colonies. After one hour of starvation in M199 with 0.5% FCS, ECs were stimulated with Ang-1 (20 ng/mL) or vehicle. In some experiments the effect of PI 3-kinase inhibitor LY294002 (25 μM; Sigma-Aldrich) was evaluated by pretreating ECs for 5 minutes before stimulation. Time-lapse videomicroscopy and image analysis were performed exactly as described.38 Cell paths were generated from centroid positions and migration parameters were computed with DIAS software (Solltech, Oakdale, IA). The difference between stimulated and unstimulated cells was reported as increase of motility speed.
GTPase and PI 3-kinase assays
GTPase pull-down assay was performed on subconfluent (2.5 × 106) or spotted confluent ECs that were starved as described above and stimulated with Ang-1 (20 ng/mL) or vehicle for the indicated times. Ang-1 concentration has been selected on preliminary dose-response experiment from 1 to 200 ng/mL. In some experiments PI 3-kinase inhibitor LY294002 (25 μM) was added 5 minutes before stimulation. At the indicated times, cells were washed with cold phosphate-buffered saline (PBS) and lysed (20 minutes) on ice in 50 mM Tris, pH 7.4, 2 mM MgCl2, 100 mM NaCl, 1% nonidet P-40 (NP-40), and 10% glycerol containing protease inhibitors. Lysates were centrifuged and equal amounts of proteins (500 μg) were incubated (60 minutes, 4°C) with 30 μg of GST fusion molecules bound to Sepharose beads. After washes, solubilized proteins were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with monoclonal antibody (mAb) anti-RhoA, anti-Rac1, or anti-Cdc42 (Santa Cruz Biotechnology). Cell lysates were treated in the same conditions to evaluate the total amounts of RhoA, Rac1, and Cdc42. Densitometry was performed with Phoretix 1D software (Nonlinear USA, Durham, NC) calculating the ratio of normalized samples with the amount of activated GTPases in 0.5% FCS vehicle-treated cells at time point 0.
PI 3-kinase activity (ELISA kit; Echelon Biosciences, Salt Lake City, UT) was assayed on antiphosphotyrosine mAb immunoprecipitates39 prepared from subconfluent, starved ECs stimulated for 2 minutes with Ang-1 (100 ng/mL; R&D Systems, Abingdon, United Kingdom).
Immunofluorescence
COS7 cells were fixed for 10 minutes in 4% paraformaldehyde in PBS, pH 7.5, permeabilized with Tween 0.1% in PBS for 10 minutes on ice, and rinsed in PBS. For RhoA-GTP, Rac1-GTP, and Cdc42-GTP staining, cells were saturated with 3% bovine serum albumin (20 minutes, 37°C) and incubated overnight at 4°C with 30 μg GST-PBD, GST-RBD, or GSTWBD. PBS-washed cells were incubated (30 minutes, 37°C) first with a mAb anti-GST (Santa Cruz Biotechnology) and then with the secondary tetramethyl-rhodamine isothiocyanate (TRITC)-conjugated mAb (Santa Cruz Biotechnology). For F-actin staining, cells were incubated (30 minutes, 37°C) with fluorescein isothiocyanate (FITC)-conjugated phalloidin (Sigma-Aldrich). To stain endogenous activated Rho GTPases, ECs or ECs carrying N17Rac1, N19RhoA, or vector alone were fixed with paraformaldehyde (3.7%; 15 minutes, room temperature), quenched for 20 minutes in 20 mM NH4Cl in PBS, and permeabilized with 0.1% saponin (Sigma-Aldrich; 30 minutes in ice bath). Blocking incubations were performed in 10% goat serum, 0.25% fish skin gelatin (Sigma-Aldrich) (1 hour, room temperature). GST-PBD, GST-RBD, or GST-WBD (30 μg) and antibodies were diluted in 10% of blocking solution and incubated for one hour at room temperature. In order to exclude interferences of cell fixation on the GTP-bound state of Rho GTPases, a pull-down assay was performed on cell lysates prepared from fixed and permeabilized cells after incubation with PBS (12 hours, 4°C).
Morphometric analysis of stained ECs was calculated by software analysis (ImageProPlus 4.0; Media Cybernetics, Silver Spring, MD) and expressed as arbitrary units. Cytoskeleton modification was expressed as percent of modified cells over the control; immunolocalization of GST-PBD and GST-RBD was quantified as fluorescence intensity.
Results
Ang-1 reorganizes actin cytoskeleton and modulates Rac1 and RhoA GTPase activity
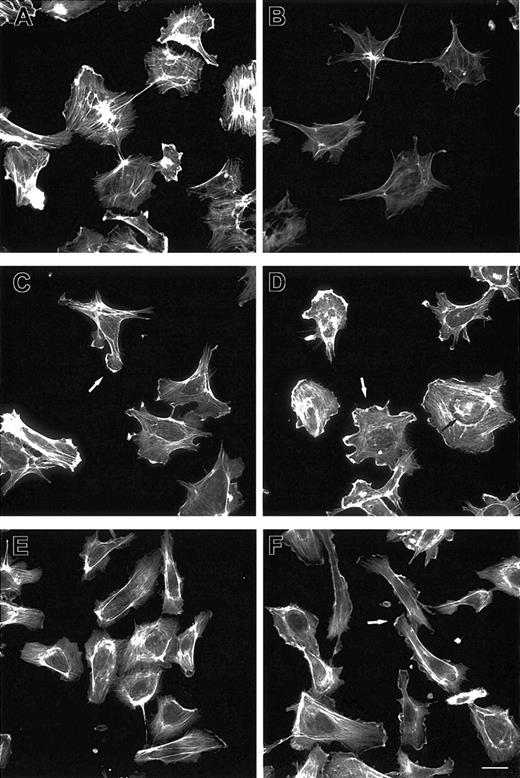
Because Ang-1 regulates EC motility,8-11 we investigated EC cytoskeleton remodeling after Ang-1 stimulation. Phalloidin staining revealed that starved cells had poorly organized F-actin compared with cells maintained in FCS (Figure 1A-B). After 2 minutes, Ang-1 induced membrane ruffling and lamellipodia extensions (Figure 1C). Lamellipodia increased in size and F-actin cables appeared along cell bodies after 5 minutes (Figure 1D) and persisted up to one hour. Prolonged incubations caused EC polarization (Figure 1E) and ruffling lamellipodia at the leading edge (Figure 1F). Table 1 shows the cell percentage responsive to Ang-1-induced cytoskeleton remodeling.
F-actin reorganization induced by Ang-1. ECs plated on gelatin-coated coverslips were maintained in 20% FCS (A) or starved for 1 hour in 0.5% FCS and stimulated with vehicle (B) or Ang-1 (20 ng/mL) for 2 (C), 5 (D), 90 (E), and 120 (F) minutes. Cells were stained with FITC-phalloidin. Bar indicates 10 μm. White and black arrows indicate lamellipodia and F-actin cables, respectively. Figure is representative of 3 experiments.
F-actin reorganization induced by Ang-1. ECs plated on gelatin-coated coverslips were maintained in 20% FCS (A) or starved for 1 hour in 0.5% FCS and stimulated with vehicle (B) or Ang-1 (20 ng/mL) for 2 (C), 5 (D), 90 (E), and 120 (F) minutes. Cells were stained with FITC-phalloidin. Bar indicates 10 μm. White and black arrows indicate lamellipodia and F-actin cables, respectively. Figure is representative of 3 experiments.
Morphometric analysis of Ang-1-mediated cytoskeleton modification and RhoGTPase activation in ECs
Parameter examined . | Unstimulated cells . | Ang-1, 2 min . | Ang-1, 5 min . |
|---|---|---|---|
| Lamellipodia, % positive cells*† | 10.0 ± 0.2 | 75.3 ± 1.2 | Not tested |
| GST-PBD fluorescence of lamellipodia-positive cells, arbitrary unit‡ | 15.6 ± 3.1 | 37.5 ± 5.5 | Not tested |
| F-actin cables, % positive cells†§ | 7.8 ± 2.1 | Not tested | 72.5 ± 2.3 |
| GST-RBD fluorescence of F-actin cable-positive cells, arbitrary unit∥ | 12.6 ± 3 | Not tested | 31.5 ± 2.2 |
Parameter examined . | Unstimulated cells . | Ang-1, 2 min . | Ang-1, 5 min . |
|---|---|---|---|
| Lamellipodia, % positive cells*† | 10.0 ± 0.2 | 75.3 ± 1.2 | Not tested |
| GST-PBD fluorescence of lamellipodia-positive cells, arbitrary unit‡ | 15.6 ± 3.1 | 37.5 ± 5.5 | Not tested |
| F-actin cables, % positive cells†§ | 7.8 ± 2.1 | Not tested | 72.5 ± 2.3 |
| GST-RBD fluorescence of F-actin cable-positive cells, arbitrary unit∥ | 12.6 ± 3 | Not tested | 31.5 ± 2.2 |
Lamellipodia were identified according to Nobes and Hall15 in fixed and permeabilized cells stained with FITC-phalloidin.
Mean ± SD of 3 individual experiments. For each experiment, 100 cells were counted.
The results are expressed as mean ± SD of 30 lamellipodia-positive cells in 3 different experiments.
Cells were fixed, permeabilized, and stained by FITC-phalloidin. A fluorescence intensity greater than 40 arbitrary units was a limit to consider cells positive for the presence of F-actin cables.
The results are expressed as mean ± SD of 30 F-actin cable-positive cells in 3 different experiments.
Because actin dynamics are mainly regulated by the Rho GTPase family,15 we studied the time-course of RhoA and Rac1 activity in Ang-1-stimulated ECs by evaluating the amount of GTP-bound Rho GTPases in pull-down experiments. The assay takes advantage of downstream effectors (Pak 1B, rhotekin, and WASP) that specifically bind the GTP-bound form of these enzymes.27
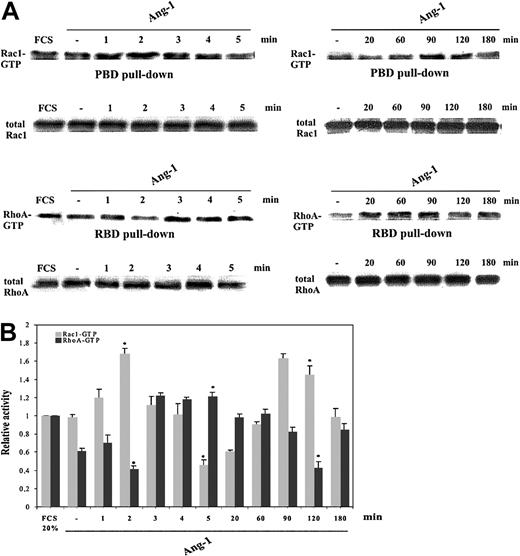
The amount of either Rac1-GTP or RhoA-GTP in subconfluent ECs cultured in the presence of 20% FCS or vehicle did not change over time (Figure 2A and not shown). Cell starvation for one hour in 0.5% FCS decreased the amount of RhoA-GTP, whereas the level of Rac1-GTP was unchanged. After 1 minute, Ang-1 increased Rac1-GTP level that peaked at 2 minutes and then dramatically dropped to about 50% of the basal activity of starved cells within 5 minutes. A second wave of Rac1 activation was observed at 90 to 120 minutes of stimulation. RhoA activity was reciprocally modulated. Actually, RhoA-GTP decreased after 2 minutes, then rose after 3 to 5 minutes, and decreased again after 120 minutes. At 180 minutes both GTPase activities fell to the basal level (Figure 2).
Modulation of Rac1 and RhoA activity induced by Ang-1. (A) Starved, subconfluent ECs stimulated with Ang-1 (20 ng/mL) or vehicle underwent pull-down assays. The leftmost lanes indicate Rho GTPase activity in the presence of FCS (20%). (B) The results of densitometric analysis of Rac1-GTP (▦) and RhoA-GTP (▪) were normalized to total Rac1 and RhoA in whole cell lysates and expressed relative to the basal value of cells maintained in 20% FCS. (Means ± SDs of 5 independent experiments; *P < .05 versus starved unstimulated cells by Student t test).
Modulation of Rac1 and RhoA activity induced by Ang-1. (A) Starved, subconfluent ECs stimulated with Ang-1 (20 ng/mL) or vehicle underwent pull-down assays. The leftmost lanes indicate Rho GTPase activity in the presence of FCS (20%). (B) The results of densitometric analysis of Rac1-GTP (▦) and RhoA-GTP (▪) were normalized to total Rac1 and RhoA in whole cell lysates and expressed relative to the basal value of cells maintained in 20% FCS. (Means ± SDs of 5 independent experiments; *P < .05 versus starved unstimulated cells by Student t test).
Ang-1 induces Rac1 and RhoA localization to specific subcellular compartments
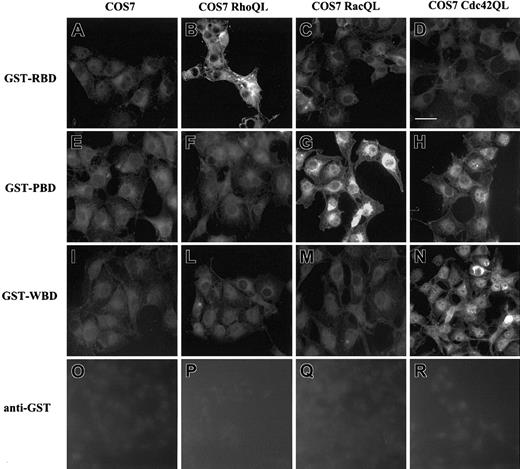
To gain insights in the subcellular localization of Rho GTPases permeabilized cells were loaded with the above GST fusion proteins followed by indirect immunofluorescence detection with Ab anti-GST.40 To test the binding specificity of RhoA-GTP, Rac1-GTP, and Cdc42-GTP to GST-RBD, GST-PBD, and GST-WBD, COS7 cells were transiently transfected with constructs of constitutively active RhoAQL, Rac1QL, and Cdc42QL. RhoAQL expression selectively increased binding of GST-RBD (Figure 3B). Similarly, the expression of Rac1QL (Figure 3G) and Cdc42QL (Figure 3H) allowed the specific binding of GST-PBD. Expression of Cdc42QL selectively increased binding of GST-WBD (Figure 3N). Anti-GST Ab did not detect any subcellular compartment (Figure 3O-R). No staining was detected following cell incubation with GST protein (data not shown). These data suggest that in our experimental conditions the intracellular staining of GST-RBD, GST-PBD, and GST-WBD specifically tracks the localization of the GTP-bound form of RhoA, Rac1, and Cdc42, as already reported in the nervous system.40
Immunolocalization of GST-RBD, GST-PBD, and GST-WBD in COS7 cells carrying RhoAQL, Rac1QL, and Cdc42QL. COS7 cells transfected with cDNA encoding RhoAQL (B,F,L,P), Rac1QL (C,G,M,Q), and Cdc42QL (D,H,N,R) were fixed, permeabilized, and incubated with GST-RBD (A-D), GST-PBD (E-H), or GST-WBD (I-N). Anti-GST Ab was used as negative control (O-R). Scale bar, 10 μm. There were 3 experiments performed with similar results.
Immunolocalization of GST-RBD, GST-PBD, and GST-WBD in COS7 cells carrying RhoAQL, Rac1QL, and Cdc42QL. COS7 cells transfected with cDNA encoding RhoAQL (B,F,L,P), Rac1QL (C,G,M,Q), and Cdc42QL (D,H,N,R) were fixed, permeabilized, and incubated with GST-RBD (A-D), GST-PBD (E-H), or GST-WBD (I-N). Anti-GST Ab was used as negative control (O-R). Scale bar, 10 μm. There were 3 experiments performed with similar results.
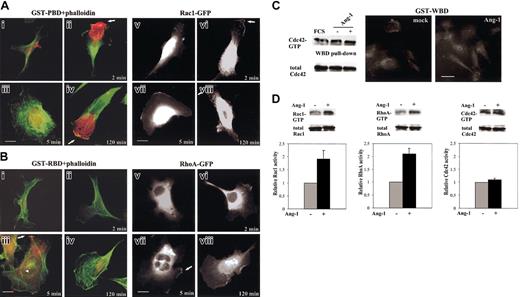
According to the time-course profile of RhoA and Rac1 activity (Figure 2B), starved ECs were stimulated with Ang-1 for 2, 5, and 120 minutes and immunolocalization assay was performed (Figure 4A-B). The immunofluorescence intensity of GST-PBD was highly positive after 2 and 120 minutes of stimulation (Figure 4Aii,iv) and barely detectable after 5 minutes (Figure 4Aiii). GST-RBD staining appeared after 5 minutes of Ang-1 treatment (Figure 4Biii). The increased Rac1-GTP was mostly concentrated at lamellipodia where it was partially colocalized with cortical F-actin at the leading edge (Figure 4Aii,iv). In Ang-1-induced EC spreading, RhoA-GTP localized mostly at the perinuclear region while a portion redistributed at the sites of membrane spreading where F-actin accumulated (Figure 4Biii). Although this immunolocalization assay is only qualitative, as paraformaldehyde is a cross-linking agent that may interfere with the stoichiometry of antigen-Ab interaction,41 a single-cell morphometric analysis of the immunofluorescence observations is reported in Table 1. To exclude the possibility that the immunolocalization assay could give false interpretations of RhoA- and Rac1-GTP localization we stimulated ECs carrying GFP-tagged Rac1 and RhoA as described above, and we looked at GFP molecule translocation (Figure 4Av-viii,Bv-viii). In starved cells, Rac1-GFP was cytosolic and enriched in the perinuclear region (Figure 4Av) as shown for other cell types.37 After stimulation, Rac1-GFP rapidly moved into lamellipodia (Figure 4Avi) and then it was shifted in the cytosol, with a small amount remaining in the plasma membrane (Figure 4Avii). A prolonged stimulation caused a new translocation from cytosol to lamellipodia rims (Figure 4Aviii). In starved cells, RhoA-GFP was diffused in the cytoplasm (Figure 4Bv). After Ang-1 challenge, a small amount of RhoA translocated to the plasma membrane (Figure 4Bvi-viii), but it was in GTP-bound form only at the specific time point of 5 minutes (Figure 4Bvii), consistent with the results obtained in pull-down experiments (Figure 2). The trafficking of GFP-tagged molecules demonstrates the ability of exogenous proteins to still bind Rho guanine nucleotide dissociation inhibitor, which is instrumental for enzyme retention in the cytosol.37
Subcellular localization of RhoA-GTP, Rac1-GTP, and Cdc42-GTP, and of GFP-tagged Rac1 and RhoA. (A) ECs (i-iv) and ECs carrying Rac1-GFP (v-viii) were starved and stimulated with vehicle (i,v) or with Ang-1 (20 ng/mL) for 2 (ii,vi), 5 (iii,vii), and 120 (iv,viii) minutes. Panels i-iv are merged images of F-actin (green) and GST-PBD (red). Arrows indicate lamellipodia. (B) ECs (i-iv) and ECs carrying RhoA-GFP (v-viii) were starved and stimulated with vehicle (i,v) or with Ang-1 (20 ng/mL) for 2 (ii,vi), 5 (iii,vii), and 120 (iv,viii) minutes. Panels i-iv are merged images of F-actin (green) and GST-RBD (red). The arrowhead and the white arrows indicate F-actin cables and ruffles, respectively. (C) Starved, subconfluent ECs were stimulated for 2 hours (or 2 minutes, not shown) with vehicle or Ang-1 (20 ng/mL) and underwent pull-down and immunolocalization assays with GST-WBD. (D) Pull-down assays for Rac1, RhoA, and Cdc42 from lysates of fixed and permeabilized ECs. The results of densitometric analysis of Rac1-GTP, RhoA-GTP, and Cdc42-GTP where normalized to total Rac1, RhoA, and Cdc42 in whole cell lysates and expressed to the basal value of unstimulated cells (mean ± SDs of 3 independent experiments). Bars indicate 10 μm. Figures are representative of at least 3 experiments.
Subcellular localization of RhoA-GTP, Rac1-GTP, and Cdc42-GTP, and of GFP-tagged Rac1 and RhoA. (A) ECs (i-iv) and ECs carrying Rac1-GFP (v-viii) were starved and stimulated with vehicle (i,v) or with Ang-1 (20 ng/mL) for 2 (ii,vi), 5 (iii,vii), and 120 (iv,viii) minutes. Panels i-iv are merged images of F-actin (green) and GST-PBD (red). Arrows indicate lamellipodia. (B) ECs (i-iv) and ECs carrying RhoA-GFP (v-viii) were starved and stimulated with vehicle (i,v) or with Ang-1 (20 ng/mL) for 2 (ii,vi), 5 (iii,vii), and 120 (iv,viii) minutes. Panels i-iv are merged images of F-actin (green) and GST-RBD (red). The arrowhead and the white arrows indicate F-actin cables and ruffles, respectively. (C) Starved, subconfluent ECs were stimulated for 2 hours (or 2 minutes, not shown) with vehicle or Ang-1 (20 ng/mL) and underwent pull-down and immunolocalization assays with GST-WBD. (D) Pull-down assays for Rac1, RhoA, and Cdc42 from lysates of fixed and permeabilized ECs. The results of densitometric analysis of Rac1-GTP, RhoA-GTP, and Cdc42-GTP where normalized to total Rac1, RhoA, and Cdc42 in whole cell lysates and expressed to the basal value of unstimulated cells (mean ± SDs of 3 independent experiments). Bars indicate 10 μm. Figures are representative of at least 3 experiments.
Because Pak 1B is also an effector of Cdc4227 we investigated the possible modulation of this enzyme as well. Data shown in Figure 4C clearly demonstrate that at the time point where Ang-1 stimulated Rac1, the amount of Cdc42-GTP bound to its specific interactor GST-WBD27 was unmodified compared with unstimulated cells. Similarly the weak GST-WBD staining found in starved ECs did not increase after Ang-1 stimulation (Figure 4C).
To rule out possible interferences due to the technical procedures of the immunolocalization assay, pull-down experiments were performed starting from lysates of ECs processed as for this assay. Figure 4D clearly demonstrates that these procedures allow Rho GTPases to still bind the effector molecules.
Rac1 and RhoA are both involved in Ang-1-induced chemokinesis
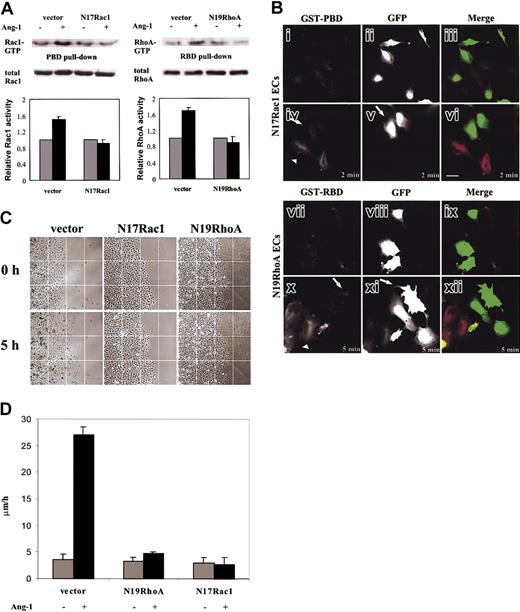
To gain insights in the role of Rac1 and RhoA activation during Ang-1-elicited motility, we infected ECs with N17Rac1 and N19RhoA dominant-negative mutants. Infection conditions per se were without effect on EC morphology and on cell cycle evaluated by propidium staining and FACS analysis (data not shown). To verify the dominant-negative effect on ECs we performed pull-down assays. When compared with cells infected with vector alone, ECs carrying mutants did not show any activation of RhoA and Rac1 after Ang-1 stimulation (Figure 5A). The dominant activity of N17Rac1 and N19RhoA on their endogenous homologs was checked through the immunolocalization assay (Figure 5B). GFPECs carrying N19RhoA and N17Rac1 were stimulated with Ang-1, and then GST-PBD and GST-RBD localization was evaluated by the immunolocalization assay. The fluorescence signal of GSTPBD (Figure 5Bi,iv) and GST-RBD (Figure 5Bvii,x) in GFP-ECs carrying N17Rac1 (Figure 5Bi-vi) and N19RhoA (Figure 5Bvii-xii), respectively, and vector alone (data not shown) did not increase after Ang-1 stimulation. On the contrary, immunolocalization of both effectors was evident in Ang-1-stimulated GFP-negative ECs that did not express the mutant molecules (Figure 5Bvi,xii).
EC chemokinesis stimulated by Ang-1. (A) GTPase activity in ECs carrying vector alone or N19RhoA and N17Rac1 was stimulated for 5 and 120 minutes, respectively, (or 2 minutes, data not shown) with Ang-1 (20 ng/mL).  indicates unstimulated cells (-); ▪ indicates Ang-stimulated cells (+) exactly as in Figure 4D. Densitometric and statistical analysis have been performed as in Figure 4D. The panel is representative of 4 experiments. (B) ECs carrying N17Rac1 (i-vi) and N19RhoA (vii-xii) were stimulated with vehicle (i-iii and vii-ix) or Ang-1 (20 ng/mL) for 2 (iv-vi) and 5 minutes (x-xii), respectively. N17Rac1 ECs were incubated with GST-PBD (i,iv) and N19RhoA ECs with GST-RBD (vii,x). GFP shows infected ECs (ii,v,viii,xi). GST-PBD staining (red) and GFP (green) are merged in panels ix and xii. Arrows and arrowheads indicate GFP-positive and GFP-negative cells, respectively. In this experiment, ECs were infected at 50% of efficiency. Scale bar equals 10 μm. (C) ECs carrying vector alone or N19RhoA and N17Rac1 were plated in confluent colonies, starved, and then stimulated with Ang-1 (20 ng/mL) or vehicle. Images were extracted at beginning and after 5 hours of stimulation by time-lapse videomicroscopy (magnification, × 100) (D) Motility was expressed as micrometers per hour as detailed in “Materials and methods.” Mean ± SD of 5 experiments.
indicates unstimulated cells (-); ▪ indicates Ang-stimulated cells (+) exactly as in Figure 4D. Densitometric and statistical analysis have been performed as in Figure 4D. The panel is representative of 4 experiments. (B) ECs carrying N17Rac1 (i-vi) and N19RhoA (vii-xii) were stimulated with vehicle (i-iii and vii-ix) or Ang-1 (20 ng/mL) for 2 (iv-vi) and 5 minutes (x-xii), respectively. N17Rac1 ECs were incubated with GST-PBD (i,iv) and N19RhoA ECs with GST-RBD (vii,x). GFP shows infected ECs (ii,v,viii,xi). GST-PBD staining (red) and GFP (green) are merged in panels ix and xii. Arrows and arrowheads indicate GFP-positive and GFP-negative cells, respectively. In this experiment, ECs were infected at 50% of efficiency. Scale bar equals 10 μm. (C) ECs carrying vector alone or N19RhoA and N17Rac1 were plated in confluent colonies, starved, and then stimulated with Ang-1 (20 ng/mL) or vehicle. Images were extracted at beginning and after 5 hours of stimulation by time-lapse videomicroscopy (magnification, × 100) (D) Motility was expressed as micrometers per hour as detailed in “Materials and methods.” Mean ± SD of 5 experiments.
EC chemokinesis stimulated by Ang-1. (A) GTPase activity in ECs carrying vector alone or N19RhoA and N17Rac1 was stimulated for 5 and 120 minutes, respectively, (or 2 minutes, data not shown) with Ang-1 (20 ng/mL).  indicates unstimulated cells (-); ▪ indicates Ang-stimulated cells (+) exactly as in Figure 4D. Densitometric and statistical analysis have been performed as in Figure 4D. The panel is representative of 4 experiments. (B) ECs carrying N17Rac1 (i-vi) and N19RhoA (vii-xii) were stimulated with vehicle (i-iii and vii-ix) or Ang-1 (20 ng/mL) for 2 (iv-vi) and 5 minutes (x-xii), respectively. N17Rac1 ECs were incubated with GST-PBD (i,iv) and N19RhoA ECs with GST-RBD (vii,x). GFP shows infected ECs (ii,v,viii,xi). GST-PBD staining (red) and GFP (green) are merged in panels ix and xii. Arrows and arrowheads indicate GFP-positive and GFP-negative cells, respectively. In this experiment, ECs were infected at 50% of efficiency. Scale bar equals 10 μm. (C) ECs carrying vector alone or N19RhoA and N17Rac1 were plated in confluent colonies, starved, and then stimulated with Ang-1 (20 ng/mL) or vehicle. Images were extracted at beginning and after 5 hours of stimulation by time-lapse videomicroscopy (magnification, × 100) (D) Motility was expressed as micrometers per hour as detailed in “Materials and methods.” Mean ± SD of 5 experiments.
indicates unstimulated cells (-); ▪ indicates Ang-stimulated cells (+) exactly as in Figure 4D. Densitometric and statistical analysis have been performed as in Figure 4D. The panel is representative of 4 experiments. (B) ECs carrying N17Rac1 (i-vi) and N19RhoA (vii-xii) were stimulated with vehicle (i-iii and vii-ix) or Ang-1 (20 ng/mL) for 2 (iv-vi) and 5 minutes (x-xii), respectively. N17Rac1 ECs were incubated with GST-PBD (i,iv) and N19RhoA ECs with GST-RBD (vii,x). GFP shows infected ECs (ii,v,viii,xi). GST-PBD staining (red) and GFP (green) are merged in panels ix and xii. Arrows and arrowheads indicate GFP-positive and GFP-negative cells, respectively. In this experiment, ECs were infected at 50% of efficiency. Scale bar equals 10 μm. (C) ECs carrying vector alone or N19RhoA and N17Rac1 were plated in confluent colonies, starved, and then stimulated with Ang-1 (20 ng/mL) or vehicle. Images were extracted at beginning and after 5 hours of stimulation by time-lapse videomicroscopy (magnification, × 100) (D) Motility was expressed as micrometers per hour as detailed in “Materials and methods.” Mean ± SD of 5 experiments.
Ang-1 has a chemokinetic activity, which results in cell scatter.10,11 Therefore, we set up a centrifugal migration assay where confluent “spot colonies” of ECs were challenged with Ang-1. After 5 hours of stimulation ECs scattered out from the colonies (Figure 5C) and increased basal locomotion speed (from 3.5 ± 1.02 μm/h to 27.02 ± 1.5 μm/h) (Figure 5D). Expression of either N19RhoA or N17Rac1 in ECs dramatically counteracted Ang-1 effect (Figure 5C-D).
In these experimental conditions, where ECs form confluent colonies, Ang-1-dependent modulation of RhoA and Rac1 activities was as similar as in subconfluent cells (data not shown).
Sequential signals triggered by Ang-1
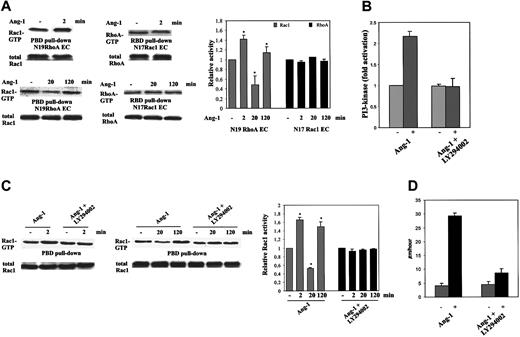
To establish whether Rac1 and RhoA are modulated in a coordinate or independent way, we analyzed GTPase activity during chemokinesis of spotted ECs carrying N17Rac1 or N19RhoA (Figure 6A). Inhibition of RhoA by N19RhoA did not affect the ability of Ang-1 to modulate Rac1. On the contrary, N17Rac1 infected cells were unable to modulate RhoA activity in response to Ang-1. These results demonstrate that Rac1 is necessary for RhoA modulation in ECs stimulated by Ang-1. This is supported by the chemotactic effect of Ang-1 in ECs expressing both N17Rac1 and RhoAQL, the active form of RhoA. The presence of RhoAQL increased the basal motility of ECs, but was also able to partially revert the inhibitory effect of N17Rac1 as well as that of N19RhoA (EC-vector: 20 ± 5 [number of migrated cells in Boyden chamber35 ]; EC-vector + Ang-1 [20 ng/mL]: 63 ± 8; EC-N17Rac1: 17 ± 6; EC-N17Rac1 + Ang1: 16 ± 3; EC-N19RhoA: 21 ± 4; EC-N119RhoA + Ang1: 23 ± 5; EC-N17Rac1/RhoAQL: 40 ± 7; EC-N17Rac1/RhoAQL + Ang-1: 58 ± 3; EC-N19RhoA/RhoAQL: 43 ± 5; EC-N19RhoA/RhoAQL + Ang-1: 64 ± 6; n = 3).
Ang-1-dependent modulation of Rac1 and RhoA in ECs carrying N17Rac1 or N19RhoA, and LY294002 effect on activation in ECs. (A) Rho GTPase activities were evaluated in chemokinesis assay by using cells carrying N17Rac1, N19RhoA, or vector. (B) Starved, subconfluent ECs were preincubated (5 minutes) with or without LY294002 (25 μM) and then stimulated for 2 minutes with Ang-1 (50 ng/mL). PI 3-kinase was measured on antiphosphotyrosine mAb immunopreciptates (the level of PI(3,4,5)P in unstimulated cells was 26.2 ± 1.2 pmol, n = 3). (C) ECs were preincubated with LY294002 as detailed in panel B and then stimulated for the indicated times with Ang-1 (20 ng/mL) in chemokinesis assay. Densitometric analysis was evaluated as in Figure 4 (mean ± SD of 5 experiments; *P < .05 versus unstimulated cells by Student t test). (D) EC chemokinesis induced by Ang-1 in the presence or absence of LY294002 (25 μM). Mean ± SD of 3 experiments.
Ang-1-dependent modulation of Rac1 and RhoA in ECs carrying N17Rac1 or N19RhoA, and LY294002 effect on activation in ECs. (A) Rho GTPase activities were evaluated in chemokinesis assay by using cells carrying N17Rac1, N19RhoA, or vector. (B) Starved, subconfluent ECs were preincubated (5 minutes) with or without LY294002 (25 μM) and then stimulated for 2 minutes with Ang-1 (50 ng/mL). PI 3-kinase was measured on antiphosphotyrosine mAb immunopreciptates (the level of PI(3,4,5)P in unstimulated cells was 26.2 ± 1.2 pmol, n = 3). (C) ECs were preincubated with LY294002 as detailed in panel B and then stimulated for the indicated times with Ang-1 (20 ng/mL) in chemokinesis assay. Densitometric analysis was evaluated as in Figure 4 (mean ± SD of 5 experiments; *P < .05 versus unstimulated cells by Student t test). (D) EC chemokinesis induced by Ang-1 in the presence or absence of LY294002 (25 μM). Mean ± SD of 3 experiments.
Direct activation of Rho GTPases by activated tyrosine kinase receptor may be dependent of PI 3-kinase.42 Ang-1 induced a 2-fold increase of PI 3-kinase activity that was inhibited by LY294002 (Figure 6B) according to previous results.43 Because this PI 3-kinase inhibitor blocked Ang-1-dependent EC migration8 and according to the results in Figure 6A we evaluated the effect of the PI 3-kinase inhibitor LY294002 on Rac1 activation and on Ang-1 chemokinetic function. Preincubation of ECs with LY294002 abolished Rac1 modulation induced by Ang-1 (Figure 6C) and inhibited Ang-1-induced basal locomotion speed (Figure 6D).
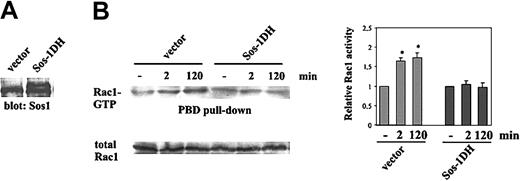
Within the guanine nucleotide exchange factors (GEFs), Sos-1 has been demonstrated to be regulated by PI 3-kinase products.44 Therefore, we examined the role of Sos-1 in Ang-1-specific activation of Rac1. PAE carrying human Tie-2 were transfected with dominant-negative Sos-1-DH (Figure 7A) and then stimulated for 2 and 120 minutes with Ang-1. Pull-down experiments show that the expression of Sos-1-DH inhibited Rac1 activation by Ang-1 compared with cells transfected with the empty vector (Figure 7B).
Sos-1-dependent activation of Rac1. (A) PAE carrying human Tie-2 were transfected with Sos-1-DH or vector alone and cell lysates were immunoblotted with an anti-Sos-1 (Santa Cruz Biotechnology) Ab. The lower and higher bands correspond to Sos-1 and Sos-1-DH, respectively.34 (B). Pull-down assay of Rac1 from starved and subconfluent cells stimulated for the indicated times with Ang-1 (50 ng/mL). Densitometric analysis evaluated as in Figure 4 is shown (mean ± SD of 3 independent experiments; *P < .05 versus unstimulated cells by t test).
Sos-1-dependent activation of Rac1. (A) PAE carrying human Tie-2 were transfected with Sos-1-DH or vector alone and cell lysates were immunoblotted with an anti-Sos-1 (Santa Cruz Biotechnology) Ab. The lower and higher bands correspond to Sos-1 and Sos-1-DH, respectively.34 (B). Pull-down assay of Rac1 from starved and subconfluent cells stimulated for the indicated times with Ang-1 (50 ng/mL). Densitometric analysis evaluated as in Figure 4 is shown (mean ± SD of 3 independent experiments; *P < .05 versus unstimulated cells by t test).
Discussion
EC motility regulated by Ang-1 is required for maturation and remodeling of nascent vessels.6,9-11 Generally, factors able to promote cell movement trigger intracellular signals that result in spatially and temporally coordinated reorganization of cytoskeletal and development of transient and definitive extracellular matrix contacts.15,45 Here we demonstrate that the migratory phenotype assumed by ECs challenged with Ang-1 is associated with modulation and discrete spatial distribution of Rho-GTPases. Actually, these enzymes are engaged in the regulation of contractility driving forces that allow cell migration.15
Ang-1 induced a very early increase in Rac1 activity and a simultaneous down-regulation of RhoA. This event occurred in the first 5 minutes and was followed by a rise of RhoA-GTP associated with a decrease of Rac1-GTP. At later time points, Rac1 gradually increased, reaching a new peak at 90 to 120 minutes, while RhoA was inversely modulated. These events paralleled the recovery of a spread phenotype, lost during cell starvation, and the acquisition of a migratory morphology. In these conditions, Ang-1 did not change the basal activity of Cdc42; moreover we cannot formally exclude a role of this enzyme in EC motility.
RhoA- and Rac1-GFP tagged molecules redistributed from the cytosol to the plasma membrane of Ang-1-stimulated ECs at specific time points, as also reported in other systems.37,46,47 To gain information on the activation state of these localized proteins, an immunolocalization technique was developed by incubating fixed and permeabilized cells with GST fusion proteins of the GTPase binding domain of specific effectors. A similar approach has been used to map activated Rho GTPases in brain section.40 When Rac1 activity peaked, ECs polarized and Rac1 translocated to developed lamellipodial extensions. There, Rac1 accumulated in the GTP-bound form. Similar observations have been done by a fluorescence resonance energy transfer-based technique in migrating fibroblasts16 and may be regulated by an adenosine diphosphate-ribosylation factor 6 (ARF6)-dependent endosomal plasma membrane recycling pathway of Rac1.48 Activated Rho GTPases may be present in the cytosol, but they interact with downstream effectors only at the membrane level.49,50 Actually, Rac effectors at the plasma membrane of the leading edge regulate a variety of functions associated with motility.51,52 One of the effectors is p21-activated kinase,52 which is a downstream Tie-2 receptor9 and regulates EC migration.53 When RhoA reached its maximal activity, its GTP-bound form was diffused in the cell body, and to some extent colocalized with F-actin in membrane ruffles and cable structures during cell spreading, as already reported in migrating ECs.14
To properly address the modulation of Rac1 and RhoA in EC motility stimulated by Ang-1, we evaluated the chemokinesis in cells carrying N19RhoA and N17Rac1. The scatter assay used showed that both enzymes were required for optimal chemokinetic activity of Ang-1.
To look for a possible cross-talk of the two GTPases, experiments were done with ECs carrying N19RhoA or N17Rac1. The observed RhoA and Rac1 modulation by Ang-1 did not occur in cells where endogenous Rac1 was inhibited. On the contrary, in N19RhoA cells stimulated by Ang-1, RhoA, but not Rac1 activity, was impaired. It has been demonstrated that Rac and Rho may act through mutually antagonistic pathways.31,54 Here, we observed that Ang-1 induced temporal Rho GTPase antagonistic modulation where the Rac1 pathway was required for RhoA modulation, as already reported in Drosophila55 and in fibroblasts.56 In the chemokinesis assay both N17Rac1 and N19RhoA cells did not respond to Ang-1. This result highlights that the balance between RhoA and Rac1 is fundamental to properly address Ang-1-induced EC motility. Furthermore, we cannot exclude that both enzymes dictate different and independent pathways necessary for cell motility.57,58
Besides the role of the DokR-Nck-Pak complex,9 Ang-1-dependent EC motility depends in part on PI 3-kinase.8 We demonstrate that Ang-1 increased the production of PI-(3,4,5) triphosphate catalyzed by PI 3-kinase and that the modulation of Rac1, as well as Ang-1-induced locomotion, was abolished by preincubating ECs with PI 3-kinase inhibitor LY294002. This suggests that activation of PI 3-kinase could determine modulation of Rac1 that in turn could counteract RhoA. This model is supported by data showing that platelet-derived growth factor induces sustained Rac activation that in turn down-regulates Rho activity.56 Furthermore, PI 3-kinase is involved in the induction of the leading edge of polarized cells and drives F-actin assembly most likely through the ability of its products to facilitate guanosine diphosphate (GDP) exchange of Rac1.45
The control of the switch from the inactive GDP-bound to active GTP-bound form of Rho GTPases is mediated by GEFs.59 By using a dominant-negative mutant of Sos-1 we demonstrated that this GEF participates in the activation of Rac1. Sos-1 belongs to the Dbl GEF family that contains a pleckstrin and a Dbl homology domain.59 It has been proposed as a model, in which these domains interact with each other through a PI 3-kinase substrate, such as PI-(4,5) biphosphate, and maintain Sos-1 in a closed and inactive form. Upon PI 3-kinase activation, Sos-1 becomes associated with PI-(3,4,5) triphosphate, and a conformational change of pleckstrin domain occurs that reduces the affinity for the Dbl homology domain that then binds Rac1.44 However we cannot exclude that other GEFs activated by PI 3-kinase products, such as Tiam1 and Vav,26,44,60 may regulate Rac1 after Ang-1 stimulation.
In summary, this paper highlights the relevance of the spatial and temporal behavior of Rac1 and RhoA in EC migration stimulated by Ang-1. We have defined an instrumental pathway for EC motility, in which Ang-1 stimulation of Tie-2 drives a PI 3-kinase/Sos-1-dependent modulation of Rac1 that influences RhoA activity.
Prepublished online as Blood First Edition Paper, June 19, 2003; DOI 10.1182/blood-2003-03-0670.
Supported by Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità (IV Programma Nazionale di Ricerca sull'AIDS-2001 and Progetto “Tumor therapy”), Compagnia di San Paolo, Ministero dell'Università e della Ricerca (60%, Cofin2002 and Fondo per gli Investimenti per la Ricerca di Base [FIRB]), and Centro Nazionale della Ricerca (CNR; Progetto Strategico Oncologia).
E.A. and E.G. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Drs G. Bockoc (University of San Diego, San Diego, CA), Y. Zheng (University of Tennessee, Memphis), A. Hall (University of London, London, United Kingdom), L. Lanfrancone, G. Scita, and M. Innocenti (European Institute of Oncology, Milano, Italy) for giving us reagents.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal