Abstract
Prothrombinase activity was tested on thrombin- and SFLLRN-activated platelets treated with RO318220, a potent inhibitor of protein kinase C. RO318220 completely inhibited platelet dense and α-granule secretion at a concentration of 20 μM but had no effect on prothrombinase activity in the presence of excess factor Va (20 nM). This indicates that protein kinase C activity and agonist-initiated secretion are not necessary for the development of a procoagulant surface. Treatment with 75 to 150 μM RO318220 potentiated platelet-supported thrombin generation up to 280% of control platelets with no change in Kd appFXa. Treated with increasing concentrations of RO318220, an increasing proportion of thrombin-stimulated platelets bound annexin V with decreasing binding sites per platelet. A lower mean forward scatter (FSC-H) of platelets treated with RO318220 suggested platelet vesiculation as a result of RO318220 treatment; however, 100 μM calpeptin pretreatment eliminated the decrease in FSC-H without affecting either the increase in platelets positive for annexin V binding, the decrease in binding sites per platelet, or the 3-fold increase in prothrombinase activity. Thus, RO318220 appears to increase prothrombinase activity by increasing platelet responsiveness to thrombin rather than by inducing platelet vesiculation. This suggests that RO318220 inhibits a signaling molecule within a negative regulatory pathway that governs platelet procoagulant surface changes. (Blood. 2003;102:2472-2481)
Introduction
Platelet agonists trigger platelet adhesion, shape change, secretion, aggregation, and procoagulant surface changes potentiating coagulation enzyme activity.1 Recently, agonist-triggered platelet signal transduction has been investigated for many platelet functions including adhesion to fibrinogen,2-5 adhesion to von Willebrand factor,6,7 adhesion to collagen,8,9 shape change,10 secretion,11 and aggregation.12,13 However, little is known about the activation steps leading to platelet-supported procoagulant enzyme activity.
Surface localization of 2 important procoagulant complexes, the factor X-activating complex and the prothrombinase complex,14,15 results in potentiation of activity by 5 (prothrombinase)16 or 6 (factor X activation) orders of magnitude.17 Platelets, as well as other blood cells,18,19 show the ability to bind components of the prothrombinase complex containing factor Xa and its cofactor factor Va. Strong agonists are required to activate platelets to express the surface changes necessary to support procoagulant activity;20 among those agonists are thrombin, collagen, and a combination of both of these agonists.21,22
Human platelets contain at least 2 categories of receptor with which thrombin interacts, the 7 transmembrane protease-activated receptors (PAR 1 and PAR 4)23,24 and glycoprotein Ib/IX/V complex.25 PAR 1 is sensitive to low thrombin concentrations (1 nM). PAR 4 requires high concentrations of thrombin (30 nM) to activate platelets26,27 in the absence of other potentiating elements such as is known to exist in murine platelets where PAR 3 presents low thrombin concentrations to PAR 4 for signaling.28,29 Glycoprotein Ib/IX/V contains a high-affinity thrombin binding site on the Ibα chain, missing in Bernard-Soulier platelets, without which low concentrations of thrombin are less effective platelet agonists,30-32 although this is still disputed.33,34
Molecular steps of signal transduction immediately subsequent to thrombin ligation of its receptors are as yet not well characterized. PAR 1 is known to be linked to heterotrimeric G proteins, Gq35 and G12/1336,37 and possibly Gi.38,39 Activation of Gq is associated with activation of phospholipase Cβ (isotypes 1, 2, and 3) resulting (through hydrolysis of phosphatidylinositol 4,5-bisphosphate2,35 ) in activation of protein kinase C and other calcium-requiring signaling molecules. More distal steps have been implicated in thrombin-stimulated platelet activation, such as phosphorylations of phosphatidylinositol-3 kinase and small guanosine 5′-triphosphate (GTP)-binding proteins.2,40 Procoagulant surface changes have been shown to require an influx of calcium41,42 and exposure of aminophospholipids normally sequestered in the inner leaflet of the cell membrane.43-45
Since the signaling steps required for thrombin stimulation of a procoagulant surface remain poorly understood, this study was undertaken to methodically investigate the requirements for expression of thrombin-stimulated platelet-supported thrombin generation. Bisindolylmaleimide IX (methanesulfonate; RO318220) is a cell-permeable pharmacologic inhibitor selective for isotypes of protein kinase C at concentrations 100- to 1000-fold below its known effects on other intracellular signaling molecules such as protein kinase A or calmodulin kinase.46-49 Since protein kinase C has been implicated in the secretion response,50 RO318220 was tested both at concentrations known to target secretion and at higher concentrations to locate other possible steps in the signaling pathway resulting in platelet expression of surface-potentiated thrombin generation.
Materials and methods
Reagents
Bovine serum albumin, buffer reagents, disodium EDTA (ethylenediaminetetraacetic acid), dimethylsulfoxide (DMSO), calcium chloride, and Sepharose 2B-CL were obtained from Sigma Chemical (St Louis, MO). Electrophoresis reagents were from Bio-Rad Laboratories (Melville, NY). Chromogenic substrate H-d-Phenylalanyl-l-pipecolyl-l-arginine-p-nitro-aniline dihydrochloride (S-2238) was purchased from DiaPharma (West Chester, OH). RO318220, GÖ6976, and calpeptin were purchased from Calbiochem (La Jolla, CA). The thrombin receptor agonist peptides, SFLLRN-amide and AYPGKF-amide, were synthesized using 9-fluorenylmethyloxycarbonyl (FMOC) chemistry on an Applied Biosystems (Foster City, CA) 430A synthesizer and reverse-phase high-performance liquid chromatography (HPLC) purified to more than 99% homogeneity.
Proteins
Factors Xa, Va, and prothrombin were obtained either from Enzyme Research Laboratories (South Bend, IN) or Haematologic Technologies (Burlington, VT). Coagulation proteins were obtained in or dissolved in buffer containing 20 mM Tris-hydroxymethane and 150 mM NaCl and dialyzed against the same buffer to remove inhibitors. Concentrations were determined by bicinchoninic acid assay (Pierce Chemical, Rockford, IL) and purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) visualized with Coomassie Brilliant Blue staining. Human α thrombin was purchased from Sigma Chemical and active-site titrated against human anti-thrombin III with residual activity assayed chromogenically with S-2238. Proteins and antibodies conjugated to fluorescein isothiocyanate (FITC) were purchased from either BDPharMingen (Los Angeles, CA) or from R&D Systems (Minneapolis, MN).
Platelet preparation
Washed, aspirin-treated, gel-filtered platelets were prepared from human whole blood as described previously51 with the following modifications. Platelet-rich plasma was incubated with 1 mM acetylsalicylic acid for 30 minutes at 37°C with prostaglandin E1 (2 μM) added 10 minutes before layering over the bovine serum albumin gradient for the wash step. Platelets were used within 30 to 60 minutes of gel filtration. The same results were obtained using platelets untreated with prostaglandin E1.
Treatment of platelets with inhibitors
RO318220, GÖ6976, and calpeptin were dissolved in DMSO to 10 mM, aliquoted into dark tubes at 10 μL/tube, and kept at -20°C until use. Inhibitors were diluted to working solutions in DMSO and maintained in the dark on ice until warmed just before addition to platelet suspensions. Stock solutions were diluted in DMSO so that addition to platelets constituted 0.5% to 1% DMSO (vol/vol). This DMSO concentration did not interfere with thrombin generation. Platelets were agitated gently upon addition of inhibitors and incubated covered at 37°C for 10 minutes prior to functional testing.
Lumiaggregometry
Platelets in an aggregometer cuvette were treated with vehicle or inhibitor and incubated within the heat block of a Chronolog Lumiaggregometer Model 400-VS (Havertown, PA) connected to a chart recorder (Amersham Pharmacia Biotech, Piscataway, NJ). Calcium chloride (5 mM) and firefly d-luciferase (422 U/mL; Chronolog) were added and monitored for 1 minute before addition of agonist. Maximum aggregation was analyzed from the analog signal as total pen deflection between platelet suspension and buffer alone (10% and 90% of chart width). Rate of aggregation was derived from the initial slope (mm/min) of the aggregation curve. Delay was measured in minutes as the elapsed time between addition of agonist and upward pen deflection beyond 10%. Adenosine triphosphate (ATP) secretion was determined from luminescence-channel pen deflection in mm (response to thrombin 1 U/mL = 100%).
Flow cytometry protocols and analyses
Washed, aspirin-treated, gel-filtered platelets (0.1 × 109/L) were treated with vehicle or RO318220 for 10 minutes and protected from light before addition of either FITC-labeled antifibrinogen antibody, FITC-labeled CD42b against glycoprotein 1bα, FITC-labeled CD62P against P-selectin, or FITC-labeled annexin V. Half of each sample was activated with thrombin (0.2 U/mL) for 15 minutes, and all were diluted and analyzed using CellQuest software (BD-PharMingen) on a FACScan flow cytometer equipped with a 488-nm-emitting laser. Platelets were gated by forward and side scatter pattern and by binding of FITC-anti-glycoprotein 1bα. Samples were analyzed by CellQuest software for positive events within and outside the platelet profile. Forward scatter data and FITC fluorescence emission in channel 1 (FL-1 = 530 nm with a bandpass = 30), after compensation for RO318220 fluorescence, were displayed logarithmically as density plots or histograms. Negative FL-1 fluorescence was defined with unlabeled platelets or platelets incubated with FITC-antirabbit IgG or with FITC-annexin V in the presence of EDTA. Quadrants (density plots) or markers (histograms) were selected for CellQuest software quantitation of positive and negative populations. For experiments investigating inhibition of calpain, platelets were pretreated with 100 μM calpeptin for 10 minutes before addition of RO318220 or vehicle.
Thrombin generation
Thrombin generation was measured as described previously52,53 with the following modifications. Washed, aspirin-treated, gel-filtered platelets (0.1 × 109/L) were incubated in wells of a microtiter plate for 10 minutes at 37°C, protected from light, and diluted with vehicle or inhibitors such that DMSO final concentration did not exceed 1% volume. Factor Xa and agonist, either thrombin (0.2 U/mL) or SFLLRN-amide (50 μM) or AYPGKF-amide (1 mM), were added and activation proceeded for 5 minutes before addition of factor Va and prothrombin (1 μM) for reactions in the presence of cofactor or prothrombin alone for reactions in its absence. Reactions were stopped after 1 minute and were analyzed for thrombin activity by kinetic measurements of chromogenic substrate S-2238 (200 μM) hydrolysis. A zero factor Xa control was used to subtract background activity or activity attributed to thrombin as agonist.
For microparticle experiments, a portion of activated platelets pretreated with inhibitors was pelleted at 800g for 20 minutes. Prothrombinase activity was assayed with both the supernatant from these samples and with the complete mixture from which they came. Activity associated with the platelet surface alone was calculated by subtracting the activity of the supernatant from the activity of the complete solution.
Data analysis
Enzyme assay results (in change in optical density per minute [mOD/min]) were converted to thrombin formed (nM/min) by comparison to a standard curve of thrombin cleavage of S-2238. Reactions performed in the absence of factor Xa were subtracted from all reactions performed in its presence to eliminate S-2238 hydrolysis by background artifact or by thrombin used as an agonist. Rates of thrombin generation were plotted using KaleidaGraph software (Synergy, Reading, PA) to derive kinetic parameters. Results from multiple experiments were pooled and analyzed for means and standard errors.
Results
To investigate platelet signaling requirements for expression of surface-potentiated thrombin generation, thrombin generation assays used albumin-washed and gel-filtered platelets treated with acetylsalicylic acid (1 mM) to prevent feedback activation mediated by arachidonic acid metabolism.54 Under the conditions chosen for these experiments, thrombin or SFLLRN-stimulated platelets supported factor Xa activation of 1 μM prothrombin to a maximum activity of 80 nM/min in the presence of added factor Va and to 15 to 25 nM/min in its absence.
Inhibiting protein kinase C: effect on aggregation and secretion
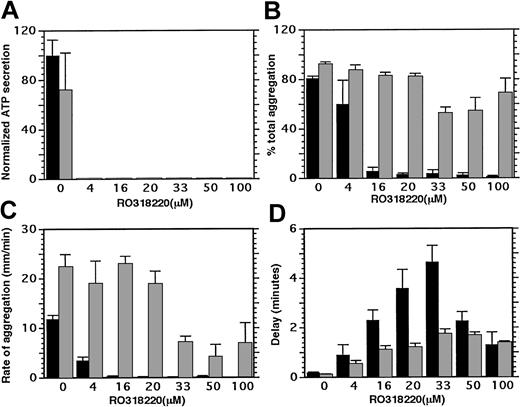
One pathway for signal transduction through PAR 1 is known to proceed through heterotrimeric G protein activation of phospholipase Cβ, whose products include diacylglycerol, a protein kinase C activator. To investigate whether protein kinase C activity provides required signals for thrombin-stimulated platelet-supported thrombin generation, RO318220 was used to inhibit protein kinase C. When RO318220 was titrated into platelet lumiaggregometry studies, 4 μM RO318220 was sufficient to inhibit ATP secretion from dense granules completely (Figure 1A) and inhibit aggregation by 25% relative to controls (Figure 1B). Thrombin-induced aggregation was completely inhibited after treatment with 20 μM RO318220 and small pen deflection was measurable only after a long delay from addition of agonist (Figure 1D). Addition of exogenous fibrinogen at 100 μg/mL had no effect on ATP secretion (Figure 1A, ▦) but restored aggregation to control levels (Figure 1B) in platelets treated with up to 20 μM RO318220. With 30 to 50 μM RO318220 treatment, exogenous fibrinogen did not completely restore platelet aggregation to control levels. With 100 μM RO318220, the delay (Figure 1D) remained abnormal, although fibrinogen appeared to restore the extent (Figure 1B) and the rate (Figure 1C) of aggregate formation to near control levels. Thus, activation of fibrinogen receptors occurred with all concentrations of RO318220 tested, although with some impairment of fibrinogen/receptor interaction at concentrations exceeding that required to inhibit secretion.
Effect of RO318220 on thrombin-stimulated platelet secretion and aggregation. Aspirin-treated, washed, and gel-filtered platelets treated with vehicle or inhibitor were incubated in a lumiaggregometer with calcium, firefly luciferase, with or without fibrinogen (100 μg/mL) before activation with thrombin (0.2 U/mL). Light transmission and ATP production were recorded for 5 to 10 minutes and analyzed for (A) ATP secretion, (B) extent of aggregation (% total), (C) initial rate of aggregation (mm/min), and (D) delay to aggregate formation (minute). ▪ indicates no fibrinogen added; and ▦, fibrinogen present at 100 μg/mL. Means and standard errors were derived from 3 to 7 experiments.
Effect of RO318220 on thrombin-stimulated platelet secretion and aggregation. Aspirin-treated, washed, and gel-filtered platelets treated with vehicle or inhibitor were incubated in a lumiaggregometer with calcium, firefly luciferase, with or without fibrinogen (100 μg/mL) before activation with thrombin (0.2 U/mL). Light transmission and ATP production were recorded for 5 to 10 minutes and analyzed for (A) ATP secretion, (B) extent of aggregation (% total), (C) initial rate of aggregation (mm/min), and (D) delay to aggregate formation (minute). ▪ indicates no fibrinogen added; and ▦, fibrinogen present at 100 μg/mL. Means and standard errors were derived from 3 to 7 experiments.
Flow cytometry was performed on control or RO318220-treated platelets, unactivated or stimulated with thrombin (0.2 U/mL), in the presence of either FITC-labeled antifibrinogen antibody or FITC-labeled CD62P against P-selectin.
Table 1 shows that treatment with RO318220 decreased secreted bound fibrinogen and prevented the fluorescence shift seen when platelets expose secreted P-selectin. Therefore, secretion from α granules was completely eliminated by 16 μM RO318220.
Flow cytometry to detect secretion in response to platelet activation
. | % gated in positive peak . | . | . | ||
|---|---|---|---|---|---|
| . | Fibrinogen . | . | P-selectin IIa, 0.2 U/mL . | ||
| RO318220, μM . | Unactivated . | IIa, 0.2 U/mL . | . | ||
| 0 | 0.2 | 30.0 | 100 | ||
| 4.4 | 0.4 | 24.2 | — | ||
| 16.7 | 0.3 | 2.4 | 0 | ||
| 33.3 | 0.3 | 1.0 | — | ||
. | % gated in positive peak . | . | . | ||
|---|---|---|---|---|---|
| . | Fibrinogen . | . | P-selectin IIa, 0.2 U/mL . | ||
| RO318220, μM . | Unactivated . | IIa, 0.2 U/mL . | . | ||
| 0 | 0.2 | 30.0 | 100 | ||
| 4.4 | 0.4 | 24.2 | — | ||
| 16.7 | 0.3 | 2.4 | 0 | ||
| 33.3 | 0.3 | 1.0 | — | ||
Gel-filtered platelets treated for 15 minutes with DMSO or RO318220 were incubated in either FITC-labeled antifibrinogen or FITC-labeled anti-P-selectin with or without prior activation by thrombin. The platelet population was defined with FITC-anti-GP1b and unlabeled platelets were used to define the negative population. Unactivated platelets showed no positive fluorescence when incubated with FITC-labeled anti-P-selectin.
—indicates not assayed.
Effect on thrombin generation
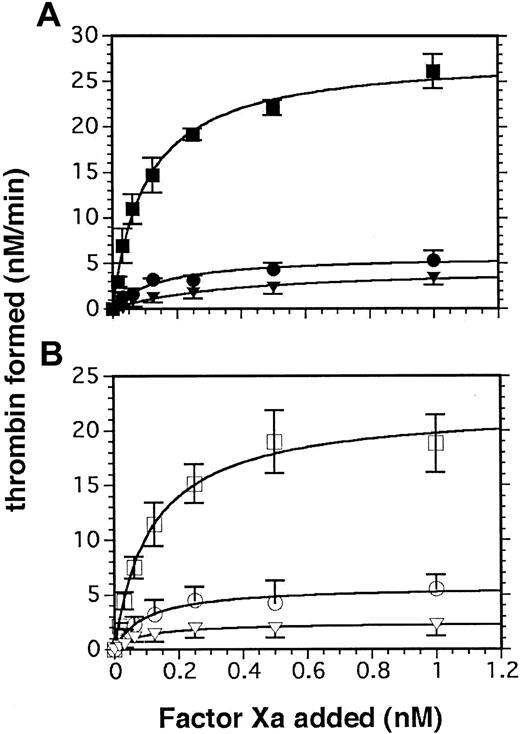
In the absence of exogenous factor Va, concentrations of RO318220 that inhibited α-granule release (a source of factor V as well as fibrinogen) inhibited thrombin generation by approximately 85% whether thrombin or SFLLRN-amide peptide was used as the agonist (Figure 2A-B; Table 2). In the presence of 2 nM exogenous factor Va, thrombin generation on platelets treated with 20 μM RO318220 was inhibited by 25% to 35% compared with DMSO-treated platelets, with agonist stimulation by either thrombin (Figure 3A) or SFLLRN peptide (Figure 3B; Table 2). Thrombin-stimulated platelets (Figure 3A) treated with 20 μM RO318220 (•) displayed less ability to support prothrombinase activity than those treated with 50 μM RO318220 (▴), whereas treatment with 20 or 50 μM RO318220 equally affected the outcome of platelets stimulated with SFLLRN (Figure 3B).
Effect of RO318220 on platelet-supported thrombin generation in the absence of added factor Va. Thrombin generation assays were performed with aspirin-treated, washed, and gel-filtered platelets incubated with vehicle or inhibitor and activated with (A) α thrombin (0.2 U/mL; closed symbols) or (B) SFLLRN (50 μM; open symbols). Thrombin generated was assayed by S-2238 hydrolysis as described in “Materials and methods.” Squares indicate control DMSO-treated platelets; circles, RO318220-treated (20 μM) platelets (3 experiments); and triangles, RO318220-treated (50 μM) platelets (6 experiments). Error bars represent SEM.
Effect of RO318220 on platelet-supported thrombin generation in the absence of added factor Va. Thrombin generation assays were performed with aspirin-treated, washed, and gel-filtered platelets incubated with vehicle or inhibitor and activated with (A) α thrombin (0.2 U/mL; closed symbols) or (B) SFLLRN (50 μM; open symbols). Thrombin generated was assayed by S-2238 hydrolysis as described in “Materials and methods.” Squares indicate control DMSO-treated platelets; circles, RO318220-treated (20 μM) platelets (3 experiments); and triangles, RO318220-treated (50 μM) platelets (6 experiments). Error bars represent SEM.
Kinetic parameters of prothrombinase supported by activated platelets treated with RO318220
. | DMSO control . | . | RO318220, 20 μM . | . | RO318220, 50 μM . | . | RO318220, 100 μM . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agonist . | Maximum activity . | Kd app . | Maximum activity . | Kd app . | Maximum activity . | Kd app . | Maximum activity . | Kd app . | ||||
| No factor Va added | ||||||||||||
| IIa (0.2 U/mL) | 27.9 (0.95) | 0.11 (0.01) | 5.8 (0.44) | 0.14 (0.03) | 4.5 (0.53) | 0.38 (0.10) | — | — | ||||
| SFLLRN (50 μM) | 22.1 (0.72) | 0.12 (0.01) | 5.7 (0.31) | 0.10 (0.02) | 2.4 (0.10) | 0.09 (0.01) | — | — | ||||
| AYPGKF (1 mM) | 14.5 (0.65) | 0.15 (0.02) | — | — | 2.3 (0.17) | 0.11 (0.03) | — | — | ||||
| 2 nM factor Va added | ||||||||||||
| IIa (0.2 U/mL) | 62.3 (1.20) | 0.09 (0.006) | 38.4 (1.04) | 0.08 (0.008) | 51.6 (1.24) | 0.06 (0.006) | 185.3 (3.5) | 0.05 (0.004) | ||||
| SFLLRN (50 μM) | 83.8 (2.26) | 0.07 (0.007) | 54.9 (1.65) | 0.07 (0.007) | — | — | 197.8 (7.7) | 0.1 (0.01) | ||||
| SFLLRN (50 μM) | 57.4 (0.74) | 0.09 (0.004) | — | — | 36.1 (1.02) | 0.06 (0.007) | — | — | ||||
| AYPGKF (1 mM) | 47.9 (0.58) | 0.07 (0.003) | — | — | 52.7 (2.52) | 0.05 (0.01) | — | — | ||||
| 20 nM factor Va added | ||||||||||||
| IIa (0.2 U/mL) | 78.5 (1.74) | 0.04 (0.004) | 74.7 (0.72) | 0.03 (0.001) | — | — | — | — | ||||
| SFLLRN (50 μM) | 86.5 (1.48) | 0.03 (0.002) | 80.7 (1.91) | 0.03 (0.003) | — | — | — | — | ||||
. | DMSO control . | . | RO318220, 20 μM . | . | RO318220, 50 μM . | . | RO318220, 100 μM . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agonist . | Maximum activity . | Kd app . | Maximum activity . | Kd app . | Maximum activity . | Kd app . | Maximum activity . | Kd app . | ||||
| No factor Va added | ||||||||||||
| IIa (0.2 U/mL) | 27.9 (0.95) | 0.11 (0.01) | 5.8 (0.44) | 0.14 (0.03) | 4.5 (0.53) | 0.38 (0.10) | — | — | ||||
| SFLLRN (50 μM) | 22.1 (0.72) | 0.12 (0.01) | 5.7 (0.31) | 0.10 (0.02) | 2.4 (0.10) | 0.09 (0.01) | — | — | ||||
| AYPGKF (1 mM) | 14.5 (0.65) | 0.15 (0.02) | — | — | 2.3 (0.17) | 0.11 (0.03) | — | — | ||||
| 2 nM factor Va added | ||||||||||||
| IIa (0.2 U/mL) | 62.3 (1.20) | 0.09 (0.006) | 38.4 (1.04) | 0.08 (0.008) | 51.6 (1.24) | 0.06 (0.006) | 185.3 (3.5) | 0.05 (0.004) | ||||
| SFLLRN (50 μM) | 83.8 (2.26) | 0.07 (0.007) | 54.9 (1.65) | 0.07 (0.007) | — | — | 197.8 (7.7) | 0.1 (0.01) | ||||
| SFLLRN (50 μM) | 57.4 (0.74) | 0.09 (0.004) | — | — | 36.1 (1.02) | 0.06 (0.007) | — | — | ||||
| AYPGKF (1 mM) | 47.9 (0.58) | 0.07 (0.003) | — | — | 52.7 (2.52) | 0.05 (0.01) | — | — | ||||
| 20 nM factor Va added | ||||||||||||
| IIa (0.2 U/mL) | 78.5 (1.74) | 0.04 (0.004) | 74.7 (0.72) | 0.03 (0.001) | — | — | — | — | ||||
| SFLLRN (50 μM) | 86.5 (1.48) | 0.03 (0.002) | 80.7 (1.91) | 0.03 (0.003) | — | — | — | — | ||||
Gel-filtered, aspirin-treated platelets were incubated with DMSO or various concentrations of RO318220 before activation and assembly with prothrombinase components. Thrombin generation was measured and analyzed as described in “Materials and methods.” Mean maximum activity is measured as nM thrombin formed/min, mean Kd app (dissociation constants) in nM, and standard errors of the means are given in parentheses.
—indicates not assayed.
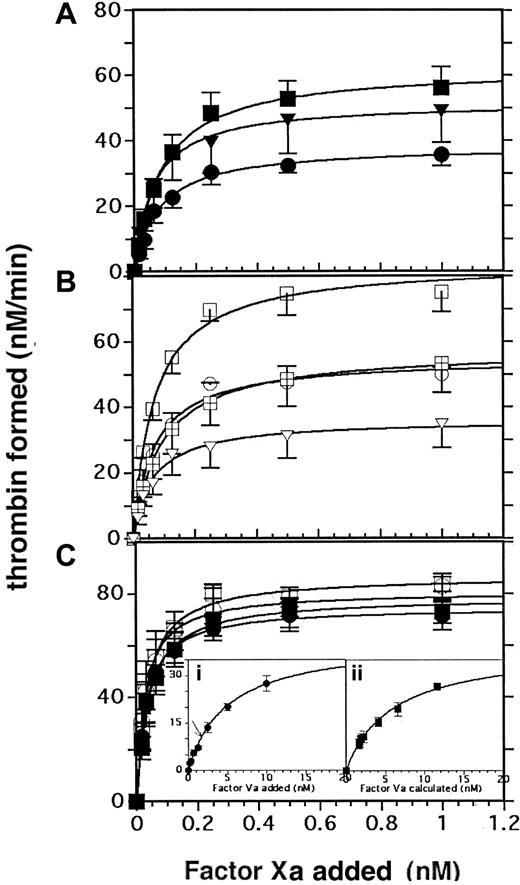
Effect of RO318220 on thrombin generation in the presence of limiting (2 nM) or excess (20 nM) exogenous factor Va. Thrombin generation assays were performed with aspirin-treated, washed, and gel-filtered platelets treated with vehicle or inhibitor and activated with (A) α thrombin (0.2 U/mL; closed symbols) or (B) SFLLRN (50 μM; open symbols). Thrombin was generated in the presence of limiting (2 nM; A-B) or excess (20 nM) factor Va (C), then was assayed by S-2238 hydrolysis as described in “Materials and methods.” Squares indicate DMSO controls; crossed squares (B), matched controls for 50 μM RO318220; circles, 20 μM RO318220; and triangles, 50 μM RO318220. The insets to panel C show factor Va titrations with 1 nM factor Xa using (i) RO318220-treated (20 μM) platelets or (ii) control platelets with the abscissa adjusted to reflect added factor Va plus 1.6 nM released platelet factor Va. Line arrow in inset i indicates the thrombin formed using control platelets with no added factor Va. Means and standard errors are derived from 3 (RO318220 20 μM) or 6 (RO318220 50 μM) experiments performed in duplicate.
Effect of RO318220 on thrombin generation in the presence of limiting (2 nM) or excess (20 nM) exogenous factor Va. Thrombin generation assays were performed with aspirin-treated, washed, and gel-filtered platelets treated with vehicle or inhibitor and activated with (A) α thrombin (0.2 U/mL; closed symbols) or (B) SFLLRN (50 μM; open symbols). Thrombin was generated in the presence of limiting (2 nM; A-B) or excess (20 nM) factor Va (C), then was assayed by S-2238 hydrolysis as described in “Materials and methods.” Squares indicate DMSO controls; crossed squares (B), matched controls for 50 μM RO318220; circles, 20 μM RO318220; and triangles, 50 μM RO318220. The insets to panel C show factor Va titrations with 1 nM factor Xa using (i) RO318220-treated (20 μM) platelets or (ii) control platelets with the abscissa adjusted to reflect added factor Va plus 1.6 nM released platelet factor Va. Line arrow in inset i indicates the thrombin formed using control platelets with no added factor Va. Means and standard errors are derived from 3 (RO318220 20 μM) or 6 (RO318220 50 μM) experiments performed in duplicate.
Factor Va titrations using secretion-inhibited platelets
To address the possibility that added factor Va was limiting leading to greater availability of factor Va to control than to RO318220-treated platelets, factor Va titrations were performed with control DMSO-treated and RO318220-treated (20 μM) platelets stimulated with thrombin (Figure 3Ci-ii). The EC50 for added factor Va was 5 nM on RO318220-treated platelets from which the release of endogenous factor Va was inhibited (Figure 3Ci). Control platelets in the absence of added factor Va supported activity equivalent to release-inhibited platelets to which 1.6 nM exogenous factor Va had been added (arrow inset i). Adjusting the X-axis of the control platelet factor Va titration curve (Figure 3Cii; factor Va calculated) to reflect the additional 1.6 nM factor Va released, the EC50 derived for factor Va was 6 nM, insignificantly different from that for release-inhibited platelets (Figure 3Ci). Furthermore, maximum activity at saturating concentrations of added factor Va was similar for both treated and control platelets (35 nM/min).
When factor Xa titrations were performed in the presence of excess (20 nM) factor Va (Figure 3C), there was no significant inhibition of the maximum response to either thrombin or SFLLRN using platelets treated with 20 μM RO318220 compared with control platelets (Table 2); therefore the 30% difference in activity noted between paired reactions performed in the presence of 2 nM (limiting) factor Va could be attributed to the difference between a total factor Va availability of 3.6 nM in control reactions versus 2 nM in RO318220-treated reactions.
RO318220 titrations reveal an additional cellular target
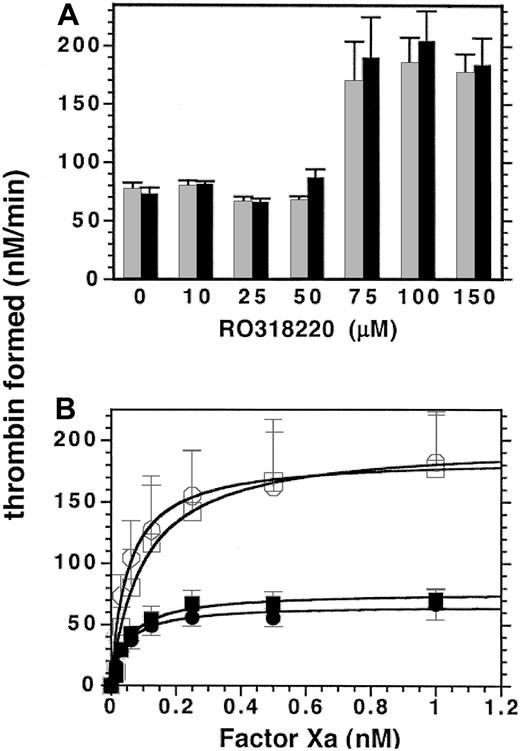
Although minimal RO318220 concentrations that totally inhibit protein kinase C-dependent secretion had no inhibitory effect on platelet-supported thrombin generation, RO318220 has been cited for inhibition of other molecules and for activation of Janus kinase 1 (JNK 1).55,56 To test for other effects of RO318220 on platelet-supported thrombin generation, RO318220 was titrated into kinetic assays of thrombin generation for comparison with DMSO-treated controls. Results are shown in Figure 4. In the presence of 2 nM factor Va, up to 50 μM RO318220 had little if any effect on thrombin generation on platelets stimulated by SFLLRN or thrombin. Surprisingly, under the same conditions, thrombin generation was enhanced by 250% to 300% using platelets treated with 75 to 150 μM RO318220 (Figure 4A). On the contrary, thrombin generation performed in the absence of added factor Va was equally inhibited at all inhibitor concentrations (data not shown), consistent with inhibition of factor Va secretion from α granules. Factor Xa titrations in the presence of 2 nM factor Va comparing control and RO318220-treated (100 μM) platelets showed a 3-fold increased maximum activity (Figure 4B). Analysis of factor Xa titrations comparing the effect of 20, 50, and 100 μM RO318220 treatment of platelets (Table 2) showed no effect on the Kd appFXa.
Effect of higher concentrations of RO318220 on platelet-supported thrombin generation. Thrombin generation assays were performed in the presence of 1 nM factor Xa and 2 nM factor Va, with aspirin-treated, washed, and gel-filtered platelets preincubated with vehicle or either (A) increasing concentrations of RO318220 or (B) 100 μM RO318220 (open symbols) and activated with either α thrombin (0.2 U/mL; A, ▦; B, •) or SFLLRN (50 μM; A, ▪; B, squares). Thrombin generation was assayed by S-2238 hydrolysis as described in “Materials and methods.” Means and standard errors were derived from 4 (A) or 3 (B) experiments.
Effect of higher concentrations of RO318220 on platelet-supported thrombin generation. Thrombin generation assays were performed in the presence of 1 nM factor Xa and 2 nM factor Va, with aspirin-treated, washed, and gel-filtered platelets preincubated with vehicle or either (A) increasing concentrations of RO318220 or (B) 100 μM RO318220 (open symbols) and activated with either α thrombin (0.2 U/mL; A, ▦; B, •) or SFLLRN (50 μM; A, ▪; B, squares). Thrombin generation was assayed by S-2238 hydrolysis as described in “Materials and methods.” Means and standard errors were derived from 4 (A) or 3 (B) experiments.
To confirm the effects of RO318220, another protein kinase C inhibitor GÖ6976 was tested for its effects on platelet-supported prothrombinase activity. There was neither inhibition nor enhancement of prothrombinase activity using platelets treated with GÖ6976 from 2.5 μM, subthreshold for inhibition of aggregation, to 150 μM, although complete inhibition of platelet aggregation occurred with 20-μM treatment.
RO318220 effect on platelet-supported prothrombinase stimulated through PAR 4
The large increase in activity seen with concentrations of RO318220 more than 50 μM suggested that a threshold had been reached at which RO318220 had inhibited a target other than the protein kinase C implicated in secretion. Although 50 μM RO318220 appeared not to affect the prothrombinase reaction with factor Va in excess, differences were noted under conditions of limiting cofactor, depending upon whether thrombin or SFLLRN was used as a platelet agonist (Figure 3A-B). Reactions stimulated with thrombin, but not with SFLLRN, showed less loss of activity after 50 μM RO318220 than after 20 μM RO318220 (Table 2). Since thrombin can activate platelets through both PAR 1 and PAR 4, while SFLLRN peptide acts only upon PAR 1, a PAR 4 peptide AYPGKF was used to stimulate platelets through PAR 4 only. Treatment with 50 μM RO318220 did not inhibit thrombin generation supported by AYPGKF-stimulated platelets in the presence of limiting (2 nM) exogenous factor Va (Table 2). These results suggested that signal transduction through PAR 4 involves a stimulatory pathway different from that of PAR 1 with a lower threshold for RO318220-targeted release from a negative regulatory pathway. They supported the hypothesis that treatment with concentrations of RO318220 in excess of those necessary to inhibit secretion increases exposure of prothrombinase binding sites by either removing some negative regulation or by triggering a pathway not usually accessible to thrombin stimulation alone.
Effect of RO318220 on aminophospholipid exposure
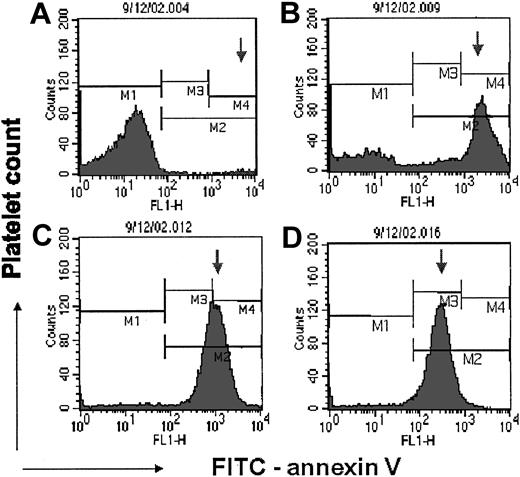
To investigate whether 100 μM RO318220 might increase prothrombinase binding sites by increasing negatively charged phospholipid exposure on thrombin-stimulated platelets, flow cytometry was performed to detect FITC-annexin V binding to RO318220-treated thrombin-activated platelets. RO318220 is a dye that fluoresces with peak emission in the FL-3 window leaking into the FL-2 window; hence, compensation was increased to eliminate overlap of RO318220 emissions in the FL-1 and FL-2 windows. A representative experiment with FITC-annexin V is shown in Figure 5. The results from 8 experiments were compiled in Tables 3 and 4. FITC-annexin V binding was positive on greater proportions with increasing RO318220 (Figure 5; Table 3). Treated with 100 μM RO318220 (Figure 5D), practically all platelets displayed some FITC-annexin V on their surface. Platelets treated with RO318220 at all concentrations tested showed fewer FITC-annexin V binding sites per platelet than controls, with the density of binding sites decreasing with increasing RO318220 (Figure 5 arrows; Table 3). These results suggested that RO318220 treatment allows thrombin to recruit surface changes on an increased population of platelets.
Effect of RO318220 on annexin V binding to thrombin-stimulated platelets. Flow cytometry detected the binding of FITC-labeled annexin V to thrombin-stimulated gel-filtered platelets treated with DMSO (A) or 20 (B), 50 (C), or 100 (D) μM RO318220. Histograms depict the platelet population negative for annexin V in M1, the control platelet population highly positive for annexin V in M4, and the arrow follows the peak positive fluorescence as it moves toward the negative marker with increasing RO318220 treatment. The platelet population was defined by FITC-anti-glycoprotein 1bα (GP1bα) binding. Negative controls containing EDTA were used to set the positive window for FITC-annexin V.
Effect of RO318220 on annexin V binding to thrombin-stimulated platelets. Flow cytometry detected the binding of FITC-labeled annexin V to thrombin-stimulated gel-filtered platelets treated with DMSO (A) or 20 (B), 50 (C), or 100 (D) μM RO318220. Histograms depict the platelet population negative for annexin V in M1, the control platelet population highly positive for annexin V in M4, and the arrow follows the peak positive fluorescence as it moves toward the negative marker with increasing RO318220 treatment. The platelet population was defined by FITC-anti-glycoprotein 1bα (GP1bα) binding. Negative controls containing EDTA were used to set the positive window for FITC-annexin V.
Effect of RO318220 on aminophospholipid exposure
. | Annexin V binding . | . | . | |
|---|---|---|---|---|
. | % platelets positive IIa, 0.2 U/mL (± SE) . | Relative fluorescence IIa, 0.2 U/mL (± SE) . | Platelets as % positive events . | |
| RO318220, μM | ||||
| 0 | 4 (1) | 2100 (550) | 88 | |
| 10 | 40 | 1113 | 97 | |
| 20 | 41 (21) | 1013 (750) | 96 | |
| 50 | 98 | 838 | — | |
| 100 | 86 (21) | 144 (90) | 100 | |
. | Annexin V binding . | . | . | |
|---|---|---|---|---|
. | % platelets positive IIa, 0.2 U/mL (± SE) . | Relative fluorescence IIa, 0.2 U/mL (± SE) . | Platelets as % positive events . | |
| RO318220, μM | ||||
| 0 | 4 (1) | 2100 (550) | 88 | |
| 10 | 40 | 1113 | 97 | |
| 20 | 41 (21) | 1013 (750) | 96 | |
| 50 | 98 | 838 | — | |
| 100 | 86 (21) | 144 (90) | 100 | |
Gel-filtered, aspirin-treated platelets treated with DMSO or RO318220 were activated with thrombin (0.2 U/mL) in the presence of FITC-labeled annexin V. The platelet population was defined by positive FITC-anti-GP1bα fluorescence. Compensation was set to minimize RO318220 fluorescence spillover into either FL-2 or FL-1. The negative gates were set with an EDTA control. Positive events were analyzed within and outside the platelet population. Results were analyzed from 5 experiments.
—indicates not assayed.
Effect of RO318220 on mean platelet forward scatter
. | . | Geometric mean FSC-H . | . | |
|---|---|---|---|---|
| Sample . | Condition . | Platelet mean . | Annexin V-positive mean . | |
| RO318220, μM | ||||
| 0 | Unlabeled | 97 | — | |
| 0 | Unact | 92 | — | |
| 0 | IIa 0.2 U/mL | 79 | 46 | |
| 10 | Unlabeled | 94 | — | |
| 10 | Unact | 101 | — | |
| 10 | IIa 0.2 U/mL | 78 | 52 | |
| 20 | Unlabeled | 104 | — | |
| 20 | Unact | 103 | — | |
| 20 | IIa 0.2 U/mL | 66 | 40 | |
| 100 | Unlabeled | 47 | — | |
| 100 | Unact | 41 | — | |
| 100 | IIa 0.2 U/mL | 56 | 61 | |
| Calpeptin, μM | ||||
| 0 | No | 610 | 353 | |
| 0 | Yes | 655 | 418 | |
| 100 | No | 288 | 288 | |
| 100 | Yes | 599 | 638 | |
. | . | Geometric mean FSC-H . | . | |
|---|---|---|---|---|
| Sample . | Condition . | Platelet mean . | Annexin V-positive mean . | |
| RO318220, μM | ||||
| 0 | Unlabeled | 97 | — | |
| 0 | Unact | 92 | — | |
| 0 | IIa 0.2 U/mL | 79 | 46 | |
| 10 | Unlabeled | 94 | — | |
| 10 | Unact | 101 | — | |
| 10 | IIa 0.2 U/mL | 78 | 52 | |
| 20 | Unlabeled | 104 | — | |
| 20 | Unact | 103 | — | |
| 20 | IIa 0.2 U/mL | 66 | 40 | |
| 100 | Unlabeled | 47 | — | |
| 100 | Unact | 41 | — | |
| 100 | IIa 0.2 U/mL | 56 | 61 | |
| Calpeptin, μM | ||||
| 0 | No | 610 | 353 | |
| 0 | Yes | 655 | 418 | |
| 100 | No | 288 | 288 | |
| 100 | Yes | 599 | 638 | |
Gel-filtered, aspirin-treated platelets were incubated with varying concentrations of RO318220 before activation with thrombin and exposure to FITC-labeled annexin V. Flow cytometry measured forward (FSC-H) versus side scatter (SSC-H) of unlabeled or labeled unactivated or activated platelets (defined by positive staining for FITC-anti-GP1bα).
—indicates not applicable; and Unact, unactivated.
However, there was no direct correlation between increased population displaying annexin V binding sites and increased procoagulant activity, and there was an inverse correlation between the density of annexin V binding sites per platelet and platelet procoagulant activity.
Effect of RO318220 on platelet fragmentation
Table 5 shows that thrombin activation caused a decreased forward scatter (FSC-H) for control platelets, indicating either smaller size or surface changes that scatter light differently. Unactivated platelets treated with up to 20 μM RO318220 showed no change in FSC-H relative to controls, while activation decreased their FSC-H similar to controls. Treatment with 100 μM RO318220 decreased the forward scatter of unactivated platelets by 40%, and activation resulted in no further reduction. Pretreatment of platelets with calpeptin to inhibit calpain-dependent microvesiculation resulted in slightly increased FSC-H of the control platelet population (Table 4) and normalized FSC-H for the platelet population treated with 100 μM RO318220, suggesting that microvesiculation was responsible for the reduced FSC-H. Forward scatter of control platelets positive for annexin V was always decreased relative to that of total control platelet population and remained so even though pretreatment with calpeptin increased their FSC-H values (Table 4) and the density of annexin V binding sites per platelet (Table 5). These latter results suggested that a portion of the FSC-H reduction could be attributed to modified surface characteristics. Pretreatment with calpeptin did not effect either the decreased density of annexin V binding sites or increased recruitment associated with 100 μM RO318220 treatment (Table 5), even though it corrected the FSC-H of these platelets (Table 4).
Effect of RO318220 on calpeptin-pretreated platelets
RO318220, μM . | Calpeptin, 100 μM . | % platelets positive . | Relative fluorescence . | % positive outside platelets . |
|---|---|---|---|---|
| 0 | No | 2.4 | 4000 | 1.7 |
| 0 | Yes | 4.9 | 8000 | 1.8 |
| 100 | No | 97.0 | 280 | 1.3 |
| 100 | Yes | 93.0 | 200 | 0.13 |
RO318220, μM . | Calpeptin, 100 μM . | % platelets positive . | Relative fluorescence . | % positive outside platelets . |
|---|---|---|---|---|
| 0 | No | 2.4 | 4000 | 1.7 |
| 0 | Yes | 4.9 | 8000 | 1.8 |
| 100 | No | 97.0 | 280 | 1.3 |
| 100 | Yes | 93.0 | 200 | 0.13 |
Gel-filtered, aspirin-treated platelets pretreated 10 minutes with 100 μM calpeptin prior to being treated with DMSO or RO318220 were activated with thrombin (0.2 U/mL) in the presence of FITC-labeled annexin V. The platelet population was defined by positive FITC-anti-GP1b fluorescence. Compensation was set to minimize RO318220 fluorescence spillover into either FL-2 or FL-1. The negative gates were set with an EDTA control. Positive events were analyzed within and outside the platelet population. Results were averaged from 3 experiments.
Effect of RO318220 on procoagulant microparticle formation
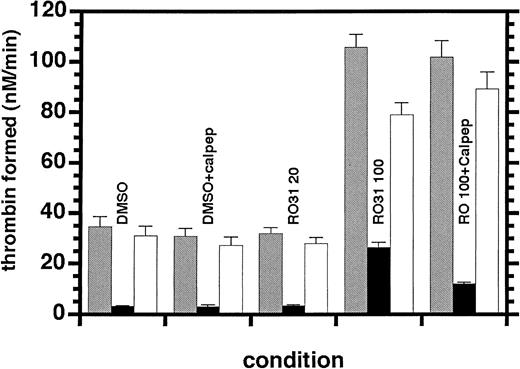
The prothrombinase potentiation seen at higher concentrations of RO318220 could result from greater microvesiculation than would normally result from the relatively low concentrations of agonists used in these assays. Microparticle formation was investigated by assaying the supernatant from activated inhibitor-treated platelets and comparing the results with the prothrombinase activity assayed in the complete activation mixture. In Figure 6, it can be seen that the activity associated with the 800g supernatant of the DMSO control platelets constituted less than 10% of the activity measured in the complete mixture. This was unchanged for platelets treated with 20 μM RO318220. When these platelets were pretreated with 100 μM calpeptin to inhibit the calpain-dependent mechanism of microvesiculation, there was no effect on this supernatant-associated prothrombinase activity that could result partially from the shear forces on centrifuged platelets. The supernatant from 100-μM RO318220-treated platelets contained 25% of the 3-fold enhanced activity of the complete mixture. When platelets were pretreated with 100 μM calpeptin before treatment with 100 μM RO318220, this supernatant-associated activity was reduced to 12% of the 3-fold enhanced total activity. Therefore, the potentiation of prothrombinase by treatment with 100 μM RO318220 may be accompanied by increased microvesiculation but is not dependent upon it.
Effect of RO318220 on microvesicle-supported thrombin generation. Thrombin generation was assayed in the presence of 1 nM factor Xa, 20 nM factor Va, and 1 μM prothrombin using the complete activation mixture (▦) or the 800g supernatant (▪) of aspirin-treated, washed, and gel-filtered platelets (half of which were preincubated with 100 μM calpeptin) before being incubated with vehicle, 20, or 100 μM RO318220 and activated with SFLLRN (30 μM). Thrombin generation was assayed by S-2238 hydrolysis as described in “Materials and methods.” Activities associated with the platelet surfaces (□) were calculated by subtraction of supernatant activities from those of the complete mixtures. Means and standard errors were derived from 8 experiments.
Effect of RO318220 on microvesicle-supported thrombin generation. Thrombin generation was assayed in the presence of 1 nM factor Xa, 20 nM factor Va, and 1 μM prothrombin using the complete activation mixture (▦) or the 800g supernatant (▪) of aspirin-treated, washed, and gel-filtered platelets (half of which were preincubated with 100 μM calpeptin) before being incubated with vehicle, 20, or 100 μM RO318220 and activated with SFLLRN (30 μM). Thrombin generation was assayed by S-2238 hydrolysis as described in “Materials and methods.” Activities associated with the platelet surfaces (□) were calculated by subtraction of supernatant activities from those of the complete mixtures. Means and standard errors were derived from 8 experiments.
Flow cytometry experiments (not requiring centrifugation and thus not subjected to those shear forces) revealed 2% to 10% of total events positive for annexin V binding fell outside of the defined platelet population of control and treated samples stimulated with 0.2 U thrombin/mL (Tables 3-4). Interestingly, calpeptin pretreatment did not appear to decrease that number for control platelets, whereas calpeptin pretreatment of 100-μM RO318220-treated samples decreased 90% of extra platelet annexin V binding (only 1.3% of positive events). These results confirmed that only minor procoagulant microvesiculation accompanied activation with low levels of thrombin with or without RO318220 treatment.
Discussion
When activated by the strong agonists thrombin or collagen, platelets undergo surface changes that support formation of the intrinsic pathway factor X activation complex and prothrombinase complex. Platelet activation comprises many bifurcating and overlapping pathways leading to specific functional expression. It is expected that distal steps leading to each functional expression would be specific to the functional end point; however, steps closer to receptor activation may also differ for a particular functional end point.
This report focuses on thrombin-induced signaling, specifically through the PAR 1 receptor that activates through a heterotrimeric Gq protein, phospholipase Cβ, whose action on phosphatidylinositol 4,5-bisphosphate initiates a bifurcated pathway through protein kinase C activation and increased cytoplasmic ionized calcium concentration. This bifurcated pathway has been implicated in platelet secretion and aggregation.35 In the expectation that these signaling events would be implicated in the platelet procoagulant response as well, RO318220 a known inhibitor of protein kinase C was used to investigate the necessity for protein kinase C signaling.
The results indicate that both conventional protein kinase C activity and molecules secreted from granules as a result of protein kinase C activity are not required for surface support of thrombin generation. Although platelet factor V sequestered in α granules and supplied by the release reaction as factor Va is a convenient source of the cofactor for platelet-supported thrombin generation, the complex can form with extracellular factor Va available in the absence of secretion. Microvesicle formation does not substantially contribute to thrombin generation when platelets are activated with thrombin at its EC50 in the absence of stirring. Finally, the data reveal a regulatory pathway that contains a molecular target for higher concentrations of RO318220 than that required for inhibition of secretion. The regulatory pathway leading to increased surface activation either precedes or parallels microvesiculation, which is also increased by treatment with high concentrations of RO318220. This regulatory pathway either provides constitutive negative regulation of surface procoagulant activity that is overcome with the combination of thrombin (or SFLLRN) and RO318220 or represents a stimulatory pathway usually inaccessible to thrombin alone.
Lumiaggregometry, used as a control to test the effect of RO318220 on platelet function, determined that RO318220 inhibits thrombin-stimulated dense granule secretion at lower concentrations (2-4 μM) than α-granule secretion (8-10 μM) without affecting activation of fibrinogen receptors integrin α2bβ3 until higher concentrations (25-50 μM).
Thrombin-generation assays showed similar kinetics to previous reports.57 The Kd appFXa derived from these results was approximately 0.08 nM, comparable with that calculated by Tracy et al.20,57 This report identifies 1.6 nM platelet factor Va released from 6 × 109 platelets/L or approximately 1 U of factor Va activity per 108 platelets, a value similar to that (0.17-0.35 U of factor Va activity per 108 platelets) measured by Kane et al.58 Factor Va titrations performed here determined the EC50 for factor Va as 5 nM, compared with the 1 nM Kd app determined in the cited reports.
There are reports in the literature that activation of integrin α2bβ3 requires signaling through heterotrimeric Gi protein mediated by adenosine diphosphate (ADP) interaction with its P2Y12 receptor, even when thrombin is the agonist.59 In the presence of added fibrinogen, platelet aggregation was unaffected by the presence of 4 μM RO318220, which completely inhibited release from dense granules. This implies normal activation of fibrinogen receptors in the absence of ADP signaling. There was, however, an increased delay to aggregate formation (Figure 1D) in the presence of added fibrinogen, suggesting either an impairment in the number of receptors activated per platelet or integrin activation by an alternate pathway.
Other reports have suggested that activation of integrin α2bβ3 is required60-62 or not required31,63 for full expression of prothrombinase activity. Physiologically, plasma fibrinogen or fibrin accumulation could occupy either integrin α2bβ3 or glycoprotein Ib, as previously suggested.60,61 However, at concentrations of RO318220 (25-50 μM) that compromised activation of integrin α2bβ3 (decreased rate and extent, increased delay of aggregation in the presence of exogenous fibrinogen) as well as inhibited fibrinogen secretion, the prothrombinase activity in the presence of excess factor Va was unaffected. The present data do not support the requirement for fibrinogen signaling through integrin α2bβ3 receptors or for fibrin interaction with glycoprotein Ib for optimal thrombin-activated platelet-supported thrombin generation.
Although thrombin generation was unaffected by RO318220 concentrations that appeared to delay activation of fibrinogen receptors, thrombin generation was 3-fold higher than control values after treatment with 100 μM RO318220. A lower-affinity RO318220 target must modulate platelet-supported thrombin generation as part of a pathway controlling exposure of prothrombinase binding sites since there was no effect on Kd appFXa. On the contrary, GÖ6976, a protein kinase C inhibitor more specific for the α and β isotypes, neither inhibited nor enhanced prothrombinase at concentrations that completely inhibited aggregation or at concentrations 8-fold above that. These data not only corroborate the conclusion that secretion and calcium-dependent protein kinase C activity is not required for platelet procoagulant activity but they suggest that either calcium-independent protein kinase C (δ or ϵ)or another RO318220 target are involved in the regulation of platelet procoagulant activity.
RO318220 potentiation of platelet prothrombinase activity was postulated to involve either an increased density of platelet binding sites for the prothrombinase complex or increased platelet responsiveness to thrombin, above the small subpopulation of platelets that has been reported to respond to thrombin with binding sites for factor IXa.64 Detection of annexin V binding studies was undertaken to differentiate between these 2 possibilities. Binding of annexin V has been used to detect exposure of aminophospholipids on the surface of activated or apoptotic blood cells and has been correlated with platelet procoagulant activity.41,51,65 We expected to find no difference in annexin V binding between 20-μM RO318220-treated and control platelets because RO318220 has no effect on prothrombinase activity at this concentration. On the other hand, we expected to find increased annexin V binding on 100-μM RO318220-treated platelets which support increased prothrombinase activity.
Although only a small number of activated control platelets bound a high density of annexin V, a greater proportion of thrombin-activated platelets bound annexin V at reduced density of binding sites per platelet when treated with increasing RO318220 concentrations. There was a lack of correlation between the effect of a given concentration of RO318820 on annexin V binding sites and on prothrombinase activity. Thus, a greater number of 10- to 20-μM-treated platelets bound less annexin V per platelet than did control platelets despite there being no difference in support of thrombin generation, while practically all 100-μM-treated platelets bound some annexin V and supported 3-fold thrombin generation compared with controls. The discrepancy between annexin V binding and procoagulant complex assembly in RO318220-treated platelets may represent differences in their respective binding requirements. Although much is known of the lipid classes required for annexin V or procoagulant protein binding, little is known of the lipid configurations required for either. It has been shown that 5% phosphatidylserine exposure is necessary for annexin V binding,66 which may be more or less than required for procoagulant complex assembly on a physiologic surface. Thrombin-stimulated platelets positive for annexin V binding were characterized by a decreased forward scatter, implying that thrombin stimulates surface changes as well as annexin V binding. RO318220 treatment appears to sensitize a greater proportion of platelets to respond to thrombin with surface changes including exposure of aminophospholipids allowing low density annexin V binding.
An increase of prothrombinase binding sites as well as a decreased forward scatter could be achieved by microvesiculation. Microvesicles originating from activated platelets or other blood cells have been implicated in unregulated procoagulant activity.67 Microvesicles can bind procoagulant proteins68-70 since they represent a portion of the activated cellular surface freed from cellular regulation in which aminophospholipidtranslocase reasserts the normally quiescent surface phenotype.21,44,71 Thrombin activation of platelets has not been associated with a large exposure of aminophospholipids or with much microvesiculation without the synergy provided by collagen.14,72 Indeed, the present results confirm that more than 90% of the prothrombinase activity associated with thrombin-activated platelets can be attributed to the platelet surface. Since the remaining prothrombinase activity is unaffected by pretreatment with 100 μM calpeptin, it may result from unpelleted small platelets rather than from microvesicles. This is in agreement with the finding73 that adherent platelets rather than microvesicles derived from adherent platelets are responsible for procoagulant activity. However, the decreased forward scatter of platelets binding annexin V suggests either the loss of membrane resulting from vesiculation and/or surface differences resulting in decreased light scatter.
Platelets treated with 100 μM RO318220 showed a decreased mean forward scatter as well as greater supernatant-associated prothrombinase activity, both of which suggest increased platelet fragmentation and/or surface changes. The 100-μM calpeptin pretreatment of 100-μM RO318220-treated platelets was sufficient to completely block calpain-dependent microvesiculation74 as suggested by the normalized FSC-H results (Table 4) and decreased by 50% the activated platelet supernatant support of prothrombinase (Figure 6). However, the total procoagulant activity of the inhibitor-treated platelet preparation was equivalent in the presence and absence of calpain-dependent microvesiculation. The 2 steps, exposure of a procoagulant surface and microvesiculation, have been previously shown to be separable and perhaps independent.42,45,75,76 The current results support the notion that RO318220-assisted conversion of a quiescent surface to a procoagulant surface precedes or parallels the formation of microvesicles.
Use of the protein kinase C selective inhibitor RO318220 has shown that protein kinase C activity directly stimulated by the Gq-coupled thrombin signaling pathway and required for secretion is not required for thrombin-triggered surface changes supporting thrombin generation. However, these data suggest that there is a RO318220 target controlling maintenance of platelet quiescence and that RO318220 inhibition of this target increases platelet responsiveness to thrombin resulting in surface changes supporting prothrombinase complex assembly.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2003-03-0734.
Supported by National Institutes of Health Research Grant HL70683 and American Heart Association Grant PA/DE 0151561U.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Grateful appreciation is due to Mariola Marcinkiewicz for excellent technical assistance, to Drs Dipali Sinha and Peter N. Walsh for constant encouragement, and to Patricia Pileggi for her kind administrative and word processing assistance. Mayumi Katuoka provided expert assistance with flow cytometry.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal