Abstract
Notch1 activity is essential for the specification of T-lineage fate in hematopoietic progenitors. Once the T-cell lineage is specified, T-cell precursors in the thymus must choose between αβ and γδ lineages. However, the impact of Notch1 signaling on intrathymic pro-T cells has not been addressed directly. To approach this issue, we used retroviral vectors to express constitutively active Notch1 in human thymocyte progenitors positioned at successive developmental stages, and we followed their differentiation in fetal thymus organ culture (FTOC). Here we show that sustained Notch1 signaling impairs progression to the double-positive (DP) stage and efficiently diverts the earliest thymic progenitors from the main αβ T-cell pathway toward development of γδ T cells. The impact of Notch1 signaling on skewed γδ production decreases progressively along intrathymic maturation and is restricted to precursor stages upstream of the pre-T-cell receptor checkpoint. Close to and beyond that point, Notch1 is not further able to instruct γδ cell fate, but promotes an abnormal expansion of αβ-committed thymocytes. These results stress the stage-specific impact of Notch1 signaling in intrathymic differentiation and suggest that regulation of Notch1 activity at defined developmental windows is essential to control αβ versus γδ T-cell development and to avoid deregulated expansion of αβ-lineage cells. (Blood. 2003;102:2444-2451)
Introduction
Notch receptors are highly conserved transmembrane proteins that regulate many cell-fate decision processes in a wide spectrum of cell types.1,2 Notch1, one of 4 family members, binds multiple ligands including Delta/Serrate and Jagged. On ligand binding, the Notch1 protein is proteolytically cleaved by a presenilin-dependent γ-secretase activity to release its intracellular domain (ICN1), which represents an activated form of Notch1 that behaves as a transcriptional regulator.2,3 Recent evidence supports a key role for Notch1 signaling at multiple steps in T-cell development.1,2 Specifically, Notch1 activity regulates T/B-lineage specification and directs T-cell lineage commitment within the thymus, likely by blocking the differentiation of a common lymphoid progenitor (CLP) into the B-cell lineage.4-8 Once the T-cell lineage is specified, a pro-T cell has to face several postcommitment decisions, including the αβ versus γδ and CD4 versus CD8 lineage choices, which take place at defined intrathymic maturation stages9 and can also be influenced by Notch1.10,11
Development of αβ T cells involves progression of early thymic precursors through a number of developmental checkpoints that have been well defined in mice and humans.12,13 Intrathymic CLPs reside within the CD44+CD25- (CD34hi in humans) subset of CD3-CD4-CD8- triple-negative (TN) thymocytes (TN1 stage). CLPs can generate B cells, natural killer (NK) cells, dendritic cells (DCs), and CD44+CD25+ (CD34lo in humans) pro-T cells, which still lack productive rearrangements at the γ, δ, and β T-cell-receptor (TCR) loci14,15 (TN2 stage). Complete γ and δ transcription and onset of TCRβ gene rearrangements occur at the downstream TN3 stage of CD44-CD25+ thymocytes (CD4 immature single-positive [CD4 ISP] in humans), which actively tran scribe the pre-TCRα (pTα) gene. Only those TN3 pre-T cells that accomplish a functional TCRβ rearrangement produce a TCRβ chain that associates with pTα and CD3 chains to form a pre-TCR complex, which signals progression to the CD44-CD25- (double-positive [DP] CD3lo in humans) of β-selected pre-T cell (TN4 stage), and results in cellular expansion and differentiation into conventional DP thymocytes. Following completion of TCRα rearrangements, DP thymocytes express the αβ TCR, undergo positive and negative selection, and finally become either CD4+ or CD8+ single-positive (SP) mature αβ T cells.
The decision to enter the γδ lineage is taken during this maturation process, likely from a T-committed αβ/γδ bipotential precursor.9,12,16 However, the particular intrathymic stage at which γδ T-lineage precursors branch off the main αβ T-cell pathway remains unclear,9 and the precise mechanisms involved in this process are poorly understood, although successful TCR rearrange ments at the γ and δ versus the β loci,16,17 basic helix-loop-helix (bHLH) transcription factors,18 interleukin 7 receptor (IL-7R) expression,19,20 and Notch1 signaling21,22 seem to be implicated. Experimental evidence for the role of Notch1 in αβ/γδ-lineage choice came initially from studies of Washburn and colleagues, who found that a reduction in the Notch1 gene dosage in bone marrow chimeric mice affected more severely αβ than γδ T-cell development, suggesting that Notch1 signaling favors αβ over γδ T-cell fate.21 However, mutant mice lacking the Notch ligand Jagged2 showed a selective defect in the generation of thymic γδ T cells, which implies that Notch signaling actually favors γδ T-cell differentiation in some situations.22 A more recent study by the Zúñiga-Pflücker group have shown that Notch signals mediated by Delta-like-1 play a key role in the generation of both αβ and γδ T cells from hematopoietic precursors.23 These contradictory results could reflect nonredundant specific functions of individual Notch family members and ligands in a particular microenvironment or developmental context and, therefore, the outcome of Notch signaling may depend on the particular experimental approach used.24 In this regard, different effects are observed when Notch1 activity is modified very early in bone marrow stem cells or later in developing thymocytes,2,25 suggesting that strict regulatory mechanisms may control Notch1 activity at defined temporal windows during T-cell development. In fact, whereas Notch1 activity is essential for T-lineage specification from bone marrow progenitors,4 it seems dispensable for the generation of both αβ and γδ T cells beyond the pre-TCR checkpoint (late TN3 stage)26 ; but αβ development is partially impaired and γδ development is unaffected when Notch1 activity is disrupted at the early TN3 stage.25 However, the impact of Notch1 signaling on pro-T cells passing through the transition from TN1 to TN3 stages has not been addressed directly. This appears an essential issue because αβ/γδ T-cell specification must be confined to this period of uncertain Notch1 dependence. To approach this question, we have used a retroviral vector to express constitutively active Notch1 in early human intrathymic precursors positioned at successive developmental stages from TN1 to TN4, and we followed their differentiation kinetics in fetal thymus organ culture (FTOC). Unexpectedly, we found that sustained Notch1 signaling upstream of the pre-TCR checkpoint impairs progression of early thymic progenitors along the αβ T-cell differentiation pathway, but simultaneously promotes their development toward γδ T cells. However, at or beyond that point Notch1 activity is not further able to favor γδ T-cell fate but promotes an abnormal expansion of αβ-lineage thymocytes.
Materials and methods
Intrathymic cell subsets
Normal human postnatal thymocytes were isolated from thymus fragments removed during corrective cardiac surgery of patients aged 1 month to 4 years, after informed consent was provided. Total thymocytes were depleted of lineage-positive cells by magnetic cell sorting (AutoMacs, Miltenyi Biotech, Bergisch Gladbach, Germany), using anti-CD3, anti-CD1a, anti-CD19, anti-CD56, and anti-HLA-DR magnetic beads. TN1 (CD34hi CD44hi) and TN2 (CD34lo CD44lo) stage thymocytes were sorted from the recovered Lin- fraction (FACStar plus, BD Biosciences, Erembodegem, Belgium) as described.27 T-committed CD4 ISP (TN3 stage) precursors were isolated from HLA-DR-, CD8-, and CD3-depleted thymocytes by immunomagnetic sorting with anti-CD4-phycoerythrin (PE) and anti-PE MicroBeads (Miltenyi Biotech). Isolation of pre-TCR+ DP thymocytes was performed after depletion of mature T cells by 2 rounds of autoMACS sorting with anti-TCRαβ (Bma031),28 provided by Dr R. Kurrle (Behringwerke, Marburg, Germany) and anti-TCRγδ (TCRδ1),29 provided by Dr M. Brenner (Brigham and Women's Hospital, Boston, MA) monoclonal antibodies (mAbs), plus goat antimouse IgG MicroBeads, and subsequent sorting of CD8αβ+ cells with anti-CD8β-PE (Coulter Immunotech, Marseille, France) plus anti-PE MicroBeads. Recovered cells displayed the DP CD3lo TCRαβ- TCRγδ- reported for β-selected pre-TCR+ thymocytes placed at the TN4 stage.14 Sorted populations were proved 99% or more pure on reanalysis.
Retroviral constructs and transduction of thymocytes
The retroviral vectors encoding the entire intracellular domain of human Notch1 (ICN1) and green fluorescent protein (GFP) from a bicistronic transcript (MigR1-ICN1), or GFP alone (MigR1-GFP), were provided by Dr J. C. Aster (Brigham and Women's Hospital, Harvard Medical School, Boston, MA).30 Retroviral supernatant was collected after 48 to 72 hours of transfection from 293T-based Phoenix-amphotropic packaging cells, provided by Dr G. Nolan (Stanford University, CA). Original studies on MigR1-ICN1-transduced cells validated the use of GFP as a surrogate marker for ICN1 expression,6 and successful ICN1 expression was also confirmed in our laboratory by Western blot analysis of ICN1-transduced Jurkat cells (not shown).
For retroviral transductions, TN1 and TN2 pro-T cells were cultured overnight with recombinant human interleukin 7 (rhIL-7; 100 IU/mL), rhIL-6 (50 IU/mL), rhIL-3 (5 IU/mL), rh stem cell factor (rhSCF; 100 IU/mL), and rh Flt3-ligand (rhFlt3-L; 50 IU/mL) from the National Institute of Biological Standards and Controls (NIBSC; Potters Bar, Hertfordshire, United Kingdom). Cells were then incubated for 7 to 8 hours with virus supernatants in plates coated with 30 μg/mL rh fibronectin fragment CH-296 (RetroNectin; Takara, Otsu, Japan). TN3 and TN4 pre-T-cell subsets were transduced immediately after isolation by overnight incubation with virus supernatants plus 100 IU/mL rhIL-7.
Hybrid human-mouse FTOC
Deoxyguanosine (dGuo)-treated thymic lobes from 15-day-old Swiss mouse embryos31 were seeded (one lobe/well) in Terasaki plates (Nunc, Roskilde, Denmark) together with ICN1- or mock-transduced progenitors (2 × 104/well). After 48 hours of culture in hanging drops (day 0 of FTOC), lobes were cultured on gelfoam/filters for up to 5 weeks.31 To assess transduction efficiencies, percentage of GFP+ cells was evaluated at day 0 by flow cytometry (EPICS Profile, Coulter Electronics, Hialeah, FL). T-cell generation was analyzed on electronically gated GFP+ cells recovered from different lobes pooled at the indicated time points. No significant differences were observed in the development of GFP- nontransduced progenitors relative to GFP+ mock-transduced controls (data not shown). For comparative purposes, absolute numbers of transduced cells recovered from lobes were normalized to 2 × 104 GFP+-transduced cells seeded per hanging drop/thymic lobe. Because cells entering the lobes were estimated to represent 10% of input cell numbers,32 normalized values correspond to 2 × 103 cells seeding each lobe. When indicated, FTOCs seeded with untransduced TN1 progenitors were treated at 12-hour intervals with 50 μM difluoro ketone γ-secretase inhibitor DFK167 (ICN Pharmaceuticals, Aurora, OH), delivered in dimethyl sulfoxide (DMSO), or the solvent DMSO alone as control.
Flow cytometry analysis
The following directly labeled mAbs were used: CD8α-PE, or CD8α-PECy5, and TCRγδ-PE-Cy5 from Caltag Laboratories (South San Francisco, CA); CD3-PE, CD5-PE, Vδ2-PE, Vγ9-PE, and CD33-PE from BD Biosciences; and CD1a-RD1, CD4-RD1, CD8β-PE, CD4-PE-Cy5, CD34-PE-Cy5, and TCRαβ-PE-Cy5 from Beckman Coulter (Marseille, France). Unlabelled Vδ1 was from Beckman Coulter and antimouse IgG1-PE was from Caltag. Data were collected on viable cells as determined by appropriate electronic gating on forward and side scatter light parameters. Several preliminary experiments based on propidium iodide (PI) staining validated the use of this approach to exclude dead cells (< 1%) in these cultures. Isotype-matched irrelevant antibodies from Caltag were used as negative controls to define background fluorescence.
Results
T-cell development of human intrathymic TN1 precursors expressing constitutively active Notch1 in human-mouse FTOC
To investigate the impact of sustained Notch1 signaling on the earliest human intrathymic precursors, CD34hi Lin- (> 99% pure) sorted thymocytes (referred to as TN1 stage) were transduced with bicistronic retroviral vectors encoding either ICN1 and GFP or GFP alone,6,30 and their T-cell developmental potential was then examined in a human-mouse (hu/mo) FTOC assay, which faithfully recapitulates thymocyte development in vitro and allows us to perform time-course studies.
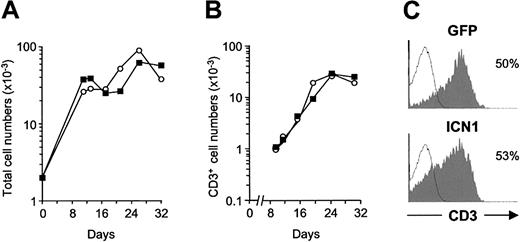
Kinetic analyses on electronically gated GFP+ cells showed that cellular reconstitution of thymic lobes with ICN1- or mock-transduced TN1 progenitors mimicked that observed with their GFP- nontransduced counterparts, so that frequencies of GFP+ cells (9.5% ± 4.5% and 10% ± 1.2 for ICN1- and mock-transduced cells, respectively, in 4 experiments) and GFP expression levels (mean fluorescence intensity [MFI] ± SD, 27.6 ± 11.9 and 48.0 ± 19.0, respectively) remained essentially unchanged throughout culture. Accordingly, absolute numbers of GFP+ cells recovered from lobes seeded with ICN1-transduced TN1 precursors were equivalent to those derived from controls at every time point analyzed (Figure 1A), indicating that sustained Notch1 signaling did not appreciably perturb the developmental kinetics or the proliferation rate of TN1 progenitors. As shown in Figure 1A, time-course phenotypic analyses revealed that, after an initial phase of cellular expansion, very few (3%-4%) TN1 progenitors had become CD3+ T cells by day 10 to 12, but CD3+ cells derived from ICN1- and mock-transduced progenitors increased gradually thereafter with overlapping kinetics and reached maximal numbers by day 24 (Figure 1B). Because no significant differences in the differentiation kinetics, absolute numbers (Figure 1B), and surface CD3 levels (Figure 1C) of ICN1- and mock-transduced CD3+ cells were detected throughout culture, our results indicate that sustained Notch1 signaling does not appreciably disturb the generation of CD3+ T-lineage cells from TN1 thymic precursors in FTOCs.
Generation of CD3+ T cells from human CD34hi thymic lymphoid precursors expressing a constitutively active form of Notch1. Development of T cells from human CD34hi thymic lymphoid precursors transduced with a retroviral vector encoding either the entire intracellular domain of human Notch1 (ICN1) and GFP from a bicistronic transcript, or GFP alone, was analyzed in a hybrid hu/mo FTOC assay. Absolute numbers of total (A) and CD3+ cells (B) derived from ICN1-(▪) or mock-transduced (○) progenitors calculated on electronically gated GFP+ cells at the indicated culture times. Absolute numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. (C) CD3 expression levels determined by flow cytometry on GFP+-gated cells derived from ICN1-transduced (ICN1) or mock-transduced (GFP) progenitors after 32 days of culture (shaded histograms). Background staining (empty histograms) was determined with isotype-matched irrelevant antibodies. Results are representative of 4 independent experiments.
Generation of CD3+ T cells from human CD34hi thymic lymphoid precursors expressing a constitutively active form of Notch1. Development of T cells from human CD34hi thymic lymphoid precursors transduced with a retroviral vector encoding either the entire intracellular domain of human Notch1 (ICN1) and GFP from a bicistronic transcript, or GFP alone, was analyzed in a hybrid hu/mo FTOC assay. Absolute numbers of total (A) and CD3+ cells (B) derived from ICN1-(▪) or mock-transduced (○) progenitors calculated on electronically gated GFP+ cells at the indicated culture times. Absolute numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. (C) CD3 expression levels determined by flow cytometry on GFP+-gated cells derived from ICN1-transduced (ICN1) or mock-transduced (GFP) progenitors after 32 days of culture (shaded histograms). Background staining (empty histograms) was determined with isotype-matched irrelevant antibodies. Results are representative of 4 independent experiments.
Sustained Notch1 signaling in TN1 intrathymic precursors favors the generation of γδ T-lineage cells in FTOCs
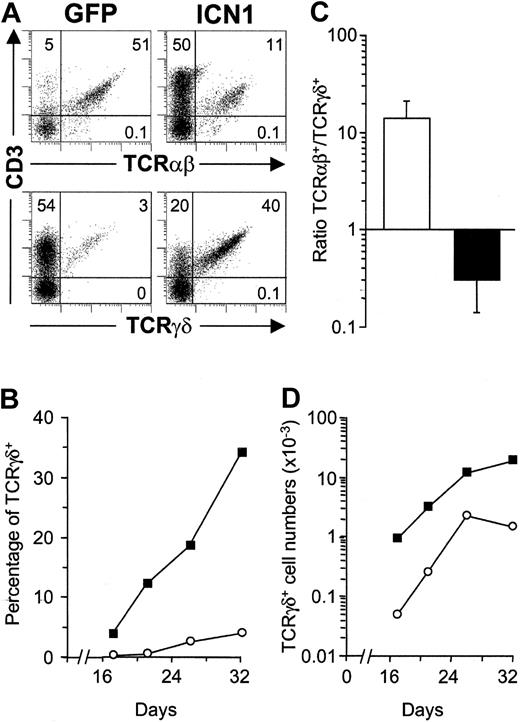
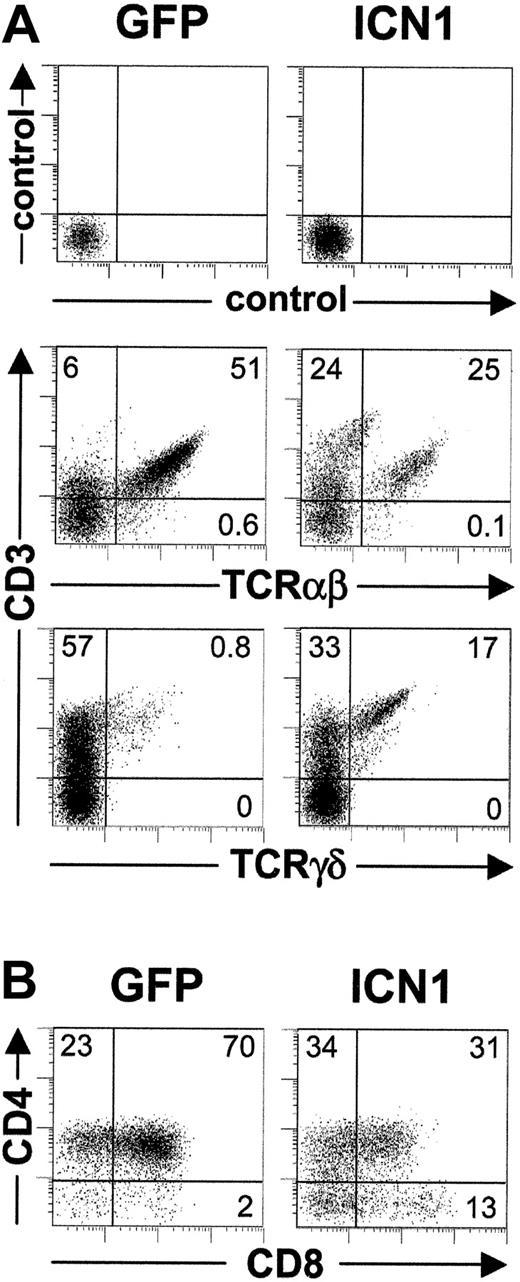
Phenotypic analyses on the coexpression of CD3 and TCR revealed striking differences between ICN1- and mock-transduced CD3+ cell progenies developing in FTOCs (Figure 2). Expectedly, most (≥ 95%) CD3+ T cells derived from TN1 controls expressed a mature αβ TCR, whereas only a minor fraction (≤ 5%) were γδ T cells (Figure 2A). In contrast, a major proportion (up to 80%) of CD3+ T cells derived from ICN1+ progenitors coexpressed a γδ TCR, whereas TCRαβ+ cells represented a minor population (up to 20% in Figure 2A). Time-course experiments revealed that the biased γδ T-cell production induced by Notch1 occurred throughout the culture period (Figure 2B) and resulted in up to 24-fold increased proportions of TCRγδ+ T cells (17.7 ± 6.2 in 4 independent experiments). More importantly, absolute γδ T-cell numbers were also markedly increased (18.5 ± 4.3-fold) in FTOCs (Figure 2D), and paralleled a simultaneous decrease (4.0 ± 1.5-fold) of TCRαβ+ cells, whereas total cell numbers remained unchanged relative to controls (Figure 4A). As a result, an inverse TCRαβ+ to TCRγδ+ ratio was consistently found in lobes reconstituted with ICN1-transduced progenitors as compared with mock-transduced controls (0.3 ± 0.2 versus 14.3 ± 6.5, respectively; Figure 2C). Despite these marked differences, both the ICN1+ and the control γδ T-cell progenies increased throughout culture with parallel kinetics (Figure 2D), indicating that no selective growth advantage was conferred by Notch1 signaling to γδ T cells. Taken together, these data suggested that sustained Notch1 signaling instructs TN1 intrathymic precursors to develop into γδ T cells at the expense of αβ T cells in FTOC.
Sustained ICN1 expression in CD34hi thymic lymphoid progenitors results in a skewed production of γδ T cells at the expense of αβ T cells. (A) Flow cytometry analysis of CD3 versus TCRαβ or TCRγδ expression on electronically gated GFP+ progenies derived from ICN1-transduced (ICN1) or mock-transduced (GFP) CD34hi thymic lymphoid progenitors at day 32 in FTOC. Numbers represent percentages in each quadrant. Percentages (B) and absolute numbers (D) of TCRγδ+ cells recovered from lobes seeded with ICN1- (▪) or GFP-transduced (○) progenitors at the indicated days of culture. (C) TCRαβ+/TCRγδ+ cell ratios recovered from mock-transduced (open bars) or ICN1-transduced (filled bars) thymic lymphoid precursors (14.3 ± 6.5 and 0.3 ± 0.2, respectively). Values are the mean ± SD of absolute cell number ratios observed at 11 different time points along FTOCs from 4 independent experiments.
Sustained ICN1 expression in CD34hi thymic lymphoid progenitors results in a skewed production of γδ T cells at the expense of αβ T cells. (A) Flow cytometry analysis of CD3 versus TCRαβ or TCRγδ expression on electronically gated GFP+ progenies derived from ICN1-transduced (ICN1) or mock-transduced (GFP) CD34hi thymic lymphoid progenitors at day 32 in FTOC. Numbers represent percentages in each quadrant. Percentages (B) and absolute numbers (D) of TCRγδ+ cells recovered from lobes seeded with ICN1- (▪) or GFP-transduced (○) progenitors at the indicated days of culture. (C) TCRαβ+/TCRγδ+ cell ratios recovered from mock-transduced (open bars) or ICN1-transduced (filled bars) thymic lymphoid precursors (14.3 ± 6.5 and 0.3 ± 0.2, respectively). Values are the mean ± SD of absolute cell number ratios observed at 11 different time points along FTOCs from 4 independent experiments.
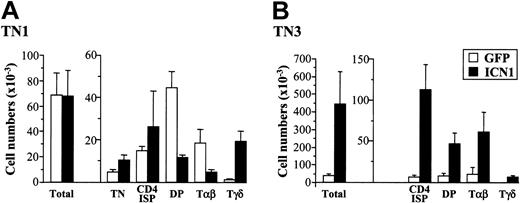
Differential effects of ICN1 expression on αβ versus γδ T-cell development from human pro-T (TN1) and pre-T (TN3) cells. Cell numbers for the indicated subsets derived from ICN1- or mock-transduced CD34hi pro-T cell precursors (TN1) and CD4 ISP T-committed pre-T cells (TN3) were determined after 20 to 30 days in a hu/mo FTOC assay. Absolute numbers were calculated as the mean ± SD of at least 14 samples from 3 to 4 independent experiments. Numbers were normalized to 2 × 103 GFP+ transduced cells/lobe.
Differential effects of ICN1 expression on αβ versus γδ T-cell development from human pro-T (TN1) and pre-T (TN3) cells. Cell numbers for the indicated subsets derived from ICN1- or mock-transduced CD34hi pro-T cell precursors (TN1) and CD4 ISP T-committed pre-T cells (TN3) were determined after 20 to 30 days in a hu/mo FTOC assay. Absolute numbers were calculated as the mean ± SD of at least 14 samples from 3 to 4 independent experiments. Numbers were normalized to 2 × 103 GFP+ transduced cells/lobe.
Enforced expression of constitutive active Notch1 impairs transition of early thymic precursors to the DP stage
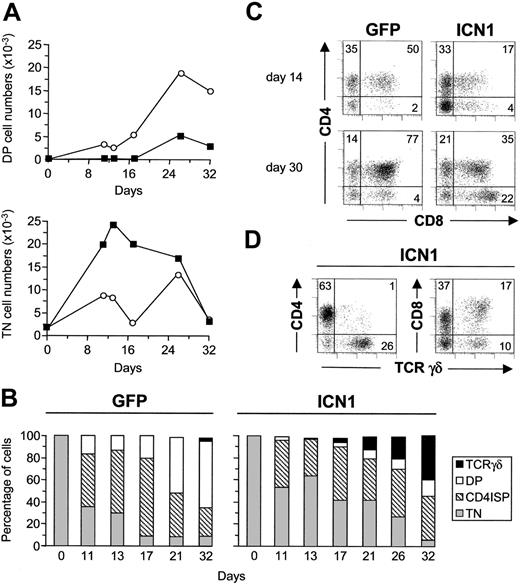
To investigate the reasons for the inefficient production of αβ T cells from ICN1-transduced precursors, we next analyzed their potential to generate DP thymocytes in FTOC. As shown in Figure 3, a gradual decrease of TN progenitors paralleled the increasing production of DP thymocytes in control lobes. However, DP thymocyte production was severely impaired in both absolute and relative terms in lobes seeded with ICN1-transduced progenitors. Absolute numbers of ICN1+ DP cells were reduced throughout culture (Figure 3A), but this reduction was particularly significant from day 20 (Figure 4A), when a major proportion (52% ± 12%) of control thymocytes has reached the DP stage (Figure 3B). As expected of a developmental blockade, the impaired DP production resulted in the accumulation of ICN1+ TN thymocytes, which was particularly significant during the first 15 days of culture (5.5 ± 3.0-fold increased TN cell numbers in 4 experiments; Figure 3A-C). However, ICN1+ TN thymocytes did not remain in the lobes as undifferentiated precursors; rather, they differentiated thereafter into γδ T-lineage cells (Figure 3B). As a whole, these data indicate that Notch1 signaling prevents TN thymocytes from progressing to the DP stage and simultaneously promotes their development into γδ T cells in FTOC.
Sustained expression of activated Notch1 impairs CD34hi thymic lymphoid precursors from progressing into the DP stage. (A) Absolute numbers of CD4+CD8+ (DP) and CD3-CD4-CD8- (TN) cells derived from ICN-transduced (▪) or mock-transduced (○) CD34hi thymic lymphoid progenitors at the indicated time points in FTOCs. Numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. (B) Percentages of TN, CD4 ISP, DP, and TCRγδ+ cells derived from ICN1- (ICN1) or mock-transduced (GFP) progenitors at the indicated time points in FTOC. (C) Flow cytometry analysis of CD4 versus CD8 expression on GFP+ progenies derived from ICN1-transduced (ICN1) or mock-transduced (GFP) progenitors after 14 and 30 days in FTOC. (D) Expression of CD4 or CD8 versus TCRγδ on ICN1-transduced progenitors after 30 days in FTOCs. Results are representative of 4 independent experiments.
Sustained expression of activated Notch1 impairs CD34hi thymic lymphoid precursors from progressing into the DP stage. (A) Absolute numbers of CD4+CD8+ (DP) and CD3-CD4-CD8- (TN) cells derived from ICN-transduced (▪) or mock-transduced (○) CD34hi thymic lymphoid progenitors at the indicated time points in FTOCs. Numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. (B) Percentages of TN, CD4 ISP, DP, and TCRγδ+ cells derived from ICN1- (ICN1) or mock-transduced (GFP) progenitors at the indicated time points in FTOC. (C) Flow cytometry analysis of CD4 versus CD8 expression on GFP+ progenies derived from ICN1-transduced (ICN1) or mock-transduced (GFP) progenitors after 14 and 30 days in FTOC. (D) Expression of CD4 or CD8 versus TCRγδ on ICN1-transduced progenitors after 30 days in FTOCs. Results are representative of 4 independent experiments.
Because transition from TN to DP thymocytes occurs in humans through a transient TN3 stage of CD4 ISP thymocytes, our data may also reflect that Notch1 signaling impairs the transition of TN to CD4 ISP. Supporting this possibility, reduced frequencies of CD4 ISP thymocytes expressing ICN1 were recovered relative to controls during the first 20 days of culture, although significant CD4 ISP cell numbers were still produced (Figure 3B). However, production of ICN1+ CD4 ISP thymocytes was relatively increased at late time points (Figure 4A), likely due to their accumulation resulting from their impaired potential to generate DP thymocytes. These data allowed us to conclude that sustained Notch1 signaling impairs progression throughout successive developmental stages along the αβ T-cell lineage but induces early thymic progenitors to develop into γδ T cells.
As shown in Figure 3D, phenotypic analyses on γδ T-cell progenies revealed that most (two thirds) γδ cells derived from ICN1+ TN1 thymocytes were CD8+ SP, whereas a minor proportion displayed the expected DN phenotype and few if any CD4+ SP TCRγδ+ cells were found. In contrast, most (∼80%) TCRγδ+ cells derived from mock-transduced progenitors were DN cells, whereas CD8+ cells represented a minor γδ T-cell fraction (data not shown). No differences, however, were observed between ICN1+ and control γδ T cells regarding expression of markers such as CD1 and CD5. More importantly, further analyses on the Vγ and Vδ gene usage of the γδ T cells generated revealed that both ICN1- and mock-transduced γδ T cells displayed frequencies of Vγ9, Vδ2, and Vδ1 gene expression equivalent to those on untransduced γδ T cells or γδ thymocytes found in vivo (data not shown), suggesting that the ICN1+ γδ T-cell progeny was representative of normal γδ T-cell development. Collectively, our results are compatible with the possibility that sustained Notch1 signaling efficiently diverts early thymic progenitors from the main αβ T-cell pathway toward development of CD8+ SP (and to a lesser extent DN) γδ T cells in FTOC.
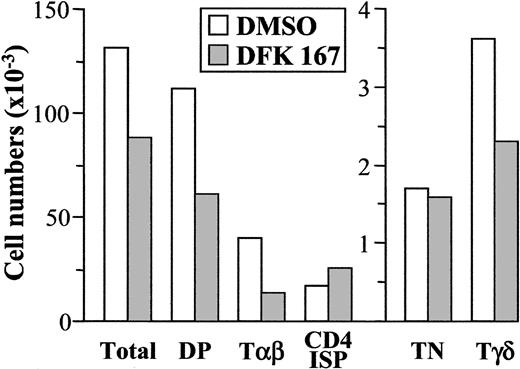
Previous studies in mice have shown that pharmacologic inhibition of γ-secretase in FTOC recapitulates the physiologic effects observed for loss or reduction of Notch1 function on murine T-cell development.33,34 Thus, this experimental system offers a unique tool to evaluate the consequences of lowering Notch1 activity on thymocyte maturation in humans. To approach this issue, the maturation of normal untransduced TN1 thymocytes was next analyzed in hu/mo FTOC treated with a dose of 50 μM of the γ-secretase inhibitor DFK167, proved to interfere with murine thymocyte development in FTOC.33 As shown in Figure 5, we found a significant reduction of total cell numbers in DFK167-treated lobes, which resulted mostly from a decreased production of DP and, consequently, TCRαβ+ thymocytes. The impaired αβ production was not accompanied by a relative increase of TCRγδ+ cells; rather, γδ T-cell generation was also decreased at similar proportions. This was a specific effect on thymocyte maturation, because CD4 ISP precursors were consistently increased and numbers of TN progenitors did not change significantly. These data suggest that lowering Notch1 activity by γ-secretase inhibitors may affect γδ development at the TN stage and also impairs the CD4 ISP to DP transition in the αβ T-cell pathway, but does not affect generation of CD4 ISP thymocytes from TN1 progenitors. Collectively, our results support a functional role for Notch1 activity in the development of both αβ and γδ T-cell lineages in humans.
Impaired development of both αβ and γδ T cells from the earliest human thymic precursors in hu/mo FTOCs treated with the γ-secretase inhibitor DFK167. Fetal murine thymic lobes seeded with normal TN1 human thymocytes were cultured in the presence of DFK167 γ-secretase inhibitor (50 μM) or DMSO, added fresh every 12 hours for 19 days. Absolute numbers of cells for the indicated subsets were normalized to 2 × 103 cells/lobe. Results are representative of 2 independent experiments.
Impaired development of both αβ and γδ T cells from the earliest human thymic precursors in hu/mo FTOCs treated with the γ-secretase inhibitor DFK167. Fetal murine thymic lobes seeded with normal TN1 human thymocytes were cultured in the presence of DFK167 γ-secretase inhibitor (50 μM) or DMSO, added fresh every 12 hours for 19 days. Absolute numbers of cells for the indicated subsets were normalized to 2 × 103 cells/lobe. Results are representative of 2 independent experiments.
Decreased susceptibility of TN2 pro-T cells to Notch1-induced γδ T-cell development
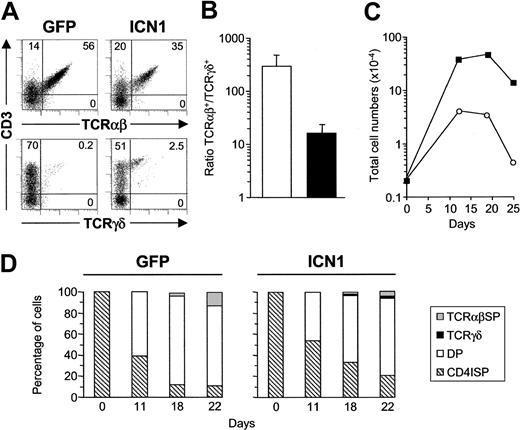
To investigate whether the Notch1-induced γδ cell fate determination effect observed in FTOC was restricted to the earliest TN1 thymocytes, we next analyzed the impact of sustained Notch1 signaling on more mature CD34lo TN2 stage pro-T cells. As observed with TN1, ICN1 expression did not significantly affect total cell yields in lobes seeded with TN2 thymocytes, ruling out a selective growth advantage of ICN1+ cells; neither did it affect the generation of total CD3+ T cells (Figure 6A). However, as shown for TN1 progenitors, Notch1 signaling promoted an increased (up to 20-fold) production of γδ T cells from TN2 thymocytes. This was coupled with a decreased generation (2- to 4-fold) of DP thymocytes and TCRαβ+ T cells (Figure 6A-B), which resulted in the production of similar proportions of αβ and γδ cells (Figure 6A). Although the net increase of γδ production from ICN1+ TN2 thymocytes was less pronounced than from TN1 progenitors (Figure 2C), a reduced αβ/γδ ratio was consistently found relative to controls. Therefore, TN2 pro-T cells are less prone to Notch1-induced γδ-lineage specification than TN1 progenitors, but still display the potential to adopt a γδ-cell fate at the expense of αβ T-cell production.
Impact of sustained ICN1 expression on T-cell development from CD34lo thymic pro-T cells. Flow cytometry analysis of either CD3 versus TCRαβ or TCRγδ expression (A) or CD4 versus CD8 expression (B) performed after 30 or 19 days of FTOC, respectively, on electronically gated GFP+ cells generated from ICN1-transduced (ICN1) or mock-transduced (GFP) CD34lo thymic pro-T cells. Results are representative of 3 independent experiments.
Impact of sustained ICN1 expression on T-cell development from CD34lo thymic pro-T cells. Flow cytometry analysis of either CD3 versus TCRαβ or TCRγδ expression (A) or CD4 versus CD8 expression (B) performed after 30 or 19 days of FTOC, respectively, on electronically gated GFP+ cells generated from ICN1-transduced (ICN1) or mock-transduced (GFP) CD34lo thymic pro-T cells. Results are representative of 3 independent experiments.
Sustained Notch1 signaling does not prevent TN3 thymocytes from adopting an αβ T-cell fate but promotes cellular expansion
The reduced impact of ICN1 expression on TN2 pro-T cells compared with TN1 progenitors would suggest that the timing of Notch1 signaling is critical for lineage choice and fate determination of developing thymocytes. To approach this issue, we next investigated the effect of Notch1 activity on more mature CD4 ISP thymocytes, which are fully T-committed progenitors positioned at the TN3 stage, just immediately upstream of the pre-TCR checkpoint.14 Importantly, CD4 ISP thymocytes represent the human equivalents of early DN3 murine thymocytes, known to be the main targets of genetic modifications driven by the lck proximal promoter.25,35 As shown in Figure 7A, γδ production derived from ICN1+ TN3 thymocytes was very low (1%-3%), but still higher than that observed from TN3 controls (0.1%-0.2%), indicating that the impact of Notch1 signaling on γδ development was very weak at the TN3 stage. Still, however, frequencies of CD3+ αβ-lineage cells, including TCRαβ+ thymocytes and CD3lo TCRαβ- TCRγδ- β-selected pre-TCR+ cells, were slightly decreased in lobes seeded with ICN1+ TN3 thymocytes (Figure 7A). Accordingly, a decreased αβ/γδ T-cell ratio was observed relative to control lobes (16.0 ± 7.6 and 296.0 ± 177.0, respectively; Figure 7B). Therefore, despite the fact that normal ex vivo isolated TN3 thymocytes have a negligible γδ developmental potential (30-fold lower than TN1 precursors), constitutive Notch1 signaling is still able to divert a minor proportion toward a γδ T-cell fate, but does not impair most TN3 progenitors from adopting an αβ T-cell fate (Figure 4B).
Sustained Notch1 signaling does not prevent CD4 ISP thymocytes from adopting an αβ T-cell fate, but enhances cellular expansion. (A) Flow cytometry analysis of CD3 versus TCRαβ or TCRγδ expression on electronically gated GFP+ cells generated from ICN1-transduced (ICN1) or mock-transduced (GFP) CD4 ISP thymic precursors after 12 days in FTOC. (B) TCRαβ+/TCRγδ+ cell ratios recovered from mock-transduced (open bars) and ICN1-transduced (filled bars) CD4 ISP thymic precursors (296 ± 177 and 16 ± 7.6, respectively). Values are the mean ± SD of absolute cell number ratios observed at 5 different time points along FTOC. (C) Absolute numbers of GFP+-gated total cells derived from ICN1- (▪) or mock-transduced (○) CD4 ISP thymocytes in FTOCs at the indicated time points. Absolute numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. (D) Percentages of CD4 ISP, DP, TCRγδ+, and TCRαβ+ SP cells derived from ICN1- (ICN1) or mock-transduced (GFP) CD4 ISP progenitors at the indicated time points in FTOCs. Results are representative of 3 independent experiments.
Sustained Notch1 signaling does not prevent CD4 ISP thymocytes from adopting an αβ T-cell fate, but enhances cellular expansion. (A) Flow cytometry analysis of CD3 versus TCRαβ or TCRγδ expression on electronically gated GFP+ cells generated from ICN1-transduced (ICN1) or mock-transduced (GFP) CD4 ISP thymic precursors after 12 days in FTOC. (B) TCRαβ+/TCRγδ+ cell ratios recovered from mock-transduced (open bars) and ICN1-transduced (filled bars) CD4 ISP thymic precursors (296 ± 177 and 16 ± 7.6, respectively). Values are the mean ± SD of absolute cell number ratios observed at 5 different time points along FTOC. (C) Absolute numbers of GFP+-gated total cells derived from ICN1- (▪) or mock-transduced (○) CD4 ISP thymocytes in FTOCs at the indicated time points. Absolute numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. (D) Percentages of CD4 ISP, DP, TCRγδ+, and TCRαβ+ SP cells derived from ICN1- (ICN1) or mock-transduced (GFP) CD4 ISP progenitors at the indicated time points in FTOCs. Results are representative of 3 independent experiments.
Although GFP expression levels of ICN1- and mock-transduced TN3 precursors remained constant throughout culture (MFI ± SD, 26.6 ± 10.2 and 61.8 ± 9.8, respectively), overexpression of ICN1 in TN3 thymocytes led to increased frequencies of GFP+ cells (12.0 ± 6.0-fold increase relative to 1.3 ± 0.4-fold increase in mock-transduced controls, from day 0 to day 30 in 4 experiments). This effect resulted in a 13.6 ± 5.3-fold increase of total ICN1+ cell numbers (Figure 4B). As shown in Figure 7C, the increased ICN1+ cell production was restricted to the first 10 to 12 days of culture, coinciding with an initial phase of cellular expansion that paralleled a modest thymocyte maturation in control lobes (about 50% CD4 ISP+, < 1% TCRγδ+, < 10% TCRαβ+ T cells; Figure 7D and data not shown). Total numbers of ICN1+ and control thymocytes remained essentially unchanged until day 18 to 20, and decreased thereafter with overlapping kinetics (Figure 7C). Therefore, Notch1 signaling may enhance the intrinsic proliferation potential of CD4 ISP thymocytes at the TN3 stage in FTOC. Accordingly, numbers of ICN1+ CD4 ISP cells were increased (16.6 ± 9.1-fold in 4 experiments) relative to controls (Figure 4B). The capacity of Notch1 to expand the CD4 ISP precursor pool resulted in a relative delay in the generation of DP thymocytes, although ICN1-transduced CD4 ISP progenitors progressed finally to the DP stage (Figure 7D) and gave rise to an enlarged production of TCRαβ+ thymocytes. TCRγδ+ cells were also increased in absolute terms compared with TN3 controls (Figure 4B). Collectively, our data indicate that most developing thymocytes that reach the CD4 ISP TN3 stage in vivo have become fully αβ committed and refractory to the γδ-cell fate switching effect induced by Notch1, but highly susceptible to ICN1-induced proliferation.
Enforced expression of activated Notch1 drives cellular expansion of β-selected pre-T cells and improves αβ T-cell generation
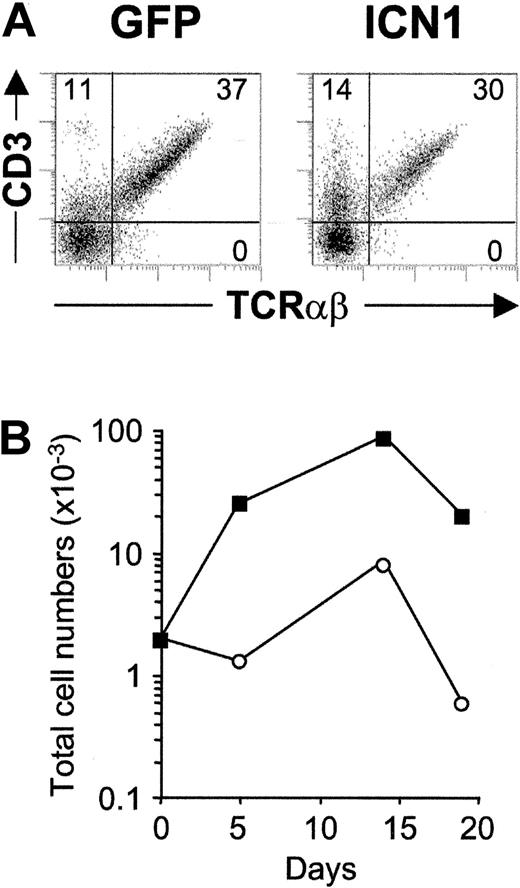
To next investigate whether Notch1 signaling could also be involved in the expansion of recent β-selected thymocytes, we directly addressed the impact of constitutive ICN1 expression on ex vivo isolated TN4 stage DP pre-T cells, which express low levels of the CD3-pre-TCR complex (DP CD3lo). As shown in Figure 8A, frequencies of TCRαβ+ cells generated in lobes seeded with ICN1-transduced DP CD3lo progenitors were similar to (or slightly lower than) those obtained in control lobes (1.1 ± 0.1-fold increased). However, frequencies of GFP+ cells increased throughout culture and paralleled a dramatic (18.6 ± 9.5-fold) increase of ICN1+ absolute cells numbers (Figure 8B), which resulted in an equivalent increase in TCRαβ+ T-cell numbers relative to controls. In contrast, γδ T-cell production was essentially undetectable in lobes seeded with ICN1- or mock-transduced TN4 thymocytes, confirming the current view that β-selected pre-T cells are irreversibly committed to the αβ T-cell lineage. We therefore concluded that enforced Notch1 signaling confers a proliferative advantage to β-selected thymocytes but does not impair their development into mature TCRαβ+ T cells nor can it induce diversion into the γδ T-cell pathway.
Enforced expression of activated Notch1 drives cellular expansion of β-selected pre-T cells and increases αβ T-cell generation. (A) Flow cytometry analysis of CD3 versus TCRαβ expression on electronically gated GFP+ cells generated from ICN1-transduced (ICN1) or mock-transduced (GFP) β-selected DP CD3lo thymocytes after 14 days in FTOC. (B) Total cell numbers of GFP+-gated cells recovered from ICN1-transduced (▪) or GFP-transduced (○) β-selected thymocytes at the indicated time points in FTOC. Absolute numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. Results are representative of 3 experiments.
Enforced expression of activated Notch1 drives cellular expansion of β-selected pre-T cells and increases αβ T-cell generation. (A) Flow cytometry analysis of CD3 versus TCRαβ expression on electronically gated GFP+ cells generated from ICN1-transduced (ICN1) or mock-transduced (GFP) β-selected DP CD3lo thymocytes after 14 days in FTOC. (B) Total cell numbers of GFP+-gated cells recovered from ICN1-transduced (▪) or GFP-transduced (○) β-selected thymocytes at the indicated time points in FTOC. Absolute numbers were normalized to 2 × 103 GFP+ transduced cells/lobe. Results are representative of 3 experiments.
Discussion
In this study, attempts to compare the impact of Notch1 signaling at individual stages during intrathymic development have shown that sustained expression of constitutively active Notch1 efficiently promotes development of human pro-T cells toward γδ T cells, but interferes with the generation of αβ T cells in FTOC. The skewed production of γδ T cells is coupled with an impaired transition to the DP stage, suggesting that it occurs at the expense of αβ T-cell differentiation. These Notch1-induced effects are shown to be dependent on the developmental stage and restricted to early intrathymic precursors positioned upstream of the pre-TCR checkpoint. Close to and beyond this point, Notch1 activity is not further able to instruct γδ differentiation but promotes an abnormal expansion of αβ-committed thymocytes, which results in an increased production of αβ T cells.
The effects of sustained Notch1 activity in early progenitors developing in FTOC resemble those well characterized previously in invertebrate development, where enforced expression of Notch promotes increased commitment of bipotent progenitors to one cell fate at the expense of the other.3,36 Similarly, ICN1 expression instructs common T/B progenitors to adopt a T-cell fate at the expense of B cells.6 Thus, our results suggest a novel role for Notch1 in γδ cell fate determination of intrathymic αβ/γδ T-cell precursors, and allowed us to identify key developmental stages at which Notch1 activity can induce γδ-lineage precursors to split off from the αβ T-cell program. This proposal contrasts with the current belief that Notch1 signaling favors αβ over γδ T-cell development,21 but it fits the recent finding that mice lacking the Notch-ligand Jagged2 have reduced γδ T-cell numbers.22 Further evidence supporting a role for Notch1 in γδ T-lineage development was recently obtained from elegant experimental approaches by Schmitt and Zúñiga-Pflücker,23 who showed that Notch signals mediated by Delta-like-1 are sufficient to promote efficient development in vitro of both αβ and γδ T-lineage cells from prethymic hematopoietic progenitors. Although a potential role for Notch signaling in αβ versus γδ commitment was not directly addressed in that study, unequivocal evidence was provided that Notch is involved in the generation of both αβ and γδ T cells, a finding that concurs with our observation that lowering Notch activity with γ-secretase inhibitors affects the generation of both T-cell lineages. Nonetheless, if Notch signaling favors γδ over αβ development as proposed in the present study, reduced Notch signaling would be expected to induce an increased αβ T-cell production at the expense of γδ T cells. In this regard, it is worth noting that increased numbers of CD4 ISP αβ-committed progenitors were recovered from lobes treated with γ-secretase inhibitors, suggesting that the TN1-to-CD4 ISP (TN3) transition was not impaired. This resulted in an increased CD4 ISP/γδ T-cell ratio relative to controls, whereas a decreased CD4ISP/γδ ratio was consistently observed in lobes seeded with TN1 progenitors overexpressing active Notch1. Although clarification of the physiologic relevance of these findings demands suitable in vivo approaches in mice, our results likely reflect the involvement of Notch signaling at multiple steps in T-cell development, including maturation toward αβ or γδ lineages from TN1 to TN3 stages as well as expansion of β-selected αβ T-lineage cells at later stages.
Collectively, the discordant developmental outcomes obtained from different studies stress the relevance of the particular experimental approach used. Therefore, a key aspect of our study relies on the observed stage-specific effect of Notch1 in γδ-cell fate determination, which was dramatic at the TN1 stage and resulted in a reversed αβ/γδ ratio, but decreased at the TN2 stage, and was hardly detectable in TN3 thymocytes. Likewise, the γδ precursor potential of normal thymocytes was found to drop progressively at sequential maturation stages (about 30-fold from TN1 to TN3), indicating that most progenitors that reach the TN3 stage are actually αβ-committed progenitors that have already lost the capacity to develop along the γδ lineage. These data allowed us to conclude that intrathymic precursors commit to the γδ lineage and branch off the αβ lineage very early in development, mostly at the TN1, and to a lesser extent at the TN2 stages, and thus the proposed role of Notch1 on γδ-cell fate determination must be restricted to this narrow developmental window. In this scenario, increased numbers of γδ thymocytes could be expected from mice reconstituted with ICN1-transduced bone marrow progenitors6,37,38 if Notch1 instructs γδ T-cell fate determination in vivo, as found in FTOC. However, no kinetic analyses on γδ T-cell production have been reported from those mice and, thus, the possibility that Notch1 signaling actually favors γδ T-cell fate early in T development cannot be formally excluded. A careful examination of γδ production in mice overexpressing Notch would also be relevant considering the reported age-dependent propensity of Notch transgenic mice to develop T cell tumors,6,39,40 which could obscure the effects of activated Notch1 on αβ/γδ-lineage choice, as has been reported for CD4/CD8-lineage commitment.41 This possibility is further supported by the fact that ICN1-promoted cellular expansion and leukemic transformation are both dependent on pre-TCR signaling,37,39,42 and restricted to αβ-lineage cells expressing a functional TCRβ chain.37 Therefore, available information derived in vivo concurs with our proposal that Notch1 signaling increases TCRαβ+ thymocyte production in FTOCs when expressed at or beyond the pre-TCR checkpoint. This explains our finding that decreased Notch signaling by γ-secretase inhibition affects maturation of αβ T cells at the TN3 stage. Reciprocally, sustained Notch1 signaling at the TN3 and TN4 stages led to increased proportions of GFP+ αβ-lineage cells, whereas equivalent GFP+ cell numbers were recovered from lobes seeded with ICN1- or mock-transduced progenitors placed at TN1 or TN2 stages. As a whole, these data argue against a selective growth advantage or survival of ICN1-transduced progenitors in FTOCs before the pre-TCR checkpoint.
Our experimental approach has proved useful to reveal the intrinsic γδ potential of yet uncommitted αβ/γδ pro-T-cell subsets, which provides an explanation for the observation that modifications of Notch1 activity driven under the control of the lck promoter at the TN2 to TN3 transition did not affect γδ T-cell production in mice.21,25 Moreover, it can explain very recent findings by De Smedt and colleagues showing that ICN1 overexpression increases γδ T-cell frequencies from whole unfractionated CD34+ human thymocytes highly enriched in TN2 stage progenitors, but does not result in the dramatic skewing shown in this study for TN1 stage thymocytes.43 In this scenario, it can be proposed that, after initial specification of T-lineage fate, there is a period of T-cell development when αβ/γδ thymic progenitors lose progressively their potential to adopt a γδ-cell fate, but simultaneously become increasingly restricted to the αβ T-cell differentiation program and commit irreversibly to the αβ lineage at the early TN3 stage, just prior to the β-selection checkpoint.
This view has important implications regarding the question of whether and to what extent signaling through either a TCRγδ or a pre-TCR complex determines the αβ/γδ-lineage choice of precursor thymocytes. To date, this crucial issue remains highly controversial,17,44 but recent evidence against an instructive role of surface receptors on αβ/γδ fate specification have been provided by Kang and coworkers in mice.20 Our finding that the human thymocyte subsets susceptible to ICN1-induced skewed γδ production (TN1 and TN2) still lack functional rearrangements at the γ, δ, and β TCR loci,14,15 would provide further support for a TCR-independent model of αβ/γδ-lineage commitment in humans. Likewise, αβ commitment must be specified in vivo prior to achievement of a functional V-D-Jβ rearrangement because most (> 95%) thymocytes at the TN3 stage are refractory to the ICN1-induced switching effect and behave as αβ-committed precursors, while still displaying a germline configuration at the TCRβ loci.14,15 Therefore, Notch1 signaling could reasonably be at least one of the factors driving the γδ-cell fate choice upstream to TCRγδ expression.
Collectively, all these results provide support for a model in which early T-committed αβ/γδ precursors that receive a sustained Notch1 signal at the TN1 (or TN2) stages are directed toward the γδ T-cell lineage, whereas those that receive no or only a weak signal would efficiently progress to the TN3 stage and differentiate into αβ T cells. This model does not preclude that Notch1 activity is also required for several nonredundant functions necessary for αβ development, such as pTα gene transcriptional upregulation45 and complete VDJβ rearrangements.25 Because both events occur in vivo at the TN3 stage,14,46 such a role for Notch1 must be confined to this particular developmental point. Nonetheless, down-regulation of Notch1 should be mandatory at or beyond the pre-TCR checkpoint (late TN3 stage) to avoid deregulated expan sion of αβ-committed precursors and T-cell leukemogenesis. Alternatively, these physiologic features may depend on additional Notch family members. A possible candidate is Notch3, which is expressed in vivo around the pre-TCR checkpoint, and has been involved in pre-TCR-dependent leukemogenesis.42 These data support the interesting possibility raised previously by Rothenberg47 that individual Notch family members are specialized for roles at different points in T-cell development. It is thus becoming increasingly evident that the impact of Notch1 signaling in T-cell development is highly dependent on signal strength, timing, and developmental context. Our strategy to approach the impact of Notch1 signaling at individual thymic precursor subsets supports a role for Notch1 in γδ T-cell development and emphasizes the requirement for exquisite control of Notch1 activity at sequential developmental stages to ensure appropriate differentiation/expansion of common αβ/γδ precursors along the αβ pathway.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-10-3261.
Supported by grants from Comisión Interministerial de Ciencia y Tecnología (SAF2001-1629), Comunidad de Madrid (CAM08.3/0021/2000), Fondo de Investigación Sanitaria (FIS00/1044), and Fundación Eugenio Rodríguez Pascual. We thank the Fundación Ramón Areces for an institutional grant to the Centro de Biología Molecular Severo Ochoa. V.G.d.Y. and M.G.-P. are supported by fellowships from Ministerio de Sanidad y Consumo (BEFI 9879336) and CAM 08.3/0021.2000, respectively.
M.G.-P. and V.G.d.Y. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs J. C. Aster for the retroviral vectors, G. Nolan for Phoenix cells, H. Spits and N. Taylor for reagents and helpful discussions, M. Brenner and R. Kurrle for mAbs, M. A. R. Marcos and P. Pereira for reading the manuscript, A. M. Martinez for technical help, and the Pediatric Cardiosurgery Units from Centro Especial Ramón y Cajal and Ciudad Sanitaria La Paz (Madrid) for the thymus samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal