Abstract
Adult murine bone marrow hematopoietic stem cells (HSCs) can be purified by sorting Hoechst 33342-extruding side population (SP) cells. Herein we investigated whether SP cells reside within embryonic tissues and exhibit hematopoietic progenitor activity. We isolated yolk sac (YS) and embryonic tissues 7.5 to 11.5 days after coitus (dpc), resolved an SP in each, and demonstrated that these SP cells exhibit distinct phenotypic and functional characteristics throughout development. YS and embryonic SP isolated 8.0 dpc expressed vascular endothelial-cadherin (VE-cadherin) and vascular endothelial receptor 2 (Flk-1), markers not expressed by bone marrow SP but expressed by endothelial cells and progenitors. SP at this stage did not express CD45 or produce hematopoietic colonies in vitro. In contrast, SP isolated 9.5 to 11.5 dpc contained a significantly higher proportion of cells expressing cKit and CD45, markers highly expressed by bone marrow SP. Furthermore, YS SP isolated 9.5 to 11.5 dpc demonstrated 40- to 90-fold enrichment for hematopoietic progenitor activity over unfractionated tissue. Our data indicate that YS and embryonic SP cells detected prior to the onset of circulation express the highest levels of endothelial markers and do not generate blood cells in vitro; however, as development progresses, they acquire hematopoietic potential and phenotypic characteristics similar to those of bone marrow SP. (Blood. 2003;102:2436-2443)
Introduction
Hematopoiesis in the mouse occurs initially in the extraembryonic yolk sac (YS) at the presomitic stage, approximately 7.5 days after coitus (dpc).1-3 The original model of hematopoiesis in the embryo proper presumed seeding of fetal liver hematopoiesis by YS hematopoietic progenitors that migrated to the fetal liver after the onset of circulation, eventually colonizing bone marrow and giving rise to adult hematopoietic stem cells (HSCs).4 The contribution of YS-derived hematopoietic progenitors to definitive adult hematopoiesis was questioned with the discovery of hematopoietic activity in the intraembryonic aorta-gonad-mesonephros (AGM) region.5 The AGM region demonstrated adult HSC activity at a developmental stage prior to the YS,6-8 and adult HSC activity in the YS was detected only after the onset of circulation6,7 and after substantial expansion of HSCs in the AGM.7 Together with the independent origin of hematopoietic activity demonstrated in the YS and AGM,8 these data suggested that the AGM region, and not the YS, is the source of HSCs that colonize fetal liver and are responsible for definitive hematopoiesis in the adult mouse. However, these data could not exclude the possibility of adult HSC activity arising independently at a later stage in the YS.
YS cells isolated at a developmental stage at which they are unable to directly engraft into the adult murine hematopoietic system could, if transplanted into newborn recipients, engraft into all hematopoietic lineages of animals undergoing transplantation and persist into adulthood; donor-derived cells from these adults could later directly engraft into other adult recipients upon bone marrow transplantation.9 The ability of YS cells to engraft into an adult environment by conditioning in a neonate environment suggested a pivotal role for the microenvironment with respect to the hematopoietic potential of YS cells. This idea was further supported by experiments in which precirculation YS cells were cultured on AGM stroma,10 resulting in their ability to directly regenerate all hematopoietic lineages of adult recipients. Enforced expression of HoxB4, a gene linked to adult HSC renewal, in YS cells also resulted in the ability to directly engraft into all hematopoietic lineages of adult recipients.11 This body of work suggested that the HSC potential of YS cells is dictated by their microenvironment, and these cells may contribute to adult definitive hematopoiesis during development.
In the adult mouse, HSCs with long-term multilineage reconstituting ability are contained in the dye-effluxing Hoechst side population (SP) of bone marrow.12,13 The dye-extruding ability of this HSC population is not confined to the mouse, as pig, rhesus monkey, and human bone marrow also contains a Hoechst SP with enriched hematopoietic ability.13 A Hoechst SP is also observed in other adult tissues, namely skeletal muscle14 and mammary gland,15 where they also demonstrate stem cell activity.14,15 Given these findings, it has been suggested that Hoechst dye efflux represents a general property of stem cells and progenitors.
To investigate whether this property of adult stem cells extends to stem cells and progenitors found in tissues supporting the earliest HSC activity in the mouse, we focused our studies on detection and characterization of a Hoechst SP in developing YS and embryo isolated 7.5 to 14.5 dpc. SP cells were detected in both YS and embryo prior to the onset of circulation (8.0 dpc) through 11.5 dpc, disappearing in YS by 14.5 dpc while remaining distinct in the embryo. SP from both tissues exhibited distinct phenotypic and functional characteristics throughout development, becoming less abundant and increasingly similar to adult bone marrow SP in phenotype and function.
Materials and methods
Isolation of yolk sac and embryonic tissues
Experiments were performed using 6- to 12-week-old female and 8- to 12-week-old male C57BL/6 mice mated overnight and checked for vaginal plugs, estimating noon of the day in which the plug was found as 0.5 dpc. The exact stage of development for each experiment was confirmed by counting embryonic somite pairs and observing embryonic anatomic features as described in the Edinburgh Mouse Atlas (http://genex.hgu.mrc.ac.uk/Databases/Anatomy/).
Pregnant females were humanely killed by cervical dislocation and their uteri dissected. YS and embryonic tissue from individual concepti were dissected, washed once in Hanks balanced salt solution (HBSS), and resuspended in a 0.05% solution of type II collagenase (Sigma, St Louis, MO) for 10 minutes (7.5 to 9.5 dpc), 15 minutes (10.5 to 11.5 dpc), or 20 minutes (12.5 to 14.5 dpc) at 37°C and then mechanically disrupted by tritration and centrifuged. After resuspension in Dulbecco modified Eagle medium (DMEM; Invitrogen/Gibco, Carlsbad, CA) at 37°C containing 2% fetal calf serum (FCS) and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), tissues were passed through a 40-μm filter and cell concentration was adjusted to 106/mL using DMEM.
Hoechst 33342 staining of yolk sac and embryonic tissues
Hoechst 33342 (Sigma) was added at a final concentration of 5 μg/mL and the suspension incubated at 37°C for 60 minutes. For some experiments, cell suspensions were preincubated with verapamil at a concentration of 50 μM for 5 minutes prior to the addition of Hoechst 33342 dye. Cell suspensions were gently mixed every 20 minutes, pelleted, and resuspended in 4°C HBSS containing 2% FCS and 2 μg/mL propidium iodide (PI).
Antibody staining for flow cytometric analysis
Antibodies were added to Hoechst-stained cells suspended in HBSS. Cells were incubated on ice for 20 minutes, pelleted, and washed with HBSS. Antibodies used were Ly6A/EPE (Sca-1), CD117PE (cKit), CD45PE, CD45FITC, CD34PE, and vascular endothelial receptor 2 (Flk-1)PE (clones D7, 2B8, 30-F11, 30-F11, RAM34, Avas 12α1, respectively; BD Biosciences/Pharmingen, San Diego, CA) at a staining concentration of 2 μg/mL (superscripts “PE” and “FITC” denote direct conjugation to phycoerythrin or fluorescein isothiocyanate). CD31 (platelet endothelial cell adhesion molecule-1 [PECAM-1]) and CD144 (vascular endothelial-cadherin [VE-cadherin]) were used at a staining concentration of 5 μg/mL (clones MEC13.3 and 11D4.1, respectively; BD Biosciences/Pharmingen) and detected using 2 μg/mL AlexaFluor 488 (Molecular Probes, Eugene, OR) secondary antibodies.
Yolk sac tissue-section immunostaining and whole mount staining
Paraffin-embedded 10-μm 10.5 dpc YS sections were baked at 65°C for 30 minutes, deparaffinized, and rehydrated with xylene, ethanol, and sterile water washes. Sections were digested with 0.02% protease XXIV for 6 minutes, rinsed with phosphate-buffered saline (PBS), and blocked with PBS containing 0.1% Tween 20 (PBS-T), 0.1% bovine serum albumin, and 10% normal goat serum for 30 minutes. Rat anti-cKit at 2 μg/mL in PBS-T (clone no. 2B8) was applied for 1 hour followed by a biotinylated goat antirat immunoglobulin G (IgG) at 1 μg/mL in PBS-T for 1 hour. Preformed avidin-biotin-horseradish peroxidase (avidin-biotin-HRP) complexes were applied for 30 minutes and detected with diamino benzidine (DAB) NiCl2 substrate (Vector Laboratories, Burlingame, CA).
For whole mount immunostaining, 10.5 dpc YS and embryos were fixed in 4% paraformaldehyde at 4°C, washed with PBS, and dehydrated with methanol. Endogenous peroxidase activity was quenched by incubation in methanol-dimethyl sulfoxide-hydrogen peroxide (methanol-DMSO-hydrogen peroxide) (4:1:1) for 5 hours, subsequent rehydration in 50% methanol-PBS and PBS, and blocking in PBS-T with 4% nonfat dry milk (PBSMT) for 1 hour. Concepti were incubated with biotinylated anti-cKit at 0.5 μg/mL in PBSMT at 4°C overnight and detected as described for antibody staining methods.
Hoechst profile detection and fluorescence-activated cell sorting
Flow cytometric analysis and fluorescence-activated cell sorting (FACS) was carried out on a triple laser cell sorter (MoFlow, Cytomation, Fort Collins, CO). For Hoechst detection, an ultraviolet (350 nm) tuned argon laser was used for excitation, and emission was detected with a 670/40 BP filter (Hoechst red) and 405/30 BP filter (Hoechst blue). A standard 488-nm argon laser was used to detect PE, FITC, and compatible AlexaDyes.
Primitive erythroid colony assay
To evaluate the ability of cells to generate primitive erythroid colonies, cells were sorted into tissue culture-treated 24-well polystyrene plates containing 500 μL of 1% methylcellulose-based media in Iscoves modified Dulbecco medium (IMDM) containing 2 mM glutamine, 15% plasma-derived serum (PDS) (Cocalico Biologicals, Reamstown, PA), 200 μg/mL iron-saturated transferrin (Sigma), 450 μM monothioglycerol (Sigma), 5% protein-free hybridoma medium (Invitrogen/Gibco), and 4 U/mL recombinant Erythropoietin (Stem Cell Technologies, Vancouver, BC, Canada).16-18 Cells were cultured at 37°C and monitored daily up to 7 days for the appearance of primitive erythroid colonies.
In vitro Methocult GF M3434 assay
To evaluate in vitro definitive hematopoietic potential, cells were sorted into tissue culture-treated 12- and 24-well polysterene plates containing 1 mL and 500 μL, respectively, of MethoCult GF M3434 (Stem Cell Technologies). Cells were cultured at 37°C for 2 weeks and colonies evaluated and scored at 3, 7, and 14 days.
Giemsa staining and flow cytometric analysis of MethoCult colonies
MethoCult cultures were diluted 4-fold with HBSS and cells centrifuged. For Giemsa staining, cells were resuspended in 300 μL HBSS and spun onto a glass slide using a CytoPro centrifuge (Wescor, Logan, UT). Cells were fixed with methanol for 5 minutes, stained with 0.02% Giemsa stain (Sigma) for 20 minutes, rinsed with deionized water, and coverslipped using glycerol.
For hematopoietic lineage analysis, cells were resuspended in 100 μL HBSS containing Ter119PE, Gr-1FITC, or B220PE antibodies (clone nos. Ter119, RB6-8C5, and RA3-6B2, respectively; BD Biosciences/Pharmingen) and analyzed using a FACScan cytometer (Becton Dickinson, Chicago, IL).
Results
Hoechst staining of yolk sac and embryonic tissue
To determine whether the SP phenotype is present at early stages of murine embryonic development, we subjected YS and embryonic tissues isolated 7.5 to 14.5 dpc to Hoechst 33342 staining using a protocol based on the original method for detecting adult bone marrow SP.12,19
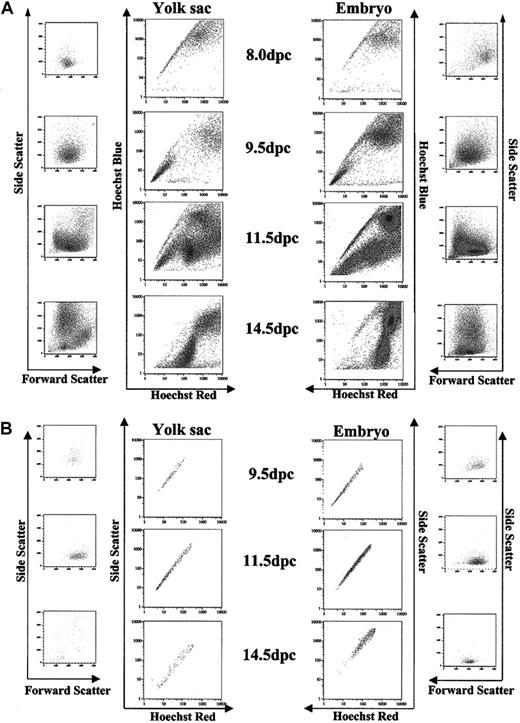
A Hoechst SP was detected in unseparated YS and embryo cells isolated at 7.5 dpc (data not shown). At 8.0 dpc, the first developmental stage at which YS and embryo could be reliably separated by dissection, Hoechst staining revealed similar SP in each tissue (Figure 1). A profile closely resembling the Hoechst profile of adult tissues, with the SP representing a smaller percentage of total cells, is present from 9.5 to 11.5 dpc. SP cells as a percentage of total viable cells decreased throughout development for YS and embryonic tissue (Table 1).
Hoechst profile of YS and embryonic tissues isolated 8.0 dpc to 14.5 dpc. (A) A Hoechst SP is present in YS isolated 8.0 to 11.5 dpc and in embryonic tissues isolated 8.0 dpc to 14.5 dpc. Accompanying forward scatter/side scatter (FS/SS) profiles indicate distribution of all events in the Hoechst profile. (B) Gated SP demonstrates homogeneity by FS/SS distribution.
Hoechst profile of YS and embryonic tissues isolated 8.0 dpc to 14.5 dpc. (A) A Hoechst SP is present in YS isolated 8.0 to 11.5 dpc and in embryonic tissues isolated 8.0 dpc to 14.5 dpc. Accompanying forward scatter/side scatter (FS/SS) profiles indicate distribution of all events in the Hoechst profile. (B) Gated SP demonstrates homogeneity by FS/SS distribution.
Hoechst SP cells as a percentage of total viable cells
Developmental stage . | Embryo, % . | YS, % . |
|---|---|---|
| 8.0 dpc | 8.3 ± 3.2 (n = 5) | 16.7 ± 2.3 (n = 6) |
| 9.5 dpc | 0.9 ± 0.8 (n = 10) | 1.6 ± 0.6 (n = 10) |
| 10.5 dpc | 3.2 ± 0.5 (n = 15) | 2.3 ± 0.5 (n = 15) |
| 11.5 dpc | 1.6 ± 0.4 (n = 18) | 1.5 ± 0.2 (n = 18) |
| 14.5 dpc | 0.2 ± 0.6 (n = 2) | Not present |
Developmental stage . | Embryo, % . | YS, % . |
|---|---|---|
| 8.0 dpc | 8.3 ± 3.2 (n = 5) | 16.7 ± 2.3 (n = 6) |
| 9.5 dpc | 0.9 ± 0.8 (n = 10) | 1.6 ± 0.6 (n = 10) |
| 10.5 dpc | 3.2 ± 0.5 (n = 15) | 2.3 ± 0.5 (n = 15) |
| 11.5 dpc | 1.6 ± 0.4 (n = 18) | 1.5 ± 0.2 (n = 18) |
| 14.5 dpc | 0.2 ± 0.6 (n = 2) | Not present |
Weighted mean values ± SEM for Hoechst SP cells as a percentage of total viable cells in YS and embryo isolated 8.0 to 14.5 dpc. The number of individual experiments from which weighted mean values were calculated is reported next to each mean.
Although an SP is detected in both YS and embryo in generally equal proportion from day 8.0 to 11.5 dpc, by 14.5 the YS SP is sparse and diffuse but remains distinct in embryonic tissues. The decline in numbers and resolvability of the SP in the YS parallels the documented decrease of hematopoeitic progenitors observed for whole YS after 12.5 dpc.
Effect of verapamil on yolk sac and embryonic Hoechst staining
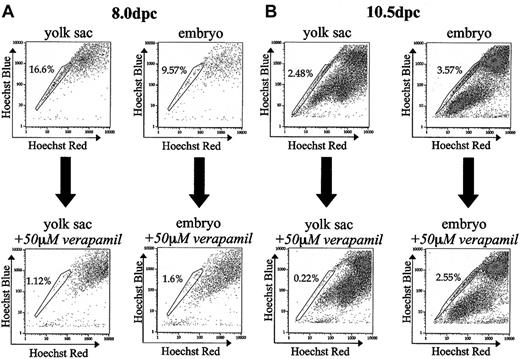
To determine if the Hoechst efflux activity observed in developing YS and embryonic cells could be blocked, as in bone marrow, by the addition of verapamil, YS and embryo preparations were stained with Hoechst in the presence of 50 μM verapamil. For YS and embryonic tissues isolated at 8.0 dpc, SP cells as a percentage of total viable cells decreased by a factor of 14.8 and 5.9, respectively, in the presence of verapamil (Figure 2A). YS SP isolated 10.5 dpc, when blocked with verapamil, decreased 11.3-fold. (Figure 2B). In contrast, embryonic tissues isolated at 10.5 dpc incubated with verapamil decreased only by a factor of 1.4 (Figure 2B). The decreased effectiveness of verapamil on 10.5 dpc embryo SP presumably reflects a variety of cell types within the SP of this dissociated tissue, each with Hoechst efflux ability likely conferred by different transporters. Different transporters conferring the SP phenotype are probably not all inhibited by verapamil or the concentration of verapamil used in our studies.
Effect of verapamil on embryonic SP. (A) Coincubation of tissues with Hoechst and 50 μM verapamil dramatically reduces the percentage of SP cells in yolk sac and embryonic tissue isolated 8.0 dpc by a factor of 14.8 and 5.9, respectively. (B) Coincubation of tissues isolated 10.5 dpc with Hoechst and 50 μM verapamil decreases the percentage of SP cells in yolk sac and embryo by a factor of 11.4 and 1.4, respectively. All verapamil experiments were performed twice.
Effect of verapamil on embryonic SP. (A) Coincubation of tissues with Hoechst and 50 μM verapamil dramatically reduces the percentage of SP cells in yolk sac and embryonic tissue isolated 8.0 dpc by a factor of 14.8 and 5.9, respectively. (B) Coincubation of tissues isolated 10.5 dpc with Hoechst and 50 μM verapamil decreases the percentage of SP cells in yolk sac and embryo by a factor of 11.4 and 1.4, respectively. All verapamil experiments were performed twice.
Characterization of SP by cell surface antigens
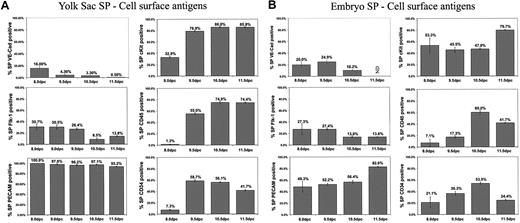
To further characterize the SP, we examined the expression of specific cell surface antigens by flow cytometry. We evaluated the expression of the pan-leukocyte marker CD45, endothelial cell/hematopoietic progenitor marker CD34, stem cell factor receptor cKit, vascular endothelial growth factor (VEGF) receptor Flk-1, cellular adhesion molecule PECAM-1, endothelial cell marker VE-cadherin, and HSC antigen Sca-1. Except for Sca-1, which was not detected, the above-mentioned proteins were found to be expressed by YS and embryonic tissue SP. The percentage of SP cells positive for these markers varied at different stages of embryonic development (Figure 3).
Cell surface antigen expression of embryonic SP. (A) Percentage of YS SP cells positive for cell surface antigens at various developmental stages. (B) Percentage of embryo SP cells positive for cells surface antigens at various developmental stages. Graphs in panels A and B represent weighted average ± SEM of 2 or more experiments. ND indicates not determined.
Cell surface antigen expression of embryonic SP. (A) Percentage of YS SP cells positive for cell surface antigens at various developmental stages. (B) Percentage of embryo SP cells positive for cells surface antigens at various developmental stages. Graphs in panels A and B represent weighted average ± SEM of 2 or more experiments. ND indicates not determined.
Analysis of flow cytometric antibody staining demonstrated the relative level of expression of the markers studied in YS SP cells isolated 10.5 dpc (Figure 4B). Of the SP cells positive for cKit, most were highly positive, with a similar distribution observed for PECAM-1+ cells. CD45+ SP cells demonstrated a wide range of expression, from highly positive to dim cells. SP cells positive for CD34 were almost all dim with only a limited number of highly positive events. Flk-1 showed only dimly positive events in the SP population.
Relative expression of cell surface antigens by embryonic SP. (A) Hoechst profile of 10.5 dpc YS. After gating of SP, a tight FS/SS distribution becomes evident for the SP cells. (B) Representative antibody staining of gated SP isolated 10.5 dpc.
Relative expression of cell surface antigens by embryonic SP. (A) Hoechst profile of 10.5 dpc YS. After gating of SP, a tight FS/SS distribution becomes evident for the SP cells. (B) Representative antibody staining of gated SP isolated 10.5 dpc.
Analysis of hematopoietic lineage markers revealed that for YS and embryonic tissue isolated 9.5 to 11.5 dpc, the SP was devoid of Ter119, Gr-1, or B220 marker expression (data not shown). YS and embryonic tissues showed very low reactivity to the B220 antibody, and reactivity to the Gr-1 antibody by YS and embryonic tissues was limited to non-Hoechst-effluxing cells. But, interestingly, for tissues isolated 9.5 to 11.5 dpc, high Ter119 expression segregated exclusively to a population below the SP (data not shown). This subpopulation was inhibited to a limited extent by verapamil (Figure 2B), in accordance with studies on the molecular basis of Hoechst extrusion by SP cells that found strong expression of the Hoechst-extruding ABC transporter Bcrp1 by Ter119+ cells in adult mice.20
Yolk sac localization of cKit+ SP cells
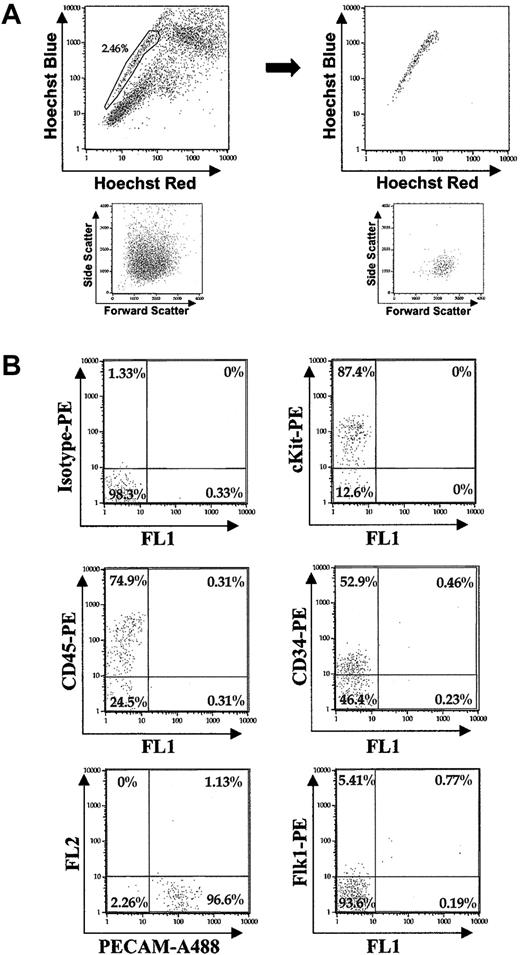
cKit staining revealed that in YS tissue isolated 10.5 dpc, all cells that stained highly positive for cKit (cKit+) fell exclusively within the SP (Figure 5A). Thus, to localize SP cells within the YS, we examined expression of cKit protein via immunohistochemical whole mount and section staining of YS isolated 10.5 dpc. Immunochemistry of YS sections revealed clusters of cKit+ cells exclusively in the mesodermal layer, within the endothelium, and around blood vessels (Figure 5B). Whole mount also revealed cKit+ cell clusters within and around the walls of blood vessels (Figure 5C).
Immunohistochemical localization of YS SP cells. (A) Dot plots: (i) 10.5 dpc YS Hoechst profile of all cells, (ii) cKit staining of 10.5 dpc YS (2.31% of all 10.5 dpc YS viable cells are cKitHI), and (iii) gating of cKitHI 10.5 dpc YS cells illustrate that they fall within the SP region. (B) cKit antibody staining of 10.5 dpc YS tissue sections finds cKitHI cells (arrowheads) in the mesodermal layer of the YS (cell nuclei DAPI [4,6 diamidino-2-phenylindole] stained; endoderm pseudocolored green). Original magnification, × 1000. (C) Whole mount staining of YS isolated 10.5 dpc for cKit demonstrates clusters of cells within and along the endothelium of large blood vessels.
Immunohistochemical localization of YS SP cells. (A) Dot plots: (i) 10.5 dpc YS Hoechst profile of all cells, (ii) cKit staining of 10.5 dpc YS (2.31% of all 10.5 dpc YS viable cells are cKitHI), and (iii) gating of cKitHI 10.5 dpc YS cells illustrate that they fall within the SP region. (B) cKit antibody staining of 10.5 dpc YS tissue sections finds cKitHI cells (arrowheads) in the mesodermal layer of the YS (cell nuclei DAPI [4,6 diamidino-2-phenylindole] stained; endoderm pseudocolored green). Original magnification, × 1000. (C) Whole mount staining of YS isolated 10.5 dpc for cKit demonstrates clusters of cells within and along the endothelium of large blood vessels.
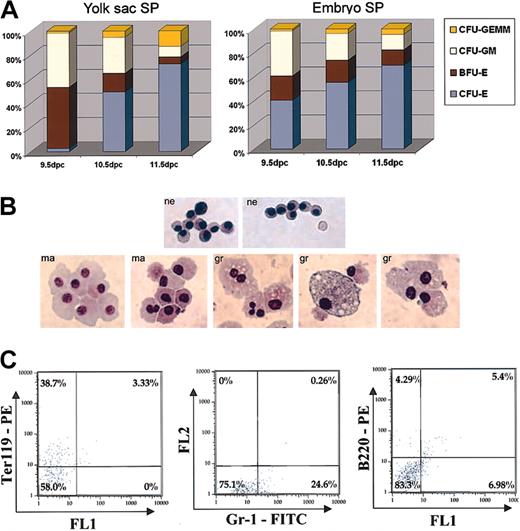
Yolk sac SP cells generate definitive erythroid and myeloid colonies in vitro
To determine the definitive hematopoietic potential of YS and embryonic SP, SP cells were sorted into MethoCult medium. This assay revealed that as development progressed, the overall trend in the different types of hematopoietic colonies generated by YS and embryonic SP changed in a similar fashion (Figure 6A). In the YS, 9.5 dpc SP cells generated an approximately equal numbers of erythroid burst-forming unit (BFU-E) and granulocyte-macrophage colony-forming unit (CFU-GM) colonies, with small numbers of erythroid colony-forming unit (CFU-E) and granulocyte, erythroid, macrophage, megakaryocyte colony-forming unit (CFUGEMM) colonies. The relative frequency of BFU-Es and CFUGMs dropped considerably in YS SP cells isolated 10.5 and 11.5 dpc. This trend was also observed for embryo SP cells, although the drop in frequency of BFU-Es was not as precipitous as in YS SP cells. The decrease in the relative frequency of BFU-Es and CFU-GMs was paralleled by an increase in relative frequency of CFU-Es and to a lesser extent CFU-GEMMs in both YS and embryo SP. YS and embryo SP cells isolated 8.0 dpc failed to generate definitive hematopoietic colonies in MethoCult medium.
Hematopoietic cell generation by SP cells in MethoCult. (A) Relative frequency of colony formation for YS and embryo SP cells 9.5 to 11.5 dpc. Experiments were performed twice in sextuplicate. (B) Giemsa staining of 14-day MethoCult colonies derived from 10.5 dpc YS SP. Most cells displayed morphology and staining consistent with nucleated erythroblast (ne) cells. A smaller percentage of cells displayed morphology and staining pattern consistent with myeloid cells: macrophages (ma), granulopoetic cells (gr). Original magnification, × 400. (C) Fourteen-day MethoCult colonies derived from 10.5 dpc YS SP stained with lineage antibodies Ter119PE, Gr-1FITC, and B220PE.
Hematopoietic cell generation by SP cells in MethoCult. (A) Relative frequency of colony formation for YS and embryo SP cells 9.5 to 11.5 dpc. Experiments were performed twice in sextuplicate. (B) Giemsa staining of 14-day MethoCult colonies derived from 10.5 dpc YS SP. Most cells displayed morphology and staining consistent with nucleated erythroblast (ne) cells. A smaller percentage of cells displayed morphology and staining pattern consistent with myeloid cells: macrophages (ma), granulopoetic cells (gr). Original magnification, × 400. (C) Fourteen-day MethoCult colonies derived from 10.5 dpc YS SP stained with lineage antibodies Ter119PE, Gr-1FITC, and B220PE.
To corroborate the identity of MethoCult colonies indicated by timing and morphology, colonies derived from 10.5 dpc YS SP cells were stained with Giemsa cytologic stain and visualized at high magnification. Most of the colony-forming cells were found to be of a size as well as staining pattern consistent with nucleated erythroblasts. A smaller proportion of stained cells was consistent with cells of myeloid lineage (Figure 6B).
To verify the lineage identity of SP-derived MethoCult colonies by cell surface antigens, MethoCult colonies generated by 10.5 dpc sorted YS SP cells were pooled and stained with antibodies specific to cells of erythroid, myeloid, and lymphoid lineages (Ter119, Gr-1, and B220, respectively). Antibody staining revealed that of the pooled colony cells, 38.7% stained positively for Ter119, 24.1% stained positively for Gr-1, and 4% stained positively for B220 (Figure 6C). These data confirmed MethoCult colony timing and morphology as well as Giemsa staining results.
To assess whether YS or embryo SP cells isolated 8.0 dpc possessed primitive erythroid colony-forming ability, cells were plated in a primitive erythroid colony assay. Whereas unfractionated whole tissue subjected to the same sorting procedure without Hoechst and gated only on the basis of viability produced primitive erythroid colonies, sorted SP cells failed to do so in 4 independent experiments, indicating that primitive erythroid colony-forming ability is not contained within the SP.
Flk-1+/CD45- yolk sac SP cells generate hematopoietic colonies
Double antibody labeling experiments show that expression of CD45 and Flk-1 is mutually exclusive in 9.5 dpc YS SP cells (data not shown). Thus, 9.5 dpc YS SP cells can be separated into Flk-1+/CD45-, Flk-1-/CD45+, and Flk-1-/CD45- fractions. To test the ability of these different YS SP fractions to individually generate definitive hematopoietic colonies, 9.5 dpc YS cells were Hoechst stained, double labeled with Flk-1 and CD45, and the 3 SP fractions individually sorted into MethoCult GM3434. CD45+ SP cells generated hematopoietic colonies in a normal time course. Flk-1+ SP cells also generated hematopoietic colonies, but the appearance of hematopoietic colonies that normally arise by day 3 (erythroid colonies) was delayed until day 6 of culture in MethoCult. Sorted Flk-1-/CD45- SP cells failed to generate any hematopoietic colonies in MethoCult.
Yolk sac SP cells are enriched for hematopoietic activity
To compare the frequency of hematopoietic progenitors within the YS SP with the frequency of progenitors within unfractionated tissue, exact numbers of SP and unfractionated cells isolated at the same time were sorted into MethoCult GM3434. SP cells were sorted according to their location within the Hoechst profile, whereas unfractionated cells, subjected to a treatment identical to SP samples but without Hoechst, were gated only on the basis of viability as indicated by PI staining. Sorted cells were cultured for 14 days at which time the total number of colonies was scored for each experiment (Table 2). YS SP at 9.5 dpc showed the highest level of enrichment of hematopoietic progenitors over whole YS tissue cells of the same time point, showing 77- and 89-fold greater ability to generate colonies in MethoCult. YS SP cells at 10.5 dpc demonstrated 26- and 41-fold enrichment of progenitors over whole tissue counterparts while 11.5 dpc YS SP showed 58- and 48-fold enrichment over whole YS. The enrichment values obtained for YS SP closely approximate the maximal enrichment possible for SP of individual experiments (Table 2, footnote), indicating that all definitive hematopoietic progenitors in YS are likely contained within the SP.
YS SP hematopoietic enrichment
. | Experiment no. 1 . | . | . | Experiment no. 2 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Yolk sac SP . | Whole yolk sac . | Enrichment . | Yolk sac SP . | Whole yolk sac . | Enrichment . | ||||
| 9.5 dpc | 77-fold | 89-fold | ||||||||
| Cells plated | 205 | 205 | 210 | 66 600 | ||||||
| Colonies formed | 78 | 1 | 34 | 120 | ||||||
| Colonies per cell | 380.4 × 10−3 | 4.9 × 10−3 | 161.9 × 10−3 | 1.8 × 10−3 | ||||||
| 10.5 dpc | 26-fold | 41-fold | ||||||||
| Cells plated | 1200 | 60 000 | 775 | 59 500 | ||||||
| Colonies formed | 150 | 284 | 134 | 254 | ||||||
| Colonies per cell | 125.8 × 10−3 | 4.7 × 10−3 | 172.9 × 10−3 | 4.2 × 10−3 | ||||||
| 11.5 dpc | 58-fold | 48-fold | ||||||||
| Cells plated | 600 | 60 000 | 1500 | 60 000 | ||||||
| Colonies formed | 62 | 107 | 109 | 90 | ||||||
| Colonies per cell | 103.3 × 10−3 | 1.8 × 10−3 | 72.6 × 10−3 | 1.5 × 10−3 | ||||||
. | Experiment no. 1 . | . | . | Experiment no. 2 . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Yolk sac SP . | Whole yolk sac . | Enrichment . | Yolk sac SP . | Whole yolk sac . | Enrichment . | ||||
| 9.5 dpc | 77-fold | 89-fold | ||||||||
| Cells plated | 205 | 205 | 210 | 66 600 | ||||||
| Colonies formed | 78 | 1 | 34 | 120 | ||||||
| Colonies per cell | 380.4 × 10−3 | 4.9 × 10−3 | 161.9 × 10−3 | 1.8 × 10−3 | ||||||
| 10.5 dpc | 26-fold | 41-fold | ||||||||
| Cells plated | 1200 | 60 000 | 775 | 59 500 | ||||||
| Colonies formed | 150 | 284 | 134 | 254 | ||||||
| Colonies per cell | 125.8 × 10−3 | 4.7 × 10−3 | 172.9 × 10−3 | 4.2 × 10−3 | ||||||
| 11.5 dpc | 58-fold | 48-fold | ||||||||
| Cells plated | 600 | 60 000 | 1500 | 60 000 | ||||||
| Colonies formed | 62 | 107 | 109 | 90 | ||||||
| Colonies per cell | 103.3 × 10−3 | 1.8 × 10−3 | 72.6 × 10−3 | 1.5 × 10−3 | ||||||
The sum of all colonies scored at 14 days was divided by the sum of all cells seeded. This calculation, carried out for each population for each individual experiment, yielded the per cell colony-forming ability of each population. Dividing the per cell colony-forming ability of the SP cells by the per cell colony-forming ability of the unfractionated tissue yielded the fold increase in the frequency of progenitors in the SP over unfractionated tissue. The maximal enrichment possible of SP over whole tissue for individual experiments is determined by calculating the inverse of SP cells as a percentage of total viable cells (ie, SP comprises 1.29% of total viable cells; inverse of 1.29% is 77; maximal enrichment is 77-fold). Each experiment was replicated in sextuplicate.
Discussion
The Hoechst SP was initially characterized in murine bone marrow and found to be highly enriched for HSCs capable of multilineage, long-term reconstitution of lethally irradiated mice.12 This dyeeffluxing population was later found in bone marrow of other species,13 as well as other tissues in the adult mouse, exhibiting progenitor and stem cell activity.14,15,21 Thus, Hoechst dye efflux may be a general property of multilineage stem cells and early progenitors.
In our studies, we posited that a Hoechst SP might be found in the earliest tissues reported to support HSC activity in the mouse. Indeed, we found that SP cells reside within YS and embryonic tissues 7.5 to 14.5 dpc and exhibit distinct phenotypic and functional characteristics throughout development. The presence of SP cells in separated YS and embryo isolated 8.0 dpc, prior to the onset of blood circulation, strongly suggests an independent origin for these cells in both sites.
Compared with bone marrow SP, the SP cells found in YS and embryonic tissues constitute a significantly larger percentage of total viable cells. Whereas the number of SP cells in bone marrow range from 0.01% to 0.05% of total viable cells,12 depending on the stage of embryonic development, YS and embryo SP range from 1% to 16% of total viable cells. If the SP is indeed representative of HSC and early progenitors, this large difference is not unexpected given that stem cells in adult bone marrow are largely quiescent, while YS and embryonic tissues are undergoing extensive growth and would thus be expected to contain a larger proportion of proliferating stem cells and progenitors.
Despite the relatively large number present, the SP cells found in the developing YS and embryo constitute a distinct and homogenous population as indicated by their tight forward scatter/side scatter (FS/SS) (Figures 1B and 4A), a reflection of homogeneity by size and morphology corroborated by microscope-aided visualization of sorted SP cells. In addition to showing homogeneity by dye efflux, size, and morphology, the percentage of Hoechst SP cells expressing specific cell surface antigens tested reflected a substantial increase over the proportion of whole tissue cells expressing these markers.
Further analysis of cell surface marker expression revealed significant differences between the SP found during embryonic development and SP found in adult tissues, as well as differences among SP cells isolated at distinct stages of development. High expression of cKit and CD45 are hallmarks of adult bone marrow SP.13 However, in YS and embryonic SP isolated 8.0 dpc, cKit is initially expressed by a percentage of SP much lower than that observed in adult bone marrow, and CD45 is almost completely absent. Instead, early SP cells exhibit a higher proportion of cells expressing markers of endothelial cells and progenitors, namely Flk-1 and VE-cadherin, which are not expressed by bone marrow SP cells. Interestingly, throughout the course of development, YS and embryonic SP become less abundant and increasingly phenotypically and functionally similar to bone marrow SP.
Comparison of the number of colonies generated by specific numbers of unfractionated YS versus SP cells by in vitro methylcellulose assays revealed that essentially all hematopoietic activity of YS was contained within the SP from 9.5 to 11.5 dpc. The colony-forming ability of sorted SP cells was compared with unfractionated cells not exposed to Hoechst to eliminate the possibility of a deleterious effect of the dye with respect to the in vitro hematopoietic potential of cells that did not efficiently extrude Hoechst. Any small discrepancy observed between the hematopoietic potential of a specific number of SP cells and a corresponding number of unfractionated cells may be due to diminished potential of SP cells themselves when exposed to Hoechst dye. The disappearance of an SP in YS occurs simultaneously with the reported disappearance of hematopoietic activity in this tissue, consistent with the SP marking early progenitor and stem cells.
The fact that YS and embryonic SP cells isolated during the onset of blood and blood vessel development express markers of the endothelial lineage is interesting in light of the fact that SP cells in YS tissue reside within the endothelium and around blood vessels. It has previously been observed that hematopoietic and vascular endothelial cells emerge in high spatial and temporal proximity in regions such as the YS and AGM.22 These observations have led to the hypothesis that these cell types emerge from common mesodermal progenitors,23,24 and data over the years have supported this hypothesis. Gene deletion studies have shown that mice lacking Flk-125 or transcription factor Tal1/SCL26,27 are defective in both hematopoietic and endothelial cell formation; clo knock-out zebrafish are also defective in generation of both of these cell types.28 Embryonic stem (ES) cells that give rise to blast colonies in semisolid medium generate both hematopoietic and adherent stromal-type cells.29,30 Although lacking direct clonogenic evidence for a common progenitor, these data demonstrate common essential genetic components of endothelial and hematopoietic cell commitment and differentiation.
More recently, it has been demonstrated, using ES cell culture and conditioned murine transplants, that CD45+ cells can emerge directly from Flk-1+/VE-cadherin+ cells, which are themselves preceded by Flk-1+/VE-cadherin- cells.31-33 These data are consistent with our observations that SP cells isolated from YS and embryo during the onset of blood and blood vessel development express VE-cadherin and Flk-1 but are CD45- and cKit-low and fail to make hematopoietic colonies in vitro. Progressive loss of endothelial marker expression parallels an increase in CD45 and cKit expression along with hematopoietic in vitro colony-forming ability. Furthermore, others have shown that Flk-1+ cells in early embryo (8.0 to 9.5 dpc) possess hematopoietic ability, whereas Flk-1+ cells after 11.5 dpc do not.34 Within this developmental time frame (9.5 to 11.5 dpc), the proportion of Flk-1+ cells within the SP is significantly decreased. Separation of 9.5 dpc YS SP cells into Flk-1+/CD45- and Flk-1-/CD45+ fractions demonstrates that SP cells expressing Flk-1 and not CD45 are capable of generating hematopoietic colonies in vitro but do so in a delayed fashion compared with Flk-1-/CD45+ SP cells isolated at the same time. The more rapid response to the hematopoietic cytokines present in our in vitro assay indicates that CD45 SP cells are more immediate hematopoietic progenitors than Flk-1 SP cells.
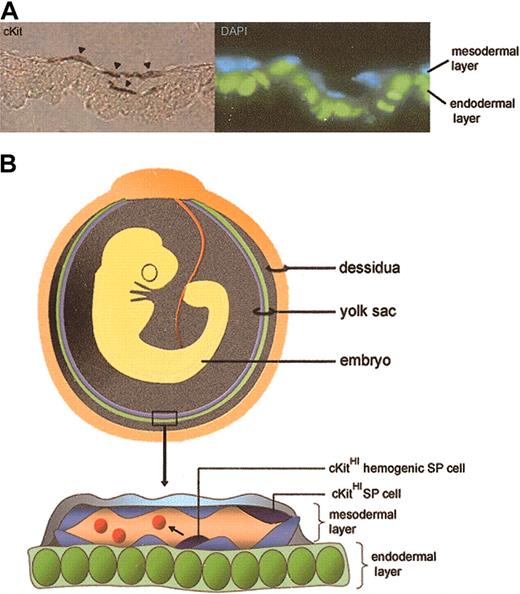
Thus, it is possible that the phenotypic characteristics of SP cells isolated at different developmental stages reveal a transition from an endothelial to a hematopoietic progenitor cell type. That is, SP cells isolated 8.0 dpc may represent primitive endothelium that forms the initial vascular plexus and precedes the onset of definitive hematopoietic activity. At later stages of development (9.5 to 11.5 dpc), SP cells within the endothelium may acquire hemogenic activity and give rise to progenitors capable of multilineage hematopoietic cell generation (Figure 7, schematic). Thus, the expression of the transporter(s) that confers the SP phenotype may be one of the first identifiable features of mesodermal progenitors that constitute hemogenic endothelium.
Schematic representation of SP cells in YS. (A) 10.5 dpc YS SP cells identified by cKit staining localize to the mesodermal layer and to clusters of cells within blood vessels (arrowheads). They exhibit endothelial and hematopoietic phenotypic characteristics and demonstrate enriched hematopoietic activity (endodermal layer pseudocolored green). Original magnification, × 1000. (B) Schematic depicting the orientation of endodermal (green) and mesodermal (blue) yolk sac layers in relation to the developing embryo. In the YS close-up, some cKitHI (dark blue) YS SP cells embedded in the endothelium acquire the ability to generate hematopoietic cells (red cells in vessel lumen). Green cells are endodermal cells; light blue cells, endothelial cells.
Schematic representation of SP cells in YS. (A) 10.5 dpc YS SP cells identified by cKit staining localize to the mesodermal layer and to clusters of cells within blood vessels (arrowheads). They exhibit endothelial and hematopoietic phenotypic characteristics and demonstrate enriched hematopoietic activity (endodermal layer pseudocolored green). Original magnification, × 1000. (B) Schematic depicting the orientation of endodermal (green) and mesodermal (blue) yolk sac layers in relation to the developing embryo. In the YS close-up, some cKitHI (dark blue) YS SP cells embedded in the endothelium acquire the ability to generate hematopoietic cells (red cells in vessel lumen). Green cells are endodermal cells; light blue cells, endothelial cells.
Prepublished online as Blood First Edition Paper, June 12, 2003; DOI 10.1182/blood-2003-01-0118.
Supported by NIH R01-HL61408, USDA 6250-51000-033, and AHA-National SDG 9930054N grants (K.K.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 5. Immunohistochemical localization of YS SP cells. (A) Dot plots: (i) 10.5 dpc YS Hoechst profile of all cells, (ii) cKit staining of 10.5 dpc YS (2.31% of all 10.5 dpc YS viable cells are cKitHI), and (iii) gating of cKitHI 10.5 dpc YS cells illustrate that they fall within the SP region. (B) cKit antibody staining of 10.5 dpc YS tissue sections finds cKitHI cells (arrowheads) in the mesodermal layer of the YS (cell nuclei DAPI [4,6 diamidino-2-phenylindole] stained; endoderm pseudocolored green). Original magnification, × 1000. (C) Whole mount staining of YS isolated 10.5 dpc for cKit demonstrates clusters of cells within and along the endothelium of large blood vessels.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2003-01-0118/6/m_h81935014005.jpeg?Expires=1769231435&Signature=g5gVE3rnhvIvvgPBDQWezX6fPvEU9go56S~PQeyfIAZK8XUkaXpU1LgzIQfnk0aX8ZTBUJO-81TVuqKxwF6zWSkci1LlbIMvC2jLsTgoNEYHNi3OEGM6yQT3VHZ9tt1~6P2hgGfiCgwC7CL80cXDybEdGX9qZPcH0rb6VFLKTiLj5GPOcxfjGD6~Xi6aZLbmB8mqumipy1lkjjVkiK3DEeCfDpc3Y-G3UxcQtvpjWi5fIqo7p9P3Y3yl7eY7bMDJLbEed1w3h9UwWBuTemJfLczwydVrflzvjwPZWpAGqjCY01FB6yN-OdY4Rhn3B5sqbyWkEpi8D4C4LcqFo6wedg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal