Abstract
Due to their ability to inhibit antigen-induced T-cell activation in vitro and in vivo, anergic T cells can be considered part of the spectrum of immunoregulatory T lymphocytes. Here we report that both murine and human anergic T cells can impair the ability of parenchymal cells (including endothelial and epithelial cells) to establish cell-cell interactions necessary to sustain leukocyte migration in vitro and tissue infiltration in vivo. The inhibition is reversible and cell-contact dependent but does not require cognate recognition of the parenchymal cells to occur. Instrumental to this effect is the increased cell surface expression and enzymatic activity of molecules such as CD26 (dipeptidyl-peptidase IV), which may act by metabolizing chemoattractants bound to the endothelial/epithelial cell surface. These results describe a previously unknown antigen-independent anti-inflammatory activity by locally generated anergic T cells and define a novel mechanism for the long-known immunoregulatory properties of these cells.
Introduction
T-cell anergy has been defined as a “cellular state in which a lymphocyte is alive but fails to display certain functional responses (including cell division and interleukin 2 [IL-2] production) when optimally stimulated through both its antigen-specific receptor and any other receptor that is normally required for full activation.”1 Anergic T cells can be generated in vitro and in vivo by various mechanisms,1,2 all involving partial or inappropriate stimulation. While losing their proliferative and effector potential, anergic T cells have long been known to be able to exert immunoregulation. The potential for anergic T cells to act as suppressor cells came first from a superantigen in vivo model in which anergic T cells acted as efficient suppressor cells in an antigen-nonspecific manner.3 More recently, murine anergic T cells either generated in vivo or rendered anergic in vitro with immobilized anti-CD3 and adoptively transferred have been shown to prolong skin allograft survival in an antigen-specific manner.4,5 In the human system, anergic CD4+ T cells were shown to exert contact-dependent and antigen-specific suppression in vitro.6,7
The mechanism by which anergic T cells exert their immunoregulatory properties appears to be indirect by altering the antigen presenting cells (APCs) immunogenicity8,9 in a cell-cell contact-dependent manner. The molecular basis for this effect is still unknown and it has been hypothesized to involve the induction of “regulatory” molecules on the anergic T cells, capable of delivering negative signals to the APC.2
It has recently become clear that the initial stages of T-cell activation are mediated by antigen-independent interactions, which establish areas of focal contact between T cells and APCs.10,11 Such interactions are initiated by chemoattractant-induced cell polarization and subsequent redistribution of adhesion molecules on the T-cell surface. These, in turn, allow T-cell receptor (TCR) interactions with the major histocompatibility complex (MHC)–peptide complexes displayed on the APC and lead to the formation of highly organized structures named immunologic synapses, which efficiently transduce antigen-initiated signals. Thus, one way of diminishing the efficiency of T-cell activation is the disruption of antigen-independent contact with the APC. In this study, we have explored the possibility that anergic T cells may act by altering the adhesive interactions between T cells and other cell types using in vitro and in vivo models of tissue infiltration by T cells. We observed that the ability of endothelial and epithelial cells to mediate migration of lymphocytes and other leukocytes, an adhesion-dependent function, is altered following noncognate interactions with anergic T cells. Similarly, in vivo contact with anergic T lymphocytes prevents infiltration of the peritoneal membrane by antigen-specific T cells in a murine peritonitis model. This effect appears to be mediated by increased surface expression by anergic T cells of the dipeptidyl-peptidase IV, CD26, which might act by metabolizing chemotactic cytokines on the endothelial/epithelial cell surface, thus altering adhesion-dependent migration, and ultimately, tissue infiltration.
Materials and methods
Mice
Male and female 4- to 8-week-old C57BL/6 mice and CBA/Ca mice were purchased from Olac Harlan (Bicester, United Kingdom) and used at 6 to 8 weeks of age.
Peptides, antibodies, and reagents
The CD26 ectopeptidase inhibitor12 human peptide YY (3-36) was purchased from Bachem UK (Meyerside, United Kingdom). The following monoclonal antibodies (mAbs) were used: antihuman CD3 (OKT3; American Type Culture Collection [ATCC], Rockville, MD), antihuman CD28 (CD28.2; BD Biosciences, Oxford, United Kingdom), antihuman CD26 (M-A261; Serotec, Kidlington, United Kingdom), antimouse CD4 (YTS 191,13 ), antimouse CD26 (H194-112; BD Biosciences), antimouse CD3 (2C11; BD Biosciences), and antimouse CD28 (MCA1363; Serotec). The human chemokine CXCL-12 (stromal-derived factor-1α [SDF-1α]) was purchased from Pepro Tech (Peterborough, United Kingdom).
Endothelial cells and epithelial cells
Endothelial cells (ECs) and renal tubular epithelial cells (RTECs) were isolated from human umbilical cord veins and tissue, respectively, and cultured as previously described.14,15 Prior to use in functional assays, human umbilical vein endothelial cells (HUVECs) and RTECs were treated with either 300 U/mL interferon-γ (IFN-γ) for 72 hours or with 10 ng/mL tumor necrosis factor α (TNF-α; Pepro Tech) to optimize migration.
T-cell clones and lines
Human CD4+ T-cell clones, HC3 and 7P.61, specific for the influenza hemagglutinin peptide HA100-115 and HA307-319 and restricted by DRB1*0101 and DRB1*0701, respectively, were generated and cultured as described.16,17 Alloreactive CD4+ T-cell lines were generated by stimulation of purified peripheral blood CD4+ T cells with allogeneic irradiated (60 Gy) peripheral blood mononuclear cells (PBMCs) and maintained in culture by weekly restimulation in the presence of recombinant IL-2 (rIL-2; 10 U/mL; Roche, Mannheim, Germany) in RPMI 1640 medium supplemented with 10% human serum (HS).
The murine CD8+ C6 T-cell clone, specific for HY peptide epitope TENSGKDI presented by H2-Kk,18 and the murine CD4+ T-cell clone B9, specific for HY peptide epitope NAGFNSNRANSSRSS and restricted by H2-Ab,19 were maintained in culture by forthnightly stimulation with peptide-pulsed splenocytes and rIL-2 (20 U/mL; Roche) in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; Globepharm, Guildford, United Kingdom), and 50 mM 2-mercaptoethanol (2-ME) (Gibco, Paisley, United Kingdom).
Isolation of human peripheral blood granulocytes
Heparinized blood from healthy adult volunteers was layered on a Ficoll-Paque gradient (Pharmacia, Uppsala, Sweden) and centrifuged for 20 minutes at 800g. The plasma and the interphase consisting mainly of lymphocytes and monocytes were discarded. Erythrocytes were eliminated from the pellet by rapid osmotic lysis. The remaining cell suspension contained more than 99% granulocytes. Cell viability was more than 98%, as determined by trypan blue exclusion (data not shown).
Anergy induction in human and murine T cells
For anergy induction by costimulation-deficient TCR triggering, T cells (alloreactive human CD4+ T-cell lines and the B9 murine CD4+ T-cell clone) were plated in 24-well plates (5 × 105/well) in the presence of plastic-bound anti-CD3 (1 μg/mL OKT3 or 10 μg/mL 2C11) in a total volume of 500 μL.20 Anergy was also induced by T-T antigen presentation. Human CD4+ T-cell clones (HC3 and 7P.61) were incubated overnight (106 T cells) in the presence of 0.7 μg/mL cognate HA peptides17 while C6 T cells (5 × 105) were incubated with 100 nM HY peptide in 24-well plates.21 Following 24-hour incubation, T cells were harvested and viable cells recovered by density gradient prior to use in functional assays. Induction of anergy was assessed by measuring 3H thymidine incorporation following antigenic rechallenge.
Resting lymphocytes and T cells activated with plastic-bound anti-CD3 (OKT3; 1 μg/mL for human T cells or 2C11 [10 μg/mL] for murine T cells) plus anti-CD28 (CD28.2; 5 μg/mL for human T cells and MCA1363 [5 μg/mL] for murine T cells) were used as a control in all the in vitro and in vivo experiments described in this paper. As both resting and activated T cells displayed the same behavior in all the experiments performed, they will be referred as to “responsive” T cells throughout the text.
Lymphocyte transmigration assays and chemotaxis assays
Transmigration assays were carried out using HUVEC or RTEC monolayers (5 × 104 cells/12-mm–diameter well) grown on a 3-μm–pore polycarbonate transwell (Costar, High Wycombe, United Kingdom), as previously described,22 and incubated overnight with anergic or responsive T cells (5 × 104/well). Following removal of the T cells, fresh T cells (5-7 × 105/well, with different antigen specificity) or granulocytes (106/well) were added into each insert and left to migrate through the monolayers. The number of migrated T cells or granulocytes was determined by counting the cells present in the well media over the next 24 hours. Results are expressed as percentage of transmigrated cells in 3 independent counts.
For chemotaxis assays with CXCL-12, naive CD4+ CD45RA+ T isolated by negative immunomagnetic selection were seeded in the top chamber of a 3-μm–pore polycarbonate transwell (Costar).23 Chemotaxis medium (RPMI 2% FCS) containing CXCL-12 (50 ng/mL), or chemotaxis medium alone, was added to the bottom chamber of the transwell, while the cell suspension was added to the top chamber. The number of migrated cells was evaluated as described in “Lymphocyte transmigration assays and chemotaxis assays.”
Peritoneal membrane infiltration by antigen-specific T cells
Male CBA/Ca mice were given an intraperitoneal injection of IFN-γ (600 U; Pepro Tech), which induces a localized inflammatory response (F.M.M.-B., unpublished observations, January 2001). As a control, an untreated female mouse was used. HY-specific C6 CD8+ T cells (5-7 × 106) previously labeled with the PKH26 Cell Linker (Sigma, Poole, United Kingdom) were injected intraperitoneally 48 hours later. After a further 24 hours, mice were killed and infiltration of the peritoneal membrane by labeled T cells was visualized by wide-field fluorescence microscopy (see “Wide-field fluorescence microscopy and flow cytometry”). The presence of fluorescently labeled cells in the peritoneal lavage, draining lymph nodes, and the spleen was assessed by cytofluorymetric analysis. In some experiments, responsive or previously anergized unlabeled CD8+ C6 (antigen-specific) or CD4+ B9 (irrelevant) T cells (4-5 106) were injected intraperitoneally 24 hours prior to the injection of C6 T cells. To assess the functional role of T-cell surface CD26, samples of anergic T cells were pretreated with anti-CD26 (5 μg/mL), anti-CD4 (5 μg/mL), or an isotype-matched antibody (5 μg/mL) for 30 minutes at room temperature (RT) and washed prior to injection. Alternatively, anergic T cells were incubated with human peptide YY (3-36, 30 nM) for 30 minutes at RT and washed before injection.
Wide-field fluorescence microscopy and flow cytometry
Peritoneal membranes were laid onto polysine microscope slides (VWR International, Lutterworth, United Kingdom), left to dry overnight, and then mounted in Vectorshield mounting medium for fluorescence with DAPI (4,6 diamidino-2-phenylindole; Vector Laboratories, Peterborough, United Kingdom). Slides were visualized with a Coolview 12–cooled camera (Photonic Science, Newbury, United Kingdom) mounted over a Zeiss Axiovert S100 microscope equipped with Metamorph software (Zeiss, Welwyn Garden City, United Kingdom). A × 10 and a × 40 0.6 objectives and a standard epi-illuminating rhodamine fluorescence filter cube were used and 12-bit image data sets were generated. Tissue infiltration was quantified by randomly selecting ten × 40-magnified fields and assessing the number of fluorescent cells in each field.
For flow cytometry, 105 cells were incubated with the indicated fluoresceinated mAb at 4°C for 30 minutes. An isotype-matched irrelevant antibody was used as a control. Stained cells were analyzed using a FACSCalibur (Becton Dickinson, Mountain View, CA).
Statistical analysis
In the in vitro experiments, comparisons between groups were made using the Student t test. To assess statistical significance in the in vivo experiments, the Mann-Whitney U test was used. All reported P values are 2-sided.
Results
Parenchymal cells fail to establish effective cell-cell interactions following exposure to anergic T cells
The cellular ability to establish effective cell-cell interactions following exposure to anergic T cells was tested by an in vitro model of lymphocyte transmigration. Cytokine-treated EC/RTEC monolayers grown on transwells (5 × 104/well) were incubated overnight with either anergic (5 × 104/well) or responsive (105/well) CD4+ T-cell lines or clones, or in medium alone. The number of responsive T cells used was doubled to compensate for the fraction of T cells that would have migrated through the monolayer overnight (about 50% of the initial input on average22 ), while the majority of anergic T cells remained in the top chamber, having lost their migratory ability.22 Following thorough removal (97% recovery on average) of the T cells, fresh T lymphocytes of similar or different specificity (7 × 105/well), or granulocytes (106/well) were seeded onto the EC monolayers. Cell migration was monitored over the next 6 to 26 hours. The following combinations were used: anergic 7P.61 CD4+ T-cell and responsive 7P.61, HC3, or alloreactive CD4+ T-cell lines, anergic allospecific CD4+ T-cell line and responsive 7P.61, or granulocytes. 7P.61 T cells were rendered anergic by T-T presentation, while the alloreactive T-cell lines were incubated on plastic-bound anti-CD3.
As it is shown in Figure 1 both T cells (Figure 1A) and granulocytes (Figure 1B) migrated less efficiently through EC monolayers exposed to anergic T cells. Incubation with responsive T cells often led to increased migration, particularly at early time points. This effect was cell-contact dependent (Figure 1C). The EC monolayer was not disrupted by anergic T cells (data not shown). In addition, culture of human RTEC monolayers with anergic T cells diminished the ability of the epithelium to mediate T-cell migration (Figure 1D). The inhibition was equally exerted by T cells rendered anergic by T-T presentation and costimulation-deficient TCR triggering and did not require cognate recognition of ECs by the anergic T cells (data not shown). Consistently, similar results were obtained using TNF-α–treated MHC class II–negative ECs (data not shown).
Anergic T cells inhibit parenchymal cell–mediated migration. (Panels A-B) EC monolayers (5 × 104/transwell) were incubated for 16 hours with either anergic (•; 5 × 104/transwell) or responsive (○; 105/well) alloreactive CD4+ T-cell lines or in medium alone (▵). T cells were subsequently removed and fresh T cells (7 × 105/well; panel A) or granulocytes (106/well; panel B) were seeded onto the EC monolayers. The results are expressed as percentage of migrated T cells at the given time points and reported as the average of 3 experiments of identical design. The bars show the standard deviations (SD). * indicates statistically significant (at least P < .01, panel A; at least P < .02, panel B) versus control cultures (EC monolayers cultured in medium). (Panel C) EC monolayers were incubated overnight with supernatants obtained from cultures of T cells in anergizing (•) or resting (○) conditions or in medium alone (▵). Following 2 washes with warm culture medium, fresh T cells (7 × 105/well) were seeded onto the EC monolayers. The results are expressed and reported as specified for panels A-B. (Panel D) TNF-α–treated RTEC monolayers (5 × 104/transwell) were incubated for 16 hours with either anergic (5 × 104/well), or responsive (105/well) CD4+ T-cell lines or clones, or in medium alone. T cells were subsequently removed and fresh T cells (7 × 105/well) were seeded onto the RTEC layers. The results are expressed and reported as specified for panels A-B. * indicates statistically significant (at least P < .05) versus control cultures (RTEC monolayers cultured in medium). (Panels E-G) EC monolayers (5 × 104/transwell) were incubated for 30 minutes at RT with an antihuman CD40 mAb (5 μg/mL, filled symbols) or with an isotype-matched mAb (open symbols). Excess antibody was then removed by gentle washing. Bound antibody was then cross-linked by addition of goat antimouse Miltenyi microbeads (30 μL/μg mAbs) during incubation with anergic (panel E) or responsive (panel F) 7P.61 T cells or in medium alone (panel G) for a further 16 hours. T cells were then removed and migration of freshly added alloreactive T cells (7 × 105/well) was monitored. In all these experiments, cell migration was monitored over the next 6 to 26 hours. The results are expressed and reported as specified for panels A-B. * indicates statistically significant (at least P < .01) versus control cultures (EC monolayers cultured in the presence of anergic T cells).
Anergic T cells inhibit parenchymal cell–mediated migration. (Panels A-B) EC monolayers (5 × 104/transwell) were incubated for 16 hours with either anergic (•; 5 × 104/transwell) or responsive (○; 105/well) alloreactive CD4+ T-cell lines or in medium alone (▵). T cells were subsequently removed and fresh T cells (7 × 105/well; panel A) or granulocytes (106/well; panel B) were seeded onto the EC monolayers. The results are expressed as percentage of migrated T cells at the given time points and reported as the average of 3 experiments of identical design. The bars show the standard deviations (SD). * indicates statistically significant (at least P < .01, panel A; at least P < .02, panel B) versus control cultures (EC monolayers cultured in medium). (Panel C) EC monolayers were incubated overnight with supernatants obtained from cultures of T cells in anergizing (•) or resting (○) conditions or in medium alone (▵). Following 2 washes with warm culture medium, fresh T cells (7 × 105/well) were seeded onto the EC monolayers. The results are expressed and reported as specified for panels A-B. (Panel D) TNF-α–treated RTEC monolayers (5 × 104/transwell) were incubated for 16 hours with either anergic (5 × 104/well), or responsive (105/well) CD4+ T-cell lines or clones, or in medium alone. T cells were subsequently removed and fresh T cells (7 × 105/well) were seeded onto the RTEC layers. The results are expressed and reported as specified for panels A-B. * indicates statistically significant (at least P < .05) versus control cultures (RTEC monolayers cultured in medium). (Panels E-G) EC monolayers (5 × 104/transwell) were incubated for 30 minutes at RT with an antihuman CD40 mAb (5 μg/mL, filled symbols) or with an isotype-matched mAb (open symbols). Excess antibody was then removed by gentle washing. Bound antibody was then cross-linked by addition of goat antimouse Miltenyi microbeads (30 μL/μg mAbs) during incubation with anergic (panel E) or responsive (panel F) 7P.61 T cells or in medium alone (panel G) for a further 16 hours. T cells were then removed and migration of freshly added alloreactive T cells (7 × 105/well) was monitored. In all these experiments, cell migration was monitored over the next 6 to 26 hours. The results are expressed and reported as specified for panels A-B. * indicates statistically significant (at least P < .01) versus control cultures (EC monolayers cultured in the presence of anergic T cells).
To investigate whether the observed effect was reversible, ECs were activated via CD40 ligation24,25 during exposure to the anergic T cells. As shown in Figure 1E-G, CD40 ligation led to a partial recovery of EC function (Figure 1E). Ligation of CD40 on EC incubated with either responsive T cells or medium did not modify their function (Figure 1F and 1G, respectively).
Anergic T cells prevent tissue infiltration in vivo
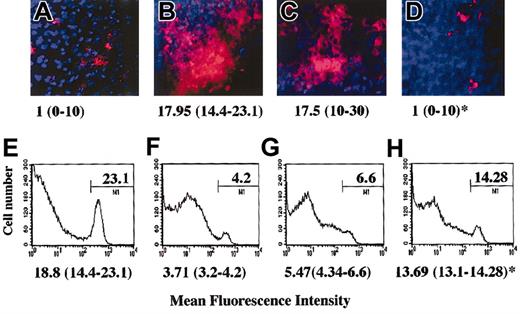
Preliminary to these experiments we observed that the anergic murine C6 or B9 T cells, similarly to their human counterpart, were capable to inhibit EC function in vitro (data not shown). As our working hypothesis is that during inflammation locally generated anergic T cells modulate the ability of surrounding parenchymal cells to establish functional cell-cell interactions that support leukocyte infiltration, a model of peritoneal membrane infiltration in which anergic T cells are administered locally by intraperitoneal injection was developed. In this model, HY-specific C6 T cells (5-7 × 106) that are injected intraperitoneally in CBA male mice previously (48 hours) treated intraperitoneally with IFN-γ promptly infiltrate the peritoneal membrane (Figure 2B,F). Untreated female mice, which displayed minimal C6 infiltration (the T cells being recoverable from the peritoneal lavage), were used as a control (Figure 2A,E). Anergic murine T cells were generated by T-T peptide presentation (clone C6, data not shown and Chai et al21 ) or by culture in the presence of immobilized anti-CD3 (clone B9, data not shown and Marelli-Berg et al22 ). As shown in Figure 2D and 2H, injection of anergic T cells (3-5 × 106) 24 hours prior to injection of the C6 T cells prevented infiltration of the peritoneal membrane by the C6 T-cell clone, and a large proportion of the labeled T cells remained in the peritoneal cavity. This was not due to competition, as responsive T cells, which promptly infiltrate the tissue, had a minimum effect on tissue infiltration by the labeled T cells (Figure 2C,G). In addition, we had previously established that anergic T cells are unable to infiltrate the peritoneal membrane while remaining localized in the peritoneal lavage (data not shown). Labeled T cells were not detectable under any of these conditions in lymph nodes or spleen (data not shown). In keeping with our in vitro observations, the specificity of anergic T cells was irrelevant to the effect observed as tissue infiltration was prevented to the same extent by either C6 (antigen-specific) or B9 (irrelevant) anergic T cells (data not shown).
Anergic T cells inhibit tissue infiltration in vivo. Male CBA mice were injected intraperitoneally with 600 U IFN-γ. As a negative control, untreated female mice were used (panels A,E). Two days later, mice received an intraperitoneal injection of either PBS (panels A-B,E-F), responsive (3-5 × 107; panels C,G), or anergic (3-5 × 107; panels D,H) B9 CD4+ T cells (4 × 106/mouse). After 24 hours, red fluorescence-labeled (PKH26) HY-specific C6 T cells (5 × 107) were also injected intraperitoneally in all the mice. The following day, the presence of fluorescently labeled cells in the peritoneal membrane (× 10 original magnification; panels A-D) and in the peritoneal lavage (panels E-H) was assessed by wide-field fluorescence microscopy and flow cytometry, respectively. The peritoneal membrane wide-field images and histograms (in which the percentage of positive cells is specified) are taken from a representative experiment; however, the median number and range of labeled cells quantified in 10 randomly selected × 40-magnified fields of the peritoneal membrane of at least 3 different animals are shown below each image (panels A-D). Similarly, the median percentage of labeled cells recovered in the peritoneal lavage of 3 different samples is specified below each representative histogram. * indicates statistically significant (P < .0001, panel D; P = .05, panel H) compared with male CBA mice (positive control).
Anergic T cells inhibit tissue infiltration in vivo. Male CBA mice were injected intraperitoneally with 600 U IFN-γ. As a negative control, untreated female mice were used (panels A,E). Two days later, mice received an intraperitoneal injection of either PBS (panels A-B,E-F), responsive (3-5 × 107; panels C,G), or anergic (3-5 × 107; panels D,H) B9 CD4+ T cells (4 × 106/mouse). After 24 hours, red fluorescence-labeled (PKH26) HY-specific C6 T cells (5 × 107) were also injected intraperitoneally in all the mice. The following day, the presence of fluorescently labeled cells in the peritoneal membrane (× 10 original magnification; panels A-D) and in the peritoneal lavage (panels E-H) was assessed by wide-field fluorescence microscopy and flow cytometry, respectively. The peritoneal membrane wide-field images and histograms (in which the percentage of positive cells is specified) are taken from a representative experiment; however, the median number and range of labeled cells quantified in 10 randomly selected × 40-magnified fields of the peritoneal membrane of at least 3 different animals are shown below each image (panels A-D). Similarly, the median percentage of labeled cells recovered in the peritoneal lavage of 3 different samples is specified below each representative histogram. * indicates statistically significant (P < .0001, panel D; P = .05, panel H) compared with male CBA mice (positive control).
Increased expression of dipeptidyl peptidase IV (DPPIV)/CD26 by anergic T cells is instrumental to the inhibition of EC function
In a search for “inhibitory” molecules expressed by anergic T cells we observed that a proportion of these displayed membrane-bound transforming growth factor β (TGFβ) and IL-10 (Figure 3A-B) and that the surface enzyme DPPIV/CD26 was strongly up-regulated on the whole T-cell population (Figure 3C). Similarly, CD26 expression was induced (although at low level) in anergic but not in responsive C6 and B9 T cells, which remained negative (Figure 3D). In functional assays, while neutralizing anti-TGFβ and/or anti-IL10 mAbs were ineffective (data not shown), antibody blockade of CD26 on anergic T cells prior to incubation with cytokine-treated EC monolayers completely prevented the inhibition of EC-mediated T-cell migration (Figure 3F) and partially that of EC-mediated granulocyte migration (Figure 3H). Antibody treatment of responsive T cells (which express intermediate levels of CD26) prior to incubation with the EC did not elicit any effect (Figure 3E,G). Treatment of anergic T cells with the anti-CD26 mAb did not restore responsiveness or motility23 in these cells (data not shown). Similar results were obtained when murine T cells were analyzed (data not shown).
Inhibition of EC function by anergic T cells is mediated by CD26. (Panels A-D) Responsive (▦) or anergic (□) human alloreactive T-cell lines were incubated with FITC-conjugated anti-TGFβ (panel A), anti–IL-10 (panel B), and anti-CD26 (panel C) mAbs. Cells were then analyzed by flow cytometry. The average median fluorescence intensity of CD26 expression by anergic T cells in 3 separate experiments was 162.68 ± 57.72 SD, while that of responsive T cells was 30.1 ± 3.8 SD. This difference was statistically significant (P < .02). Expression of CD26 by murine responsive and anergic T cells was also compared (clone B9; panel D). The average median fluorescence intensity of CD26 expression by murine anergic T cells in 3 separate experiments was 5.6 ± 0.8, while that of responsive T cells was 1.5 ± 2.1 SD. This difference was statistically significant (P < .05). (Panels E-H) Responsive (105/well; panels E,G) and anergic (5 × 104/well; panels F,H) T-cell lines were treated for 30 minutes at 4°C with an anti-CD26 antibody or with an isotype-matched control mAb and washed prior to being added to TNF-α–treated EC monolayers grown on 12-mm–diameter transwells (5 × 104/well). As a control, EC monolayers were incubated in medium alone. T cells were subsequently removed and fresh 7P.61 T cells (7 × 105/well; panels E-F) or granulocytes (106/well; panels G-H) were seeded onto the EC monolayers. The results are expressed as percentage of migrated T cells at the given time points and are reported as the average of 3 experiments of identical design. The bars show the SD. * indicates statistically significant (at least P < .03, panel F; at least P < .03, panel H) versus control cultures (EC monolayers cultured with anergic T cells).
Inhibition of EC function by anergic T cells is mediated by CD26. (Panels A-D) Responsive (▦) or anergic (□) human alloreactive T-cell lines were incubated with FITC-conjugated anti-TGFβ (panel A), anti–IL-10 (panel B), and anti-CD26 (panel C) mAbs. Cells were then analyzed by flow cytometry. The average median fluorescence intensity of CD26 expression by anergic T cells in 3 separate experiments was 162.68 ± 57.72 SD, while that of responsive T cells was 30.1 ± 3.8 SD. This difference was statistically significant (P < .02). Expression of CD26 by murine responsive and anergic T cells was also compared (clone B9; panel D). The average median fluorescence intensity of CD26 expression by murine anergic T cells in 3 separate experiments was 5.6 ± 0.8, while that of responsive T cells was 1.5 ± 2.1 SD. This difference was statistically significant (P < .05). (Panels E-H) Responsive (105/well; panels E,G) and anergic (5 × 104/well; panels F,H) T-cell lines were treated for 30 minutes at 4°C with an anti-CD26 antibody or with an isotype-matched control mAb and washed prior to being added to TNF-α–treated EC monolayers grown on 12-mm–diameter transwells (5 × 104/well). As a control, EC monolayers were incubated in medium alone. T cells were subsequently removed and fresh 7P.61 T cells (7 × 105/well; panels E-F) or granulocytes (106/well; panels G-H) were seeded onto the EC monolayers. The results are expressed as percentage of migrated T cells at the given time points and are reported as the average of 3 experiments of identical design. The bars show the SD. * indicates statistically significant (at least P < .03, panel F; at least P < .03, panel H) versus control cultures (EC monolayers cultured with anergic T cells).
Anergic T cells metabolize chemokines via CD26
CD26 has been shown to metabolize and inactivate chemokines such as CXCL-12.26-28 We first tested the possibility that anergic T cells display increased chemokine metabolism. CXCL-12 (50 ng/mL) was incubated overnight at 37°C, 7% CO2 in chemotaxis medium alone, or in the presence of either responsive (106/mL) or anergic (106/mL) human alloreactive CD4+ T cells. In addition, chemotaxis medium alone, or containing either responsive or anergic T cells incubated overnight at 37°C 7% CO2 were used as a control. Supernatants were then obtained from each sample by centrifugation and their ability to induce migration of CXCL-12–responsive CD45RA+ T cells (2 × 106/well)23 was measured. Incubation in the presence of anergic T-cell lines completely abrogated the chemotactic activity of CXCL-12, while resting T cells had only a modest effect (Figure 4A-C). To establish whether increased CD26 activity was instrumental to this effect, responsive and anergic T cells were incubated with a blocking anti-CD26 antibody (1 μg/mL) for 30 minutes and washed prior to incubation with CXCL-12, as described in this paragraph. As it is shown in Figure 4D, loss of chemotactic activity by CXCL-12 was prevented by CD26 blockade on anergic T cells, while CD26 blockade on responsive T lymphocytes had little effect on CXCL-12–mediated chemotaxis. This effect was still evident 24 hours later, although reduced due to a loss of chemotactic gradient (Figure 4E). This observation, however, ruled out the possibility that a factor released by the anergic T cells might either be toxic to naive T cells or alter their motility.
Anergic T cells metabolize chemokines via CD26. CXCL12 was added to chemotaxis medium (RPMI 2% FCS) at a concentration of 50 ng/mL and incubated overnight at 37°C 7% CO2 in the absence of T cells (panel A) or in the presence of responsive (panel B) or anergic human CD4+ T cells (panel C). As a control, chemotaxis medium (RPMI 2% FCS) alone, or containing either responsive (106/mL) or anergic (106/mL) human T cells, was used. The results are expressed as percentage of migrated naive T cells after 6 hours and are reported as the average of at least 3 experiments of identical design. The bars show the SD. * indicates statistically significant (P < .005) versus control cultures (CXCL12 incubated with anergic T cells). (Panels D-E) In some experiments responsive (R) and anergic (A) T cells (106/sample) were treated with an antihuman CD26 antibody (5 μg/mL) for 30 minutes and washed prior to incubation with CXCL12 as described in “Anergic T cells metabolize chemokines via CD26.” Supernatant was then obtained from each sample (as specified below each column) by centrifugation and the chemotactic activity was assessed monitoring the migration of purified CD45RA+ T cells (2 × 106/well) through a transwell after 6 (panel D) and 24 (panel E) hours. The results are expressed as specified for panels A-C. * indicates statistically significant (P < .006, panel D; P < .001, panel E) versus control cultures (CXCL12 incubated with anergic T cells).
Anergic T cells metabolize chemokines via CD26. CXCL12 was added to chemotaxis medium (RPMI 2% FCS) at a concentration of 50 ng/mL and incubated overnight at 37°C 7% CO2 in the absence of T cells (panel A) or in the presence of responsive (panel B) or anergic human CD4+ T cells (panel C). As a control, chemotaxis medium (RPMI 2% FCS) alone, or containing either responsive (106/mL) or anergic (106/mL) human T cells, was used. The results are expressed as percentage of migrated naive T cells after 6 hours and are reported as the average of at least 3 experiments of identical design. The bars show the SD. * indicates statistically significant (P < .005) versus control cultures (CXCL12 incubated with anergic T cells). (Panels D-E) In some experiments responsive (R) and anergic (A) T cells (106/sample) were treated with an antihuman CD26 antibody (5 μg/mL) for 30 minutes and washed prior to incubation with CXCL12 as described in “Anergic T cells metabolize chemokines via CD26.” Supernatant was then obtained from each sample (as specified below each column) by centrifugation and the chemotactic activity was assessed monitoring the migration of purified CD45RA+ T cells (2 × 106/well) through a transwell after 6 (panel D) and 24 (panel E) hours. The results are expressed as specified for panels A-C. * indicates statistically significant (P < .006, panel D; P < .001, panel E) versus control cultures (CXCL12 incubated with anergic T cells).
CD26 enzymatic activity mediates inhibition of tissue infiltration by anergic T cells
To establish the role of CD26 activity in the anti-inflammatory effects of anergic T cells in vivo, 3 × 106 to 5 × 106 anergic CD4+ B9 T cells were incubated either with a blocking anti-CD26 mAb or with an isotype-matched mAb for 30 minutes at RT before intraperitoneal injection into mice treated intraperitoneally with IFN-γ 24 hours earlier. To exclude the possibility that CD26 signaling modified other intrinsic properties of anergic T cells, the naturally occurring peptide YY peptide (3-36) containing the X-X-Pro N-terminal motif known to inhibit DPPIV enzymatic activity was also employed12 to treat anergic T cells prior to injection (30 nM for 30 minutes at room temperature). The following day, PKH26-labeled C6 T cells (5-7 × 106) were injected intraperitoneally. Following a further 24-hour incubation, the presence of labeled T cells in the peritoneal membrane and lavage was analyzed as described in “Materials and methods.” As shown in Figure 5, pretreatment of anergic T cells with anti-CD26 mAbs (Figure 5D,J) or with the YY peptide (Figure 5F,L) prevented the inhibitory effect of anergic T cells on C6 T-cell infiltration of the peritoneal membrane, which appeared extensive. Pretreatment with an isotype-matched mAb resulted in minimal interference with the anti-inflammatory effect of anergic T cells (Figure 5E,K). The possibility that antibody binding to T cells could interfere with their effect on the peritoneal membrane permeability was excluded by pretreating the cells with an anti-CD4 antibody that binds CD4+ B9 T cells. This treatment did not affect the anti-inflammatory activity of anergic T cells (data not shown). Anti-CD26 antibody or YY peptide treatment did not lead to the recovery of responsiveness or motility by anergic T cells (data not shown).
CD26 activity mediates the anti-inflammatory properties of anergic T cells in vivo. Untreated female mice (negative control; panels A,G) and IFN-γ–treated male mice (positive control; panels B,H) were injected with an equal volume of sterile saline containing no T cells. Alternatively, IFN-γ–treated male mice were injected with either untreated anergic B9 T cells (3-5 × 106/mouse; panels C,I), or preincubated with an antimouse CD26 mAb (5 μg/mL; panels D,J), or with an isotype-matched control (5 μg/mL; panels E,K), or with 30nM human YY peptide (3-36) (panels F,L) for 30 minutes at room temperature and subsequently washed. The following day, fluorescently labeled HY-specific C6 T cells (5-7 × 106) were injected intraperitoneally. Following a further 24-hour incubation, the presence of red-fluorescent T cells in the peritoneal membrane (panels A-F) and lavage (panels G-L), as well as the lymph nodes and spleen (data not shown), was assessed. The median number and range of labeled cells quantified in 10 randomly selected × 40-magnified fields of the peritoneal membrane of at least 3 different animals are shown below each image (panels A-D), which is taken from a representative experiment. The median percentage of labeled cells recovered in the peritoneal lavage of 3 different samples is specified below each representative histogram (in which the percentage of positive cells is indicated). * indicates statistically significant (P < .0001 for both panel D and J) compared with anergic T cells treated with isotype-matched control mAbs or with untreated anergic T cells; **, statistically significant (P < .0001, panel F; P < .05, panel L) compared with untreated anergic T cells.
CD26 activity mediates the anti-inflammatory properties of anergic T cells in vivo. Untreated female mice (negative control; panels A,G) and IFN-γ–treated male mice (positive control; panels B,H) were injected with an equal volume of sterile saline containing no T cells. Alternatively, IFN-γ–treated male mice were injected with either untreated anergic B9 T cells (3-5 × 106/mouse; panels C,I), or preincubated with an antimouse CD26 mAb (5 μg/mL; panels D,J), or with an isotype-matched control (5 μg/mL; panels E,K), or with 30nM human YY peptide (3-36) (panels F,L) for 30 minutes at room temperature and subsequently washed. The following day, fluorescently labeled HY-specific C6 T cells (5-7 × 106) were injected intraperitoneally. Following a further 24-hour incubation, the presence of red-fluorescent T cells in the peritoneal membrane (panels A-F) and lavage (panels G-L), as well as the lymph nodes and spleen (data not shown), was assessed. The median number and range of labeled cells quantified in 10 randomly selected × 40-magnified fields of the peritoneal membrane of at least 3 different animals are shown below each image (panels A-D), which is taken from a representative experiment. The median percentage of labeled cells recovered in the peritoneal lavage of 3 different samples is specified below each representative histogram (in which the percentage of positive cells is indicated). * indicates statistically significant (P < .0001 for both panel D and J) compared with anergic T cells treated with isotype-matched control mAbs or with untreated anergic T cells; **, statistically significant (P < .0001, panel F; P < .05, panel L) compared with untreated anergic T cells.
Discussion
Anergic T cells can exert immunoregulation by reducing the immunogenicity of APCs they come into contact with.4,5,7 The molecules involved in anergic T-cell–mediated immune regulation remain undefined. In undertaking the present study we reasoned that, in a manner similar to the antigen-independent stage of T-cell activation and synapse formation,10,11 leukocyte transmigration and tissue infiltration require effective adhesive interactions with the vascular endothelium as well as other parenchymal components. This led to the hypothesis that contact with anergic T cells might prevent/disrupt the ability to establish cell-cell interactions. We here report that exposure to anergic T cells reduces substantially the ability of parenchymal cells to establish effective cell-cell interactions with leukocytes. This effect does not require cognate recognition of the parenchymal cells as it occurs following interaction with anergic T cells with irrelevant specificity and correlates with up-regulation/induction of CD26 (DPPIV) expression, which confers on anergic T cells the ability to metabolize chemokines. CD26 is a surface-bound dipeptidyl-peptidase capable of cleaving N-terminal dipeptides from polypeptides, including chemokines,26,27 with a proline or an alanine at second position. Albeit extensively studied, the role of CD26 in T-cell biology has not yet been clarified. CD26 has been attributed direct costimulatory activity as well as protection of T cells from adenosine-induced apoptosis.26,27 More recently, CD26 has attracted great interest due to its ability to inactivate or alter the specificity of many chemokines, including CXCL10 IFNγ-inducible protein 10 kDa (IP-10) and CXCL12,28 and has been described to be preferentially expressed by T helper-1 (Th-1)29 and T regulatory-1 (Tr-1) cells.30 Several explanations can account for our observation that although CD26 is expressed at significant levels also by responsive T cells, in this form it is not inhibitory. First, it might be simply a quantitative effect. Second, it is known that CD26 activity is increased by polymerization.31,32 It is possible that this molecule is expressed in a more “active” form by anergic T cells. Third, other DPPIV molecules (such as attractin33 ) that share antigenic determinants with CD26 (and thus might not be discriminated by antibodies33 ) can be expressed by anergic T lymphocytes. Finally, CD26 might be required for the inhibitory effect but another factor/molecule specifically expressed by anergic T cells is also needed. Further studies will be required to clarify this issue.
The most likely mechanism by which CD26 enzymatic activity exerts its effect in our system is by metabolizing cell surface–bound chemoattractants.34 In support of this hypothesis, we have shown that anergic T cells reduce CXCL12 activity and that this effect is partly prevented by CD26 blockade. Other surface-bound enzymes with similar properties, such as cathepsin-G35 and attractin,36 which are expressed on the T-cell surface where they are metabolically active, might be involved in this effect. Interestingly, the T-cell surface enzyme attractin (DPPT-L) that shares many properties with CD26, including the regulation of T-cell–APC interactions, had been hypothesized to regulate T-cell activation by down-regulating chemokine/cytokine activity by proteolytic modification following the induction of cluster formation.33,36 These opposing effects on T-cell activation are probably determined by the balance of membrane-expressed to soluble attractin.33
How may this mechanism contribute to physiologic immunoregulation in vivo? The hypothesis that we have promoted is that antigen presentation by tissue parenchymal cells leads to the generation of immunoregulatory anergic T cells and that this is a key event in the control of autoimmunity induced as a byproduct of ongoing inflammation.37 This hypothesis is supported by numerous observations of anergy induction by costimulation-deficient antigen-presenting cells37 including epithelial cells.38,39 This is likely to be a prominent event, in vivo, once tissue inflammation is well established and parenchymal cell MHC class II expression has been induced. In addition, increasing evidence suggests that T-cell anergy may arise in vivo as a result of homeostatic mechanisms involving T-T antigen presentation (R.I.L., unpublished observations, January 1999) and CTLA-4 ligation.40 In this context, newly generated anergic T cells may contribute to the containment of the inflammatory process by metabolizing chemoattractants within the tissue, thus reducing tissue infiltration. Our observations that anergic T cells are immobile and remain localized and die in the site where they have been generated make it unlikely that anergic T cells can exert chemokine metabolism at the luminal side of the endothelium (ie, leukocyte transendothelial migration). Finally, chemokine metabolism may contribute to reducing the immunogenicity of tissue-resident APCs by rendering them unable to induce polarization and adhesion of interacting T cells. This possibility remains to be tested.
Prepublished online as Blood First Edition Paper, May 29, 2003; DOI 10.1182/blood-2003-02-0637.
Supported in part by the British Heart Foundation (Grant PG/2001014).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. Rudd and A. George for critical review of the manuscript and S. Puhalla for her help with wide-field fluorescence microscopy.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal