Abstract
Human natural killer (NK) and NK T cells play an important role in allogeneic bone marrow (BM) transplantation and graft-versus-leukemia (GVL) effect. The mechanisms by which these cells home to the BM and spleen are not well understood. Here we show that treatment of these cells with pertussis toxin and neutralizing antibodies to the chemokine receptor CXCR4 inhibited homing of the cells to the BM, but not the spleen, of NOD/SCID mice. The retention of NK and NK T cells within the spleen and BM was dependent on Gαi signaling and CXCR4 function. The chemokine receptors CXCR4 and CXCR3 are expressed predominantly on the cell surface of NK T cells. Following activation with interleukin-2 (IL-2), the levels of CXCR4 on NK and NK T cells decreased significantly. Treatment of cells with IL-2 inhibited their migration in response to CXCL12 and their homing and retention in the BM and spleen of NOD/SCID mice. In contrast to CXCR4, the expression levels of the chemokine receptor CXCR3 and the migration of cells in response to CXCL9 and CXCL10 increased after IL-2 treatment. Thus, down-regulation of CXCR4 and up-regulation of CXCR3 may direct the trafficking of cells to the site of inflammation, rather than to hematopoietic organs, and therefore may limit their alloreactive potential.
Introduction
Human natural killer (NK) cells are predominantly large granular lymphocytes (LGLs), the majority of which express CD16 and CD56 cell-surface antigens. A small subset of NK cells lacks CD16 and expresses high levels of CD56 and CD94. Another major CD56+ population is composed of T cells that express the CD3 antigen. The human peripheral blood lymphocyte (PBL) NK T-cell population is enriched with CD8+ T cells of effector phenotypes. NK and NK T cells can mediate cytolysis of tumor cells and virus-infected cells.1,2 To improve responses against metastatic tumors, a variety of adoptive cellular strategies have been tested. Among the techniques most studied and developed is the use of lymphokine-activated killer (LAK) cells. LAK cells express surface markers characteristic of NK cells, including CD56 and CD16, and rarely express the common T-cell marker CD3.3,4 The LAK activity is induced from a population of resting lymphoid cells by in vitro exposure of these cells to a supraphysiologic concentration of IL-2 (500-1000 IU/mL).3,4 NK and NK T cells are also key effector cells mediating the graft-versus-leukemia (GVL) effect, which is used to control minimal residual disease (MRD) and for the reinduction of remission in chronic myelogenous leukemia patients who relapse after allogeneic stem cell transplantation.1,5-8 These cells are also important for low-intensity conditions and nonmyeloablative allogeneic stem cell transplantation, which are currently being performed not only in hematologic malignancies, but also in solid tumors such as renal cell carcinoma.9 In hematogenic metastasis, such as that of prostate cancer, breast cancer, and neuroblastoma, the malignant cells home, arrest, and develop in the BM.10,11 In order to extract their function, NK and NK T cells must reach the BM. However, the mechanism that regulates the trafficking of these cells to the BM is unknown.
Chemokines and their receptors have an essential role in the recruitment and tissue localization of cells from the immune system.12 NK cells have mainly been reported to express chemokine receptors from the CXC family, such as CXCR1-4 and CXC3R1. In contrast, CD56+, CD16–, CD3+ NK T cells have been shown to express the chemokine receptors CCR1, CCR2, CCR5, and CCR6 from the CC family and the chemokine receptors CXCR 3, CXCR4, and CXCR6 from the CXC family.13,14 The chemokine receptor CXCR4 and its ligand, CXCL12, have been shown to play a crucial role in the trafficking and tissue localization of human hematopoietic stem cells and breast tumor cells to hematopoietic organs.15,16 This makes the chemokine-receptor pair CXCL12/CXCR4 of particular interest in investigating their own role in the homing of NK and NK T cells to the BM microenvironment. Here we show that the homing of NK and NK T cells to the BM was dependent upon G protein signaling and CXCR4 and was inhibited by IL-2 treatment. Upon activation of these cells with IL-2, CXCR4 expression was reduced, whereas the expression of CXCR3 on the cell surface of both subsets of cells increased. Down-regulation of CXCR4 expression reduced the migration of NK and NK T cells in response to CXCL12, whereas their migration in response to the CXCR3 ligands CXCL9 and CXCL10 increased significantly. This study suggests that under physiologic conditions or in ex vivo activation of NK and NK T cells in which IL-2 is involved, the trafficking of NK and NK T cells to the BM and spleen will be inhibited.
Materials and methods
In vitro experimental procedures
Human mononuclear cells (MNCs) were isolated from donor blood buffy coats obtained from the Blood Bank, Hadassah Ein-Kerem. NK and NK T cells were purified from the MNCs by immunomagnetic positive selection using the MACS system (Miltenyi Biotech, Bergicsh Gladbach, Germany) according to the manufacturer's instructions. Peripheral NK clones that stained positive for CD56 and CD16 and negative for CD3 were collected and cloned in the presence of IL-2 as previously described.17
The purity of the cells was determined before use by immunostaining for CD56 and CD16. For immunostaining, 2 × 105 cells were resuspended in staining buffer (phosphate-buffered saline [PBS], 0.1% bovine serum albumin [BSA], 0.01% sodium azide) and incubated with 1% human plasma for 20 minutes at 4°C. Cells were then stained with human-specific, direct-labeled antibodies (Abs) and incubated for 30 minutes at 4°C. After staining, cells were washed in the same buffer and analyzed by FACScalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA), using CellQuest software. Immediately after purification, cells were suspended in RPMI 1640 supplemented with 10% low-endotoxin fetal calf serum (FCS; Gibco BRL Life Technologies, Grand Island, NY) and 1% glutamine, sodium piruvate, and penicillin-streptomycin (all from Biological Industries, Beit Haemek, Israel). In order to get lymphokine-activated killer (LAK) cells, purified resting lymphoid cells isolated from peripheral blood mononuclear cells were first plated for 2 hours in tissue-culture flasks (Corning, Corning, NY), to allow the adherence of monocytes. To remove the B cells, the nonadherent cells were then passed through nylon mesh columns. The cells collected were further separated on Percoll gradient to enrich for LGL precursors. The fraction of cells that contained the LGLs was treated with 1000 IU/mL IL-2 (Proleukine; Chiron, Amsterdam, The Netherlands), plated at 5 × 105/mL in 24-well tissue-culture plates (Corning) and cultured at 37°C, 5% CO2 atmosphere, for 5 days.
Antibodies, fluorescence-activated cell sorter (FACS) analysis, and cell migration
The purity of the cells was determined before use by immunostaining for CD56 and CD16. The following directly conjugated antihuman mAbs were used in this study: CD3 Cy-Q, B-B11 (IgG1; IQ Products, Groningen, The Netherlands), CD16 FITC, B-E16 (IgG2A; IQ Products), CD56 PE, MOC-1 (IgG1; IQ Products), (IgG2A; R&D Systems, Minneapolis, MN), CXCR3 PE, 49801.111 (IgG1; R&D Systems), CXCR4 PE, 12G5 (IgG2A; R&D Systems), CXCR1, and 42705.11 (IgG2A; R&D Systems). Cell sorting was performed on a FACStar Plus (Becton Dickinson Immunocytometry Systems).
Two major CD56+ subpopulations of NK and NK T cells were sorted by gating on CD56+CD16+ and CD56+CD3+ cells (Figure 1Aii). Chemotaxis experiments with human NK and NK T cells were assayed by transwell migration assay (Costar, Cambridge, MA; 6.5-mm diameter, 5-μm pore size). Briefly, 100 μL chemotaxis buffer (RPMI 1640, 1% FCS) containing 2 × 105 NK cells was added to the upper chamber, and 0.6 mL chemotaxis buffer with or without chemokines was added to the bottom chamber. Cells migrating within 4 hours to the bottom chamber of the transwell were counted for 30 seconds using FACScalibur (Becton Dickinson Immunocytometry Systems). The following human chemokines were used in this study: recombinant stromal-derived factor CXCL12 (R&D Systems) and monokines induced by γ-interferon (CXCL9) and CXCL10 (R&D Systems).
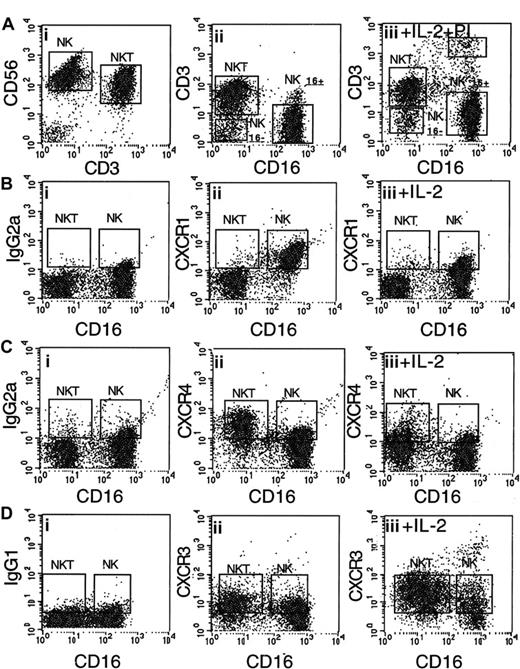
Regulation of the expression of chemokine receptors CXCR1, CXCR3, and CXCR4 on purified CD56+CD16+ NK and CD56+CD3+ NK T cells. (A) (i) CD56+ cells were purified from peripheral blood lymphocytes. (ii) Three major subpopulations of CD56+ NK cells were found: CD56+CD3+CD16– NK T cells (35%), CD56+CD16+CD3– NK cells (60%), and CD56+CD16–CD3– NK cells (5%). (B) CD56+CD16+ NK cells, but not NK T cells, express CXCR1 (ii); after 48 hours' incubation with IL-2 (1000 IU/mL), the cell-surface expression of CXCR1 is down-regulated (iii). (C) CD56+CD3+ NK T cells, but not NK cells, express high levels of CXCR4 (ii); after 48 hours' incubation with IL-2, the cell-surface expression of CXCR4 is down-regulated on both types of cells (iii). (D) NK and NK T cells express low levels of CXCR3 (ii); after 48 hours' incubation with IL-2, the cell-surface expression of CXCR3 is up-regulated on both NK and NK T cells (iii). IgG2APE and IgG1PE were used as control antibodies (panels Bi, Ci, and Di).
Regulation of the expression of chemokine receptors CXCR1, CXCR3, and CXCR4 on purified CD56+CD16+ NK and CD56+CD3+ NK T cells. (A) (i) CD56+ cells were purified from peripheral blood lymphocytes. (ii) Three major subpopulations of CD56+ NK cells were found: CD56+CD3+CD16– NK T cells (35%), CD56+CD16+CD3– NK cells (60%), and CD56+CD16–CD3– NK cells (5%). (B) CD56+CD16+ NK cells, but not NK T cells, express CXCR1 (ii); after 48 hours' incubation with IL-2 (1000 IU/mL), the cell-surface expression of CXCR1 is down-regulated (iii). (C) CD56+CD3+ NK T cells, but not NK cells, express high levels of CXCR4 (ii); after 48 hours' incubation with IL-2, the cell-surface expression of CXCR4 is down-regulated on both types of cells (iii). (D) NK and NK T cells express low levels of CXCR3 (ii); after 48 hours' incubation with IL-2, the cell-surface expression of CXCR3 is up-regulated on both NK and NK T cells (iii). IgG2APE and IgG1PE were used as control antibodies (panels Bi, Ci, and Di).
In vivo experimental procedures
NOD/SCID mice (NOD/LtSz PrKdcscid/PrKdcscid) are maintained under defined flora conditions at the Hebrew University Animal Pathogen Free Facility. The Animal Care Committee of the Hebrew University approved all the experiments. Human CD56+ cells (90%-97% purity, 5 × 106 cells per mouse) in 0.25 mL RPMI with 10% FCS were injected into the tail veins of 6-week-old mice. Prestaining of cells was done by using carboxyfluorescein diacetate, succinimidyl ester (CFDA SE) Cell Tracer Kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Mice were killed 1, 4, 24, and 72 hours after transplantation and cells from the blood, spleen, liver, and femur and tibia bones were collected and treated with an RBC lysis solution (0.155 M NH4Cl, 0.01 M KHCO3, 0.01 mM EDTA [ethylenediaminetetraacetic acid], pH 7.4). Liver tissue was homogenized through a steel sieve (Sigma, St Louis, MO; 50 mesh) into PBS (Gibco, Paisley, Scotland). Hepatic lymphocytes were washed 3 times at 900 rpm for 5 minutes following isolation onto Ficoll-Paque Plus (Amersham Biosciences, Uppsala, Sweden), then washed and resuspended with PBS.
A single-cell suspension was then stained with human-specific, direct-labeled antibody for CD3 and analyzed by FACS. For blocking chemokine-dependent migration, homing, and retention, cells were preincubated with 1 μg/mL pertussis toxin (PTX) at 37°C for 3 hours (List Biological Laboratories, Campbell, CA) and then used for in vitro and in vivo assays. For the blocking of CXCR4 function, NK and NK T cells were preincubated with 10 μg/mL mouse antihuman CXCR4 mAb 12G5 (IgG2a) (R&D Systems) or a control mouse IgG2a antibody (R&D Systems) at 37°C for 1 hour and then used for in vitro and in vivo assays.
Results
In vitro IL-2 activation of NK and NK T cells differentially regulates CXCR4 and CXCR3 cell-surface expression and function
Human NK cells express CD16 and CD56 cell-surface antigens and do not express the T-cell marker CD3 (CD56+CD16+CD3– cells), whereas NK T cells do not express the CD16 molecule but do express the CD56 and CD3 markers (CD56+CD16–CD3+ cells). Most of the human NK T cells express the CD8 antigen but do not express the CD4 antigen.18 The purification of CD56+ NK and NK T cells was performed, as described in “Materials and methods,” from the peripheral blood of healthy donors. Three major distinct subsets were identified in the purified population of CD56+ cells: (1) NK T cells that express the CD3 antigen (Figure 1A) and constitute approximately 40% of the purified cells; (2) NK cells that do not express the CD3 antigen but do express the CD16 antigen and that constitute approximately 55% of the purified cells (Figure 1A); and (3) NK cells that do not express the CD3 and CD16 antigens and constitute approximately 5% of the purified cells. CD56+CD16+ cells did not express the T-cell CD3 marker and were defined as NK cells. CD56+CD16– cells expressed CD3 and were defined as NK T cells (Figure 1Aiii). Activation of CD56+ cells with IL-2 (1000 IU/mL) did not change the relative percentage of each subpopulation and did not stimulate significant cell death (Figure 1Aiii).
Our study was focused on the role of the CXC receptor family in the trafficking of resting and IL-2–activated human CD56+CD16– NK and CD56+CD16–CD3+ NK T cells to the BM. CXCR1, CXCR3 and CXCR4 were detected on the cell surface of resting and IL-2–activated human NK and NK T cells (Figure 1B-D). These chemokine receptors were expressed differentially by NK and NK T cells, and their surface expression was differentially regulated upon IL-2 activation. The expression of CXCR1 and CXCR4 was first tested on purified CD56+CD16+ NK and CD56+CD3+ NK T cells. The chemokine receptor CXCR1 was expressed at higher levels on the cell surface of NK cells (97%) than on NK T cells (9%; Figure 1Biii). Following activation with IL-2 for 2 days, the levels of CXCR1 expression on NK cells decreased significantly: only 32% of NK cells expressed CXCR1 (Figure 1Biii). The chemokine receptor CXCR4 was expressed at higher levels on the cell surface of NK T cells than on NK cells (Figure 1Cii). About 68% of NK T cells and only 20% of NK cells expressed CXCR4 in a resting state. Following activation with IL-2, the levels of CXCR4 expression on NK and NK T cells decreased significantly: 33% of activated NK T cells and about 6% of activated NK cells expressed CXCR4 (Figure 1Ciii). The chemokine receptor CXCR3 is expressed on activated T cells and is involved in the trafficking of these cells to the site of inflammation.19,20 Like CXCR4, the chemokine receptor CXCR3 was also expressed at higher levels on NK T cells than on NK cells (Figure 1Cii). In contrast to CXCR4, the expression levels of CXCR3 increased after IL-2 treatment on both NK and NK T cells (Figure 1Ciii). In contrast to CD56+CD16+ NK cells, the CD56+CD16CD3– NK subset expressed high levels of CXCR4. Similar to CD16+ NK and NK T cells, upon activation with IL-2 or IL-15 CD56+CD16– NK cells down-regulated CXCR4 expression and up-regulated CXCR3 cell-surface expression (J.H., O.W., D. Goldman-Wohl, et al, unpublished data, November 2002).
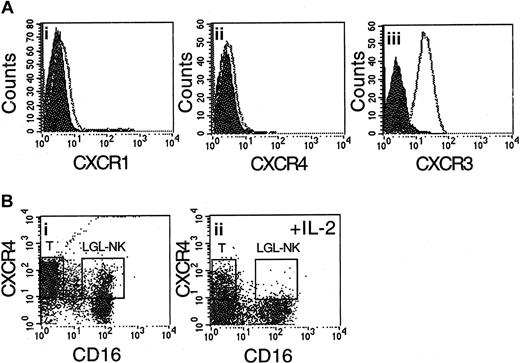
The levels of CXCR1 and CXCR4 cell-surface expression on IL-2–activated CD56+CD16+ primary NK cell lines purified by negative selection17 were also very low (Figure 2A). Increased cell surface expression of CXCR3 in these cells is also shown in Figure 2A. The major effector population in LAK cells is CD56+CD16+ large granular lymphocytes (LGLs).21 These cells are induced from a population of resting lymphoid cells by in vitro exposure of these cells to a supraphysiologic concentration of IL-2 (1000 IU/mL). Following activation with IL-2, CD56+CD16+ LGLs, as well as the remaining CD3+ cells, down-regulated CXCR4 expression (Figure 2B).
CD56+CD16+ NK cell lines, as well as LAK cells, do not express the chemokine receptor CXCR4. (A) CD56+CD16+ cells grown in the presence of IL-2 (50-100 IU/mL) for 4 weeks (NK cell lines) do not express the chemokine receptors CXCR1 (i) and CXCR4 (ii), but do express the chemokine receptor CXCR3 (iii). (B) After activation with a supraphysiologic concentration of IL-2 (1000 IU/mL), the major effector population of LAK cells, CD56+CD16+ large granular lymphocytes (LGLs) as well as T cells, down-regulate CXCR4 expression (ii). Staining of control resting lymphoid cells with antibodies against CXCR4 and CD16 is shown in panel i.
CD56+CD16+ NK cell lines, as well as LAK cells, do not express the chemokine receptor CXCR4. (A) CD56+CD16+ cells grown in the presence of IL-2 (50-100 IU/mL) for 4 weeks (NK cell lines) do not express the chemokine receptors CXCR1 (i) and CXCR4 (ii), but do express the chemokine receptor CXCR3 (iii). (B) After activation with a supraphysiologic concentration of IL-2 (1000 IU/mL), the major effector population of LAK cells, CD56+CD16+ large granular lymphocytes (LGLs) as well as T cells, down-regulate CXCR4 expression (ii). Staining of control resting lymphoid cells with antibodies against CXCR4 and CD16 is shown in panel i.
The migration potential of human CD16+ NK and CD3+ NK T cells in response to the chemokine CXCL12 was tested in vitro in a transwell migration assay. In all donors tested, resting CD56+ cells migrated in response to a chemotactic gradient of CXCL12 (Figure 3A). Upon activation with IL-2 (1000 IU/mL), their migration potential was reduced dramatically (Figure 3A). The effect of IL-2 on the migration of CD56+ cells was dependent on the concentration of IL-2 (Figure 3B). In contrast to CXCL12, the migration of IL-2–activated CD56+ cells toward the CXCR3 ligands CXCL9 and CXCL10 increased significantly (Figure 3C). FACS-sorted CD3+ NK T cells that express high levels of CXCR4 migrated better in response to CXCL12, in a dose-dependent manner, than FACS-sorted CD16+ NK cells (Figure 3D). CD56+CD16+ NK cells grown in the presence of IL-2 (100 IU/mL) for 3 weeks (NK cell lines), as well as CD56+CD16+ LGLs grown in the presence of IL-2 (1000 IU/mL) for 5 days, also failed to migrate in response to CXCL12 (data not shown). Following activation with IL-2, the response of NK and NK T cells to CXCL12 was reduced significantly (Figure 3E). The migration of CD56+ cells in response to CXCL12 was dependent on Gαi signaling and was inhibited by PTX and CXCR4 neutralizing antibodies (Figure 3F).
CD56+CD3+CD16– NK T cells migrate better in response to CXCL12 than do CD56+CD16+CD3– NK cells. (A) The migration of CD56+ cells in response to the chemokine CXCL12 (100-500 ng/mL) is inhibited by pretreatment of cells with 1000 IU/mL IL-2 for 48 hours. (B) Incubation of cells with IL-2 at concentrations of 100, 500, and 1000 IU/mL significantly reduced the migration of these cells (at 100 ng/mL) toward CXCL12. Index of migration indicates the fold increase in the number of migrating cells in response to CXCL12 vs control. (C) IL-2–activated CD56+ cells show increased percentage of migration in response to CXCL9 and CXCL10, the ligands for CXCR3. (D) Sorted CD3+CD16– NK T cells migrate better, in a dose-dependent manner, in response to CXCL12 than do CD16+ NK cells. (E) A marked reduction in the migration toward CXCL12 after activation with IL-2 is shown for the sorted NK and NK T cells. (F) The migration of CD56+ cells toward CXCL12 can be completely inhibited by incubating the cells with pertussis toxin (PTX) or neutralizing anti-CXCR4 antibodies. The results represent the average of at least 3 independent experiments ± SE. *P < .05.
CD56+CD3+CD16– NK T cells migrate better in response to CXCL12 than do CD56+CD16+CD3– NK cells. (A) The migration of CD56+ cells in response to the chemokine CXCL12 (100-500 ng/mL) is inhibited by pretreatment of cells with 1000 IU/mL IL-2 for 48 hours. (B) Incubation of cells with IL-2 at concentrations of 100, 500, and 1000 IU/mL significantly reduced the migration of these cells (at 100 ng/mL) toward CXCL12. Index of migration indicates the fold increase in the number of migrating cells in response to CXCL12 vs control. (C) IL-2–activated CD56+ cells show increased percentage of migration in response to CXCL9 and CXCL10, the ligands for CXCR3. (D) Sorted CD3+CD16– NK T cells migrate better, in a dose-dependent manner, in response to CXCL12 than do CD16+ NK cells. (E) A marked reduction in the migration toward CXCL12 after activation with IL-2 is shown for the sorted NK and NK T cells. (F) The migration of CD56+ cells toward CXCL12 can be completely inhibited by incubating the cells with pertussis toxin (PTX) or neutralizing anti-CXCR4 antibodies. The results represent the average of at least 3 independent experiments ± SE. *P < .05.
In vitro IL-2 activation of NK and NK T cells inhibits their CXCR4-dependent homing to the BM of NOD/SCID mice
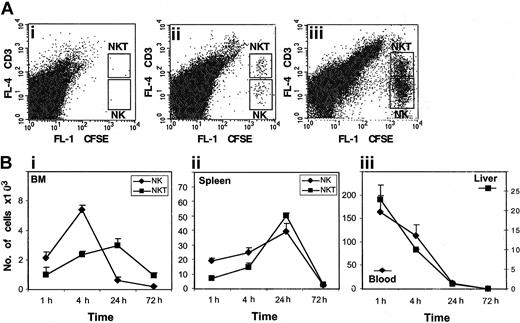
The function of the CXCR4/CXCL12 receptor/ligand pathway was associated mainly with the circulation of naive immune cells such as stem cells, B cells, and T cells15 under physiologic conditions. In contrast to CXCR4, the chemokine receptor CXCR3 and its ligand were associated mainly with the trafficking of activated T cells to inflammatory sites.21-23 We therefore further studied the role of CXCR4 in the in vivo homing (1-4 hours) and accumulation (ie, retention and survival; 4-72 hours) of resting or activated NK and NK T cells to the BM and spleen of NOD/SCID mice. Purified CD56+ cells were prestained with the fluorescent dye CFDA SE and injected intravenously into nonirradiated NOD/SCID mice. NK and NK T cells from the blood, BM, spleen, and liver were collected 1, 4, 24, and 72 hours following transplantation. The number of NK and NK T cells was then tested by staining the cells with antibodies to the CD3 antigen (Figure 4A) and by counting the stained cells by FACS. In the first 4 hours following injection, we could detect homing of both NK and NK T cells to the BM; however, after 24 hours, NK T cells accumulated in the BM at higher numbers than NK cells (Figure 1B). The number of NK and NK T cells homing and accumulating in the spleen during the first 24 hours was similar (Figure 4B). As illustrated in Figure 4B, the number of human CD56+ cells in the blood and liver gradually decreased, and when homing to the BM and spleen was completed after 24 hours, almost no CD56+ cells were detected in the blood and liver (Figure 4B). Seventy hours following transplantation, we could not detect cells in any of the tissues tested (Figure 4B). We therefore concluded that in the spleen and BM, NK and NK T cells homed, were retained, and survived for the first 24 hours following transplantation.
Twenty-four hours after transplantation, NK and NK T cells accumulate in the spleen and BM, but not in the blood or liver, of NOD/SCID mice. CD56+ cells loaded with the fluorescent dye CFDA SE were injected into the tail veins of NOD/SCID mice. At 1, 4, 24, and 72 hours after injection, cells from the BM, spleen, liver, and blood were isolated. (A) The numbers of CD3+ NK T cells and NK cells in the BM (ii) and spleen (iii) were counted by FACS. Cells from the BM of uninjected mice are shown as a control (i). (B) The kinetics of homing (1 and 4 hours) and accumulation (24 and 72 hours) of NK and NK T cells detected in the BM (i) and spleen (ii). The presence of CD56+ cells in the blood and liver of injected mice is shown in panel iii. The results represent the average of 3 independent experiments ± SE.
Twenty-four hours after transplantation, NK and NK T cells accumulate in the spleen and BM, but not in the blood or liver, of NOD/SCID mice. CD56+ cells loaded with the fluorescent dye CFDA SE were injected into the tail veins of NOD/SCID mice. At 1, 4, 24, and 72 hours after injection, cells from the BM, spleen, liver, and blood were isolated. (A) The numbers of CD3+ NK T cells and NK cells in the BM (ii) and spleen (iii) were counted by FACS. Cells from the BM of uninjected mice are shown as a control (i). (B) The kinetics of homing (1 and 4 hours) and accumulation (24 and 72 hours) of NK and NK T cells detected in the BM (i) and spleen (ii). The presence of CD56+ cells in the blood and liver of injected mice is shown in panel iii. The results represent the average of 3 independent experiments ± SE.
The process of homing from the blood to the tissues was finalized 24 hours following transplantation, and in the liver, cells were accumulating at high numbers; shortly after injection, and by a process of cell death, these cells rapidly disappeared from the liver. To further test the role of CXCR4 in the homing of NK and NK T cells to the BM of NOD/SCID mice, cells were pretreated with either PTX or neutralizing antibodies to CXCR4, and a control antibody. Treatment of both NK and NK T cells with PTX, an inhibitor of chemokine receptor signaling, or with anti-CXCR4 antibodies significantly inhibited the homing (4 hours after injection) and accumulation (24 hours after injection) of both NK and NK T cells to the BM (Figure 5A). In contrast, 4 hours following injection, the homing of NK and NK T cells to the spleen was not dependent on CXCR4 or PTX. The accumulation of cells within the spleen was also dependent on Gαi signaling and CXCR4 (Figure 5B). These results suggest a role for the CXCR4/CXCL12 pathway in the homing of NK and NK T cells to the BM, but not the spleen, and a role for CXCR4 in the accumulation (ie retention and/or survival) of NK and NK T cells within the BM and the spleen.
The homing of NK and NK T cells to the BM, but not the spleen, of NOD/SCID mice is dependent on Gαi signaling and CXCR4. (A) The homing (4 hours) and accumulation (24 hours) of NK and NK T cells to the BM were reduced when cells were treated with either PTX or anti-CXCR4 neutralizing antibody. (B) The homing (4 hours) of NK and NK T cells to the spleen is not dependent on Gαi signaling and CXCR4. The accumulation (24 hours) of NK and NK T cells to the spleen was reduced when cells were treated with either PTX or anti-CXCR4 neutralizing antibody. The results represent the average of 3 independent experiments ± SE. *P < .05.
The homing of NK and NK T cells to the BM, but not the spleen, of NOD/SCID mice is dependent on Gαi signaling and CXCR4. (A) The homing (4 hours) and accumulation (24 hours) of NK and NK T cells to the BM were reduced when cells were treated with either PTX or anti-CXCR4 neutralizing antibody. (B) The homing (4 hours) of NK and NK T cells to the spleen is not dependent on Gαi signaling and CXCR4. The accumulation (24 hours) of NK and NK T cells to the spleen was reduced when cells were treated with either PTX or anti-CXCR4 neutralizing antibody. The results represent the average of 3 independent experiments ± SE. *P < .05.
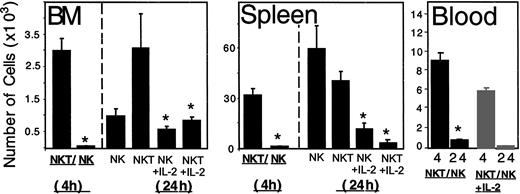
As illustrated in Figure 3D, activation of NK and NK T cells with IL-2 (1000 IU/mL) significantly reduced the migration of these cells toward CXCL12. In correlation, treatment of NK T or NK cells with IL-2 dramatically reduced the homing and accumulation of these cells to the BM and spleen (Figure 6). We further tried to exclude the possibility that this reduction in the number of cells migrating in response to CXCL12 and homing to the BM of NOD/SCID mice was due to cell death. In order to do so, we tested the percentage of dead cells in the IL-2–activated CD56+ cells before transplantation and their numbers in the blood 4 hours after injection. Treatment of CD56+ cells with IL-2 (1000 u/mL) did not induce significant cell death and did not change the relative percentage of cells from each subpopulation (Figure 1Aiii). A significant reduction (35%) in the number of activated CD56+ cells in the blood was found 4 hours after transplantation (Figure 6). This may be due to the rapid entry of cells into the liver, which was already reported to constitutively express the chemokine CXCL9, the ligand for CXCR3, and the chemokine CCL3, the ligand for CCR5.23 The inhibition in the homing of activated CD56+ cells in the blood to the BM and spleen can be explained by the down-regulation of CXCR4 on IL-2–activated NK and NK T cells.
The homing and accumulation of IL-2–activated NK and NK T cells to the BM and spleen is significantly reduced. The number of untreated or IL-2 (1000 IU/mL)–treated NK and NK T cells in the BM and spleen of NOD/SCID mice 4 and 24 hours after transplantation is shown, along with the number of untreated or IL-2 (1000 IU/mL)–treated CD56+ cells in the blood of these mice. The results represent the average of 3 independent experiments ± SE. *P < .05.
The homing and accumulation of IL-2–activated NK and NK T cells to the BM and spleen is significantly reduced. The number of untreated or IL-2 (1000 IU/mL)–treated NK and NK T cells in the BM and spleen of NOD/SCID mice 4 and 24 hours after transplantation is shown, along with the number of untreated or IL-2 (1000 IU/mL)–treated CD56+ cells in the blood of these mice. The results represent the average of 3 independent experiments ± SE. *P < .05.
Discussion
The idea of tissue-specific lymphocyte trafficking has become a fundamental concept in the study of adapted and acquired immunity. Lymphocyte recirculation into lymphoid and nonlymphoid tissues is tightly regulated by the expression of particular adhesion molecules and chemoattractant receptors on lymphocytes, combined with the spatial and temporal expression of ligands for these receptors by a variety of tissue cells.24 Human NK and NK T cells constitute one of the major lymphocyte subsets that play an important role in both innate and adaptive immune responses against a broad variety of infections and tumors. Three main subpopulations of NK cells have been identified in humans: CD56+CD16+CD3– NK cells, CD56+CD16–CD3– NK cells, and CD56+CD16–CD3+ NK T cells. In order to mediate their cytolitic function effectively, these cells must be recruited to the site of infected or neoplastic cells. Moreover, in order to regulate the adaptive immune response, cytokine-secreting NK and NK T cells must be in intimate proximity to antigen-stimulated T and/or B cells.1,2 Thus, understanding the mechanism responsible for the homing of NK cells into hematopoietic organs, tumors, and areas of inflammation is of great relevance to human health and disease.
The expression of chemokine receptors by human NK cells remains a subject of controversy. Two of the most comprehensive published studies in this area provide contradictory results, particularly with respect to the CXC chemokine receptors. Campbell et al found that while most human NK cells express high levels of CXCR1, the chemokine receptors CXCR4, CX3CR, CXCR2, and CXCR3 were expressed at lower levels.14 In contrast, Inngjerdingen et al found that purified resting human NK cells expressed CXCR4, but not CXCR1, CXCR2, CXCR3, or CX3CR.25 The published data are most consistent with respect to NK cell expression of the CC chemokine receptors. While CCR1, CCR2, CCR3, CCR4, CCR5, CCR6, CCR7, CCR8, and CCR9 are not expressed at significant levels on the cell surface of most resting human NK cells, CCR2 and CCR5 are expressed after in vitro activation with IL-2 or IL-15.14,25 The results obtained from our group corroborate those of Campbell et al with respect to expression of CCR1, CCR2, CCR3, CCR5, CCR6, CCR7, CXCR1, and CX3CR1 on the resting CD56+ cells and expression of CCR7 on IL-2–activated cells (data not shown). However, we could not detect significant cell-surface expression of CCR2, CCR5, or CCR7 on NK and NK T cells following IL-2 activation (data not shown). In fact, we found that while cell-surface expression of CXCR3 and CXCR4 on resting NK cells was low, these receptors were expressed mainly on resting NK T cells.
CXCR4 is the chemokine receptor for CXCL12, a CXC chemokine that is highly expressed by BM stromal cells and other organs.26-28 It has been found that within the human BM, CXCL12 is expressed mainly by immature bone-forming osteoblasts as well as by BM endothelial and stromal cells.15,27 CXCL12 mediates chemokinesis and chemotaxis in a variety of cell types expressing CXCR4, including monocytes and macrophages, B and T lymphocytes, platelets and megakaryocytes, and CD34+ hematopoietic stem cells.28-30 CXCR4 and its ligand, CXCL12, play a central role in the homing and retention of hematopoietic stem cells as well as maturing and mature lymphoid and myeloid cells to the BM and fetal liver. In the absence of CXCR4, B cells and myeloid precursors may fail to localize properly in association with stromal cells and exit the BM prematurely, before full differentiation and proliferation have taken place.31 Human and murine CXCL12 are cross-reactive and differ in one amino acid.32 Indeed, it has recently been shown that human stem cell engraftment of NOD/SCID mice is dependent on the expression of CXCL12 and CXCR4.15 CXCL12 has also been demonstrated to induce the arrest of rolling lymphocytes and CD34+ hematopoietic stem cells under flow conditions, a critical step for recruitment of these cells to the BM 15,33
We therefore studied the role of CXCR4 in the in vivo homing of NK and NK T cells to the BM and spleen of NOD/SCID mice. NOD/SCID mice are severely deficient in both adaptive and innate immunity. The lack of an adaptive immune system results from an inability of SCID mice to express rearranged antigen receptors and to produce mature T and B lymphocytes. NOD mice have multiple defects in innate immunity: they are deficient in NK cell activity and display defects in myeloid development and function.34 Owing to these defects, NOD/SCID mice are unable to reject allogeneic and xenogeneic organ grafts and have been used to examine the in vivo homing, engraftment, and growth patterns of normal and neoplastic human hematopoietic cells.35 Functional in vivo assays for human stem cell homing and BM repopulation have been developed based on intravenous injection of human cells into sublethally irradiated NOD/SCID mice. Animal studies suggest that engraftment is more complete in irradiated recipients, owing to improved access for transplanted cells to supportive niches within the BM and an increased leukocyte interaction with endothelium.36,37 However, stem cell homing may not require the upregulation of adhesion molecules above their constitutive levels. A recent study found that total body irradiation causes profound changes in BM vessels and alters the molecular interaction between microvascular endothelial cells and circulating stem cells. It alters the composition of endothelial adhesion molecules that mediate rolling, and it reduces blood flow and microvascular diameters in the BM. In addition, the irradiation-induced chemoattractants appeared to be distinct from CXCL12.38
Based on these results, we chose to use nonirradiated NOD/SCID mice to study the in vivo role of CXCR4 in the trafficking of human NK and NK T cells to the hematopoietic organs such as the BM and spleen. The cells were injected into the tail veins of mice, the animals were killed 4 hours after the injection, and the cells from the BM, spleen, liver, and blood were collected. In this study we provide evidence that chemokines in general and CXCR4 in particular are involved in the homing of human resting NK and NK T cells to the BM, but not to the spleen. The accumulation (ie, retention or survival) of NK T and NK cells in the spleen and BM is mediated by CXCR4. NK T cells accumulate in greater numbers than NK cells in the BM, but not in the spleen. The fact that NK T cells express high levels of CXCR4, migrate well in response to CXCL12, and home and are retained in the BM of NOD/SCID mice in a CXCR4-dependent manner may explain why human NK T cells are proportionally more plentiful in the bone marrow (20%-30%) than in the spleen (3%) and blood (1%-4%) of humans.1,2
Upon activation with IL-2, NK and NK T cells down-regulated the cell-surface expression of CXCR4. Data obtained from studies by other groups that tested regulation of CXCR4 expression on T cells are contradictory. While some investigators found that activated T lymphocytes down-regulated CXCR4 expression,28,39 others have shown the induction in functional surface expression of CXCR4 on T cells following IL-2 activation.40 Activation of the 3 main subpopulations of NK cells with IL-15 or IL-2 down-regulated most of the chemokine receptors expressed by resting NK cells, including CXCR4, CXCR1, and CX3CR1, and upregulated the expression of CXCR3 and CCR5 (data not shown). These results suggest that CXCR3 and CCR5 are the major chemokine receptors expressed on LAK and activated NK and NK T cells. The chemokine receptor CXCR3 and its ligands, CXCL10 and CXCL9, are thought to be involved in the inflammation processes of the liver, intestine, brain, and synovium. Enhanced expression of CXCL10 was detected in the livers of patients with chronic hepatitis C virus infection.22 Furthermore, CXCL9, the ligand for CXCR3, and CCL3, the ligand for CCR5, were shown to be essential for NK cell inflammation in livers during murine cytomegalovirus (MCMV) infection.23 The fact that the cell-surface expression of CXCR3 and CXCR4 is regulated by IL-2 and IL-15 activation suggests a role for these chemokine receptors in regulating the trafficking of NK and NK T cells during physiologic and inflammatory conditions.
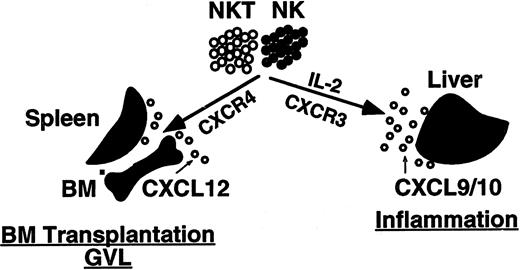
To test this hypothesis, we further studied the in vitro migration and in vivo trafficking of activated NK and NK T cells. The migration potential of resting and IL-2–activated human NK and NK T cells in response to the chemokine CXCL12 was first tested in vitro in a transwell migration assay. In all donors tested, CD56+ cells migrated in response to a chemotactic gradient of CXCL12. Sorted NK T cells that expressed high levels of CXCR4 migrated better in response to CXCL12 than did NK cells. Following activation with IL-2, the response of NK and NK T cells to CXCL12 was significantly reduced. This finding correlates with down-regulation in cell-surface expression of CXCR4 upon activation with IL-2. Our results provide evidence that chemokines in general and CXCR4 in particular are involved in the homing of human resting NK and NK T cells to the BM. The proposed model for the in vivo trafficking of human NK and NK T cells under resting and activated conditions is presented in Figure 6. Upon activation with IL-2 or IL-15, NK and NK T cells down-regulated the cell-surface expression of CXCR4 and up-regulated the cell-surface expression of the chemokine receptor CXCR3. The decreased expression of CXCR4 may promote mobilization of the cells from hematopoietic organs to the peripheral blood; the increased expression of CXCR3 may direct the migration and accumulation of these cells to the sites of inflammation, such as an infected liver, where CXCL9 or CXCL10, the ligands for CXCR3, are expressed (Figure 7).
Model for trafficking of NK and NK T cells in vivo. Under normal conditions, the homing of NK and NK T cells to the BM is Gαi- and CXCR4-dependent. The homing of NK and NK T cells to the BM is important for both BM transplantation and the GVL effect. Upon activation with IL-2, NK and NK T cells up-regulate the cell-surface expression of the receptor CXCR3 and down-regulate the expression of CXCR4. This may direct the cells to sites of inflammation, such as virally infected livers, where the chemokines CXCL9 and CXCL10 are highly expressed.
Model for trafficking of NK and NK T cells in vivo. Under normal conditions, the homing of NK and NK T cells to the BM is Gαi- and CXCR4-dependent. The homing of NK and NK T cells to the BM is important for both BM transplantation and the GVL effect. Upon activation with IL-2, NK and NK T cells up-regulate the cell-surface expression of the receptor CXCR3 and down-regulate the expression of CXCR4. This may direct the cells to sites of inflammation, such as virally infected livers, where the chemokines CXCL9 and CXCL10 are highly expressed.
IL-2 induces proliferation of T lymphocytes and NK cells and the production of interferon-γ; it also results in the induction of lymphokine-activated killer (LAK) cells against previously NK-resistant cell preparations and cell lines. It has been established that the majority of LAK precursor cells as well as LAK effector cells are phenotypically identical to NK cells. The LAK precursors express surface markers characteristic of NK cells, including CD56 and CD16, and rarely express CD3 and CD5, common T-cell markers.41 The LAK activity is induced from a population of resting lymphoid cells by in vitro exposure of these cells to a supraphysiologic concentration of IL-2 (500-1000 IU/mL).42 LAK cytolytic activity is directed primarily against malignant tumor cells, including freshly isolated autologous or allogeneic tumor cells, cultured tumor cell lines, leukemic blasts, and xenogenic tumor cells.43,44 Based on their efficient, major histocompatibility complex (MHC)–nonrestricted cytolytic activity against a broad range of tumor cells, recent clinical studies have employed LAK cells in the adoptive immunotherapy of melanoma, renal cell carcinoma, and non-Hodgkin lymphoma. We speculate that owing to activation of NK and NK T cells and changes in their chemokine receptors' repertoire, the homing and activity of these cells will be limited to the tumor cells that express CXCR3 ligands. NK and NK T cells have also been used in adoptive immunotherapy after BM transplantation (BMT). The use of delayed lymphocyte infusion after allogeneic BMT resulted in less graft-versus-host disease (GVHD) with a significant graft-versus-leukemia (GVL) response, which is used to control minimal residual disease (MRD) and for the reinduction of remission in chronic myelogenous leukemia patients who relapse after allogeneic BMT.5-7
Our results showing down-expression of CXCR4 on NK and NK T cells upon IL-2 activation, and reduced homing of IL-2–activated NK and NK T cells to the BM, suggest that ex vivo preactivation of NK and NK T cells with IL-2, which increases their cytolytic capability, can reduce the BM homing potential of these cells and may therefore limit their alloreactive potential as an immunotherapeutic tool against hematologic tumors. Understanding how chemokines regulate NK and NK T-cell homing and retention in the BM may thus lead to the development of novel therapeutics for the treatment of hematologic malignancies and solid tumors.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2002-10-3293.
Supported by grants from the Israel Ministry of Health (grant no. 5052) and the Israel Cancer Research Fund (grant no. 20020085).
K.B. and A.N. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank T. Lapidot and D. Zipori, Weizmann Institute of Science, for their helpful comments on the manuscript and Khaitovich Raya and Mery Clausen, Hadassah Hospital, for technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal