Abstract
Reduced-intensity conditioning may reduce transplantation-related mortality in high-risk adults undergoing hematopoietic transplantation. We investigated unrelated donor umbilical cord blood (UCB) transplantation after such conditioning in 43 patients (median age, 49.5 years; range, 22-65 years) with a primary end point of donor engraftment. The first 21 patients received busulfan 8 mg/kg, fludarabine 200 mg/m2, and 200 cGy of total body irradiation (Bu/Flu/TBI). Subsequent patients (n = 22) received cyclophosphamide 50 mg/kg, fludarabine 200 mg/m2, and 200 cGy TBI (Cy/Flu/TBI). UCB grafts (93%) were 1-2 HLA antigen–mismatched with the recipient and contained a median cryopreserved cell dose of 3.7 × 107 (range, 1.6 × 107-6.0 × 107) nucleated cells per kilogram of recipient body weight (NC/kg). Graft versus host disease (GVHD) prophylaxis was cyclosporin A to day 180 plus mycophenolate mofetil to day 30. The cumulative incidence of sustained donor engraftment was 76% (95% confidence interval [CI], 56%-96%) for Bu/Flu/TBI recipients and 94% (95% CI, 84%-100%) for Cy/Flu/TBI recipients. The median day of neutrophil recovery (at least 0.5 × 109/L) for engrafting Bu/Flu/TBI recipients was 26 days (range, 12-30 days) and for Cy/Flu/TBI recipients was 9.5 days (range, 5-28 days). Incidence of grades III-IV acute GVHD was 9% (95% CI, 1%-17%), and survival at 1 year was 39% (95% CI, 23%-56%). These data demonstrate that 0-2 antigen mismatched UCB is sufficient to engraft most adults after reduced-intensity conditioning and is associated with a low incidence of severe acute GVHD.
Introduction
Bone marrow transplantation (BMT) may be curative in a variety of malignant and nonmalignant hematopoietic disorders.1 However, many adults receiving conventional high-dose myeloablative conditioning regimens, particularly recipients of unrelated donor (URD) BMT for diseases other than chronic myelogenous leukemia (CML), experience high transplantation-related mortality (TRM) with this approach.2,3 Therefore, “reduced-intensity” or “nonmyeloablative” regimens are being investigated for older patients or those with poor performance status, organ dysfunction, or extensive prior therapy using either related or unrelated volunteer donors.4-12 Immunosuppression without myeloablation can be sufficient to achieve complete donor chimerism, and a graft versus malignancy (GVM) effect can be demonstrated in both hematologic and nonhematologic malignancies.4-13 However, most patients do not have an available HLA-matched sibling donor. Because 0-2 donor-recipient HLA antigen disparity appears to be more tolerable in URD umbilical cord blood transplantation (UCBT) than in URD BMT, UCB offers the opportunity to extend the donor pool with a low risk of severe acute graft versus host disease (GVHD).14-17 Also, UCB grafts can be available substantially more quickly than URD BM.18 However, reduced UCB alloreactivity could compromise the ability of UCB to engraft after reduced-intensity conditioning. Therefore, we investigated a pilot study of URD UCBT after reduced-intensity conditioning in adults not eligible for conventional conditioning with the hypothesis that alloreactive T cells in UCB are sufficient to effect donor engraftment after reduced-intensity conditioning.
Patients, materials, and methods
URD UCBT was considered if HLA-compatible related (5 or 6 out of 6 HLA-A, B, DRB1 matches) or unrelated BM donors (6 out of 6 HLA-A, B, DRB1 matches) were not available or the transplantation was deemed to be urgent (required within 3 months of referral). Criteria for a reduced-intensity preparative regimen (rather than conventional high-dose conditioning) were (1) age at least 45 years; (2) extensive prior therapy (high-dose conditioning and autologous transplantation, or more than 12 months of alkylator chemotherapy, or more than 6 months of alkylator chemotherapy with extensive radiation); and/or (3) poor performance status,19 including serious concurrent medical condition, serious organ dysfunction, and/or invasive mold infection within 60 days.
Treatment plan
A dose-intensity de-escalation approach was used. The first 21 patients received conditioning with busulfan 2 mg/kg orally every 12 hours for 4 doses (days –8 to –7), fludarabine 40 mg/m2 daily for 5 days (days –6 to –2), and a single dose of 200 cGy of total body irradiation (day –1) (Bu/Flu/TBI). Diphenylhydantoin was given to Bu/Flu/TBI recipients on days –9 to 0. Subsequent patients (n = 22) received an identical regimen except for a substitution of busulfan with a single dose of cyclophosphamide 50 mg/kg on day –6 (Cy/Flu/TBI). One Cy/Flu/TBI patient with myelodysplasia (MDS) without prior chemotherapy also received antithymocyte globulin (ATG) 15 mg/kg every 12 hours for 6 doses on days –3 to –1.
All patients received cyclosporin A (CSA) from day –3 for a minimum of 6 months (aiming for a trough blood level of more than 200 μg/L). In addition, all patients received mycophenolate mofetil 15 mg/kg twice daily either orally or intravenously from days –3 to +30. Granulocyte colony-stimulating factor (G-CSF) (Filgrastim; Amgen, Thousand Oaks, CA) was administered to all patients at 5 μg/kg/d after transplantation when the total white cell count (WCC) fell below 2.5 × 109/L until the absolute neutrophil count (ANC) was more than 2.5 × 109/L for 2 days. The treatment protocol was approved by the Institutional Review Board of the University of Minnesota. Written informed consent was obtained from all patients prior to transplantation.
Patient characteristics
Patient characteristics are summarized in Table 1. Bu/Flu/TBI patients (n = 21) underwent transplantation between July 2000 and September 2001, and Cy/Flu/TBI recipients (n = 22) underwent transplantation between October 2001 and September 2002. All patients had high-risk or advanced-stage hematologic malignancy. Consistent with their high-risk or advanced disease, 19 patients (44%) had received extensive prior therapy and 24 (55%) had poor performance status. Thirty-two patients (74%) were aged at least 45 years with 26 (60%) of 43 patients having at least 2 of the 3 criteria warranting a reduced-intensity transplantation approach.
Characteristics of adult URD UCBT recipients
Characteristic . | Bu/Flu/TBI . | Cy/Flu/TBI . |
|---|---|---|
| No., n | 21 | 22 |
| Median age, y (range) | 49 (22-65) | 49 (24-58) |
| Median weight, kg (range) | 75 (55-109) | 73 (51-119) |
| Median time from diagnosis to transplantation (range) | 661 d (105 d - 8 y) | 575 d (102 d - 7 y) |
| Recipient CMV-positive, n (%) | 9 (43) | 7 (32) |
| Diagnosis | ||
| AML, n (%) | 10 (48) | 8 (36) |
| CR1, high risk,* n | 3 | 2 |
| CR2, n | 4 | 6 |
| Not in remission, n | 3 | 0 |
| MDS, n (%) | 2 (9) | 1 (5) |
| Refractory anemia with excess blasts, n | 2 | 0 |
| CMML, n | 0 | 1 |
| Advanced myelofibrosis, n (%) | 0 | 1 (5) |
| CML, n (%) | 2 (9) | 2 (9) |
| Accelerated/prior auto Tx, n | 1 | 0 |
| Postblast crisis, n | 1 | 2 |
| ALL, n (%) | 0 | 3 (14) |
| CR1 (Ph+), n | 0 | 2 |
| Fifth relapse, n | 0 | 1 |
| Hodgkin disease, n (%) | 2 (9) | 0 |
| Relapsed after auto Tx, n | 1 | 0 |
| Primary refractory, n | 1 | 0 |
| NHL, n (%) | 5 (24) | 6 (27) |
| Relapsed DLC in second PR, n | 3 | 1 |
| At least PR3 or refractory, n | 1 | 4 |
| Mantle cell in PR1, n | 1 | 1 |
| Plasma cell leukemia, n (%) | 0 | 1 (5) |
| Indication for reduced-intensity conditioning | ||
| Age at least 45 y, n (%) | 15 (71) | 17 (77) |
| Extensive prior therapy, n (%) | 9 (43); 7 prior auto Tx | 10 (45); 5 prior auto Tx |
| Poor performance status, n (%) | 14 (67) | 10 (45) |
Characteristic . | Bu/Flu/TBI . | Cy/Flu/TBI . |
|---|---|---|
| No., n | 21 | 22 |
| Median age, y (range) | 49 (22-65) | 49 (24-58) |
| Median weight, kg (range) | 75 (55-109) | 73 (51-119) |
| Median time from diagnosis to transplantation (range) | 661 d (105 d - 8 y) | 575 d (102 d - 7 y) |
| Recipient CMV-positive, n (%) | 9 (43) | 7 (32) |
| Diagnosis | ||
| AML, n (%) | 10 (48) | 8 (36) |
| CR1, high risk,* n | 3 | 2 |
| CR2, n | 4 | 6 |
| Not in remission, n | 3 | 0 |
| MDS, n (%) | 2 (9) | 1 (5) |
| Refractory anemia with excess blasts, n | 2 | 0 |
| CMML, n | 0 | 1 |
| Advanced myelofibrosis, n (%) | 0 | 1 (5) |
| CML, n (%) | 2 (9) | 2 (9) |
| Accelerated/prior auto Tx, n | 1 | 0 |
| Postblast crisis, n | 1 | 2 |
| ALL, n (%) | 0 | 3 (14) |
| CR1 (Ph+), n | 0 | 2 |
| Fifth relapse, n | 0 | 1 |
| Hodgkin disease, n (%) | 2 (9) | 0 |
| Relapsed after auto Tx, n | 1 | 0 |
| Primary refractory, n | 1 | 0 |
| NHL, n (%) | 5 (24) | 6 (27) |
| Relapsed DLC in second PR, n | 3 | 1 |
| At least PR3 or refractory, n | 1 | 4 |
| Mantle cell in PR1, n | 1 | 1 |
| Plasma cell leukemia, n (%) | 0 | 1 (5) |
| Indication for reduced-intensity conditioning | ||
| Age at least 45 y, n (%) | 15 (71) | 17 (77) |
| Extensive prior therapy, n (%) | 9 (43); 7 prior auto Tx | 10 (45); 5 prior auto Tx |
| Poor performance status, n (%) | 14 (67) | 10 (45) |
CMV indicates cytomegalovirus; AML, acute myelogenous leukemia; CR, complete remission; MDS, myelodysplasia; CMML, chronic myelo-monocytic leukemia; CML, chronic myelogenous leukemia; auto Tx, autologous transplantation; ALL, acute lymphoblastic leukemia; Ph+, Philadelphia chromosome positive; NHL, non-Hodgkin lymphoma; DLC, diffuse large cell; and PR, partial remission.
AML: CR1 (high-risk) defined by prior myelodysplasia (MDS) or requiring at least 3 induction cycles.
UCB grafts
UCB units were obtained from the National Cord Blood Program in New York; the St Louis Cord Blood Bank; the National Heart, Lung, and Blood Institute (NHLBI) Cord Blood Transplantation Study (COBLT); and Netcord. UCB grafts had at least 4 of 6 HLA-A, B, DRB1 antigens that were matched to the recipient and had a cryopreserved cell dose of at least 1.5 × 107 nucleated cells per kilogram of recipient body weight (NC/kg). Patients underwent transplantation with a single UCB if the unit had a cell dose of at least 3.5 × 107 NC/kg. Patients with a single unit with a cell dose less than 3.5 × 107 NC/kg were eligible for transplantation with two 4 to 6 of 6 HLA-A, B, DRB1 matched UCB units if available.
HLA typing was performed using the standard 2-stage complement-dependent microcytotoxicity assay, and antigens were assigned as defined by the World Health Organization HLA nomenclature committee. HLA-DRB1 type was determined by hybridization of polymerase chain reaction (PCR)–amplified DNA with sequence-specific oligonucleotide probes with sequencing if needed. High-resolution class II typing results for HLA-DRB1 was used to determine the selection of all UCB units. UCB grafts were thawed using the method of Rubinstein et al.20
Graft characteristics are summarized in Table 2. Nine Bu/Flu/TBI patients (43%) and 15 Cy/Flu/TBI patients (68%) received grafts consisting of 2 UCB units. Overall, the median total cryopreserved nucleated cell dose was 3.7 × 107 NC/kg (range, 1.6 × 107-6.0 × 107 NC/kg). Bu/Flu/TBI recipients received grafts with a significantly smaller median infused cell dose of 2.6 × 107 NC/kg (range, 1.6 × 107-3.8 × 107 NC/kg) compared with 3.2 × 107 NC/kg (range, 1.1 × 107-5.1 × 107 NC/kg) in Cy/Flu/TBI recipients (P = .04). However, there was no significant difference in the CD34+ cell dose in the 2 patient cohorts (P = .39). UCB grafts were 1-2 HLA-A, B, DRB1 antigen mismatched to the patient in 40 (93%) of 43, with 3 patients receiving grafts that were 6 of 6 matched to the recipient.
Graft characteristics
Characteristic . | Bu/Flu/TBI; N = 21 . | Cy/Flu/TBI; N = 22 . |
|---|---|---|
| Cryopreserved TNC dose, × 107 NC/kg (range) | 3.3 (2.3-5.1) | 4.0 (1.6-6.0) |
| Infused TNC dose, × 107 NC/kg (range) | 2.6 (1.6-3.8) | 3.2 (1.1-5.1) |
| Infused CD34+ dose, × 105/kg (range) | 3.7 (1.1-8.1) | 4.3 (1.1-10.3) |
| Infused CD3+ dose, × 108/kg (range) | 0.06 (0.02-0.15) | 0.05 (0.02-0.12) |
| Graft 1-2 HLA antigen mismatched (%) | 20 (95) | 20 (91) |
| Graft consisted of 2 units (%) | 9 (43) | 15 (68) |
Characteristic . | Bu/Flu/TBI; N = 21 . | Cy/Flu/TBI; N = 22 . |
|---|---|---|
| Cryopreserved TNC dose, × 107 NC/kg (range) | 3.3 (2.3-5.1) | 4.0 (1.6-6.0) |
| Infused TNC dose, × 107 NC/kg (range) | 2.6 (1.6-3.8) | 3.2 (1.1-5.1) |
| Infused CD34+ dose, × 105/kg (range) | 3.7 (1.1-8.1) | 4.3 (1.1-10.3) |
| Infused CD3+ dose, × 108/kg (range) | 0.06 (0.02-0.15) | 0.05 (0.02-0.12) |
| Graft 1-2 HLA antigen mismatched (%) | 20 (95) | 20 (91) |
| Graft consisted of 2 units (%) | 9 (43) | 15 (68) |
TNC indicates total nucleated cells.
Donor chimerism analysis
Donor chimerism was determined serially on marrow and/or blood samples on days +21 to +28, +50 to +60, +100, +180, +360, and annually after transplantation and as clinically indicated. Chimerism analysis was done using quantitative PCR of informative polymorphic variable number tandem repeat (VNTR) or short tandem repeat (STR) regions in the recipient and donor.21,22 Peripheral blood specimens were separated into neutrophil and mononuclear lymphoid fractions provided the total white cell count was more than 1.0 × 109/L. Posttransplantation DNA from the recipient was amplified with fluorescent PCR primers for markers found to distinguish donor(s) from recipient alleles. The fluorescent PCR products were separated either by gel electrophoresis on an Applied Biosystems 373 Sequencer or by capillary electrophoresis on an Applied Biosystems 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). The GeneScan software package (Applied Biosystems) was used to correlate allele peak areas to the percentage of donor or recipient DNA. Chimerism values had an accuracy of ± 5%.
Statistical analysis
The primary end point was donor engraftment. Other end points included neutrophil and platelet recovery, acute GVHD (grades II-IV and III-IV), chronic GVHD, relapse, TRM, disease-free survival (DFS), and overall survival. Other outcomes evaluated included causes of death. Event time for neutrophil recovery was the date of transplantation to the first of 3 consecutive days with neutrophil recovery to at least 0.5 × 109/L. Primary donor engraftment was defined as neutrophil recovery associated with donor engraftment within the first month after transplantation. Sustained donor engraftment was defined as sustained neutrophil recovery and donor hematopoiesis beyond day +42 after transplantation. Complete donor chimerism was considered to be marrow reconstitution of donor origin of at least 90%. Patients were censored from the engraftment analysis if they died or had persistent leukemia/early relapse within the first 28 days after transplantation. The cumulative incidence of engraftment was calculated by treating deaths from other causes as competing risks.23
Diagnosis of acute and chronic GVHD was based on standard clinical criteria with histopathologic confirmation where possible.24 The maximal grade of GVHD was determined by independent reviewers who evaluated the cases retrospectively. The cumulative incidence of acute and chronic GVHD was calculated by treating deaths from other causes as competing risks.23 The statistical end points of survival and DFS were estimated by the Kaplan-Meier method.25 Event times were measured from date of transplantation to date of death or date of last contact. Event times were analyzed as of November 2002. The median follow-up for the Bu/Flu/TBI recipients is 1.9 years (range, 1.2-2.3 years) and for the Cy/Flu/TBI recipients is 0.6 years (range, 0.2-1.0 years).
Results
Hematopoietic recovery and donor chimerism
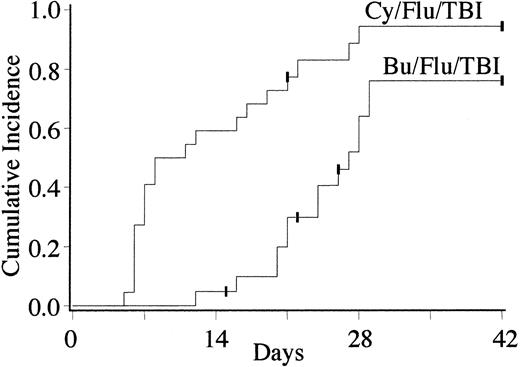
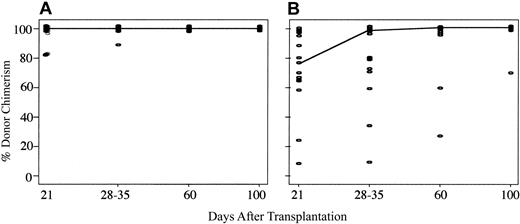
Three of the 21 Bu/Flu/TBI patients were not evaluable for donor engraftment due to early death from relapse (n = 2) or infection (n = 1). Of the 18 evaluable Bu/Flu/TBI recipients, the cumulative incidence of primary donor engraftment was 88% (95% confidence interval [CI], 72%-100%) at a median of 26 days after transplantation (range, 12-30 days). Fourteen patients displayed sustained donor engraftment for a cumulative incidence of 76% (95% CI, 56%-96%) (Figure 1). Bu/Flu/TBI patients with sustained donor engraftment demonstrated rapid and complete myeloid and lymphoid donor chimerism. The median myeloid donor chimerism of 100% (range, 84%-100%) in day +21 BM biopsy (Figure 2), and all patients had 100% lymphoid chimerism in day +28 to +35 blood samples. All engrafting patients without BM relapse were complete donor chimeras beyond 1 month after transplantation.
Cumulative incidence of sustained donor engraftment with 0-2 antigen mismatched UCB after Bu/Flu/TBI or Cy/Flu/TBI conditioning (P < .01).
Cumulative incidence of sustained donor engraftment with 0-2 antigen mismatched UCB after Bu/Flu/TBI or Cy/Flu/TBI conditioning (P < .01).
Comparison of myeloid donor chimerism in patients with sustained donor engraftment after reduced-intensity UCBT using either Bu/Flu/TBI or Cy/Flu/TBI conditioning. Achievement of complete donor chimerism was rapid for Bu/Flu/TBI recipients (A), being a median of 100% at day 21 in BM. In contrast, because the Cy/Flu/TBI regimen (B) was less myelosuppressive, myeloid donor chimerism was initially mixed, taking until day 60 to reach a median of 100%.
Comparison of myeloid donor chimerism in patients with sustained donor engraftment after reduced-intensity UCBT using either Bu/Flu/TBI or Cy/Flu/TBI conditioning. Achievement of complete donor chimerism was rapid for Bu/Flu/TBI recipients (A), being a median of 100% at day 21 in BM. In contrast, because the Cy/Flu/TBI regimen (B) was less myelosuppressive, myeloid donor chimerism was initially mixed, taking until day 60 to reach a median of 100%.
Twenty-one of the 22 Cy/Flu/TBI patients were evaluable for engraftment. The single unevaluable patient died on day +21 from pneumonitis with evidence of donor engraftment. The cumulative incidence of primary donor engraftment for the Cy/Flu/TBI regimen was 94% (95% CI, 84%-100%) with neutrophil recovery occurring at a median of 9.5 days (range, 5-28 days). A single patient with advanced myelofibrosis without prior combination chemotherapy had primary graft failure with autologous recovery. No Cy/Flu/TBI patient had secondary graft failure. Therefore, the cumulative incidence of sustained donor chimerism with Cy/Flu/TBI was 94% (95% CI, 84%-100%) (Figure 1). This was significantly higher than seen with the Bu/Flu/TBI regimen (P = < .01).
Due to temporary persistence of autologous hematopoiesis early after transplantation, engrafting Cy/Flu/TBI recipients demonstrated more gradual attainment of myeloid donor chimerism than seen with the Bu/Flu/TBI regimen, with a median of 78% (range, 8%-100%) in day +21 BM (Figure 2). The median myeloid chimerism in blood at days 50 to 60 after transplantation for Cy/Flu/TBI recipients was 100% (range, 26%-100%), with all patients except 1 having complete donor chimerism by day +100. In contrast, attainment of complete lymphoid donor chimerism was rapid in engrafting Cy/Flu/TBI patients, with the median being 100% by day +28 to +35 (range, 10%-100%).
Of the 4 Bu/Flu/TBI patients with failure of donor engraftment, 2 had primary graft failure with autologous recovery and 2 had early secondary graft failure at days +22 and +35. These patients had diagnoses of advanced MDS or secondary acute myelogenous leukemia (AML) without prior combination chemotherapy (n = 2), or AML in relapse (n = 1) and relapsed non-Hodgkin lymphoma (NHL) (n = 1), both without chemotherapy within the 4 months prior to transplantation. The single Cy/Flu/TBI patient with primary graft failure had advanced myelofibrosis without prior combination chemotherapy. Overall, all patients who received either combination chemotherapy in the 4 months preceding transplantation or a prior autologous transplantation (n = 31) had sustained donor engraftment. This compared with failure of sustained donor engraftment in 2 of 4 patients whose prior combination chemotherapy was more than 4 months preceding transplantation and in 3 of 3 patients who had never received prior combination chemotherapy. One patient with myelodysplasia and no prior combination chemotherapy had ATG added to Cy/Flu/TBI conditioning and had full donor engraftment.
The cumulative incidence of platelet engraftment to 2 × 109/L by day +180 was 24% (95% CI, 6%-42%) for Bu/Flu/TBI recipients and 80% (95% CI, 57%-100%) for Cy/Flu/TBI recipients (P < .01).
GVHD
The cumulative incidence of grades II-IV and grades III-IV acute GVHD was 44% (95% CI, 28%-62%) and 9% (95% CI, 1%-17%) for the entire group (Figure 3), respectively, with no differences between Bu/Flu/TBI and Cy/Flu/TBI regimens (data not shown). The 4 patients with grades III-IV acute GVHD had involvement of skin, gut, and liver. Two responded to corticosteroids and cyclosporine, 1 responded to ATG, and the other had refractory disease that was fatal.
Cumulative incidence of grades II-IV and III-IV acute GVHD after 0-2 antigen mismatched UCBT using reduced-intensity conditioning.
Cumulative incidence of grades II-IV and III-IV acute GVHD after 0-2 antigen mismatched UCBT using reduced-intensity conditioning.
Thus far, 8 patients have developed chronic GVHD for a cumulative incidence of 21% (95% CI, 8%-34%) at 1 year. Onset was de novo in 2 patients, quiescent in 5, and progressive in 1. Organs involved were eyes (n = 2), mouth (n = 7), skin (n = 7), gut (n = 5), and liver (n = 1), with 1 patient having autoimmune hemolysis. All patients, except for 1 patient with progressive onset, have responded to corticosteroids.
TRM, relapse, and survival
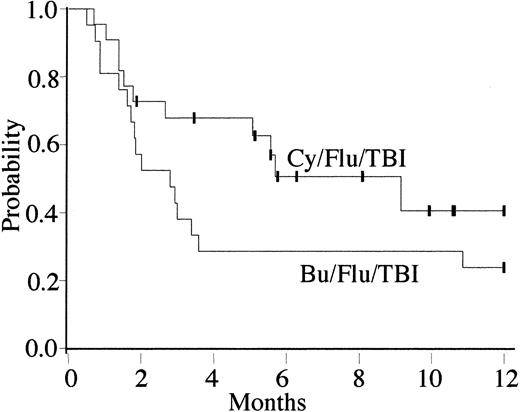
Treatment-related mortality at day 100 after transplantation was 48% (95% CI, 26%-70%) for Bu/Flu/TBI recipients and 28% (95% CI, 10%-46%) for Cy/Flu/TBI patients. For the entire group, the overall survival and DFS at 1 year were 39% (95% CI, 23%-56%) and 31% (95% CI, 15%-47%), respectively. Bu/Flu/TBI recipients had probabilities of disease-free survival of 38% (95% CI, 17%-59%) at day 100 and 24% (95% CI, 6%-42%) at 1 year. Cy/Flu/TBI recipients have probabilities of disease-free survival of 68% (95% CI, 48%-88%) at day 100 and 41% (95% CI, 15%-76%) at 1 year (Figure 4).
Kaplan-Meier estimates of disease-free survival for recipients of UCBT with Bu/Flu/TBI and Cy/Flu/TBI conditioning. Overall, 5 Bu/Flu/TBI and 11 Cy/Flu/TBI recipients were alive and disease-free at 1 year after transplantation, giving a probability of disease-free survival of 24% (95% CI, 6%-42%) and 41% (95% CI, 15%-76%) at 1 year, respectively. The differences between the 2 regimens were not significant (P = .15).
Kaplan-Meier estimates of disease-free survival for recipients of UCBT with Bu/Flu/TBI and Cy/Flu/TBI conditioning. Overall, 5 Bu/Flu/TBI and 11 Cy/Flu/TBI recipients were alive and disease-free at 1 year after transplantation, giving a probability of disease-free survival of 24% (95% CI, 6%-42%) and 41% (95% CI, 15%-76%) at 1 year, respectively. The differences between the 2 regimens were not significant (P = .15).
Overall, the predominant cause of death has been infection and organ failure (n = 13), with all TRM occurring prior to day +100. Graft failure and severe acute GVHD have accounted for 1 death each. Nine patients have died from relapse or progressive disease. Total nucleated cell dose and CD34+ cell dose were not associated with differences in survival (data not shown).
Discussion
Reduced-intensity preparative regimens are being investigated as a method to reduce regimen-related toxicity so the opportunity for a GVM effect may be extended to patients not fit for conventional conditioning.4-13 Because many patients do not have an available related or unrelated marrow donor, we have investigated reduced-intensity conditioning with UCBT. The Bu/Flu/TBI regimen was associated with rapid and complete donor chimerism in most patients. However, while the intensity of Bu/Flu/TBI is less than conventional high-dose conditioning, it resulted in prolonged neutropenia. Furthermore, 4 cases of failure of donor engraftment were seen. Therefore, in an attempt to achieve equivalent or greater immunosuppression with less myelosuppression, busulfan was substituted with a single dose of cyclophosphamide. In most patients, the Cy/Flu/TBI regimen was associated with rapid neutrophil recovery. Therefore, this approach can ameliorate the problem of delayed neutrophil recovery seen with myeloablative UCBT. Further, Cy/Flu/TBI has been associated with complete donor engraftment in most recipients, including rapid attainment of complete lymphoid chimerism.
This study did not involve randomization of patients between the 2 conditioning regimens. Therefore, it is not possible to draw firm conclusions concerning the impact of each preparative regimen on the incidence of sustained donor engraftment, which may have been also affected by the confounding factors of patient and graft variables. However, it is clear that the busulfan-based regimen is associated with prolonged neutropenia whereas Cy/Flu/TBI can permit early neutrophil recovery in many patients. This advantage, combined with a high incidence of donor engraftment, is reason to continue investigation of the Cy/Flu/TBI regimen. The engraftment results with the Cy/Flu/TBI regimen are consistent with findings of murine studies suggesting that the immunoablation associated with the combination of cyclophosphamide and fludarabine is conducive to donor engraftment in the setting of HLA mismatch.26,27
These data demonstrate that despite the low total nucleated CD34+ and T-cell dose and reduced alloreactivity associated with UCB, UCBT using reduced-intensity conditioning is associated with sustained donor engraftment in most adult patients. Interestingly, graft failure was restricted to patients without recent pretransplantation combination chemotherapy or prior autologous transplantation. While it is difficult to distinguish the impact of the underlying diagnosis (eg, myelodysplasia or myelofibrosis) from that of prior therapy on engraftment, these data may suggest that the immunosuppression resulting from pretransplantation therapy aids engraftment. A similar finding in sibling donor transplantation after nonmyeloablative conditioning has been reported by McSweeney et al.10
The donor engraftment after Cy/Flu/TBI is comparable to that reported after UCBT using traditional myeloablative regimens.16 In comparison to the engraftment using reduced-intensity conditioning and other hematopoietic stem cell sources, Giralt et al9 reported only 2 of 76 patients having graft failure after either related donor or URD BMT. In a smaller series, Nagler et al11 reported complete donor engraftment in 15 of 16 recipients of URD BMT, with partial donor chimerism in the remaining patient. More recently, Chakraverty et al12 reported initial full donor chimerism in 85% of recipients of URD BMT, with a number of patients subsequently developing mixed chimerism. Overall, prospective studies with similar conditioning regimens with control for such factors as diagnosis and prior therapy will be required to substantiate any differences in the engrafting potential of different hematopoietic stem cell (HSC) sources after reduced-intensity conditioning.
Notable in this group of adult patients is the low incidence of severe (grades III-IV) acute GVHD despite the HLA disparity of the UCB graft. These results are lower than those reported after UCBT using traditional myeloablative regimens.16 This low incidence of acute GVHD may represent an important advantage of UCB over URD BM. In the report by Giralt et al,9 the incidence of severe acute GVHD was 39%, accounting for 11 of 40 deaths. This compares with an incidence of less than 10%, accounting for only 1 death, in our UCB series. Nonetheless, the TRM in our study is high despite the reduced-intensity conditioning and low incidence of severe GVHD. While this is not unexpected given the high-risk patient population enrolled in this pilot study, and is in keeping with other reports of similar patient populations,9 further efforts to reduce the hazards in the early posttransplantation period are warranted. Transplantation in patients with high-risk malignancies using reduced-intensity regimens may be more effective if initiated prior to chemorefractory disease, extensive prior therapy, and its associated complications. Furthermore, for patients with refractory disease or very poor performance status, nontransplantation options may be more appropriate.
Ultimately, the success of a reduced-intensity regimen is dependent on the ability of the transplanted cells to engraft and exert GVM effects. It is premature to assess the potency of UCB GVM in this study that was designed to evaluate the engraftment potential of UCB using reduced-intensity conditioning in adults. However, given that pediatric UCBT series have not observed increased relapse risk as compared with BMT,17,28,29 continued investigation of this HSC source is warranted. One potential disadvantage of UCBT is that there is no access to donor lymphocyte infusions (DLIs). However, while reports of successful therapy of persistent or relapsed malignancies other than CML exist, overall the efficacy of DLIs in treating patients with diagnoses other than CML has been limited. The advantages of URD UCB—namely, tolerance of 0-2 antigen HLA disparity, speed of availability, and low incidence of severe acute GVHD—may outweigh the disadvantage of lack of DLIs.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-11-3337.
Supported in part by grants from the National Cancer Institute PO1-CA65493 (J.E.W.), R01 HL 63452 (B.R.B.), and the Children's Cancer Research Fund (J.E.W., J.N.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal