Abstract
Low-density lipoprotein (LDL) oxidation mediated by a variety of catalysts in atherosclerotic lesions plays a crucial role in the genesis and evolution of atherosclerotic plaques. In this study we focused on oxidative properties of hemoglobin (Hb)–modified LDL because Hb is present in atherosclerotic lesions. Under low oxygen tensions Hb was previously found to modify apolipoprotein B100 with covalent binding of Hb fragments and formation of electronegative LDL particles (LDL–). Here we show that HbLDL is highly susceptible to oxidation, but is not cytotoxic to vascular cells, as was found for LDL– isolated from human plasma. HbLDL and LDL– have similar levels of oxidized lipid products and low uptake rates; however, the virtual absence of HbLDL-induced toxicity depends on a marked adaptive oxidative stress response. This was evidenced by a time- and dose-dependent induction of heme oxygenase (HO-1). Cell survival was significantly decreased in the presence of HO-1 inhibitor, tin protoporphyrin (SnPPIX). HO-1 induction by HbLDL increased resistance of cells to toxic doses of hemin or t-BuOOH. The high sensitivity to oxidation and HO-1 induction was largely dependent on lipid hydroperoxides and heme associated with HbLDL. Reduction of pre-existing lipid peroxides using ebselen delayed HbLDL kinetics and inhibited HO-1 induction. Moreover, heme inactivation or its degradation inhibited HO-1 induction and provided an additive inhibitory effect to ebselen. We conclude that Hb-catalyzed reactions may modulate vascular cell survival and oxidative stress adaptation due to the presence of peroxides and heme, thus providing a possible mechanism for the evolution of atherosclerotic and hemorrhagic lesions.
Introduction
Oxidized low-density lipoprotein (LDL) is considered an important factor in the initiation and progression of atherosclerotic plaques and has been shown to elicit inflammatory processes and lipid accumulation within the arterial wall. Several types of oxidation mechanisms have been identified including reactions catalyzed by free transition metal ions, oxidative enzymes (reduced nicotinamide adenine dinucleotide phosphate [NADPH] oxidase, lipoxygenase, inducible macrophage-type nitric oxide synthase [iNOS], myeloperoxidase; reviewed in Berliner and Heinecke1 ) and transition metal-containing enzymes such as ceruloplasmin2 or hemoglobin (Hb).3 Each catalyst mediates a distinct pattern of LDL modification that may impart specific biologic properties that contribute to atherogenesis.
Heme-containing proteins, especially Hb, are present abundantly in vivo. Moreover, the levels of free Hb and its oxidized species increase significantly in blood and the arterial wall during inflammatory conditions. The role of Hb in the development of atherosclerosis may be important in the light of recent findings that associate intraplaque hemorrhage and the formation of unstable plaques with higher lipid content.4-6 To date the role of Hb in these processes remains poorly understood. Hb catalyzes LDL modification and lipid peroxidation3,7 analogously to 15-lipoxygenase.8 However, at low oxygen tensions (comparable to tensions in atherosclerotic lesions) Hb oxidation causes a marked and specific modification of LDL-apolipoprotein B100 (apoB100).7 This is accompanied by the formation of minimally oxidized LDL with electronegative properties identical to LDL–, an electronegative LDL subfraction isolated from human plasma.9 The ability of oxidized Hb to generate large amounts of LDL–7,9 is unique because auto-oxidation or metal-catalyzed oxidations of LDL typically yield low proportions of LDL–. A potentially important feature of HbLDL is the presence of an intrinsic heme moiety that is tightly associated with apoB100, possibly from the cross-linking reactions between LDL-apoB100 and Hb fragments,7,10 or very strong hydrophobic interaction with the particle core.11,12 Heme is catalytically active and may initiate oxidation of lipids associated with LDL particles and cellular membranes. Heme also induces stress response proteins in vascular cells, particularly heme oxygenase (HO-1).13 We hypothesized that high LDL– levels and the presence of catalytically active heme in this uniquely modified LDL endow it with pro-oxidant and proatherogenic potential. In this report we provide evidence that HbLDL strongly promotes LDL oxidative modification and induces HO-1 due to its lipid peroxide (LOOH) and heme content. However, HbLDL lacks cytotoxic properties described for other forms of oxidized LDL, such as copper-oxidized LDL (CuLDL) and plasma LDL–, and elicits increased resistance of cells to oxidative insult associated with HO-1 induction.
Materials and methods
All reagents were from Sigma (St Louis, MO), unless otherwise indicated, and were used at analytical grade. 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) fluorescent dye was purchased from Molecular Probes (Eugene, OR), tin protoporphyrin (SnPPIX) from Frontier Scientific (Logan, UT), polyclonal antibody against HO-1 from StressGen (San Diego, CA), and monoclonal β-actin antibody from Sigma. Polyclonal ferritin antibody was from Bethyl Laboratories (Montgomery, TX). Recombinant HO-1 was purchased from Sigma and assessed for activity as described (see “Reduction of LOOH and degradation or inhibition of heme”). Ebselen, 2-phenyl-1,2-benzisoselenazol-3(2H)-one, was a generous gift from Dr F. Ursini (University of Padova, Italy).
Plasma modification and LDL isolation
Human plasma was obtained from the blood bank (University of Southern California Hospital), pooled from 2 to 3 donors. Sucrose was added to plasma at a final concentration of 0.6%, and the pooled plasma was aliquoted into cryotubes that were stored at –80°C. These samples were used for experiments within 3 to 4 months. This has been shown not to change significantly the oxidative properties and physicochemical parameters of LDL.14 Oxidation of LDL in plasma with Hb was performed as described previously.7 Briefly, human methemoglobin (metHb; 100 μM) was oxidized with 6-fold excess of H2O2 and the reaction mixture was immediately added to plasma containing 1000 U/mL catalase to degrade remaining H2O2. The reaction was carried out at 37°C under argon, and LDL was subsequently isolated by ultracentrifugation using sodium bromide gradient according to published procedures.15 The isolated LDL fraction was dialyzed, sterile filtered (0.2 μm), and stored at 4°C. This LDL was used for experiments within 2 weeks. Protein was measured using modified Lowry method with Bio-Rad DC assay (Bio-Rad, Hercules, CA).
LDL oxysterol content was determined using a quantitative gas chromatography–mass spectrometry (GC-MS) method16 and was used in conjunction with LOOH determinations as a measure of lipid peroxidation. To further assess the level of LDL modification, the relative electrophoretic mobility (REM) of normal LDL (nLDL) and HbLDL was analyzed on agarose gels using LIPO Gel Kits (Beckman, Fullerton, CA) and LDL– was separated on a UNO Q1 column (Bio-Rad) using anion exchange chromatography–high-performance liquid chromatography (HPLC).17
LDL oxidation and pro-oxidant activity of HbLDL
Control or unmodified LDL (nLDL), HbLDL, before and after ebselen pretreatment, and in the presence or absence of 10 μM KCN, was diluted to 0.25 mg/mL and incubated in phosphate-buffered saline (PBS) with 10 μM CuSO4 for up to 6 hours at 22°C. Formation of conjugated dienes was monitored continuously at 234 nm as described previously.18 For auto-oxidation studies, the LDL samples were incubated under air in open cuvettes and conjugated diene formation measured at 234 nm. The CuLDL was prepared as described and is referred to as extensively oxidized LDL or CuLDL.
To assess the pro-oxidant catalytic activity of HbLDL, mixtures of nLDL and HbLDL were incubated at 37°C for 20 hours and REM determined on agarose gels. nLDL and HbLDL alone were incubated in parallel with the mixtures. In other experiments, nLDL/HbLDL mixtures were oxidized with 10 μM Cu2+ and the level of oxidation was assessed as described.
Reduction of LOOH and degradation or inhibition of heme
Ebselen, an organic seleno compound bearing glutathione peroxidase activity, effectively reduces lipid peroxides to alcohols in the presence of glutathione.19 One milligram of HbLDL or nLDL, CuLDL and LDL– were individually incubated with 50 μM ebselen and 3 mM glutathione in 0.5 mL PBS for 1 hour at 37°C. LOOH content before and after ebselen treatment was monitored according to a previously described method20 using t-BuOOH as calibration standard.
To degrade associated heme, 500 μg HbLDL was incubated with 1 μg HO-1 enzyme in the presence of 100 μM desferrioxamine to bind free iron. HO-1 enzymatic activity was determined by bilirubin release from hemin using an NADPH-generating system.21 The reaction mixture consisted of 1 to 5 μg HO-1, 50 μM hemin, 2 mg/mL rat liver cytosol, 3 U glucose-6-phosphate dehydrogenase, 1 mM glucose-6-phosphate, 2 mM NADP+, 1 mM MgCl2 in 0.15 mL of 0.1 M potassium buffer, pH 7.4. The reaction was stopped after a 30-minute incubation at 37°C, bilirubin extracted with benzene, and measured according to Turcanu et al.22 One microgram of HO-1 enzyme yielded about 3 pmol bilirubin/min from 50 μM hemin.
To inhibit heme, HbLDL was incubated with 10 μM NaCN for 30 minutes at room temperature followed by extensive dialysis to remove the excess NaCN. The binding of CN– to heme was confirmed spectrophotometrically. Additional studies showed no cytotoxicity with NaCN-treated HbLDL and LDL.
Cell culture
Rabbit aortic endothelial cells (RAECs) and J774 A4 macrophages (MPHs) were used for cell culture studies. RAECs were grown in Dulbecco modified Eagle medium (DMEM) with 15% fetal bovine serum (FBS), supplemented with 50 μg/mL gentamycin, 20 μg/mL heparin, and 20 μg/mL endothelial cell growth supplement (ECGS). Cultures were maintained from passage 16 to 23. MPHs were grown in DMEM with 10% FBS. All cells were grown in a humidified atmosphere (5% CO2, 95% air) at 37°C and were used for experiments within 2 days after subcultivation.
Cytotoxicity studies
RAECs and MPHs were grown in 12-well dishes in normal media. LDLs (nLDL, HbLDL, CuLDL) were added at concentrations of 10 to 200 μg/mL into FBS-free medium with or without 10 μM SnPPIX and incubated with cells for 24 hours. At the end of the incubation period, cells were washed twice with PBS, collected in complete media, and replated into 24-well dishes. After 6 hours nonadherent cells were removed by washing, and the number of attached cells was determined using a Coulter counter (Beckman Coulter, Fullerton, CA). The replating efficiency was calculated as a ratio of attached cells to the number of cells added after treatment as described previously.23 Data are presented as percent of nLDL response. For adaptation studies cells were pretreated with 200 μg/mL nLDL or HbLDL overnight (the predetermined adaptation period), followed by exposure to toxic doses of t-BuOOH or hemin (representing 2 forms of oxidant stress). The cells were replated after 6 hours, and the plating efficiency was determined as described.
For measurements of apoptosis, cells were grown in 12-well dishes, treated with 200-μg/mL concentrations of nLDL, HbLDL, CuLDL, and HbLDL in the presence of 10 μM SnPPIX for 20 hours, and washed twice with PBS. Apoptotic cells were stained with annexin V and propidium iodide using an apoptosis detection kit (PharMingen, BD Biosciences, San Diego, CA). The number of apoptotic cells was measured by flow cytometry.
DiI-LDL uptake by RAECs and MPHs
Before treatment with DiI-labeled LDLs, cells were grown in 12-well dishes and starved for 2 hours by reduction of FBS in the media to 2%. DiI-LDL was prepared by overnight incubation of LDL at 37°C with DiI at a final concentration of 50 ng DiI/μg LDL.24 DiI-LDL was added to the wells at a concentration of 10 μg/mL and after 2 hours cells were washed extensively. Isopropanol (2 mL) was added to each well, the plates were incubated 30 minutes at room temperature, and the extract was recovered for fluorescence measurements at 523/563 nm (excitation/emission [ex/em]). Cell-associated fluorescence was calculated using a DiI standard curve based on the initial fluorescence units measured for the stock solution. Fixed cells were stained with 1% Giemsa stain, the protein content read at 590 nm, and cell content normalized on the basis of protein levels.
Western immunoblotting
RAECs and MPHs were grown to 80% confluence in standard medium, after which LDLs were added in FBS-free medium at desired concentrations. After 3, 6, 12, and 24 hours, cells were washed twice, scraped from the dishes, pelleted, and lysed by sonication using 1% sodium dodecyl sulfate (SDS) buffer (30 seconds at 4°C). Samples were run on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using 12% polyacrylamide gels, and the proteins were then transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were incubated overnight with antibodies to HO-1 (1:2000) or ferritin (1:1000) and β-actin (1:20 000). The bands were detected by enhanced chemiluminescence (ECL) using corresponding secondary antibodies. Densitometry was performed using Scion Image software (Scion, Frederick, MD) and computed on the basis of β-actin levels.
Statistical analysis
At least 3 separate experiments were performed each in triplicate and the data are represented as mean ± SD. Significance was calculated using unpaired or paired Student t test and set at P < .05.
Results
Oxidative susceptibility and pro-oxidant activity of HbLDL
A high susceptibility to auto-oxidation was found for HbLDL as measured via the kinetics of conjugated diene formation (Figure 1A). This is in agreement with high levels of protein modification and slightly elevated levels of lipid peroxidation products found in HbLDL. In fact, HbLDL contained markedly elevated proportions of LDL– as previously demonstrated.7 Similarly to LDL–, the REM of HbLDL was increased compared to nLDL (Figure 2B). LOOH levels were modestly increased and were similar to the levels found in plasma LDL– (Table 1). Thiobarbituric acid–reactive substances (TBARSs) were also slightly elevated, as was reported previously.7 The levels of 7α-hydroxy-, 7β-hydroxy-, α- and β-epoxy-, 20-hydroxy-, and 7-ketocholesterols, calculated as percent of total cholesterol, were significantly increased from 5.9% ± 0.107% in nLDL to 11% ± 0.1% in HbLDL. The levels are similar to those for LDL–, but substantially lower than those formed after copper-mediated oxidation.17
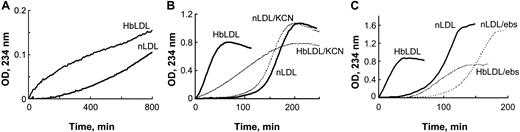
Increased oxidizability of HbLDL: effect of heme binding and LOOH reduction. (A) Auto-oxidation of nLDL and HbLDL. LDL samples were diluted to 0.25 mg/mL and conjugated diene formation was monitored at 234 nm at 2-minute intervals at 22°C using a Beckman DU-650 spectrophotometer (Beckman Coulter). (B) Copper-mediated oxidation of nLDL and HbLDL in the absence and presence of 10 μM KCN. (C) The effect of ebselen on copper-mediated oxidation of nLDL and HbLDL. nLDL and HbLDL were pretreated with ebselen in the presence of glutathione as described in “Materials and methods.” The conjugated dienes were measured at 234 nm.
Increased oxidizability of HbLDL: effect of heme binding and LOOH reduction. (A) Auto-oxidation of nLDL and HbLDL. LDL samples were diluted to 0.25 mg/mL and conjugated diene formation was monitored at 234 nm at 2-minute intervals at 22°C using a Beckman DU-650 spectrophotometer (Beckman Coulter). (B) Copper-mediated oxidation of nLDL and HbLDL in the absence and presence of 10 μM KCN. (C) The effect of ebselen on copper-mediated oxidation of nLDL and HbLDL. nLDL and HbLDL were pretreated with ebselen in the presence of glutathione as described in “Materials and methods.” The conjugated dienes were measured at 234 nm.
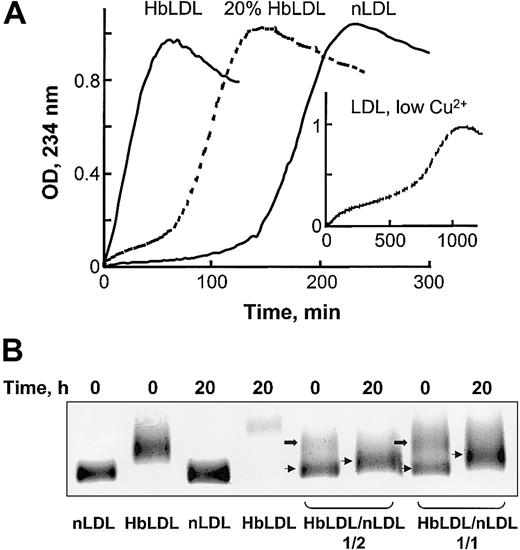
Oxidative susceptibility of HbLDL/LDL mixtures. (A) Copper-mediated oxidation kinetics of nLDL, HbLDL, or a combination of nLDL and 20% HbLDL. CuSO4 (10 μM) was added to LDL samples (0.25 mg protein/mL) and conjugated dienes were monitored at 234 nm for the indicated intervals. Inset shows LDL oxidation kinetics using 0.5 μM copper (low Cu2+, as described in Ziouzenkova et al25 ). (B) Agarose gel electrophoresis of HbLDL/LDL mixtures. HbLDL was mixed with nLDL in 1:2 and 1:1 ratios and incubated at 37°C for 20 hours. For control experiments nLDL and HbLDL alone were incubated in parallel with the mixtures. After incubation, REM was determined using LIPO Gel Kit. Lower bands represent nLDL (thin arrows) and upper bands represent HbLDL (block arrows).
Oxidative susceptibility of HbLDL/LDL mixtures. (A) Copper-mediated oxidation kinetics of nLDL, HbLDL, or a combination of nLDL and 20% HbLDL. CuSO4 (10 μM) was added to LDL samples (0.25 mg protein/mL) and conjugated dienes were monitored at 234 nm for the indicated intervals. Inset shows LDL oxidation kinetics using 0.5 μM copper (low Cu2+, as described in Ziouzenkova et al25 ). (B) Agarose gel electrophoresis of HbLDL/LDL mixtures. HbLDL was mixed with nLDL in 1:2 and 1:1 ratios and incubated at 37°C for 20 hours. For control experiments nLDL and HbLDL alone were incubated in parallel with the mixtures. After incubation, REM was determined using LIPO Gel Kit. Lower bands represent nLDL (thin arrows) and upper bands represent HbLDL (block arrows).
Lipid peroxide levels in LDL before and after ebselen treatment
. | LOOH levels, nmol/mg apoB100 . | . | |
|---|---|---|---|
. | Before ebselen treatment . | After ebselen treatment . | |
| nLDL | ND | ND | |
| HbLDL | 31.3 ± 10.3 | ND | |
| CuLDL | 55 ± 16 | ND | |
| LDL- | 29.3 ± 6.5 | ND | |
. | LOOH levels, nmol/mg apoB100 . | . | |
|---|---|---|---|
. | Before ebselen treatment . | After ebselen treatment . | |
| nLDL | ND | ND | |
| HbLDL | 31.3 ± 10.3 | ND | |
| CuLDL | 55 ± 16 | ND | |
| LDL- | 29.3 ± 6.5 | ND | |
LDL was incubated with ebselen in the presence of glutathione for 1 hour at 37°C. Then ebselen was removed by gel filtration. LOOH levels were determined according to Muller et al.19 Data are mean ± SD.
ND indicates not detectable.
In the presence of an oxidation catalyst like 10 μM CuSO4, HbLDL underwent rapid propagation of lipid peroxidation without an evident lag period (Figure 1B), unlike the oxidation of nLDL by CuSO4 that followed after a distinct lag phase (∼ 2 hours). To examine the contribution of heme associated with HbLDL, oxidation was measured in the presence of KCN, which binds strongly to heme iron and prevents formation of hypervalent oxyheme complexes. In the presence of KCN, the rate of conjugated diene formation in HbLDL was significantly reduced (P < .001, n = 3), whereas the oxidation rate for nLDL was decreased to a minor extent, suggesting participation of heme iron during the peroxidation of HbLDL. To explore the role of LOOH in HbLDL oxidation, the LOOH in HbLDL was reduced by incubation with the organic selenoperoxidase, ebselen. This treatment yielded HbLDL with little or no detectable LOOH (Table 1) and resulted in an oxidation kinetics profile similar to that of nLDL (Figure 1C).
HbLDL facilitated oxidation in HbLDL/LDL mixtures. In the presence of 10 μM Cu2+, LDL preparations containing 20% HbLDL also showed higher susceptibility to oxidation as compared to nLDL alone, evident by a decrease in the lag phase and a rapid onset of the propagation of lipid peroxidation (Figure 2A). LDL mixed with HbLDL exhibited a biphasic pattern of oxidation where the production of conjugated dienes started after a short propagation phase followed by lag phase and then a second prolonged propagation phase. A similar pattern of oxidation was previously described25 for nLDL oxidized using low (< 0.5 μM) Cu2+ concentrations (Figure 2A, insert). This mode of oxidation purportedly involves catalysis of LOOH decomposition associated with high-affinity binding sites for Cu2+.26 Furthermore, HbLDL effectively triggered oxidation of nLDL, analogously to the pro-oxidant effect previously described for LDL–.27 Different proportions of HbLDL were mixed with nLDL and incubated for up to 20 hours at 37°C. Prior to incubation, 2 distinct bands were evident on agarose gels, representing nLDL (lower bands, arrows) and HbLDL (upper bands, block arrows; Figure 2B). After incubation, the LDL mixture became oxidized with a marked increase in REM for the band representing nLDL and a loss or diffusion of the upper band. Higher levels of oxidation and REM were seen at a 1:1 HbLDL/nLDL ratio (REM = 1.23) as compared to a 1:2 ratio (REM = 1.14). Incubation of LDL samples alone greatly increased the REM for HbLDL but not nLDL.
HbLDL is not toxic to RAECs and MPHs
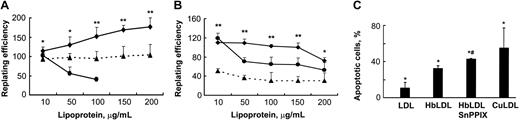
A range of HbLDL concentrations (10-200 μg/mL) was used for cytotoxicity experiments. These samples contained 10% LDL– (1-20 μg). LDL– isolated from human plasma typically comprises about 5% of LDL and has been reported to be cytotoxic to RAECs at a concentration as low as 20 μg/mL.15 CuLDL was used as a positive control for cytotoxic potential, whereas nLDL was used to determine background levels of toxicity and to serve as a negative control. The cells were incubated with nLDL, HbLDL, and CuLDL for 24 hours. After replating the attached cells were counted and the replating efficiency was calculated as described in “Materials and methods.” Surprisingly, HbLDL was not toxic to RAECs compared with CuLDL that was highly toxic at 100 μg/mL, exhibiting 100% toxicity at higher doses (Figure 3A). Moreover, HbLDL dose dependently increased cell survival in RAECs, producing a significant increase in the surviving fraction up to the maximum treatment dose. For MPHs, HbLDL treatments produced higher survival rates with only moderate toxicity occurring at a 200-μg/mL dose (Figure 3B). This dose of HbLDL induced significant apoptosis as measured by total annexin V and propidium iodide staining (Figure 3C). CuLDL caused a substantially larger apoptotic effect (Figure 3C). Staining of HbLDL-treated RAECs showed no increase in apoptosis, which agrees well with the cell survival data.
Effect of HO-1 inhibition on vascular cell viability by HbLDL. (A-B) Cells grown in 12- or 24-well dishes were incubated for 24 hours in the presence of increasing concentrations of different LDL preparations, washed, and replated. After 6 hours attached cells were counted and the replating efficiency was calculated as described in “Materials and methods.” Survival of RAECs (A) and MPHs (B) after treatments by HbLDL (♦), CuLDL (•), and HbLDL in the presence of 10 μM SnPPIX (▴). Data are presented as mean percent survival ± SD relative to the effect of nLDL treatment at the same concentration. Results are from at least 3 different experiments performed in triplicate. *P < .05; **P < .005. (C) Apoptotic cell number in MPHs after treatment with 200 μg/mL concentration of indicated LDLs. After treatment with LDLs, MPHs were stained with annexin V and propidium iodide and apoptotic cell number was measured using flow cytometry. *P < .05 compared with nLDL, #P < .01 compared with HbLDL.
Effect of HO-1 inhibition on vascular cell viability by HbLDL. (A-B) Cells grown in 12- or 24-well dishes were incubated for 24 hours in the presence of increasing concentrations of different LDL preparations, washed, and replated. After 6 hours attached cells were counted and the replating efficiency was calculated as described in “Materials and methods.” Survival of RAECs (A) and MPHs (B) after treatments by HbLDL (♦), CuLDL (•), and HbLDL in the presence of 10 μM SnPPIX (▴). Data are presented as mean percent survival ± SD relative to the effect of nLDL treatment at the same concentration. Results are from at least 3 different experiments performed in triplicate. *P < .05; **P < .005. (C) Apoptotic cell number in MPHs after treatment with 200 μg/mL concentration of indicated LDLs. After treatment with LDLs, MPHs were stained with annexin V and propidium iodide and apoptotic cell number was measured using flow cytometry. *P < .05 compared with nLDL, #P < .01 compared with HbLDL.
The presence of heme and low levels of lipid peroxidation products in HbLDL represent 2 possible factors that may induce HO-1 in cells, conferring resistance to oxidant injury.28 To investigate the possible involvement of cellular HO-1 activation that could account for the lack of HbLDL toxicity, the HO-1 inhibitor SnPPIX was used. SnPPIX significantly decreased the numbers of surviving cells in both cell types (Figure 3) under the same treatment conditions (36% ± 7.7% and 64% ± 5.3%, respectively, for MPHs and RAECs; average values calculated as a percentage of HbLDL for all doses). Furthermore, the number of apoptotic cells produced by HbLDL significantly increased in the presence of SnPPIX (Figure 3C).
HbLDL uptake by vascular cells is impaired
The uptake of HbLDL was studied in RAECs and MPHs and compared to the uptake of nLDL and CuLDL. Cell-associated fluorescence was significantly lower after incubation of both cell types with DiI-HbLDL, as compared to treatments with DiI-nLDL. This was not related to Hb-mediated quenching of the probe because the rate of fluorescence decay was similar for labeled HbLDL and LDL. In RAECs the HbLDL uptake was 4 times lower than for nLDL (0.032 ± 0.003 versus 0.14 ± 0.008 μg LDL/mg protein). In MPHs the rate of uptake was half that found for nLDL after 2 hours of incubation (0.18 ± 0.03 versus 0.36 ± 0.04 μg LDL/mg protein). DiI-CuLDL uptake was markedly higher in MPHs than uptake of nLDL (0.71 ± 0.2 versus 0.36 ± 0.04 μg LDL/mg protein), consistent with the active and unregulated uptake of highly oxidized forms of LDL by scavenger receptors and the predominance of these receptors in macrophages.29 In contrast, a modest uptake of DiI-CuLDL was found for RAECs (0.058 ± 0.008 μg LDL/mg protein) that is consistent with the absence of major types of scavenger receptors on this cell type.
HbLDL induces HO-1 in RAECs and MPHs
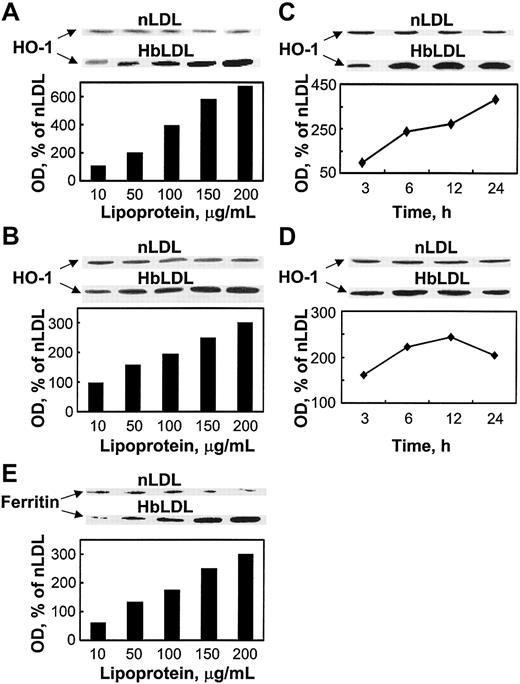
The findings above suggest the involvement of HO-1 activity in conferring oxidant damage resistance following exposure to HbLDL. Indeed, HbLDL dose dependently increased HO-1 protein levels over a 24-hour interval in RAECs and MPHs (Figure 4 A-B). nLDL treatments had no effect on HO-1 levels. Treatment of RAECs and MPHs with 100 μg/mL HbLDL resulted in progressive HO-1 induction over time (Figure 4C-D). The HO-1 levels remained significantly elevated at 24 hours for both cell types. Ferritin expression also dose dependently increased in parallel to HO-1 induction by HbLDL in MPHs (Figure 4E) but not in RAECs (data not shown).
HO-1 and ferritin induction by HbLDL. Dose-dependent induction of HO-1 by HbLDL in RAECs (A) and MPHs (B); time course of HO-1 induction in RAECs (C) and MPHs (D); ferritin (heavy chain) induction in MPHs (E). The diagrams represent corresponding optical densities normalized on β-actin levels, calculated as percent of nLDL and presented graphically. Cells grown in 12-well dishes were treated with increasing amounts of LDL and HbLDL for 24 hours, or in the case of time-course studies with 100 μg/mL LDL-protein, for indicated time intervals. HO-1, ferritin, and β-actin from lysates were visualized by immunoblotting after SDS-PAGE on 12% polyacrylamide gels. Experimental details are described in “Materials and methods.”
HO-1 and ferritin induction by HbLDL. Dose-dependent induction of HO-1 by HbLDL in RAECs (A) and MPHs (B); time course of HO-1 induction in RAECs (C) and MPHs (D); ferritin (heavy chain) induction in MPHs (E). The diagrams represent corresponding optical densities normalized on β-actin levels, calculated as percent of nLDL and presented graphically. Cells grown in 12-well dishes were treated with increasing amounts of LDL and HbLDL for 24 hours, or in the case of time-course studies with 100 μg/mL LDL-protein, for indicated time intervals. HO-1, ferritin, and β-actin from lysates were visualized by immunoblotting after SDS-PAGE on 12% polyacrylamide gels. Experimental details are described in “Materials and methods.”
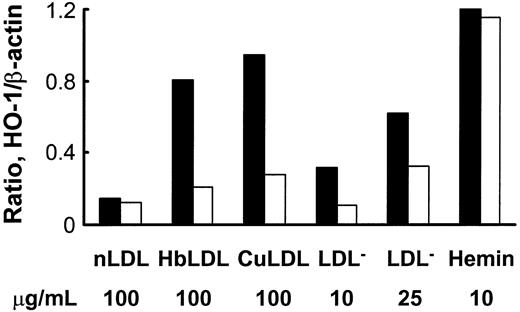
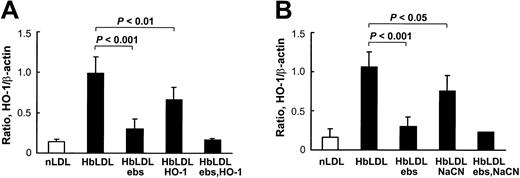
HbLDL produced a potent HO-1–inducing effect that was comparable to induction by 100 μg/mL CuLDL or 10 μM hemin (Figure 5), the latter being a strong HO-1 inducer.30 HO-1 induction following treatments with 10 μg/mL LDL– was much lower than treatments with 100 μg/mL HbLDL containing similar LDL– levels (Figure 5). However, increasing levels of induction were found using a 25-μg/mL LDL– dose, suggesting that other factors, such as LOOH or heme, may contribute to HO-1 induction. Reduction of LDL-associated LOOH with ebselen (Table 1) largely inhibited HO-1 induction in RAECs after 24 hours of treatment by HbLDL, CuLDL, and LDL–, whereas no differences were found for the inducing potential of nLDL and hemin (Figure 5). Although no detectable levels of LOOH were observed in HbLDL after ebselen treatment, HO-1 induction was not completely abolished (70% reduction; Figure 6). Degradation of heme associated with HbLDL using exogenous HO-1 or heme binding with NaCN caused an approximate 30% reduction in HO-1 induction (Figure 6A-B). The ability of HbLDL to induce HO-1 was essentially abolished after pretreatments with both ebselen and exogenous HO-1 or ebselen and NaCN (Figure 6A-B).
Effect of ebselen treatment on HO-1 induction by different oxidized LDLs or hemin. LDLs (1 mg) were incubated with 50 μM ebselen in the presence of 3 mM glutathione, dialyzed, and LOOH levels measured. RAECs were incubated with indicated concentrations of nLDL, HbLDL, CuLDL, LDL–, and hemin, with (□) or without (▪) ebselen pretreatment. After 24 hours cells were harvested and HO-1 protein was visualized by immunoblotting. Densities were calculated and data normalized based on β-actin levels. The figure shows one representative picture reflecting typical changes.
Effect of ebselen treatment on HO-1 induction by different oxidized LDLs or hemin. LDLs (1 mg) were incubated with 50 μM ebselen in the presence of 3 mM glutathione, dialyzed, and LOOH levels measured. RAECs were incubated with indicated concentrations of nLDL, HbLDL, CuLDL, LDL–, and hemin, with (□) or without (▪) ebselen pretreatment. After 24 hours cells were harvested and HO-1 protein was visualized by immunoblotting. Densities were calculated and data normalized based on β-actin levels. The figure shows one representative picture reflecting typical changes.
Inhibition of HO-1 induction in RAECs. (A) Effect of pretreatment of HbLDL by ebselen and HO-1 enzyme. (B) Effect of pretreatment of HbLDL by ebselen and NaCN. HbLDL samples were pretreated with ebselen or HO-1 enzyme or NaCN to respectively reduce LOOH and degrade/inhibit heme. The LDL was then added to cells for 24 hours and HO-1 levels were analyzed as described. Details of pretreatment conditions are provided in “Materials and methods.”
Inhibition of HO-1 induction in RAECs. (A) Effect of pretreatment of HbLDL by ebselen and HO-1 enzyme. (B) Effect of pretreatment of HbLDL by ebselen and NaCN. HbLDL samples were pretreated with ebselen or HO-1 enzyme or NaCN to respectively reduce LOOH and degrade/inhibit heme. The LDL was then added to cells for 24 hours and HO-1 levels were analyzed as described. Details of pretreatment conditions are provided in “Materials and methods.”
HbLDL induces resistance to heme and oxidant stress
A role for HO-1 as an antioxidant defense and oxidant stress–response enzyme has been proposed from several studies.31,32 To determine if HbLDL treatment of RAECs and MPHs conferred resistance to oxidant stress, a series of adaptation experiments were performed. Pretreatment with HbLDL produced an adaptive response to oxidant challenge with hemin and t-BuOOH. The 500% and 150% greater resistance was found against 50 μM hemin in RAECs and MPHs, respectively (Table 2). Lower but significantly increased adaptive responses were found using t-BuOOH as the oxidant challenge for both cell types (Table 2). The induction of resistance corresponded to the interval of induction of HO-1.
Effect of HbLDL pretreatment on RAEC and MPH oxidative stress resistance
. | RAECs . | . | MPHs . | . | ||
|---|---|---|---|---|---|---|
. | nLDL . | HbLDL . | nLDL . | HbLDL . | ||
| No treatment | 0.82 ± 0.23 | 1.1 ± 0.39* | 0.49 ± 0.11 | 0.4 ± 0.1† | ||
| Hemin, 50 μM | 0.055 ± 0.015 | 0.31 ± 0.22* | 0.1 ± 0.03 | 0.15 ± 0.09† | ||
| t-BuOOH, 200 μM | 0.34 ± 0.16 | 0.63 ± 0.31‡ | 0.16 ± 0.096 | 0.2 ± 0.14† | ||
| t-BuOOH, 500 μM | 0.08 ± 0.018 | 0.17 ± 0.13† | 0.045 ± 0.019 | 0.098 ± 0.07‡ | ||
. | RAECs . | . | MPHs . | . | ||
|---|---|---|---|---|---|---|
. | nLDL . | HbLDL . | nLDL . | HbLDL . | ||
| No treatment | 0.82 ± 0.23 | 1.1 ± 0.39* | 0.49 ± 0.11 | 0.4 ± 0.1† | ||
| Hemin, 50 μM | 0.055 ± 0.015 | 0.31 ± 0.22* | 0.1 ± 0.03 | 0.15 ± 0.09† | ||
| t-BuOOH, 200 μM | 0.34 ± 0.16 | 0.63 ± 0.31‡ | 0.16 ± 0.096 | 0.2 ± 0.14† | ||
| t-BuOOH, 500 μM | 0.08 ± 0.018 | 0.17 ± 0.13† | 0.045 ± 0.019 | 0.098 ± 0.07‡ | ||
Cells were pretreated overnight with 200 μg/mL nLDL or HbLDL, and then treated with hemin or t-BuOOH for 6 hours. Cell survival was determined as described in “Materials and methods.” Plating efficiencies calculated as described for LDL and HbLDL are presented.
P < .05.
P < .5.
P < .1.
Discussion
We have previously shown that Hb-mediated oxidation of LDL produces a marked increase in the proportions of the LDL– subfraction and that this oxidation is accompanied by binding of heme (or a Hb fragment) to LDL.7 Our present findings show that the specific composition of HbLDL, for example, particle-associated heme, low levels of LOOH, and increased LDL– levels endow it with pro-oxidant potential; however, this does not lead to increased cytotoxicity. Surprisingly, treatments with HbLDL improved vascular cell survival and resistance in response to oxidative stress. The mechanism underlying the conferred resistance is dependent, in part, on HO-1 expression, and influenced in an additive manner by the LOOH and heme associated with HbLDL. These findings demonstrate unique properties for HbLDL that can sustain cell survival via the induction of an oxidative stress response, despite the accumulation of oxidized products.
Increased LOOH levels and presence of heme in HbLDL endow it with pro-oxidant properties. Increased oxidizability for HbLDL and higher rates of conjugated diene formation were observed for HbLDL without adding a catalyst (Figure 1A). However, in the presence of Cu2+, HbLDL oxidation propagates rapidly without a distinct lag phase. The fast rate of propagation appears to depend on the presence of a heme moiety, because it is markedly reduced in the presence of KCN (Figure 1B) or by treatment with HO-1 enzyme (data not shown). Heme proteins likely facilitate lipid peroxidation12 and may alter particle structure and the conformation of apoB100 to a form that is more susceptible to oxidation.33 It is also possible that HbLDL-associated heme catalyzes a rapid decomposition of peroxides by interaction with various particles via a pseudo-lipoxygenase reaction.34,35 This reaction was previously described for membrane-associated LOOH, wherein decomposition of heme and of peroxides was rapid if a suitable catalyst was present.36 Although the absolute levels of lipid peroxidation products contained in HbLDL are low, they are sufficient to promote oxidation. This is evident from the behavior of HbLDL after ebselen treatment (Figure 1C) where oxidation rates and pro-oxidant capacity are diminished but not entirely lost in the absence of LOOH.
The heme moiety and LOOH associated with HbLDL provides a high reactivity to these particles and the capacity to trigger further oxidative modification of other LDL particles. For example, oxidation of LDL mixed with 20% HbLDL proceeded at faster rates and with shorter lag times than nLDL oxidation (Figure 2A). This manner of oxidation has been previously shown for LDL–. Accordingly, HbLDL has a high proportion of LDL–7 (Figure 2B). The REM for HbLDL and LDL– was less than that of CuLDL and was more uniform in terms of particle charge distribution (data not shown). Notably, the oxidation kinetics of HbLDL/LDL mixtures had 2 distinct propagation phases, suggesting that modified HbLDL particles undergo a specific and limited oxidation first and then facilitate further oxidation of other “nLDL” particles. This was supported by the electrophoretic analysis of HbLDL/LDL mixtures where the band for nLDL showed an increased REM (Figure 2B). Interestingly, a biphasic type of oxidation is also found in native LDL containing small endogenous proportions of LDL– when oxidation is initiated with low Cu2+ concentrations, suggesting increased binding or affinity for Cu2+ or the ability of heme to mediate oxidative decomposition reactions.25
LDL oxidation by copper, myeloperoxidase, or by reactive nitrogen species yields particles that are toxic to vascular cells.37,38 Cytotoxicity with oxidized LDL and LDL– has been attributed to different lipid oxidation products contained in the particle with particular emphasis given to oxysterols, LOOH, reactive aldehydes, and lysophosphatidylcholine.39-42 CuLDL is considered cytotoxic because it is enriched in all these lipid oxidation products. Greater toxicity is also accounted for by the enhanced uptake of CuLDL that facilitates accumulation of toxic materials into cells. Despite the presence of oxysterols, peroxides, and heme, HbLDL produced no apparent toxicity to RAECs and minimal toxicity to MPHs. This cytotoxicity was largely attributed to apoptosis (Figure 3C); however, the degree of apoptosis was substantially lower than that caused by CuLDL. Strikingly, LDL oxidized in the presence of metHb under high oxygen tension displays increased toxicity toward endothelial cells.43 At high oxygen tension, LOOH levels were increased to 100 nmol/mg protein43 versus 30 nmol/mg protein in our HbLDL samples (Table 1). It must be noted that oxidized lipid fractions in HbLDL are much lower than in CuLDL based on LOOH, TBARS, and oxysterol content. Total peroxidation products in HbLDL are comparable to in vivo LDL–. The latter has been shown to exert considerable toxicity toward vascular cells,15 albeit less than CuLDL. HbLDL and LDL– have comparable uptake rates; however, the former produces less severe injury and oxidant stress to cells. Based on the levels of lipid oxidation products in HbLDL versus the degree of adaptive response to oxidant stress, the overall toxic effects may not be sufficient to decrease cell survival, particularly in the wake of a strong oxidant stress/adaptive response.
HO-1 induction is one of the adaptive responses to oxidative stress and its protective role is attributed in part to the products of its enzymatic reaction. HO-1 induction is largely directed to the degradation of heme with the formation of bilirubin and biliverdin, CO, and free iron. Bilirubin is an antioxidant,44 whereas CO is a vasodilator at low concentrations.45 It modulates electron-transport reactions in a variety of ways that can produce either pro-oxidant or antioxidant effects.46 Free iron is sequestered rapidly by ferritin, which is coinduced with HO-1.30 Recent reports of a child with HO-1 deficiency47 and studies with HO-1 knockout mice48 demonstrate a critical role of HO-1 in mediating cell survival and resistance to oxidative stress.
HO-1 is induced in cells by several stress factors such as hemin, H2O2, CuLDL, and mildly oxidized LDL.49-51 HbLDL rapidly and strongly induces HO-1 in a dose- and time-dependent manner in RAECs and MPHs (Figure 4). The catalytic interaction of LOOH and heme in HbLDL provides a potent HO-1 induction (Figure 6A-B) that is much higher than that of LDL– and comparable to that of CuLDL (Figure 5). Moreover, both heme and the LOOH derived from HbLDL are important for adaptive HO-1 induction. This HO-1 induction in MPHs was also accompanied by coinduction of ferritin (Figure 4E), whereas the potent induction of HO-1 in RAECs was not associated with further increases in ferritin levels, suggesting that responses to iron may differ among these cell types. HO-1/ferritin and other antioxidant/defense systems may be sufficient to confer cytoprotection when confronted with an acute oxidant stress caused by HbLDL. Indeed, significant reduction in survival and increased apoptosis in MPHs were found following treatments with HbLDL when HO-1 activity was blocked with SnPPIX (Figure 3). Although CuLDL induces HO-1, it is far more toxic than HbLDL by virtue of the higher levels of various toxic oxidation products that can overwhelm antioxidant defenses and cell responses. Therefore, the balance between the expression of antioxidant defense systems and the burden of toxic products influences cell survival.
In conclusion, HbLDL represents a reactive species of LDL that can form in the circulation or arterial walls during inflammatory conditions in the presence of free Hb. The unique manner by which LDL is oxidatively modified by Hb contributes to high levels of circulating LDL– associated with hemodialysis and possibly other hemorrhage-related pathologies. Prevalence of cytoprotective and adaptive responses with HbLDL may also potentially affect atherogenesis by increasing the survival of MPHs that gradually accumulate products derived from oxidized LDL. Specifically, unstable atherosclerotic plaques characterized by high numbers of lipid-laden MPHs are prone to rupture. Whether generation of HbLDL can trigger formation of unstable plaques remains an interesting possibility.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-01-0293.
Supported in part by the National Institutes of Health grant HL50350 (A.S.), Fogarty International Fellowship F05 TW05340 (L.A.), and an American Heart Association award 1176-FI1 (O.Z.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank R. Mottahedeh of the University of California at Los Angeles for providing us with rabbit aortic endothelial cells. We also greatly appreciate the editorial help of R. Tupy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal