Abstract
Hypoxic stress plays a role in pathophysiologic states such as myocardial infarction and cerebral vascular events as well as in normal physiologic conditions including development and hematopoiesis. Members of the hypoxia inducible factor (HIF) family function as transcriptional regulators of genes involved in the hypoxic response. After generating adult mice that globally lack endothelial PAS domain protein 1 (EPAS1, also known as HIF-2α/HRF/HLF/MOP3), the second member of the HIF family, characterization of the hematopoietic cell population indicated that the loss of EPAS1/HIF-2α resulted in pancytopenia. Using bone marrow reconstitution experiments of lethally irradiated hosts, we have defined the extent and site of hematopoietic impairment in the EPAS1/HIF-2α null mice. These data suggest a critical role for EPAS1/HIF-2α in maintaining a functional microenvironment in the bone marrow for effective hematopoiesis.
Introduction
Development of hematopoietic cells involves interactions between pluripotent hematopoietic stem cells and the microenvironment in which the stem cells are housed. This microenvironment consists of a variety of stromal cell types including vascular endothelial cells, adipocytes, and bone-lining cells. These cells modulate hematopoietic development through complex mechanisms involving growth factor production as well as through cell-cell interactions mediated by cell surface molecules. Disruption of hematopoiesis may be caused by abnormalities in either stem cell or stromal cell function.
Hematopoiesis occurs under relatively hypoxic conditions in the bone marrow.1 Vascular endothelial growth factor (VEGF), a putative target gene for hypoxia-responsive HIF-1α–containing2 and EPAS1/HIF-2α–containing3-6 hypoxia inducible factor (HIF) transcription factor complexes, is induced by hypoxia and has been implicated in hematopoietic development.7,8 Knockout mice lacking ARNT/HIF-1β, the obligate dimerization partner for HIF-1α and EPAS1/HIF-2α, die in utero and exhibit low levels of VEGF in most of the embryo.9 Hematopoietic differentiation assays with ARNT/HIF-1β null embryoid bodies suggest VEGF deficiency as a cause for the observed hematopoietic defect.10
The ARNT/HIF-1β null embryoid body experiments suggest a cell-extrinsic defect for progenitor cell proliferation consistent with a stromal cell source.11 Expression of ARNT/HIF-1β has been noted in murine bone marrow primary stromal cell cultures as well as in stromal cell lines.12 The ARNT/HIF-1β knockout data are consistent with other experiments that implicate a role for the VEGF receptor Flk-1, another potential target gene for HIF-1α or EPAS1/HIF-2α, in the in vitro generation of early hematopoietic cells.13,14
The ARNT/HIF-1β data reveal that HIF complexes may be essential for hematopoietic development. If so, then either HIF-1α or EPAS1/HIF-2α might be responsible for the ARNT/HIF-1β phenotype because HIF family α members (HIF-1α or EPAS1/HIF-2α) confer biologic specificity to the HIF heterodimer complexes, whereas the β members (such as ARNT/HIF-1β) confer biologic activity. Chimera experiments with HIF-1α/Rag2 null mice suggest a role for HIF-1α in B-lymphocyte development, but not global hematopoiesis.15 Therefore, if HIF complexes are indeed essential for hematopoiesis, then EPAS1/HIF-2α would appear to be a leading candidate for the HIF α member responsible for this biologic role.
After crossing isogenic heterozygous 129S6/SvEvTac EPAS1/HIF-2α knockout mice with heterozygous C57BL/6J EPAS1/HIF-2α knockout mice generated using speed congenics,16 we obtained partial survival of adult F1 hybrid (129S6/SvEvTac: C57BL/6J) knockout mice. The use of this F1 genetic background results in the prevention or forestalling of the embryonic lethality observed in either of the parental inbred mouse strains carrying the EPAS1/HIF-2α null allele (Y.O. and J.A.G., unpublished data, March 2003). The resultant F1 hybrid mice exhibit significant perinatal mortality, most prominent within the first day or 2 of life. However, a fraction of the null mice survive such that by 1 month of age approximately 20% of the expected number remain alive (J.A.G., unpublished data, June 2002). The surviving EPAS1/HIF-2α null mice exhibit multiorgan pathology including pancytopenia, hepatomegaly, cardiac hypertrophy, and other pathologic features. In this report, we describe data obtained from hematologic studies performed on EPAS1/HIF-2α null mice and wild-type littermates using transplantation techniques. These experiments have allowed us to determine the site of the hematopoietic defect in EPAS1/HIF-2α null mice and have led us to conclude that EPAS1/HIF-2α is essential for hematopoietic development in mice.
Materials and methods
Animal studies
The EPAS1/HIF-2α knockout mutation and strain generation was previously described.6 The EPAS1/HIF-2α knockout strain was maintained by crossing with wild-type 129S6/SvEvTac mice (Taconic Labs, Germantown, NY). A congenic strain containing the EPAS1/HIF-2α knockout allele was generated using a speed congenic protocol by repeated back-crossing with C57BL/6J wild-type mice (Jackson Laboratories, Bar Harbor, ME). F1 hybrid wild-type, heterozygous EPAS1/HIF-2α knockout (het) or homozygous EPAS1/HIF-2α knockout (null) mice were obtained by crossing heterozygous EPAS1/HIF-2α knockout isogenic 129S6/SvEvTac mice with heterozygous EPAS1/HIF-2α knockout congenic (N8) C57BL/6J mice. Homozygous and heterozygous EPAS1/HIF-2α mice were identified using a polymerase chain reaction protocol with tail DNA preparations as described previously.6 Mice were housed in a standard 12/12 light-dark cycle and were fed standard chow ad lib. All experimental procedures were performed under protocols approved by the UTSWMC Institutional Animal Care and Research Committee.
Histologic studies
For β-galactosidase staining, wild-type, heterozygous EPAS1/HIF-2α knockout or homozygous EPAS1/HIF-2α knockout mice were perfused with 4% paraformaldehyde (Sigma Chemical, St Louis, MO)/phosphate-buffered saline (PBS) by cardiac perfusion techniques. Select organs were removed and postfixed for 1 additional hour prior to subsequent manipulations. Peripheral blood smear samples were air-dried on glass slides prior to further processing. Bone marrow was left in situ prior to Giemsa or β-galactosidase staining as previously described6 and then was embedded in paraffin for further sectioning. Giemsa staining was performed on decalcified femur bone sections.
Laboratory studies
Tail vein samples were collected from each mouse into heparinized capillary tubes for spun hematocrit determinations. For complete blood cell counts, blood samples were collected from anesthetized mice via retro-orbital punctures just before they were humanely killed. Automated cell counts were performed by an outside facility (Lab Corp, Research Triangle, NC).
Transplantation studies
To assess for stem cell function, bone marrow cells from 1-month-old EPAS1/HIF-2α null or wild-type littermate F1 hybrid (129S6/SvEvTac: C57BL/6J, CD45.2+) male mice were dispersed in PBS and passed through a cell strainer. Lethally irradiated (900 rad total in 2 split doses of 500 and 400 rad) Ptprca (CD45.1+; Jackson Laboratories) male recipient mice were each injected intravenously with 107 (first transplantation experiment) bone marrow cells via the tail vein (first transplantation experiment, n = 7 mice/genotype).
To assess for microenvironment effects, a similar protocol was followed using B6-Ptprca male mice as bone marrow donors and 1-month-old EPAS1/HIF-2α null or wild-type littermate F1 hybrid (CD45.2+) male mice as recipients (second transplantation experiment, n = 3 mice/genotype) with the exception that irradiated mice were injected with 106 bone marrow cells. Because EPAS1/HIF-2α null mice are a rate-limiting step for these and other experiments, the sample size for these second series of transplantation experiments was smaller than in the first set of experiments.
Immune cell studies
Where indicated, bone marrow, spleen, thymus, and lymph node samples from postfixed mice were obtained for subsequent use in flow cytometric (fluorescence-activated cell sorting [FACS]) analyses using monoclonal antibodies (BD PharMingen, San Diego, CA). Splenic, thymic, and lymph node cells were dispersed in PBS or media by gentle teasing using 2 frosted glass slides and passed through a nylon mesh prior to FACS analysis. The cells were pelleted by centrifugation and resuspended in ammonium chloride buffer to lyse red blood cells (RBCs). After neutralization with fetal calf serum (FCS), cells were pelleted by centrifugation, resuspended in fresh media, filtered through a nylon mesh, and resuspended in media. Cells were counted and FcR blocking buffer was added for incubation at 4°C for at least 15 minutes prior to antibody staining.
Bone marrow cells were obtained from femurs and tibias by flushing with PBS or media using a 27-gauge needle. Bone marrow cells were filtered through a nylon mesh, pelleted by centrifugation, resuspended in ammonium chloride lysis buffer, and processed for FACS analysis. For peripheral blood cell analyses, samples were obtained via retro-orbital puncture and were dispersed in PBS. Cells were pelleted by centrifugation, resuspended in ammonium chloride lysis buffer, and processed for FACS analysis.
Staining of cells in general was carried out at 4°C for 15 to 20 minutes. Equivalent numbers of cells were first incubated with the following antibodies: rat IgG2b anti–c-KIT (fluorescein isothiocyanate [FITC]), rat IgG2a anti–Sca-1 (FITC), rat IgG2b anti–Ter-119 (biotinylated), rat IgG2b anti–GR-1 (biotinylated), rat IgG2a anti-B220 (biotinylated), rat IgG2b anti–Mac-1 (biotinylated), mouse IgG2a anti-NK (biotinylated), rat IgG2a anti-CD4 (phycoerythrin [PE]), rat IgG2a anti-CD8 (FITC), hamster IgG anti-CD3 (FITC), mouse IgG2a anti-CD45.1 (PE), and mouse IgG2a anti-CD45.2 (FITC). For biotinylated antibodies, after washing the cells the rinsed cells were incubated with streptavidin. After the staining process was completed, the cells were fixed in 0.5% paraformaldehyde and filtered through nylon mesh prior to FACS analyses.
FACS analyses were performed on a Becton Dickinson FACS Calibur (Becton Dickinson, San Jose, CA) with collection of 104 gated events. To assess for the efficacy of the adopted transfer, comparisons were made between CD45.1 and CD45.2 cell populations. To assess lineage-specific development in the donor population, the ratio of lineage-specific donor-derived cells to total donor-derived cells was calculated.
Molecular studies
Bone marrow aspirates were obtained from freshly killed EPAS1/HIF-2α null or wild-type littermate mice. Total RNA was prepared using a commercially available reagent (RNA-Stat60; Tel-Test, Friendswood, TX). Pools were obtained consisting of 3 to 4 mice matched for age, sex, and genotype. First-strand cDNA was generated from each pool of RNA and used in subsequent reverse transcription–polymerase chain reactions (RT-PCRs) with gene-specific primers. The forward (F) and reverse (R) gene-specific primer pairs used are as follows: β-actin (F-5′-TGGAATCCTGTGGCATCCATGAAAC-3′, R-5′-TAAAACGCAGCTCAGTAACAGTCCG-3′), VEGF-A (F-5′-CTGTGCAGGCTGCTGTAACG-3′, R-5′-GTTCCCGAAACCCTGAGGAG-3′), VEGF-B (F-5′-GATCCAGTACCCGAGCAGTC-3′,R-5′-GCACCTACAGGTGTCTGGGT-3′), VEGF-C (F-5′-CAAGGCTTTTGAAGGCAAAG-3′, R-5′-TGCTGAGGTAACCTGTGCTG-3′), VEGF-D (F-5′-CTCCAGGAACCCACTCTCTG-3′, R-5′-TCCTGGCTGTAGAGTCCCTG-3′), neuropilin (F-5′-GACTTCCAGCTCACAGGAGG-3′, R-5′-AGAGCCGGACATGTGATACC-3′), Flt-1 (F-5′-AACCCCGGAGTATGCCACACCTGA-3′,R-5′-GTCCCGCCTCCTTGCTTTTACTCG-3′), Flk-1 (F-5′-AGAACACCAAAAGAGAGAGGAACG-3′, R-5′-GCACACAGGCAGAAACCAGTAG-3′), uPA (F-5′-CATGCCTCCCTTCCCCCTACCTT-3′, R-5′-AGCCCCCATTTTTCCCCTGAT-3′), uPAR (F-5′-GCGGCTGCTGCTGCTGCTGTT-3′,R-5′-AGGCCCTGGCTCCCGCTGAA-3′), fibronectin (F-5′-GGGGCTGGCGCTGTGACAACT-3′, R-5′-TCTAACGGCATGAAGCACTCA-3′), VCAM (F-5′-CAGCTAAATAATGGGGAACTG-3′,R-5′-GGGCGAAAAATAGTCCTTG-3′), and VE-cadherin (F-5′-TTGCCCAGCCCTACGAACCTAAAG-3′,R-5′-ACCACCGCCCTCCTCATCGTAAGT-3′).
Sampling of the RT-PCRs was performed after specified cycle intervals for use in a Southern blotting protocol. The linear range of amplification (and relative copy number) was determined by examination of the curve obtained by plotting signal intensity to cycle number. Input cDNA was adjusted to obtain equivalent signal intensities for the control gene (β-actin). A single cycle number within the linear range of each gene series was used for comparative purposes. The null/wild-type β-actin ratio was calculated as a normalization factor for each set of RT-PCRs. After normalization, a ratio of wild-type–null signal intensities was calculated and plotted relative to the wild-type signal intensity (set at 1) for each gene of interest. The data presented for each gene represent the mean for data obtained from 3 independent pools of RNA.
Results
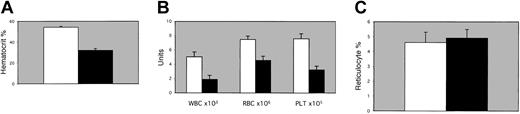
Spun hematocrit levels were significantly reduced in 1-month-old EPAS1/HIF-2α null mice as opposed to wild-type littermates under normoxic conditions (Figure 1A). Complete blood cell counts indicated that the major cell lineages were depressed proportionately (Figure 1B). RBC indices suggested a normocytic anemia with an inappropriate reticulocyte response (Figure 1C), whereas serum analyses and bone marrow preparations indicated normal bone marrow iron stores (data not shown). In addition, all lineages within the white blood cell population were depressed in approximately equal levels (data not shown).
Hematologic studies. (A) Spun hematocrits, (B) complete blood cell counts for leukocytes (WBC), erythrocytes (RBC), and platelets (PLT), or (C) percent reticulocytes were determined for peripheral blood from 1-month-old EPAS1/HIF-2α null mice (▪) or age- and sex-matched wild-type littermates (□). Spun hematocrits and individual blood cell counts were significant for P < .01 by one-tailed t test or by χ2 analyses. Error bars indicate SEM.
Hematologic studies. (A) Spun hematocrits, (B) complete blood cell counts for leukocytes (WBC), erythrocytes (RBC), and platelets (PLT), or (C) percent reticulocytes were determined for peripheral blood from 1-month-old EPAS1/HIF-2α null mice (▪) or age- and sex-matched wild-type littermates (□). Spun hematocrits and individual blood cell counts were significant for P < .01 by one-tailed t test or by χ2 analyses. Error bars indicate SEM.
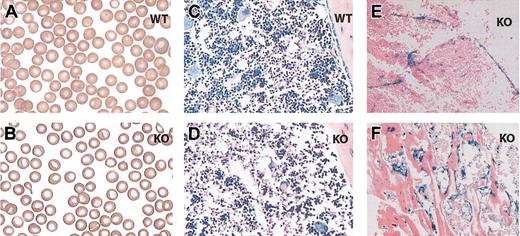
Examination of the blood smear revealed subtle differences including basophilic abnormalities and central hypolucency (Figure 2A-B). Histologic examination of the bone marrow demonstrated hypocellularity without evidence of increased fibrosis or fatty infiltrates (Figure 2C-D; data not shown). Histologic examination of bone sections stained for the surrogate marker for EPAS1/HIF-2α expression in this knockout strain, β-galactosidase activity,17 was noted in select cells within the bone marrow stroma including vascular endothelial, bone-lining cells, and possibly other adventitial cells (Figure 2E-F). EPAS1/HIF-2α null mice did not exhibit splenomegaly indicating that sequestration of hematopoietic cells within the spleen is not a causative factor for the observed pancytopenia.
Histologic studies. Blood smear from a 1-month-old wild-type (A) or EPAS1/HIF-2α null (B) mouse. Bone marrow sample preparations from a 1-month-old wild-type (C) or EPAS1/HIF-2α null (D-F) mouse stained with Giemsa stain (C-D) or assayed for β-galactosidase activity (E-F). The samples stained for β-galactosidase activity demonstrate examples of positively stained vascular endothelial cells (E), bone-lining cells (F), and possibly other adventitial cells (E-F). WT indicates wild-type; and KO, EPAS1/HIF-2α null. Original magnifications, × 100 (A-B) and × 20 (C-F).
Histologic studies. Blood smear from a 1-month-old wild-type (A) or EPAS1/HIF-2α null (B) mouse. Bone marrow sample preparations from a 1-month-old wild-type (C) or EPAS1/HIF-2α null (D-F) mouse stained with Giemsa stain (C-D) or assayed for β-galactosidase activity (E-F). The samples stained for β-galactosidase activity demonstrate examples of positively stained vascular endothelial cells (E), bone-lining cells (F), and possibly other adventitial cells (E-F). WT indicates wild-type; and KO, EPAS1/HIF-2α null. Original magnifications, × 100 (A-B) and × 20 (C-F).
To further characterize the bone marrow population, we performed FACS analysis using cell surface markers for specific hematopoietic lineages. We did not observe any proportional differences when sorting using cell surface markers for stem cells (cKIT, Sca-1), erythrocytes (Ter-119), granulocytes (GR-1), monocytes (B220), macrophages (Mac-1), natural killer cells (NK 1.1), or T cells (CD4, CD8) (data not shown). Thus, a deficiency in EPAS1/HIF-2α results in a global decrease in peripheral blood counts, but multilineage maturation processes within the marrow remain normal. This suggests that the hematopoietic defect in the EPAS1/HIF-2α null mice may be due to impaired production of hematopoietic cells in the bone marrow, either as a consequence of alterations in function of stem cells or of the hematopoietic microenvironment.
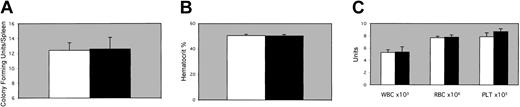
To address the source of the hematopoietic defect in the EPAS1/HIF-2α null mice, 2 sets of transplantation experiments were performed. In the first set, bone marrow cells prepared from an EPAS1/HIF-2α null mouse or a wild-type littermate were used to reconstitute irradiated wild-type recipient mice. Analysis of spleen colony-forming units (CFU-Ss) in the irradiated recipients at 8 days after transplantation demonstrated no significant difference between the 2 groups (Figure 3A). In a second group of mice receiving transplants in a similar manner, spun hematocrits, peripheral blood counts, and FACS analysis of cells derived from bone marrow, spleen, thymi, and lymph nodes were performed 2 months after transplantation. Similar distributions of peripheral cells were observed in irradiated recipients receiving bone marrow cells from either an EPAS1/HIF-2α null or wild-type littermate mouse as determined by both spun hematocrits (Figure 3B) as well as complete blood cell counts (Figure 3C).
Transplantation studies. EPAS1/HIF-2α null donors. (A) CFU-S frequency 8 days after transplantation in irradiated recipient mice receiving bone marrow from either a 1-month-old male EPAS1/HIF-2α null (▪) or wild-type littermate (□) mouse. (B-C) Spun hematocrits (B) or complete blood cell count results (C) for leukocytes (WBC), erythrocytes (RBC), and platelets (PLT) 30 days after transplantation in irradiated recipient mice receiving bone marrow from either an EPAS1/HIF-2α null (▪) or wild-type littermate (□) mouse. No significant differences were noted for CFU-Ss, spun hematocrits, or complete blood cell counts. Error bars indicate SEM.
Transplantation studies. EPAS1/HIF-2α null donors. (A) CFU-S frequency 8 days after transplantation in irradiated recipient mice receiving bone marrow from either a 1-month-old male EPAS1/HIF-2α null (▪) or wild-type littermate (□) mouse. (B-C) Spun hematocrits (B) or complete blood cell count results (C) for leukocytes (WBC), erythrocytes (RBC), and platelets (PLT) 30 days after transplantation in irradiated recipient mice receiving bone marrow from either an EPAS1/HIF-2α null (▪) or wild-type littermate (□) mouse. No significant differences were noted for CFU-Ss, spun hematocrits, or complete blood cell counts. Error bars indicate SEM.
FACS analyses confirmed that most cells in the periphery originated from the donor mice (Table 1). No significant differences were observed in the FACS profiles for bone marrow, splenic, thymic, lymph node, or peripheral hematopoietic cells derived from EPAS1/HIF-2α null or from wild-type littermate bone marrow donors (Table 1). The complexity of the stem cell–derived resident cell populations within accessory hematopoietic organs also is unaffected by loss of the EPAS1/HIF-2α gene (Table 1).
FACS analyses of hematopoietic tissues from EPAS1 null or wild-type littermate donor (CD45.2)/B6-Ptprc recipient (CD45.1) mice
Tissue and epitope . | WT ± SEM . | KO ± SEM . |
|---|---|---|
| Bone marrow | ||
| CD45.2, % total cells | 92.3 ± 0.8 | 93.3 ± 0.2 |
| CD45.1, % total cells | 2.2 ± 0.5 | 0.8 ± 0.1 |
| CD3, % CD45.2 | 2.8 ± 0.3 | 2.5 ± 0.3 |
| B220, % CD45.2 | 28.3 ± 1.8 | 29.0 ± 1.0 |
| MAC-1, % CD45.2 | 69.5 ± 2.5 | 67.7 ± 3.0 |
| GR-1, % CD45.2 | 58.0 ± 1.7 | 55.6 ± 2.3 |
| TER-119, % CD45.2 | 3.4 ± 0.5 | 4.6 ± 0.9 |
| C-KIT, % CD45.2 | 3.4 ± 0.6 | 2.7 ± 0.3 |
| SCA-1, % CD45.2 | 6.4 ± 0.8 | 7.0 ± 0.7 |
| Spleen | ||
| CD45.2, % total cells | 86.1 ± 1.2 | 88.1 ± 2.2 |
| CD45.1, % total cells | 7.1 ± 0.9 | 6.9 ± 1.2 |
| CD3, % CD45.2 | 42.5 ± 1.3 | 45.4 ± 3.1 |
| B220, % CD45.2 | 51.9 ± 2.5 | 50.9 ± 3.4 |
| NK 1.1, % CD45.2 | 3.1 ± 0.7 | 4.2 ± 0.5 |
| Thymus | ||
| CD45.2, % total cells | 98.3 ± 0.5 | 97.4 ± 0.9 |
| CD45.1, % total cells | 0.3 ± 0.1 | 0.3 ± 0.1 |
| CD8, % CD45.2 | 87.0 ± 1.5 | 89.0 ± 1.1 |
| CD4, % CD45.2 | 94.1 ± 1.8 | 95.0 ± 0.6 |
| Lymph node | ||
| CD45.2, % total cells | 86.0 ± 0.8 | 89.0 ± 1.4 |
| CD45.1, % total cells | 13.5 ± 0.8 | 10.4 ± 1.5 |
| B220, % CD45.2 | 14.5 ± 1.5 | 16.9 ± 5.7 |
| CD8, % CD45.2 | 30.5 ± 2.4 | 28.8 ± 2.3 |
| CD4, % CD45.2 | 56.6 ± 2.7 | 52.9 ± 2.9 |
| Peripheral blood | ||
| CD45.2, % total cells | 91.2 ± 2.1 | 91.5 ± 1.2 |
| CD45.1, % total cells | 7.9 ± 2.06 | 7.6 ± 1.2 |
| MAC-1, % CD45.2 | 7.1 ± 1.4 | 5.5 ± 1.7 |
Tissue and epitope . | WT ± SEM . | KO ± SEM . |
|---|---|---|
| Bone marrow | ||
| CD45.2, % total cells | 92.3 ± 0.8 | 93.3 ± 0.2 |
| CD45.1, % total cells | 2.2 ± 0.5 | 0.8 ± 0.1 |
| CD3, % CD45.2 | 2.8 ± 0.3 | 2.5 ± 0.3 |
| B220, % CD45.2 | 28.3 ± 1.8 | 29.0 ± 1.0 |
| MAC-1, % CD45.2 | 69.5 ± 2.5 | 67.7 ± 3.0 |
| GR-1, % CD45.2 | 58.0 ± 1.7 | 55.6 ± 2.3 |
| TER-119, % CD45.2 | 3.4 ± 0.5 | 4.6 ± 0.9 |
| C-KIT, % CD45.2 | 3.4 ± 0.6 | 2.7 ± 0.3 |
| SCA-1, % CD45.2 | 6.4 ± 0.8 | 7.0 ± 0.7 |
| Spleen | ||
| CD45.2, % total cells | 86.1 ± 1.2 | 88.1 ± 2.2 |
| CD45.1, % total cells | 7.1 ± 0.9 | 6.9 ± 1.2 |
| CD3, % CD45.2 | 42.5 ± 1.3 | 45.4 ± 3.1 |
| B220, % CD45.2 | 51.9 ± 2.5 | 50.9 ± 3.4 |
| NK 1.1, % CD45.2 | 3.1 ± 0.7 | 4.2 ± 0.5 |
| Thymus | ||
| CD45.2, % total cells | 98.3 ± 0.5 | 97.4 ± 0.9 |
| CD45.1, % total cells | 0.3 ± 0.1 | 0.3 ± 0.1 |
| CD8, % CD45.2 | 87.0 ± 1.5 | 89.0 ± 1.1 |
| CD4, % CD45.2 | 94.1 ± 1.8 | 95.0 ± 0.6 |
| Lymph node | ||
| CD45.2, % total cells | 86.0 ± 0.8 | 89.0 ± 1.4 |
| CD45.1, % total cells | 13.5 ± 0.8 | 10.4 ± 1.5 |
| B220, % CD45.2 | 14.5 ± 1.5 | 16.9 ± 5.7 |
| CD8, % CD45.2 | 30.5 ± 2.4 | 28.8 ± 2.3 |
| CD4, % CD45.2 | 56.6 ± 2.7 | 52.9 ± 2.9 |
| Peripheral blood | ||
| CD45.2, % total cells | 91.2 ± 2.1 | 91.5 ± 1.2 |
| CD45.1, % total cells | 7.9 ± 2.06 | 7.6 ± 1.2 |
| MAC-1, % CD45.2 | 7.1 ± 1.4 | 5.5 ± 1.7 |
FACS profiles of bone marrow, splenic, thymic, or lymph node hematopoietic cells from irradiated B6-Ptprca recipient (CD45.1+) mice reconstituted with EPAS1/HIF-2α null or wild-type littermate donor (CD45.2+) BM HSCs. WT indicates wild-type; KO, knockout.
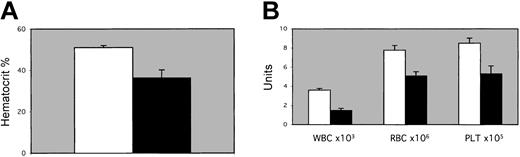
In the second set of transplantation experiments, we analyzed the function of the microenvironment in EPAS1/HIF-2α null mice. EPAS1/HIF-2α null or wild-type littermates were irradiated and repopulated with wild-type donor bone marrow cells. Spun hematocrits performed 1 month after transplantation indicated a difference in hematocrit values of EPAS1/HIF-2α null compared to wild-type littermate recipient mice (Figure 4A). Significant differences were observed in the peripheral blood cell counts for the irradiated EPAS1/HIF-2α null recipients as opposed to wild-type recipients (Figure 4B).
Transplantation studies. EPAS1/HIF-2α null recipients. (A) Spun hemat ocrits or (B) complete blood cell count results for leukocytes (WBC), erythrocytes (RBC), and platelets (PLT) 30 days after transplantation in recipient EPAS1/HIF-2α null (▪) or wild-type littermate (□) mice. Differences were all significant for P < .05 (spun hematocrits, red blood cell counts, and white blood cell counts) or P < .10 (platelets) as assessed by one-tailed t test or by χ2 test. Error bars indicate SEM.
Transplantation studies. EPAS1/HIF-2α null recipients. (A) Spun hemat ocrits or (B) complete blood cell count results for leukocytes (WBC), erythrocytes (RBC), and platelets (PLT) 30 days after transplantation in recipient EPAS1/HIF-2α null (▪) or wild-type littermate (□) mice. Differences were all significant for P < .05 (spun hematocrits, red blood cell counts, and white blood cell counts) or P < .10 (platelets) as assessed by one-tailed t test or by χ2 test. Error bars indicate SEM.
Long-term engraftment of the donor B6-Ptprca CD45.1+ cells into the EPAS1/HIF-2α null recipient differed as compared with wild-type littermate recipient, also reflected by a potentially significant difference in the peripheral blood compartment (Table 2). However, the engrafted donor B6-Ptprca CD45.1+ bone marrow cells developed to near equivalent percentages for most cell lineages in all hematopoietic tissues, regardless of whether of wild-type or EPAS1/HIF-2α knockout origin (Table 2). Whether the difference in CD8 population of the lymph node reflects a biologically relevant finding or instead is the consequence of the small sample size examined remains to be addressed.
FACS analyses of hematopoietic tissues from B6-Ptprc donor (CD45.1)/EPAS1 null or wild-type littermate recipient (CD45.2) mice
Tissue and epitope . | WT ± SEM . | KO ± SEM . |
|---|---|---|
| Bone marrow | ||
| CD45.1, % total cells | 47.2 ± 7.8* | 26.4 ± 11.4* |
| CD45.2, % total cells | 46.0 ± 6.8* | 66.6 ± 11.2* |
| CD3, % CD45.1 | 2.8 ± 0.5 | 2.6 ± 0.3 |
| B220, % CD45.1 | 20.3 ± 1.5 | 19.2 ± 2.2 |
| MAC-1, % CD45.1 | 74.4 ± 2.5 | 69.6 ± 4.7 |
| GR-1, % CD45.1 | 64.0 ± 3.3 | 61.2 ± 4.0 |
| TER-119, % CD45.1 | 3.5 ± 0.4 | 4.1 ± 1.5 |
| C-KIT, % CD45.1 | 2.5 ± 0.6 | 5.6 ± 3.5 |
| SCA-1, % CD45.1 | 7.0 ± 1.7 | 12.5 ± 3.5 |
| Spleen | ||
| CD45.1, % total cells | 52.1 ± 3.6 | 41.3 ± 5.1 |
| CD45.2, % total cells | 35.2 ± 3.5 | 49.4 ± 7.9 |
| CD3, % CD45.1 | 40.2 ± 2.6 | 48.7 ± 9.3 |
| B220, % CD45.1 | 60.2 ± 7.0 | 50.6 ± 9.7 |
| NK 1.1, % CD45.1 | 6.2 ± 0.1 | 5.9 ± 3.0 |
| Thymus | ||
| CD45.1, % total cells | 83.8 ± 7.8 | 71.1 ± 13.3 |
| CD45.2, % total cells | 11.7 ± 5.6 | 11.5 ± 5.0 |
| CD8, % CD45.1 | 88.3 ± 1.8 | 90.7 ± 1.4 |
| CD4, % CD45.1 | 94.5 ± 0.6 | 94.9 ± 1.0 |
| Lymph node | ||
| CD45.1, % total cells | 58.8 ± 4.0 | 51.7 ± 4.6 |
| CD45.2, % total cells | 40.7 ± 4.1 | 47.9 ± 4.4 |
| B220, % CD45.1 | 11.3 ± 3.8 | 15.4 ± 4.8 |
| CD8, % CD45.1 | 30.4 ± 2.3** | 20.7 ± 2.5** |
| CD4, % CD45.1 | 56.1 ± 4.0 | 52.6 ± 4.1 |
| Peripheral blood | ||
| CD45.1, % total cells | 65.8 ± 6.7** | 50.1 ± 6.7** |
| CD45.2, % total cells | 29.8 ± 4.4** | 48.1 ± 7.0** |
| MAC-1, % CD45.1 | 13.3 ± 2.4 | 9.6 ± 2.8 |
Tissue and epitope . | WT ± SEM . | KO ± SEM . |
|---|---|---|
| Bone marrow | ||
| CD45.1, % total cells | 47.2 ± 7.8* | 26.4 ± 11.4* |
| CD45.2, % total cells | 46.0 ± 6.8* | 66.6 ± 11.2* |
| CD3, % CD45.1 | 2.8 ± 0.5 | 2.6 ± 0.3 |
| B220, % CD45.1 | 20.3 ± 1.5 | 19.2 ± 2.2 |
| MAC-1, % CD45.1 | 74.4 ± 2.5 | 69.6 ± 4.7 |
| GR-1, % CD45.1 | 64.0 ± 3.3 | 61.2 ± 4.0 |
| TER-119, % CD45.1 | 3.5 ± 0.4 | 4.1 ± 1.5 |
| C-KIT, % CD45.1 | 2.5 ± 0.6 | 5.6 ± 3.5 |
| SCA-1, % CD45.1 | 7.0 ± 1.7 | 12.5 ± 3.5 |
| Spleen | ||
| CD45.1, % total cells | 52.1 ± 3.6 | 41.3 ± 5.1 |
| CD45.2, % total cells | 35.2 ± 3.5 | 49.4 ± 7.9 |
| CD3, % CD45.1 | 40.2 ± 2.6 | 48.7 ± 9.3 |
| B220, % CD45.1 | 60.2 ± 7.0 | 50.6 ± 9.7 |
| NK 1.1, % CD45.1 | 6.2 ± 0.1 | 5.9 ± 3.0 |
| Thymus | ||
| CD45.1, % total cells | 83.8 ± 7.8 | 71.1 ± 13.3 |
| CD45.2, % total cells | 11.7 ± 5.6 | 11.5 ± 5.0 |
| CD8, % CD45.1 | 88.3 ± 1.8 | 90.7 ± 1.4 |
| CD4, % CD45.1 | 94.5 ± 0.6 | 94.9 ± 1.0 |
| Lymph node | ||
| CD45.1, % total cells | 58.8 ± 4.0 | 51.7 ± 4.6 |
| CD45.2, % total cells | 40.7 ± 4.1 | 47.9 ± 4.4 |
| B220, % CD45.1 | 11.3 ± 3.8 | 15.4 ± 4.8 |
| CD8, % CD45.1 | 30.4 ± 2.3** | 20.7 ± 2.5** |
| CD4, % CD45.1 | 56.1 ± 4.0 | 52.6 ± 4.1 |
| Peripheral blood | ||
| CD45.1, % total cells | 65.8 ± 6.7** | 50.1 ± 6.7** |
| CD45.2, % total cells | 29.8 ± 4.4** | 48.1 ± 7.0** |
| MAC-1, % CD45.1 | 13.3 ± 2.4 | 9.6 ± 2.8 |
FACS profiles of bone marrow, splenic, thymic, and lymph node hematopoietic cells from EPAS1/HIF-2α null or wild-type littermate recipient (CD45.2+) mice reconstituted with B6-Ptprca donor (CD45.1+) BM HSCs. Statistical comparisons made pair-wise by 2-tailed t tests revealed significant findings at P ≤ .05 (*) or P ≤ .10 (**).
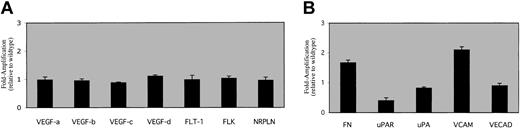
To begin to address the molecular nature of the defect in the EPAS1/HIF-2α null mice, we performed RT-PCR analyses on bone marrow preparations from knockout mice and their wild-type littermates. We chose VEGF and VEGF receptors as candidate genes for analysis because prior studies have implicated HIF members as being essential for VEGF or VEGF receptor expression. We observed only minor differences (0.8- to 1.11-fold differences) in VEGF member (A, B, C, D) or VEGF receptor (Flt-1, Flk-1, Nrpln) steady-state transcription when examining RNA derived from total bone marrow cells (Figure 5A), suggesting that global abnormalities in VEGF expression are not present in the bone marrow of EPAS1/HIF-2α null mice.
Molecular studies. RT-PCR data were obtained as described using EPAS1/HIF-2α null bone marrow cDNA and graphed relative to the wild-type gene of interest signal (set at one). The data are presented for a panel of genes involved in (A) VEGF production (VEGF A, B, C, D)and VEGF signaling (Flt-1, Flk-1, Nrpln), or (B) endothelial cell signaling (FN, uPAR, uPA, VCAM, VECAD), as well as the internal control (β-actin). Differences in FN, uPAR, and VCAM levels were all significant for P < .05 as assessed by 2-tailed t test. No other significant differences between genotypes were noted for the other gene products. Error bars indicate SEM.
Molecular studies. RT-PCR data were obtained as described using EPAS1/HIF-2α null bone marrow cDNA and graphed relative to the wild-type gene of interest signal (set at one). The data are presented for a panel of genes involved in (A) VEGF production (VEGF A, B, C, D)and VEGF signaling (Flt-1, Flk-1, Nrpln), or (B) endothelial cell signaling (FN, uPAR, uPA, VCAM, VECAD), as well as the internal control (β-actin). Differences in FN, uPAR, and VCAM levels were all significant for P < .05 as assessed by 2-tailed t test. No other significant differences between genotypes were noted for the other gene products. Error bars indicate SEM.
Development and migration of immature progenitors and mature cells from the bone marrow involves a variety of cells and cellular factors including several residing on the vascular endothelial cell surface.18-20 We therefore determined the mRNA levels of several key candidate genes involved in stem cell development or migration as a preliminary examination of changes in gene expression in EPAS1/HIF-2α null bone marrow. Expression of the mRNA encoding vascular cell adhesion molecule (VCAM), urokinase-type plasminogen activator receptor (uPAR), and fibronectin (FN), and to a lesser extent vascular endothelial cadherin (VECAD) and urokinase-type plasminogen activator (uPA), are substantially altered in the bone marrow of EPAS1/HIF-2α null mice (Figure 5B).
Discussion
The transplantation experiments performed with EPAS1/HIF-2α null mice were informative for several reasons. The first set of transplantation experiments, intended to assess the effects of the EPAS1/HIF-2α null mutation on the reimplantation capacity of bone marrow hematopoietic stem cells (BM HSCs), reveal that EPAS1/HIF-2α null donor bone marrow cells effectively repopulate irradiated wild-type recipients. The cell count results and lineage-specific FACS analyses demonstrate that immature progenitors as well as BM HSCs derived from EPAS1/HIF-2α null mice have no defect in migration, homing, or maturation. What remains to be determined is whether or not the functional aspects of mature hematopoietic cells derived from EPAS1/HIF-2α BM HSCs are unperturbed. Long-term transplantation and stimulation protocols will be needed to further characterize the effects of the EPAS1/HIF-2α null mutation on mature hematopoietic cell function.
The second set of transplantation experiments, intended to assess the effects of the EPAS1/HIF-2α null mutation on the function of the bone marrow microenvironment, indicate the pancytopenia in EPAS1/HIF-2α null mice is attributable to defects in bone marrow microenvironment function or to systemic effects of the EPAS1/HIF-2α null mutation. The hypocellular bone marrow observed in EPAS1/HIF-2α null mice would support a global decrease in maturation as a causative factor for the pancytopenia. Although engraftment of donor cells is decreased in hematologic compartments of the EPAS1/HIF-2α null recipient, the FACS analyses reveal that lineage-specific maturation is normal or else is globally impaired as a result of the microenvironment defects. The expression pattern of EPAS1/HIF-2α as revealed by the surrogate marker β-galactosidase, suggests that a likely candidate cell population responsible for the functional deficits may be a subset of cells comprising the bone marrow stromal cell compartment.
The molecular nature of the hematopoietic defect in EPAS1/HIF-2α null mice does not appear to be due to decreases in VEGF or VEGF receptor mRNA levels. This suggests that EPAS1/HIF-2α is not involved in regulation of these genes within the bone marrow compartment or that HIF-1α may be functionally redundant for EPAS1/HIF-2α in this setting. We cannot rule out an effect of the EPAS1/HIF-2α mutation on VEGF or VEGF receptor expression in a subset of bone marrow stromal cells that are essential for hematopoietic development.8 With respect to results obtained from ARNT/HIF-1β knockout mice and embryoid bodies, a deficiency in VEGF levels may play an accessory role in impaired hematopoietic development because ARNT/HIF-1β is also the obligate partner for HIF-1α a prominent regulator of VEGF expression. However, the results presented herein clearly demonstrate that pancytopenia may result from a deficiency in EPAS1/HIF-2α alone without significant changes in global bone marrow VEGF or VEGF gene expression.
In contrast to the RT-PCR results examining VEGF or VEGF receptor mRNA levels, EPAS1/HIF-2α null mice do exhibit more marked differences in expression of mRNA encoding cell surface molecules implicated in hematopoietic development. These include increases in VCAM-1 and FN as well as decreases in uPAR and uPA mRNA levels.
VECAD, a transmembrane protein involved in endothelial cell-cell interactions and in regulating transendothelial migration of hematopoietic stem cells in a VCAM-1–dependent manner,21 is minimally affected in EPAS1/HIF-2α null mice. However, mRNA levels for the interactive partner of VECAD, VCAM-1, are increased in bone marrow from EPAS1/HIF-2α null bone mice. Increased VCAM-1 levels have been associated with decreased circulating hematopoietic progenitor cells22 and are restricted to stromal reticular cells and endothelial cells lining bone marrow sinusoids.23 The interaction of VCAM-1 and FN with the integrin very late activation 4 (VLA-4) modulates the proliferation as well as the retention and homing of hematopoietic progenitor cells in and to the bone marrow.24-29 VCAM-1 is induced by a number of cytokines and other growth factors that mediate their effects through oxidative stress signaling.30-32 Future experiments will be needed to determine if alterations in cytokine, growth factor, or oxidative stress signal transduction are involved in the induction of VCAM-1 in EPAS1/HIF-2α null mice.
Levels of uPAR are notably depressed in EPAS1/HIF-2α null mice, whereas uPA levels are more moderately depressed. A role for uPAR has been defined in a number of cellular processes including cell adhesion, integrin-dependent migration,33-35 and signal transduction.36,37 Expression of the uPAR gene is regulated by hypoxia as well as by hypoxia mimetics.38-40 The decreased levels of uPAR observed in EPAS1/HIF-2α null mice in this respect are particularly abnormal because the anemia seen in EPAS1/HIF-2α null mice likely produces a more profound hypoxic stress in the bone marrow. Thus, these results suggest that EPAS1/HIF-2α may be a key regulator for uPAR gene expression in vivo. They also indicate that the absence of EPAS1/HIF-2α cannot be compensated by the more ubiquitously expressed HIF-1α.
The hematopoietic defect observed in the EPAS1/HIF-2α null mice may be secondary to the dysregulated receptors and extracellular matrix proteins identified in this study.18,24,41-49 A functional defect in the vascular endothelial stromal cell component may produce a block in exit of hematopoietic cells from the bone marrow or may cause a global decrease in the maturation process.21,41 Further characterization of the EPAS1/HIF-2α–expressing hematopoietic stromal cell population will clarify its role in normal and abnormal stromal cell biology18 and may be of particular relevance to leukemia and other blood dyscrasias.50-53
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-02-0448.
Supported by funds from the National Institutes of Health (NIH)/National Heart, Lung and Blood Institute (J.A.G.), NIH/National Institute of Mental Health Conte Center (J.A.G.), American Heart Association-Texas Affiliate (J.A.G.), and Donald W. Reynolds Foundation (J.A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Daniel Foster, Sandy Williams, and Steve McKnight for suggestions and support; Jim Richardson and John Shelton for assistance with histologic analyses; and Alok Das, Lingzhi Wang, and Arti Gaur for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal