Abstract
Prospective analysis of the importance of the plasma levels of β-2 microglobulin (B2M) in 553 patients with myelodysplastic syndrome (MDS) found that B2M is an independent prognostic variable for survival with weighted significance second only to the karyotype. The incorporation of the B2M covariate into risk assessment of MDS patients added significantly to the power of the IPSS to stratify MDS patients into risk categories. Our results further document that the 2 objectively measured covariates that display the highest power to predict survival, that is, karyotype and B2M, can alone be used for risk stratification. While the results must be verified in an independent and comparable population, our data strongly recommend routine measurement of B2M in patients with MDS.
Introduction
The variable outcomes of myelodysplastic syndrome (MDS) have encouraged the development of numerous algorithms to predict prognosis. The most commonly used system is the International Prognostic Scoring System (IPSS).1 Prognostic heterogeneity still exists, particularly in patients with low or intermediate risks. Here we report that levels of β-2 microglobulin are a valuable prognostic factor in MDS.
Study design
Study group
From 1994 through mid-2001, 923 patients with MDS were referred to M. D. Anderson Cancer Center, and levels of β-2 microglobulin (B2M) were measured prospectively in 660 patients (71.5%). Survival of 660 patients with available B2M was similar to that of 263 patients lacking this information. Of these 660 patients, 2 groups were excluded from subsequent analyses: (1) 50 patients with serum creatinine levels higher than 1.5 mg/dL, because impaired renal function could elevate B2M2 ; (2) 57 patients with chronic myelomonocytic leukemia (CMML) and white blood cell (WBC) counts of more than 12 × 109/L (CMML, proliferative), regardless of levels of B2M or serum creatinine, to comply with the IPSS, which excluded these patients.1 The remaining 553 patients provided the database for this analysis. At the time of referral, B2M and karyotype were recorded along with other hematologic, biochemical, and clinical covariates.3 Diagnosis was established using French-American-British (FAB) criteria,4 and the IPSS score was computed.1 B2M was measured in a similar proportion of patients in each FAB group (range, 65%-72%) and IPSS group (range, 74%-89%). Serum B2M was quantified by a radioimmunoassay (Pharmacia β2 Micro Ria; Pharmacia Diagnostic, Uppsala, Sweden). Median follow-up in the 288 patients remaining alive was 9 months (range, 0-87 months). Patients provided written informed consent. The study was approved by the University of Texas M. D. Anderson Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Treatment
After referral, patients received supportive care with or without hematopoietic factors (n = 202), single-agent chemotherapy (n = 35), immunosuppressive therapy (n = 33), single new investigational agents (n = 30), and intensive therapy (n = 271). Intensive chemotherapy regimens have been described in detail.5 For 21 patients, information about treatment was not available.
Statistical analyses
Univariate analysis of prognostic factors. Covariates, analyzed for association with survival, are described in Table 1. Karyotype was grouped according to IPSS criteria.1 For all covariates measured on a numerical scale, we investigated the nature of their association with survival by martingale residual plot analysis.6 Survival time was calculated from the time of referral. Time-to-event curves were obtained using the Kaplan-Meier method and compared using the log-rank test.
Univariate analysis for survival
. | Patients, n . | Patients, % . | Median survival, mo . | P . |
|---|---|---|---|---|
| Cytogenetics* | < .0001 | |||
| Good | 262 | 50 | 27 | |
| Intermediate | 108 | 20 | 22 | |
| Poor | 159 | 30 | 7 | |
| Sex | .9073 | |||
| Female | 188 | 34 | 16 | |
| Male | 365 | 66 | 15 | |
| Age | .0217 | |||
| 60 y or younger | 185 | 33 | 18 | |
| Older than 60 y | 368 | 67 | 13 | |
| Hemoglobin | .0024 | |||
| Less than 10 g/dL | 355 | 64 | 13 | |
| 10 g/dL or greater | 198 | 36 | 19 | |
| Absolute neutrophil count | .9314 | |||
| Less than 1.5 K/μL | 291 | 53 | 16 | |
| 1.5 K/μL or greater | 262 | 47 | 14 | |
| Platelets | < .0001 | |||
| Less than 100 K/μL | 370 | 67 | 12 | |
| 100 K/μL or more | 183 | 33 | 28 | |
| BM blasts, %† | .0056 | |||
| Less than 5 | 181 | 33 | 27 | |
| 5-10 | 149 | 27 | 14 | |
| 11-20 | 145 | 26 | 12 | |
| 21-30 | 76 | 14 | 16 | |
| B2M | < .0001 | |||
| 2 mg/L or less | 158 | 29 | 32 | |
| Greater than 2 mg/L | 395 | 71 | 13 | |
| Intensive treatment | .0150 | |||
| Yes | 271 | 49 | 14 | |
| No | 282 | 51 | 18 | |
| Creatinine level | .2249 | |||
| Less than 1 mg/dL | 308 | 56 | 16 | |
| 1 mg/dL or greater | 245 | 44 | 14 |
. | Patients, n . | Patients, % . | Median survival, mo . | P . |
|---|---|---|---|---|
| Cytogenetics* | < .0001 | |||
| Good | 262 | 50 | 27 | |
| Intermediate | 108 | 20 | 22 | |
| Poor | 159 | 30 | 7 | |
| Sex | .9073 | |||
| Female | 188 | 34 | 16 | |
| Male | 365 | 66 | 15 | |
| Age | .0217 | |||
| 60 y or younger | 185 | 33 | 18 | |
| Older than 60 y | 368 | 67 | 13 | |
| Hemoglobin | .0024 | |||
| Less than 10 g/dL | 355 | 64 | 13 | |
| 10 g/dL or greater | 198 | 36 | 19 | |
| Absolute neutrophil count | .9314 | |||
| Less than 1.5 K/μL | 291 | 53 | 16 | |
| 1.5 K/μL or greater | 262 | 47 | 14 | |
| Platelets | < .0001 | |||
| Less than 100 K/μL | 370 | 67 | 12 | |
| 100 K/μL or more | 183 | 33 | 28 | |
| BM blasts, %† | .0056 | |||
| Less than 5 | 181 | 33 | 27 | |
| 5-10 | 149 | 27 | 14 | |
| 11-20 | 145 | 26 | 12 | |
| 21-30 | 76 | 14 | 16 | |
| B2M | < .0001 | |||
| 2 mg/L or less | 158 | 29 | 32 | |
| Greater than 2 mg/L | 395 | 71 | 13 | |
| Intensive treatment | .0150 | |||
| Yes | 271 | 49 | 14 | |
| No | 282 | 51 | 18 | |
| Creatinine level | .2249 | |||
| Less than 1 mg/dL | 308 | 56 | 16 | |
| 1 mg/dL or greater | 245 | 44 | 14 |
Data available for 529 patients.
Data available for 551 patients.
Multivariate analysis The Cox proportional hazards regression model followed by backward selection was used to assess the ability of covariates to independently predict survival. The predictive variables were assigned weights, using the regression coefficients from the final Cox proportional hazards model multiplied by 8 and rounded to the nearest whole number. The ratios between the resulting numbers were used to produce risk categories in analogy to the IPSS.1 The scoring system thus observed (modified IPSS) was compared with the IPSS and with a model based on karyotype and B2M only.
Results and discussion
Median survival of the 553 MDS patients was 15 months (95% confidence interval [CI], 12.8-17.7 months). Some of their characteristics are summarized in Table 1. Ninety-two patients (17%) had refractory anemia (RA), 50 (9%) refractory anemia and ringed sideroblasts (RARS), 176 (32%) refractory anemia with excess of blasts (RAEB), 184 (33%) refractory anemia with excess of blasts transformed (RAEBt), and 51 (9%) “nonproliferative” CMML. Only 11% had an IPSS classification of “low,” with the remaining patients approximately equally divided between “intermediate-1,” “intermediate-2,” and “high” (Table 2, Figure 1C).
Stratification of patients within IPSS subset by B2M level
IPSS category . | n . | Median survival, mo (95% CI) . | P . |
|---|---|---|---|
| Low risk | .05 | ||
| B2M 2 mg/L or less | 19 | NR (31.9, NR) | |
| B2M greater than 2 mg/L | 42 | 53 (11.8, NR) | |
| Intermediate-1 | < .01 | ||
| B2M 2 mg/L or less | 45 | NR (31.9, NR) | |
| B2M greater than 2 mg/L | 118 | 15 (12.6, 18.3) | |
| Intermediate-2 | .13 | ||
| B2M 2 mg/L or less | 50 | 16 (10.0, 32.7) | |
| B2M greater than 2 mg/L | 116 | 12 (8.1, 13.6) | |
| High | < .01 | ||
| B2M 2 mg/L or less | 36 | 16 (11.0, NR) | |
| B2M greater than 2 mg/L | 106 | 8 (5.3, 12.5) |
IPSS category . | n . | Median survival, mo (95% CI) . | P . |
|---|---|---|---|
| Low risk | .05 | ||
| B2M 2 mg/L or less | 19 | NR (31.9, NR) | |
| B2M greater than 2 mg/L | 42 | 53 (11.8, NR) | |
| Intermediate-1 | < .01 | ||
| B2M 2 mg/L or less | 45 | NR (31.9, NR) | |
| B2M greater than 2 mg/L | 118 | 15 (12.6, 18.3) | |
| Intermediate-2 | .13 | ||
| B2M 2 mg/L or less | 50 | 16 (10.0, 32.7) | |
| B2M greater than 2 mg/L | 116 | 12 (8.1, 13.6) | |
| High | < .01 | ||
| B2M 2 mg/L or less | 36 | 16 (11.0, NR) | |
| B2M greater than 2 mg/L | 106 | 8 (5.3, 12.5) |
NR indicates not reached.
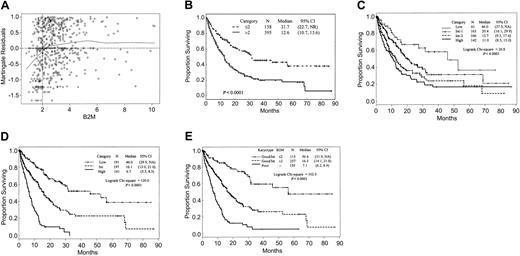
Effect of B2M levels on survival. (A) Martingale residual plot analysis of B2M. The line Y = 0 corresponds to the underlying hazard of death before accounting for the effect of B2M. Deviations from this line indicate the effect of B2M on this hazard. In particular, residuals of less than 0 correspond to better expected survival and residuals of more than 0 to worse expected survival. These residuals are analogous to residuals estimated in linear regression. (B) Survival of 553 MDS patients by B2M. (C) Survival of patients according to their risk assignment by IPSS. (D) Survival by modified IPSS. (E) Survival by combination of B2M and karyotype (Kaplan-Meier curves).
Effect of B2M levels on survival. (A) Martingale residual plot analysis of B2M. The line Y = 0 corresponds to the underlying hazard of death before accounting for the effect of B2M. Deviations from this line indicate the effect of B2M on this hazard. In particular, residuals of less than 0 correspond to better expected survival and residuals of more than 0 to worse expected survival. These residuals are analogous to residuals estimated in linear regression. (B) Survival of 553 MDS patients by B2M. (C) Survival of patients according to their risk assignment by IPSS. (D) Survival by modified IPSS. (E) Survival by combination of B2M and karyotype (Kaplan-Meier curves).
The median B2M level was 2.6 mg/L (range, 0.8-10.2 mg/L). It differed significantly neither among the FAB groups (RA, 2.5 mg/L; RARS, 2.6 mg/L; RAEB, 2.6 mg/L; RAEBt, 2.7 mg/L; nonproliferative CMML, 2.9 mg/L) nor among IPSS risk groups (low, 2.4 mg/L; intermediate-1, 2.7 mg/L; intermediate-2, 2.8 mg/L; high, 2.7 mg/L). The martingale residuals failed to identify an association between serum creatinine levels and survival (data not shown), 1 mg/dL being the best value separating patients according to survival (Table 1). The martingale residual plot for B2M identified the optimal cutpoint as 2mg/L (Figure 1A, Table 1). Use of other cutpoints could also stratify the population, but with lower discriminatory power.3 These findings led us to group patients according to dichotomous levels of B2M (≤ 2 g/L vs > 2 g/L), which stratified all patients (Figure 1B) and patients within each IPSS category (Table 2) and FAB category3 into subgroups with better or worse outcome, thus proving the independent prognostic value of B2M as a new biologic feature in assessing survival. The differences reached statistical significance in all but the intermediate-2 IPSS (Table 2), RA, and RAEBt groups.3 Univariate analysis identified cytogenetics, age, hemoglobin level, platelet count, bone marrow (BM) blasts, intensive treatment, and B2M as significantly associated with survival (Table 1).
Patients who received intensive treatment had significantly shorter survivals than other patients (12.9 months vs 18.3 months; Table 1), likely owing to selection of patients with RAEB and RAEBt for intensive chemotherapy. This association was lost in multivariate analysis. We failed to find an association between intensive treatment and levels of B2M (P = .18). The median value of B2M was 1.7 mg/L both in the subgroup receiving and in the subgroup not receiving intensive treatment; high B2M was present in 70% of patients who received intensive chemotherapy and in 73% of patients who did not. Patients with high-level B2M had shorter survival than patients with low B2M whether they received intensive treatment (11.2 months vs 18.8 months; P < .01) or were managed more conservatively (13.4 months vs > 31.9 months; P < .01). Thus, high B2M affects survival negatively, regardless of treatment.
Within karyotype categories, high B2M was associated with shorter survival both in patients with good/intermediate karyotype (Figure 1E) and in 159 patients with poor cytogenetics (6.3 months [95% CI, 5.2,8.3] vs 10.0 months [95%CI, 7.4,16.5]; P = .01). Only 35 patients (22%) with poor cytogenetics had low B2M levels.
Multivariate analysis confirmed that B2M added significant and new prognostic information to that provided by IPSS. We next examined how B2M might be incorporated into a modified IPSS (M-IPSS). The prognostic martingale residual plot suggested that marrow blasts be considered as a binary variable (< 5% vs ≥ 5%). The observation that pretreatment absolute neutrophil count (ANC) was not prognostic (Table 1) prompted us to regard anemia and thrombocytopenia as one category vs others rather as done in IPSS. With similar survival, IPSS cytogenetic categories “good” and “intermediate” were combined (Table 1). We then considered the following for inclusion in the Cox model: poor vs good/intermediate cytogenetics; B2M 2 mg/L or less vs more than 2 mg/L; BM blasts less than 5% vs 5% or more; and anemia plus thrombocytopenia vs others. In the resulting final model, karyotype was the best predictor of survival, followed by B2M, cytopenias, and percentage of BM blasts (Table 3). By combining risk scores, we stratified patients into 3 risk categories (good, intermediate, and poor; scores 0-6, 7-11, and 12-20, respectively; Table 3). Within the subgroup of high-risk M-IPSS patients, only 17 were included without contribution of high B2M. Figure 1D illustrates the prognostic impact of M-IPSS. Simple addition of B2M value to the IPSS (which forced the original IPSS cutoffs despite their lack of significance in our population) did improve the stratification of patients (not shown). However, the results remained inferior to those described for M-IPSS.
Associations between survival time and patient characteristics identified using backward selection procedure (Cox proportional hazards model*)
. | χ2 . | P . | Hazard ratio (95% CI) . | Parameter . | Score . |
|---|---|---|---|---|---|
| Poor karyotype | 62.8 | < .0001 | 3.0 (2.3-3.9) | 1.08 | 9† |
| B2M greater than 2 mg/L | 24.0 | < .0001 | 2.2 (1.6-2.9) | 0.77 | 6‡ |
| Platelet count less than 100 K/μL and hemoglobin level less than 10 g/dL | 8.5 | .0036 | 1.5 (1.1-2.9) | 0.38 | 3§ |
| 5% or more BM blasts | 3.4 | .0652 | 1.3 (1.0-1.7) | 0.27 | 2∥ |
. | χ2 . | P . | Hazard ratio (95% CI) . | Parameter . | Score . |
|---|---|---|---|---|---|
| Poor karyotype | 62.8 | < .0001 | 3.0 (2.3-3.9) | 1.08 | 9† |
| B2M greater than 2 mg/L | 24.0 | < .0001 | 2.2 (1.6-2.9) | 0.77 | 6‡ |
| Platelet count less than 100 K/μL and hemoglobin level less than 10 g/dL | 8.5 | .0036 | 1.5 (1.1-2.9) | 0.38 | 3§ |
| 5% or more BM blasts | 3.4 | .0652 | 1.3 (1.0-1.7) | 0.27 | 2∥ |
Likelihood ratio χ2, 115.7; n = 529.
1.08 × 8 = 9.0.
0.77 × 8 = 6.0.
0.38 × 8 = 3.0.
0.27 × 8 = 2.0.
Serum levels of B2M have prognostic significance in lymphomas,7-12 multiple myeloma,13 chronic lymphocytic leukemia,14,15 Philadelphia-positive chronic myelogenous leukemia,16 CMML,17 and acute lymphocytic leukemia.18 B2M was the only variable predicting the response of high-risk MDS to intensive chemotherapy.3 The prognostic importance of B2M may be related to its role in the immunological response of the host to the malignancy.19,20 Since most circulating B2M is derived from the cellular surface,21 increased levels may relate to increased cell turnover, increased tumor mass, or both. The correlation of B2M with apoptotic activity in MDS is being investigated.
In summary, we report that incorporation of B2M into risk assessment of patients with MDS improved the discriminatory power of the IPSS. Karyotype and B2M display the highest power in predicting survival and can alone be used for stratification of MDS patients. While these results must be verified in an independent, comparable population of MDS patients with normal serum creatinine levels, our data strongly recommend routine measurements of B2M in patients with MDS.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-10-3264.
Supported in part by Salners Family Fund for Leukemia Research (M.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Vivian Bush for assistance in preparation of this report.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal