Abstract
The TEL/PDGFβR oncogenic fusion protein is the product of the t(5;12)(q33; p13) translocation recurrently found in patients with chronic myelomonocytic leukemia (CMML). To investigate the coupling of molecular signaling events activated by TEL/PDGFβR to functional responses, we expressed TEL/PDGFβR in interleukin 3 (IL-3)–dependent BaF/3 cells using the tetracycline-regulated expression system. Induction of TEL/PDGFβR expression led to increased cell survival following IL-3 withdrawal and constitutive activation of protein kinase B (PKB), signal transducer and activator of transcription 5 (STAT5), extracellular signal-regulated kinases 1/2 (ERK1/2), Jun N-terminal kinases 1/2 (JNK1/2), and p38 mitogenactivated protein kinase (MAPK) pathways. However, inducible expression of TEL/PDGFβR failed to generate factor-independent cells, whereas constitutive expression of TEL/PDGFβR did, albeit at low frequency, suggesting the duration of TEL/PDGFβR expression is important for transformation. Surprisingly, in cells induced to express TEL/PDGFβR, IL-3–dependent growth was dramatically reduced as a result of increased apoptosis of cells receiving combined IL-3 and TEL/PDGFβR signals. We demonstrate that TEL/PDGFβR expression augmented IL-3–induced activation of PKB, STAT5, ERK1/2, p38, and JNK1/2. Inhibition of neither phosphoinositide-3 kinases nor p38 MAPKs reduced the inhibition of IL-3–driven proliferation observed when TEL/PDGFβR was expressed. However, inhibition of MEKs or JNKs partially reversed the combined inhibitory effects of TEL/PDGFβR and IL-3 on proliferation and survival. These results suggest that the combination of TEL/PDGFβR and IL-3–induced signals activate apoptosis through ERK and JNK MAPK-dependent pathways. Given that in vivo hematopoietic cells are in contact with a variety of cytokines, our results have important implications for cellular responses in the pathogenesis of CMML.
Introduction
Tyrosine kinase fusion proteins are a growing family of oncoproteins implicated in the pathogenesis of hematopoietic malignancies. This family is exemplified by BCR/ABL1,2 and the family of TEL/tyrosine kinase fusions.2-4 Patients with a variant of chronic myelomonocytic leukemia (CMML) have a recurring t(5;12)(q33; p13) chromosomal translocation,4 which results in the formation of a fusion protein termed TEL/PDGFβR. CMML is a myelodysplastic syndrome and is characterized clinically by dysplastic monocytosis, splenomegaly, hypercellularity of the bone marrow, and progression to an acute myeloid leukemia, which often proves fatal. In a murine bone marrow transplantation model, expression of TEL/PDGFβR induces a polyclonal myeloproliferative disease, that closely resembles t(5;12) CMML, thus supporting the notion that TEL/PDGFβR is the oncoprotein involved in this disorder.5 The 154 amino-terminal residues of TEL/PDGFβR are derived from the pointed (PNT) domain of the ets-like transcription factor TEL, with the carboxy-terminal region composed of the transmembrane and tyrosine kinase domains of platelet-derived growth factor β receptor (PDGFβR).4 Both the PNT domain and tyrosine kinase activity of TEL/PDGFβR are essential for its transforming activities5-8 with Tyr579 and Tyr581 of the PDGFβR portion of TEL/PDGFβR critical for the development of the myeloproliferative disease.5 Mice given transplants with bone marrow transduced with versions of TEL/PDGFβR containing mutations at these 2 tyrosine residues developed mediastinal lymphomas with a latency of several months.5
Expression of TEL/PDGFβR in cytokine-dependent cells has been reported to render such cell lines cytokine independent,7,9 with the PNT domain of TEL and the tyrosine kinase activity of the cytoplasmic domain of PDGFβR playing essential roles.6,7 The PNT domain mediates TEL/PDGFβR multimerization in the absence of ligand, leading to constitutive activation of PDGFβR tyrosine kinase activity and phosphorylation of tyrosine residues in the kinase domain.7-9 This enables TEL/PDGFβR to associate with signaling molecules important for mitogenesis including phospholipase Cγ, Src homology phosphatase 2 (SHP-2), phosphoinositide 3–kinases (PI3Ks), and Ras-guanosine triphosphate (GTPase)–activating protein.7 TEL/PDGFβR also activates nuclear factor κB (NF-κB),10 signal transducer and activator of transcription 1 and 5 (STAT1, STAT5),11 Jun N-terminal kinase/stress-activated protein kinases (JNK/SAPKs)12 and PI3K/protein kinase B (PKB).13 The contribution of these signals to the process of transformation is still not fully resolved, but the PI3K pathway plays an important role in regulation of TEL/PDGFβR-induced proliferation.13
We have taken a different approach to investigating the transforming activity of TEL/PDGFβR and have used a regulated expression system to express TEL/PDGFβR in interleukin 3 (IL-3)–dependent BaF/3 cells. We find TEL/PDGFβR expression partially protects cells from apoptosis on withdrawal of IL-3. But, rather than augmenting proliferation in response to IL-3, TEL/PDGFβR expression leads to a dramatic decrease in IL-3–induced proliferation, which we show is due to increased apoptosis. The extracellular signal–regulated kinase (ERK) and JNK signaling pathways appear to play a role in this response, because inhibition of either ERK, and to a lesser extent JNKs, leads to increased survival of TEL/PDGFβR-expressing cells in the presence of IL-3. These results have important implications for the in vivo functional responses of hematopoietic cells expressing the TEL/PDGFβR fusion protein.
Materials and methods
Generation of TEL/PDGFRβ pUHD10-3 constructs
Cell culture, generation, and selection of transfectants
Murine IL-3–dependent BaF/3 cells expressing the tetracycline transactivator (BaF/3-tTA), were a kind gift from Dr A. Mui (DNAX, Palo Alto, CA)16 and were cultured and electroporated (10 μg DNA at 960 μF, 450 V for 1 × 107 cells) as previously described.17 After a 48-hour recovery in media containing IL-3, cells were placed under selection in the presence of IL-3, 650 μg/mL hygromycin B (Calbiochem, Nottingham, United Kingdom), 1.5 μg/mL puromycin (Sigma, Poole, Dorset, United Kingdom), and 2 μg/mL tetracycline (Tet; Sigma). Clones were picked after 10 to 14 days, expanded in media containing IL-3, and screened for inducible expression of TEL/PDGFβR. Cells were washed 3 times in 1 × Hanks buffered saline solution (HBSS, Invitrogen, Paisley, United Kingdom), containing 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Invitrogen) and plated at 1 × 105 cells/mL in the absence or presence of 2 μg/mL Tet. After 24 hours, cells were washed once in phosphate-buffered saline (PBS; Invitrogen), lysed, and immunoblotted with 20 or 40 μg protein used per sample. Clones showing low basal and consistent inducible expression of the protein of interest were selected for further study.
To generate BaF/3 constitutively expressing TEL/PDGFβR, 107 cells were electroporated with 10 μg pcDNA3.1/TEL/PDGFβR.7 After 48 hours of recovery in media containing IL-3, cells were plated in either IL-3 plus 1 mg/mL G418 (Invitrogen) or with G418 alone. Clones were picked after 7 to 10 days and plates maintained for 4 weeks prior to final scoring.
Induction of expression in bulk cultures and cell stimulations
TEL/PDGFβR expression was induced over 24 hours as described (see “Cell culture, generation, and selection of transfectants”). IL-3 stimulations and cell lysis were carried out, as described previously.18 Protein concentrations were determined using the Bradford assay, according to the manufacturer's recommendations (Bio-Rad, Hemel Hempstead, Herts, United Kingdom) and equivalent amounts of protein used for immunoblotting.
Cytokine-dependent proliferation assay (XTT assays)
XTT (sodium 3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate; Sigma) bioreduction assays to assess cell growth in response to IL-3 were performed as previously described17,19 with doses of recombinant murine IL-3 (rmIL-3; 0.5 pg/mL to 2 ng/mL; R&D Systems, Abingdon, Oxfordshire, United Kingdom) set up in triplicate in RPMI (Invitrogen) in the presence or absence of 2 μg/mL Tet. Then, 25 μL of a solution containing 1 mg/mL XTT and 25 μM phenazine methosulfate (Sigma) was added per well for the final 4 hours of the 72-hour incubation. The soluble formazan product was measured at 450 nm. The PI3K inhibitor LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; Calbiochem),20 the MEK1/2 inhibitor U1026 (1,4-diamino-2,3-dicyano-1,4-bis(2-aminophynyltio)butadiene; Sigma),21 the JNK1/2 inhibitor SP600125 (anthra[1,9-cd]pyrazol-6(2H)-one; Biomol, Exeter, Devon, United Kingdom),22,23 and the p38 inhibitor SB202190 (4-[4-(4-fluorophenyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]phenol; Tocris, Avonmouth, United Kingdom)24 were dissolved in dimethyl sulfoxide (DMSO) and aliquots stored at –80°C.
Measurement of mitochondrial membrane potential
To measure the mitochondrial membrane potential (ΔΨM), transfectants were induced for 24 hours, washed 3 times in HBSS, and resuspended at 1 × 104 cells/mL in the presence or absence of Tet in RPMI containing either 0 or 20 pg/mL rmIL-3. Cells were incubated at 37°C for 24 to 48 hours, washed once in PBS, and incubated for 15 minutes in 10 nM 3′,3′-dihexyloxacarbocyanine iodide (DiOC6; Sigma).25-27 After one PBS wash, 10 000 events were analyzed by flow cytometry using a FACS Vantage System (Becton Dickinson, Oxford, United Kingdom) on parameter FL1.
Assessment of effect of inhibitors on TEL/PDGFβR kinase activity
Clones induced to express TEL/PDGFβR were washed and incubated with mitogen-activated protein kinase (MAPK) inhibitors or DMSO (Sigma) alone for 90 minutes. Levels of tyrosine phosphorylation of cellular proteins were analyzed by immunoblotting of cell lysates and TEL/PDGFβR autophosphorylation assessed following immunoprecipitation with 1 μg anti-PDGFβR antibody (06-498; Upstate Biotechnology, Botolph Clayton, Bucks, United Kingdom) as described previously.18 In vitro kinase assays were performed on immunoprecipitated TEL/PDGFβR in the presence or absence of MAPK inhibitors, as described,6 with the exception that radiolabeled adenosine triphosphate (ATP) was replaced with unlabeled ATP and phosphorylation assessed by antiphosphotyrosine immunoblotting.
SDS-PAGE and immunoblotting
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were carried out as described previously.28,29 Primary antibodies used were: antiphosphotyrosine antibody 4G10 at 0.1 μg/mL (05-321; Upstate Biotechnology); 1:1000 for antibodies against phosphorylated forms of ERK1 and ERK2 (9101; Cell Signaling Technology, Hitchin, Herts, United Kingdom), STAT5a/b (05-495; Upstate Biotechnology), and Ser473PKB (9271; Cell Signaling Technology) and 1:2000 for JNKs (9251; Cell Signaling Technology) and p38 MAPK (9211; Cell Signaling Technology); 0.5 μg/mL for anti-panSTAT5 (sc-835; Santa Cruz Biotechnology, Santa Cruz, CA), anti-panERK1 (sc-93; Santa Cruz Biotechnology), anti SHP-2 (sc-280; Santa Cruz Biotechnology), and anti-PDGFβR (06-498; Upstate Biotechnology); 1:1000 for anti-panp38 (9212, Cell Signaling Technology) and anti-panJNK (9252; Cell Signaling Technology). Secondary antibodies conjugated to horseradish peroxidase were used at 0.05 μg/mL (Dako, Glostrup, Denmark). Immunoblots were developed using enhanced chemiluminescence (ECL; Amersham Pharmacia, Amersham, United Kingdom) and Kodak X-AR 5 film (Eastman Kodak, Rochester, NY) and stripped and reprobed using standard conditions.30
Results
Generation of transfectants inducibly expressing TEL/PDGFβR
We wanted to investigate the coupling of TEL/PDGFβR-induced molecular signals to functional responses relating to transformation of hematopoietic cells, that is, proliferation and survival, and for this reason we chose to use the tetracycline-regulated expression system. This system allows the consequences of expressing a protein to be directly compared, within the same clone, to the situation where the protein is not expressed, thus overcoming many of the problems associated with clonal variation in constitutively expressing lines. We have successfully used this expression system in the laboratory to investigate coupling of signaling pathways to functional responses.17,31,32 Clones of BaF/3 cells expressing TEL/PDGFβR under the control of the tetracycline-sensitive transactivator, tTA, were generated. In the presence of Tet there is no expression and on the removal of Tet expression is induced. One hundred fifty antibiotic-resistant clones were obtained per transfection (selected in IL-3 and antibiotics) and 48 clones were screened for TEL/PDGFβR expression induced on removal of Tet. Fifty percent of clones screened demonstrated regulated expression of TEL/PDGFβR. We selected clones demonstrating low basal expression and reliable inducible expression. Clones expressing different maximal levels of TEL/PDGFβR on Tet removal (2 independent clones in each case) were used for further analyses, examples of which are shown in Figure 1A. The blot was reprobed with antibodies against SHP-2 to demonstrate equivalence of loading.
Inducible expression of TEL/PDGFβR leads to constitutive activation of signaling pathways. Cells were incubated for 24 hours in the presence (+) or in the absence (–) of 2 μg/mL Tet. (A) Samples were directly removed for immunoblotting. (B-C) Cells were either removed directly for immunoblotting (+ serum) or were washed 3 times with 1 × HBSS and serum starved for 1 hour prior to preparation of cell lysates (– serum). Twenty or 40 μg cell extract was immunoblotted using the following antibodies: panel A and lower part of panel B, a polyclonal antibody against PDGFβR that recognizes the TEL/PDGFβR fusion; panel B upper part, 4G10 to detect tyrosine-phosphorylated proteins; and panel C phosphospecific antibodies recognizing activated forms of STAT5, PKB, ERK, JNK, and p38. The same immunoblots were stripped and reprobed with anti-STAT5, anti-PKB, anti-ERK, anti-SHP-2, or anti-p38 antibodies as loading controls, respectively. For comparison of phospho-p38 in the presence of serum, see Figure 7.
Inducible expression of TEL/PDGFβR leads to constitutive activation of signaling pathways. Cells were incubated for 24 hours in the presence (+) or in the absence (–) of 2 μg/mL Tet. (A) Samples were directly removed for immunoblotting. (B-C) Cells were either removed directly for immunoblotting (+ serum) or were washed 3 times with 1 × HBSS and serum starved for 1 hour prior to preparation of cell lysates (– serum). Twenty or 40 μg cell extract was immunoblotted using the following antibodies: panel A and lower part of panel B, a polyclonal antibody against PDGFβR that recognizes the TEL/PDGFβR fusion; panel B upper part, 4G10 to detect tyrosine-phosphorylated proteins; and panel C phosphospecific antibodies recognizing activated forms of STAT5, PKB, ERK, JNK, and p38. The same immunoblots were stripped and reprobed with anti-STAT5, anti-PKB, anti-ERK, anti-SHP-2, or anti-p38 antibodies as loading controls, respectively. For comparison of phospho-p38 in the presence of serum, see Figure 7.
TEL/PDGFβR is a constitutively active tyrosine kinase, so we examined the effect of expressing TEL/PDGFβR on basal levels of tyrosine phosphorylated proteins both in the presence of serum and IL-3 and in cells washed and placed in serum/cytokine-free conditions for 1 hour. The results shown in Figure 1B demonstrate that expressing TEL/PDGFβR elevates the tyrosine phosphorylation of a number of proteins in BaF/3 cells, with similar results in the presence and absence of serum. TEL/PDGFβR associates constitutively with a number of signaling molecules7 and induces activation of specific signaling pathways.10-13 Given that we see increased tyrosine phosphorylation on expression of TEL/PDGFβR we examined if this also led to constitutive activation of downstream signaling pathways. Phospho-specific antibodies that recognize critical phosphorylation sites necessary for the activation of signaling molecules involved in the PI3K, STAT5, ERK, JNK, and p38 MAPK pathways were used to assess the level of activation of various signaling pathways in cells expressing TEL/PDGFβR. The results shown in Figure 1C demonstrate that expression of TEL/PDGFβR elevates signaling through all pathways examined, with similar effects observed in the presence and absence of serum. Consistent with previous reports10-13 we show activation of PKB, STAT5a/b, and JNK1/2 when TEL/PDGFβR is inducibly expressed in BaF/3 cells. We also demonstrate constitutive activation of the ERK and p38 MAPK pathways.
Expression of TEL/PDGFβR enhances cell survival in the absence of IL-3
Previous reports have documented that constitutive expression of TEL/PDGFβR confers factor independence to BaF/3 and 32D cells.5-7,10,13 We attempted on a number of occasions to culture the transfectants we derived in the absence of both Tet (so expressing TEL/PDGFβR) and IL-3. However, no cells grew in a factor-independent manner and within 7 days all cells were dead (data not shown). To examine if constitutive expression of TEL/PDGFβR could transform our BaF/3 cells to factor independence we expressed TEL/PDGFβR using the vector pcDNA3.17 and protocols similar to those used previously,7,33 with the exception that we derived individual clones. Following a 48-hour period of recovery in IL-3, half the transfected cells were plated in IL-3 plus G418, whereas the remaining transfectants were plated in the presence of G418 lacking IL-3. Thirty-three clones were obtained in the presence of IL-3 and G418, but no clones were obtained in G418 alone, even after prolonged selection (4 weeks). Fifty percent of clones screened from the IL-3 and G418 conditions expressed detectable levels of TEL/PDGFβR (Figure 2A), resulting in elevated tyrosine phosphorylation of cellular proteins (Figure 2B). The levels of TEL/PDGFβR expression were generally lower than the maximal levels we obtained using the Tet system (compare lanes 2 and 8 in Figure 2A). We performed viability assays to examine whether any of the clones constitutively expressing TEL/PDGFβR could grow in the absence of IL-3, examples of which are shown in Figure 2D. Eight days after IL-3 withdrawal only one clone (termed cTP14) contained viable cells. Although fewer than 2% of cTP14 cells remained alive, these survivors could be expanded in the absence of IL-3 over a further 2-week period. These factor-independent variants (termed cTP14FI) express TEL/PDGFβR (Figure 2E, lower panel) and exhibit enhanced levels of tyrosine phosphorylation of cellular proteins (Figure 2E, upper panel). Thus, our BaF/3 cells can become fully factor independent, but this appears to be a relatively rare event and requires constitutive expression of TEL/PDGFβR over a prolonged period.
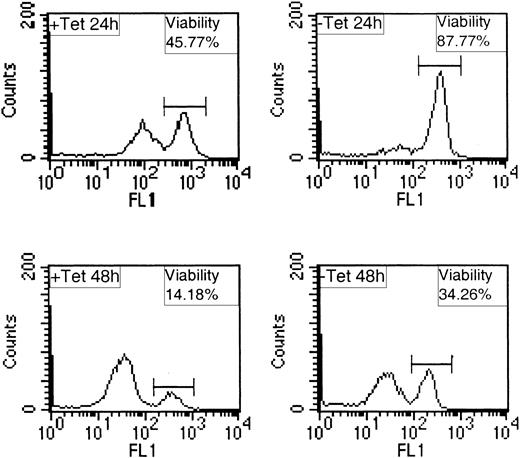
Constitutive expression of TEL/PDGFβR in BaF/3 cells. BaF/3 tTA cells were transfected with pcDNA3.1 encoding TEL/PDGFβR. (A) Immunoblot showing an example of expression of TEL/PDGFβR in antibiotic-resistant clones selected. (B) The same blot as in panel A was stripped and reprobed to detect tyrosine phosphorylated proteins. (C) The same blot as in panels A and B was stripped and reprobed with anti–SHP-2 antibodies to assess equality of loading. (D) Constitutively expressing clones cTP11, cTP14, and cTP15 or parental BaF/3 tTA were washed and incubated for 8 days in media lacking IL-3. The percentage of viable cells was determined at the times indicated using trypan blue exclusion. (E) BaF/3 tTA (BaF/3) or constitutively expressing factor-independent clone cTP14FI was incubated for 1 hour in the presence or absence of serum prior to preparation of cell lysates. Immunoblotting was performed with 4G10 to detect tyrosine phosphorylated proteins (pTyr) and the blot was stripped and reprobed to with anti-PDGFβR antibodies to detect TEL/PDGFβR.
Constitutive expression of TEL/PDGFβR in BaF/3 cells. BaF/3 tTA cells were transfected with pcDNA3.1 encoding TEL/PDGFβR. (A) Immunoblot showing an example of expression of TEL/PDGFβR in antibiotic-resistant clones selected. (B) The same blot as in panel A was stripped and reprobed to detect tyrosine phosphorylated proteins. (C) The same blot as in panels A and B was stripped and reprobed with anti–SHP-2 antibodies to assess equality of loading. (D) Constitutively expressing clones cTP11, cTP14, and cTP15 or parental BaF/3 tTA were washed and incubated for 8 days in media lacking IL-3. The percentage of viable cells was determined at the times indicated using trypan blue exclusion. (E) BaF/3 tTA (BaF/3) or constitutively expressing factor-independent clone cTP14FI was incubated for 1 hour in the presence or absence of serum prior to preparation of cell lysates. Immunoblotting was performed with 4G10 to detect tyrosine phosphorylated proteins (pTyr) and the blot was stripped and reprobed to with anti-PDGFβR antibodies to detect TEL/PDGFβR.
As an alternative functional response related to transformation, we investigated whether inducible expression of TEL/PDGFβR could extend cell survival in the absence of IL-3. To do this, we examined the mitochondrial membrane potentials of cells within a population using the indicator DiOC6. DiOC6 stains cells that have an intact mitochondrial membrane potential (viable cells) and weakly stains cells whose mitochondrial membrane potential is depolarized (apoptotic cells).27,32 We found that following IL-3 withdrawal, expression of TEL/PDGFβR led to a consistent, dramatic, and significant enhancement in cell survival. A representative experiment is presented in Figure 3, with average effects summarized in Table 1. At 24 hours following IL-3 withdrawal, cells maintained in Tet were approximately 50% viable, whereas those expressing TEL/PDGFβR were 82% viable. These results are consistent with the notion that expression of TEL/PDGFβR provides a survival signal to BaF/3 in the absence of IL-3.
Expression of TEL/PDGFβR protects BaF/3 from apoptosis following IL-3 withdrawal. BaF/3 clone TP2 was incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours, washed, and incubated for a further 24 or 48 hours (+ or – Tet) with no IL-3. Cells were stained for 15 minutes with 10 nM Di0C6 and 10 000 events were analyzed per sample by FACS on FL1 channel.
Expression of TEL/PDGFβR protects BaF/3 from apoptosis following IL-3 withdrawal. BaF/3 clone TP2 was incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours, washed, and incubated for a further 24 or 48 hours (+ or – Tet) with no IL-3. Cells were stained for 15 minutes with 10 nM Di0C6 and 10 000 events were analyzed per sample by FACS on FL1 channel.
Effect of expression of TEL/PDGFβR on viability of BaF/3 transfectants in the absence of IL-3
. | % viable cells . | . | |
|---|---|---|---|
| Conditions . | 24 h . | 48 h . | |
| + Tet (no TEL/PDGFβR) | 49.3 ± 3.3 | 11.4 ± 2.1 | |
| - Tet (TEL/PDGFβR) | 81.4 ± 2.5* | 33.9 ± 3.4† | |
. | % viable cells . | . | |
|---|---|---|---|
| Conditions . | 24 h . | 48 h . | |
| + Tet (no TEL/PDGFβR) | 49.3 ± 3.3 | 11.4 ± 2.1 | |
| - Tet (TEL/PDGFβR) | 81.4 ± 2.5* | 33.9 ± 3.4† | |
The average percentage of viable cells for each condition was calculated from data generated in 3 independent experiments using 2 independent TEL/PDGFβR-expressing clones. Means and SEMs are shown. Two-tailed paired Student t test was used to determine the significance between the samples with and without tetracycline at the same time points.
P < .01.
P < .02.
Expression of TEL/PDGFβR decreases cell proliferation and viability in the presence of IL-3
In light of these results, we reasoned that perhaps instead of TEL/PDGFβR rendering cells independent of an external growth stimulus (IL-3 in this case), expression of TEL/PDGFβR would have additive effects on cell growth in the presence of IL-3, such that there is a reduction in the dose of IL-3 required for a maximal proliferative signal. To investigate this we performed IL-3 dose-response assays with individual clones (expressing varying maximal levels of TEL/PDGFβR in the absence of Tet) and measured reduction of XTT as an indicator of metabolic activity relating to cell growth. To our surprise, rather than enhancing proliferation in response to IL-3, expression of TEL/PDGFβR led to a dramatic reduction in IL-3–induced proliferation as shown in Figure 4. This effect was most noticeable in clones expressing the highest levels of TEL/PDGFβR (Figure 4A and second independent clone, not shown), was lower in clones expressing an intermediate level of TEL/PDGFβR (Figure 4B), and not apparent in clones expressing low levels of TEL/PDGFβR (Figure 4C). Similar effects were observed when proliferation assays were performed in serum-free media (data not shown). This effect is specific to expression of TEL/PDGFβR because Tet does not affect the growth of BaF/3 transfected with empty response plasmid, shown in Figure 4D and previously.17 Interestingly, the proliferative response of the factor-independent line cTP14FI to IL-3 is considerably reduced when compared to parental BaF/3 cells (Figure 4E), consistent with the results shown in Figure 4A-B. The dose dependence of this response on TEL/PDGFβR levels can be observed when levels of TEL/PDGFβR expression are controlled by incubating in the presence of different Tet concentrations (Figure 4F). In the presence of Tet concentrations higher than 500 ng/mL expression is undetectable and cells proliferate well. Doses of less than 50 ng/mL Tet allow higher levels of expression that resulted in decreased proliferation.
Expression of TEL/PDGFβR dose dependently inhibits IL-3–induced proliferation. XTT bioreduction metabolic assays using 1000 cells/well were set up as described in “Materials and methods.” (A-D) The closed squares represent cells incubated in the presence of 2 μg/mL Tet; the closed diamonds represent cells incubated in the absence of Tet. The mean values with SDs are plotted for each point. In all cases readings obtained in the absence of IL-3 were on average 0.19 to 0.37 absorbance units. The inserts in each panel show the levels of TEL/PDGFβR expression 48 hours after induction. (E) IL-3 dose-response characteristics of constitutive TEL/PDGFβR clone cTP14FI compared with BaF/3 tTA. Errors were plotted but are too small to be visible for cTP14FI. (F) Clone TP2 was cultured with different Tet concentrations or no Tet as indicated and XTT bioreduction assay performed with 2 ng/mL IL-3. The inserted immunoblot demonstrates levels of expression of TEL/PDGFβR in this experiment.
Expression of TEL/PDGFβR dose dependently inhibits IL-3–induced proliferation. XTT bioreduction metabolic assays using 1000 cells/well were set up as described in “Materials and methods.” (A-D) The closed squares represent cells incubated in the presence of 2 μg/mL Tet; the closed diamonds represent cells incubated in the absence of Tet. The mean values with SDs are plotted for each point. In all cases readings obtained in the absence of IL-3 were on average 0.19 to 0.37 absorbance units. The inserts in each panel show the levels of TEL/PDGFβR expression 48 hours after induction. (E) IL-3 dose-response characteristics of constitutive TEL/PDGFβR clone cTP14FI compared with BaF/3 tTA. Errors were plotted but are too small to be visible for cTP14FI. (F) Clone TP2 was cultured with different Tet concentrations or no Tet as indicated and XTT bioreduction assay performed with 2 ng/mL IL-3. The inserted immunoblot demonstrates levels of expression of TEL/PDGFβR in this experiment.
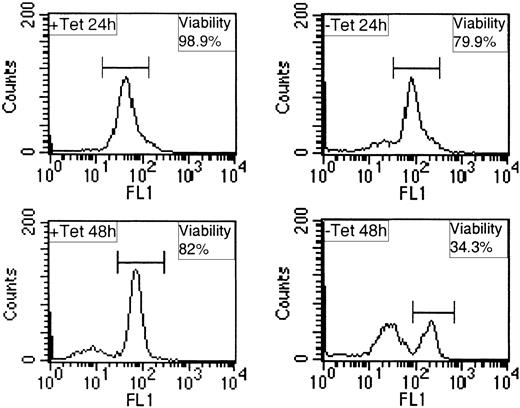
Given these rather surprising results we next examined the levels of viable and apoptotic cells in such populations by measuring mitochondrial membrane integrity using DiOC6 and flow cytometry. A dose of 20 pg/mL IL-3 was chosen for these analyses because it allows significant proliferation of BaF/3, but is 10-fold lower than the dose of IL-3 that induces maximal proliferation (Figure 4A). Representative data from these analyses are shown in Figure 5 and are summarized in Table 2. In the presence of Tet cell viability is high, on average 94% at 24 hours and 82% at 48 hours. When TEL/PDGFβR is expressed, on average the viability declined to 86% at 24 hours and to 38% at 48 hours. These results indicate that the in the presence of IL-3, expression of TEL/PDGFβR increases apoptosis of BaF/3, accounting for the effects we observe on IL-3–induced proliferation. These results are in complete contrast to the protective effect expression of TEL/PDGFβR has on BaF/3 survival following IL-3 withdrawal and suggest the presence of cytokine is modulating the functional response to TEL/PDGFβR.
Expression of TEL/PDGFβR increases apoptosis in the presence of IL-3. BaF/3 clone TP2 was incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours, washed, and incubated for a further 24 or 48 hours (+ or – Tet) with 20 pg/mL IL-3. Cells were stained for 15 minutes with 10 nM Di0C6 and 10 000 events were analyzed per sample by FACS on channel FL1.
Expression of TEL/PDGFβR increases apoptosis in the presence of IL-3. BaF/3 clone TP2 was incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours, washed, and incubated for a further 24 or 48 hours (+ or – Tet) with 20 pg/mL IL-3. Cells were stained for 15 minutes with 10 nM Di0C6 and 10 000 events were analyzed per sample by FACS on channel FL1.
Effect of expression of TEL/PDGFβR on viability of BaF/3 transfectants in the presence of 20 pg/mL IL-3
. | % viable cells . | . | |
|---|---|---|---|
| Conditions . | 24 h . | 48 h . | |
| + Tet (no TEL/PDGFβR) | 94.8 ± 0.8 | 82.0 ± 4.4 | |
| - Tet (TEL/PDGFβR) | 86.1 ± 3.1* | 37.9 ± 9.4† | |
. | % viable cells . | . | |
|---|---|---|---|
| Conditions . | 24 h . | 48 h . | |
| + Tet (no TEL/PDGFβR) | 94.8 ± 0.8 | 82.0 ± 4.4 | |
| - Tet (TEL/PDGFβR) | 86.1 ± 3.1* | 37.9 ± 9.4† | |
The average percentage of viable cells for each condition was calculated from data generated in 4 independent experiments using 2 independent TEL/PDGFβR-expressing clones. Mean and SEMs are shown. Two-tailed paired Student t test was used to determine the significance between the samples with and without Tet at the same time points.
P < .05.
P < .01.
Combined effects of TEL/PDGFβR expression and IL-3 on intracellular signaling cascades
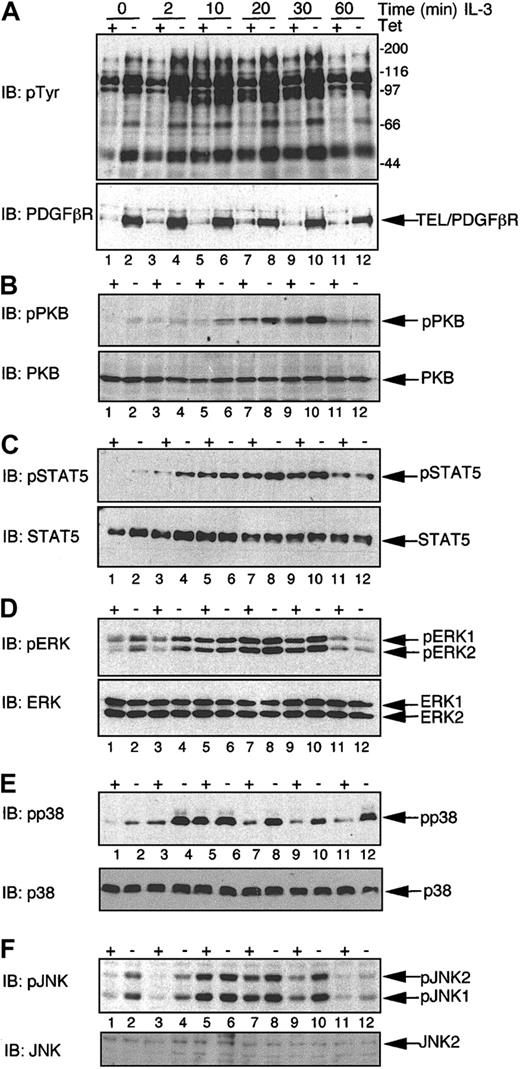
We were interested in defining the signaling cascades regulating the apparently opposing functional effects of TEL/PDGFβR in the absence and presence of IL-3. Transfectants were induced to express TEL/PDGFβR by removal of Tet or were maintained in the presence of Tet for control purposes. Cells were washed and deprived of serum and cytokine for 1 hour prior to stimulation with or without IL-3 for differing periods of time and cell extracts prepared. We have shown previously that the presence of Tet does not affect IL-3–induced phosphorylation on tyrosine, PKB, or ERKs.17,31 Expression of TEL/PDGFβR augmented IL-3–induced tyrosine phosphorylation (Figure 6A). We then used the same phosphospecific antibodies to examine the effects that a combination of TEL/PDGFβR and IL-3 has on activation of key signaling pathways. TEL/PDGFβR expression enhances the phosphorylation of Ser473 of PKB induced by IL-3 (Figure 6B, upper panel). Similarly, TEL/PDGFβR expression enhances STAT5a/b phosphorylation observed at early times following IL-3 treatment (compare lanes 3 and 4 in Figure 6C). We also examined effects on levels of activation of the MAPK family of serine/threonine kinases, as shown in Figure 6D-F. Expression of TEL/PDGFβR enhanced phosphorylation of all MAPKs at early times following IL-3 stimulation (2-minute time points, compare lanes 3 and 4 in each panel). In the case of ERK phosphorylation, TEL/PDGFβR expression appears to augment the maximal levels of ERK1/2 phosphorylation seen in response to IL-3. In the case of p38 and JNK, maximal levels of phosphorylation induced by IL-3 stimulation (observed at 10 minutes) are similar in the presence and absence of TEL/PDGFβR expression. However, TEL/PDGFβR prolongs this phosphorylation, leading to a more sustained activation of both p38 and JNK than is normally observed for IL-3 stimulation. Therefore, TEL/PDGFβR appears to enhance the responsiveness of BaF/3 to IL-3 and prolongs activation of members of the MAPK family.
Expression of TEL/PDGFβR augments IL-3–induced signaling cascades. BaF/3 clone TP2 was incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours. Cells were washed free of IL-3 and serum and incubated in serum-free media for 1 hour prior to being stimulated for the indicated period of time (in minutes) with 10 ng/mL rmIL-3 or left untreated (0). Whole cell lysates were prepared and 20 or 40 μg protein/sample was immunoblotted (IB) with the following antibodies: (A) Upper panel, 4G10 specific for tyrosine-phosphorylated proteins and in the lower panel anti-PDGFβR. (B) Antiphospho Ser473 of PKB (pPKB, upper panel); this blot was stripped and reprobed with a pan-PKB antibody (PKB, lower panel). (C) Antibody specific for phosphotyrosine 694/699 of STAT5a/b (pSTAT5, upper panel); this immunoblot was stripped and reprobed with an anti-STAT5 antibody to assess loading (STAT5, lower panel). (D) An antibody specific for dually phosphorylated ERK 1 and 2 (pERK1/2, upper panel); this blot was stripped and reprobed with an anti-panErk antibody (ERK, lower panel). (E) An antibody specific for dually phosphorylated p38 MAPK (pp38); this blot was stripped and reprobed with an anti-panp38 antibody (p38, lower panel). (F) An antibody specific for dually phosphorylated JNK1 and 2 (pJNK); this blot was stripped and reprobed with an anti-panJNK antibody (JNK2, lower panel). Positions of the phosphorylated and nonphosphorylated proteins are indicated, as are the molecular weight standards, in kilodaltons.
Expression of TEL/PDGFβR augments IL-3–induced signaling cascades. BaF/3 clone TP2 was incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours. Cells were washed free of IL-3 and serum and incubated in serum-free media for 1 hour prior to being stimulated for the indicated period of time (in minutes) with 10 ng/mL rmIL-3 or left untreated (0). Whole cell lysates were prepared and 20 or 40 μg protein/sample was immunoblotted (IB) with the following antibodies: (A) Upper panel, 4G10 specific for tyrosine-phosphorylated proteins and in the lower panel anti-PDGFβR. (B) Antiphospho Ser473 of PKB (pPKB, upper panel); this blot was stripped and reprobed with a pan-PKB antibody (PKB, lower panel). (C) Antibody specific for phosphotyrosine 694/699 of STAT5a/b (pSTAT5, upper panel); this immunoblot was stripped and reprobed with an anti-STAT5 antibody to assess loading (STAT5, lower panel). (D) An antibody specific for dually phosphorylated ERK 1 and 2 (pERK1/2, upper panel); this blot was stripped and reprobed with an anti-panErk antibody (ERK, lower panel). (E) An antibody specific for dually phosphorylated p38 MAPK (pp38); this blot was stripped and reprobed with an anti-panp38 antibody (p38, lower panel). (F) An antibody specific for dually phosphorylated JNK1 and 2 (pJNK); this blot was stripped and reprobed with an anti-panJNK antibody (JNK2, lower panel). Positions of the phosphorylated and nonphosphorylated proteins are indicated, as are the molecular weight standards, in kilodaltons.
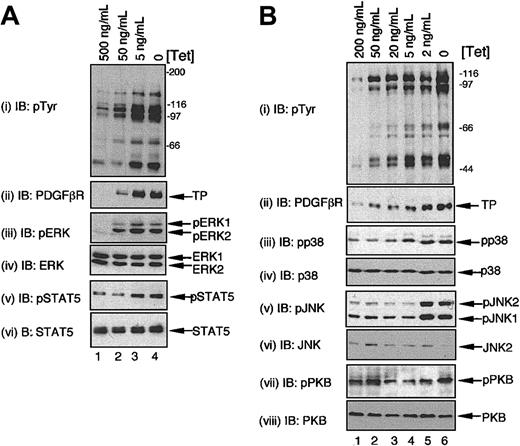
We were interested in examining whether the combined signals of TEL/PDGFβR and IL-3 led to increases in the steady-state levels of activity of these signaling cascades, because this would more closely reflect the situation in growing cells. Therefore, cells were grown in the presence of IL-3 and induced to express TEL/PDGFβR at different levels by varying the concentration of Tet. Cell lysates were prepared directly from these cultures, without any starvation step. Steady-state levels of phosphorylation of STAT5, ERK1/2 (Figure 7A), JNK1/2, p38 MAPKs, and to a much lesser extent PKB (Figure 7B) were all increased in cells grown in IL-3 and expressing TEL/PDGFβR compared to the levels in cells growing in IL-3, but expressing no or very low levels of TEL/PDGFβR. Thus, the sustained high-level activation of these pathways observed in cells receiving the combined signals of IL-3 and TEL/PDGFβR correlates with the detrimental effects we have observed on proliferation and survival.
Expression of TEL/PDGFβR augments steady-state levels of MAPK signaling pathways in cells grown in IL-3. BaF/3 clone TP2 was cultured in optimal levels of IL-3 for 24 hours in the presence of different concentrations of Tet or with no Tet as indicated and samples prepared directly. Panels A and B represent independent experiments. Immunoblotting was performed to assess the activation status of STAT5, PKB, and the different MAPKs using phosphospecific antibodies as described in Figure 6. The same immunoblots were reprobed as indicated to assess equality of loading.
Expression of TEL/PDGFβR augments steady-state levels of MAPK signaling pathways in cells grown in IL-3. BaF/3 clone TP2 was cultured in optimal levels of IL-3 for 24 hours in the presence of different concentrations of Tet or with no Tet as indicated and samples prepared directly. Panels A and B represent independent experiments. Immunoblotting was performed to assess the activation status of STAT5, PKB, and the different MAPKs using phosphospecific antibodies as described in Figure 6. The same immunoblots were reprobed as indicated to assess equality of loading.
Activation of MAPKs is involved in the decrease in cell viability observed with the combination of IL-3 and TEL/PDGFβR-induced signaling
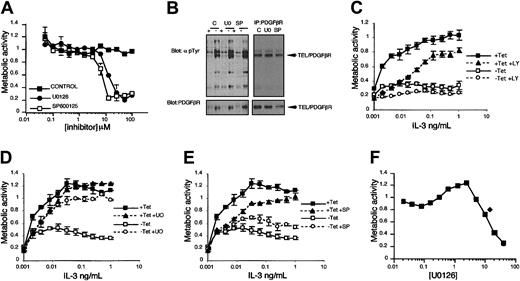
We wanted to investigate whether the augmented activation of the signaling pathways we identified are functionally related to the effects on cell proliferation and survival we observe when cells receive the combination of TEL/PDGFβR and IL-3–induced signals. Therefore, we made use of a number of small molecule inhibitors that target key components of these signaling cascades. First, we investigated whether inhibition of any of these pathways could rescue the detrimental effect that TEL/PDGFβR has on cell proliferation in the presence of IL-3. IL-3 dose-response assays were performed in the presence and absence of Tet and in the presence and absence of inhibitors. We carefully chose doses of inhibitors we determined to give submaximal inhibition (Figure 8A), that did not affect the kinase activity of TEL/PDGFβR (Figure 8B and data not shown), and that were consistent with doses used in previously published studies.22-24,34,35 Inhibition of the PI3K/PKB pathway with 5 μM LY294002 did not reverse the effects of TEL/PDGFβR expression on proliferation induced by IL-3; rather, incubation with LY294002 reduced proliferation still further, as shown in Figure 8C. Incubation with 5 μM U0126, which suppresses activation of MEK1/2,24 had little effect on proliferation in the presence of Tet at the highest doses of IL-3, but did reduce proliferation at lower doses of IL-3. Interestingly, this dose of U0126 led to a significant reversal of the inhibitory effect of TEL/PDGFβR expression, such that proliferation was almost restored to levels seen in cells with Tet (Figure 8D). U0126 (10 μM) also reversed the inhibitory effect of TEL/PDGFβR expression on IL-3–induced proliferation and similar effects were observed when the unrelated MEK inhibitor PD98059 was used (data not shown). Interestingly, incubation of the factor-independent clone cTP14FI with U0126 led to an increase in IL-3–driven proliferation, supporting a role for ERKs in the inhibitory effects observed (Figure 8F). Inhibition of JNK1/2, by incubation with 5 μM of the selective inhibitor SP600125,22,23 caused a modest decrease in proliferation of cells maintained in Tet at lower levels of IL-3 (Figure 8E). A modest recovery in proliferation was observed in cells expressing TEL/PDGFβR at this dose of SP600125. Inhibition of p38 activity with SB202190 did not lead to any recovery in proliferation in cells expressing TEL/PDGFβR (data not shown). These results indicate that the enhanced signaling observed through the ERK pathway and possibly the JNK cascade, but not via p38 MAPKs, contribute to the inhibitory effects on BaF/3 proliferation when cells receive a TEL/PDGFβR signal in combination with IL-3.
Inhibition of ERK and JNK MAPKs, but not PI3Ks, partially reverses the inhibitory effect of expression of TEL/PDGFβR on IL-3–induced proliferation. XTT bioreduction metabolic assays were set up as described in “Materials and methods” using 1000 (A,C-E) or 3000 (F) cells/well. (A) BaF/3 tTA cells were cultured in 1 ng/mL rmIL-3 and doses of inhibitors indicated and an XTT was performed. (B) Tyrosine phosphorylation of cellular proteins and TEL/PDGFβR (upper panels) were assessed in cells incubated for 1 hour with DMSO alone (C), 10 μM U0126 (UO), or 5 μM SP600125 (SP). Blots were reprobed with anti-PDGFβR antibodies (lower panels); note SP lane is underloaded. (C-E) BaF/3 clone TP2 was used. ▪ represents cells incubated in the presence of 2 μg/mL Tet; ▴, cells incubated in the presence of 2 μg/mL Tet and either (C) 5 μM LY294002, (D) 5 μM U0126, or (E) 5 μM SP600125. □ indicates cells incubated in the absence of Tet; ○, cells incubated in the absence of Tet and either (C) 5 μM LY294002, (D) 5 μM U0126, or (E) 5 μM SP600125. In all cases readings obtained in the absence of IL-3 were on average 0.19 to 0.32 absorbance units. (F) cTP14FI cells were incubated with 500 pg/mL IL-3 alone (♦) or with different doses of U0126 (▪). The mean values with SDs are plotted for each point.
Inhibition of ERK and JNK MAPKs, but not PI3Ks, partially reverses the inhibitory effect of expression of TEL/PDGFβR on IL-3–induced proliferation. XTT bioreduction metabolic assays were set up as described in “Materials and methods” using 1000 (A,C-E) or 3000 (F) cells/well. (A) BaF/3 tTA cells were cultured in 1 ng/mL rmIL-3 and doses of inhibitors indicated and an XTT was performed. (B) Tyrosine phosphorylation of cellular proteins and TEL/PDGFβR (upper panels) were assessed in cells incubated for 1 hour with DMSO alone (C), 10 μM U0126 (UO), or 5 μM SP600125 (SP). Blots were reprobed with anti-PDGFβR antibodies (lower panels); note SP lane is underloaded. (C-E) BaF/3 clone TP2 was used. ▪ represents cells incubated in the presence of 2 μg/mL Tet; ▴, cells incubated in the presence of 2 μg/mL Tet and either (C) 5 μM LY294002, (D) 5 μM U0126, or (E) 5 μM SP600125. □ indicates cells incubated in the absence of Tet; ○, cells incubated in the absence of Tet and either (C) 5 μM LY294002, (D) 5 μM U0126, or (E) 5 μM SP600125. In all cases readings obtained in the absence of IL-3 were on average 0.19 to 0.32 absorbance units. (F) cTP14FI cells were incubated with 500 pg/mL IL-3 alone (♦) or with different doses of U0126 (▪). The mean values with SDs are plotted for each point.
Inhibition of MAPKs increases cell survival of cells receiving an IL-3 and TEL/PDGFβR signal
These results indicate that ERK and possibly JNK MAPKs play roles in transducing the signals that lead to the decrease in proliferation observed when cells receive signals from both IL-3 and TEL/PDGFβR. We wanted to determine whether these same pathways were also responsible for the increase in apoptosis we observe when TEL/PDGFβR is expressed in the presence of IL-3. Transfectants were induced to express TEL/PDGFβR for 18 hours and then incubated in 20 pg/mL IL-3 in the presence or absence of Tet and 10 μM U0126 or 5 μM SP600125 and samples were taken at 24 and 48 hours. DiOC6 staining and flow cytometry were used to quantify the number of viable and apoptotic cells. Representative traces are shown in Figure 9 and summarized in Figure 9D. Reflecting the alterations in proliferation observed when signaling via ERKs or JNKs were reduced, both U0126 and SP600125 reversed the detrimental effect the combination of expression of TEL/PDGFβR and IL-3 had on cell viability. In the presence of TEL/PDGFβR expression, on average, approximately 38% of cells were viable after 48 hours. Incubation of TEL/PDGFβR-expressing cells with U0126 increased the number of viable cells to, on average, 52% (P ≤ .0005). Interestingly, U0126 had the opposite effect on cell viability in cells incubated in IL-3 that had not been induced to express TEL/PDGFβR. Here, we observed a decrease in cell viability from 93% to 75% (P ≤ .005). When the JNK inhibitor, SP600125, was added to the cells, the number of viable cells increased to, on average, 48% (P ≤ .005). These results strongly imply ERK and also possibly JNK MAPK family members in transduction of proapoptotic signals in the context of signaling through IL-3 and TEL/PDGFβR-stimulated pathways, but also that MAPK pathways are important for proliferation in response to IL-3 alone, consistent with previous reports.31,36
Inhibition of ERK and JNK MAPKs partially protects cells from apoptosis induced by the combination of IL-3 and TEL/PDGFβR signals. Cells were incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours, washed, and incubated for a further 24 or 48 hours (+ or – Tet) with (A) 20 pg/mL IL-3, (B) 20 pg/mL IL-3 with 10 μM U0126, or (C) 20 pg/mL IL-3 with 5 μM SP600125. Cells were stained for 15 minutes with 10 nM Di0C6 and 10 000 events analyzed per sample by FACS on channel FL1. (D) Bar chart summarizes the effects of TEL/PDGFβR expression on IL-3–induced survival in the presence of U0126 and SP600125. Data from 4 independent experiments using clones TP2 and TP15 were used. Mean and SEM are plotted for each treatment and 2-tailed paired Student t tests were applied to the data and values of significance indicated (P).
Inhibition of ERK and JNK MAPKs partially protects cells from apoptosis induced by the combination of IL-3 and TEL/PDGFβR signals. Cells were incubated in the presence (+ Tet) or absence (– Tet) of 2 μg/mL Tet for 24 hours, washed, and incubated for a further 24 or 48 hours (+ or – Tet) with (A) 20 pg/mL IL-3, (B) 20 pg/mL IL-3 with 10 μM U0126, or (C) 20 pg/mL IL-3 with 5 μM SP600125. Cells were stained for 15 minutes with 10 nM Di0C6 and 10 000 events analyzed per sample by FACS on channel FL1. (D) Bar chart summarizes the effects of TEL/PDGFβR expression on IL-3–induced survival in the presence of U0126 and SP600125. Data from 4 independent experiments using clones TP2 and TP15 were used. Mean and SEM are plotted for each treatment and 2-tailed paired Student t tests were applied to the data and values of significance indicated (P).
Discussion
We have investigated the coupling of molecular signaling pathways activated by the oncogenic fusion protein, TEL/PDGFβR, to functional responses associated with transformation primarily using a system allowing regulated expression of TEL/PDGFβR. We observed 2 different responses of the transfectants, which were dependent on the absence or presence of IL-3. Expression of TEL/PDGFβR partially protected cells from apoptosis following withdrawal of IL-3 and this correlated with the constitutive activation of a number of intracellular signaling pathways. Somewhat surprisingly, we made the novel observation that TEL/PDGFβR expression did not augment IL-3–induced proliferation, but rather caused a dramatic reduction in growth that was dependent on the level of TEL/PDGFβR expressed. Increased apoptosis accounted for this decrease in proliferation and hyperactivation of the ERK cascade by the combined signals of TEL/PDGFβR and IL-3 was involved in this response. These observations have important implications in the pathogenesis of CMML.
The dramatic effects we observe on proliferation and survival when cells receive the combined signals from TEL/PDGFβR and IL-3 have not been previously reported because studies have focused largely on the use of factor-independent derivatives. Our experimental evidence shows that cells receiving both TEL/PDGFβR and IL-3 signals demonstrate increased short-term and steady-state levels of activation of ERK1/2, JNK1/2, and p38 MAPKs. Treatment with suboptimal doses of the 2 independent MEK inhibitors, U0126 or PD98059, increased proliferation and survival of cells receiving combined TEL/PDGFβR and IL-3 signals, suggesting that a reduction in ERK activity is sufficient to alter the balance between proapoptotic and growth-promoting signals. This situation is reminiscent of the effects of constitutively active Ras. A transient and relatively weak activation of Ras, and thus ERKs, is coupled to cell proliferation, whereas constitutive activation of Ras throughout the cell cycle has been linked to a proapoptotic response, via MAPK-dependent pathways.37,38 In T lymphocytes, expression of constitutively active Ras results in increased apoptosis, most likely via up-regulation of FasL.39 In other situations, constitutive activation of Ras leads to increased expression of cyclin-dependent kinase inhibitors, p21WAF1 and p16INK4a, leading to cell cycle arrest. This apoptotic response may act as a protective mechanism against uncontrolled high levels of Ras/MAPK signals37 and our results are consistent with this hypothesis. ERK activation has not been detected in factor-independent variants of either 32D or BaF/3 expressing TEL/PDGFβR.33 One potential explanation for the difference in our results is that the process of transformation to factor independence selects against cells maintaining ERK activation. We have shown that chronic hyperactivation of this pathway contributes to apoptosis, hence TEL/PDGFβR constitutive expressors may need to down-regulate the ERK pathway to survive, meaning ERK activation would not be detectable.
The functional role of JNKs in survival versus apoptosis is controversial and may well be a cell-type dependent response.40 Suboptimal doses of SP600125 modestly reversed the inhibitory effect that the combination of TEL/PDGFβR and IL-3 had on proliferation and cell survival, although not to such a great extent as inhibition of MEKs. Interestingly, spontaneous apoptosis of approximately 15% of cells constitutively expressing TEL/PDGFβR has been reported, which is reduced to 5% when JNK activation was impaired by expression of a dominant-negative form of MKK4.12 Our findings are consistent with an involvement of JNKs in apoptosis resulting from expression of TEL/PDGFβR. However, the functional involvement of JNKs downstream of other oncogenic fusion proteins appears distinct. JNKs appear to be involved in BCR-ABL–mediated transformation,41 whereas JNKs play no role in transformation by TEL-JAK2.35
Although the inhibition of ERKs, and to some extent JNKs, reversed the effects that combined signaling through TEL/PDGFβR and IL-3 had on proliferation and survival, we cannot exclude the possibility that augmented STAT5 activation also plays a functional role. Because inhibition of ERKs only leads to a partial recovery in survival in cells receiving combined IL-3 and TEL/PDGFβR signals, it is possible that the constitutive activation of STAT5 we observe also contributes to this apoptotic response. The report that a constitutively active form of STAT5 can transform BaF/3 to factor independence and in the presence of IL-3 these cells undergo apoptosis42 supports this possibility.
In contrast to previous reports describing the generation of factor-independent cells resulting from expression of TEL/PDGFβR,6,7,10,12,33 using Tet-regulated expression of TEL/PDGFβR we have been unable to derive factor-independent lines from our clones, despite TEL/PDGFβR affording a transient protection from apoptosis on IL-3 withdrawal. All previous studies constitutively expressed TEL/PDGFβR, selected transfectants in IL-3 and antibiotics for 10 to 14 days, and then derived cells able to grow without IL-3.6,7,10,12,33 We have ruled out the possibility that our BaF/3 cells behave differently, because using the same expression vector and conditions described in Carroll et al,7 we have generated cells constitutively expressing TEL/PDGFβR and derived a factor-independent variant from one of the 8 clones tested. After 8 days without IL-3, viable cells, representing less than 2% of the cell population, remained in the cTP14 clone, with factor-independent cells derived over the next 2 to 3 weeks. Thus, short-term TEL/PDGFβR activity (Tet-regulated model) enhanced survival on cytokine withdrawal, whereas prolonged exposure to TEL/PDGFβR (constitutive expression) was necessary for factor independence, which occurred at low frequency. This is reminiscent of earlier work with a conditional BCR/ABL variant, where short-term BCR/ABL activity only provided a survival signal to factor-dependent cells.43 Prolonged exposure (10-30 days) to BCR/ABL was required for full transformation, suggesting a requirement for additional genetic events.43 Factor-independent BaF/3 variants have been derived from cells inducibly expressing BCR/ABL,44 but if continuous expression of TEL/PDGFβR is required for full factor independence, then the short-term (2-4 days) expression we can achieve in our high- and intermediate-expressing inducible clones, before they initiate apoptosis (2 days onward), may be insufficient to allow for transformation. We report that inducible expression of TEL/PDGFβR leads to activation of a similar complement of signaling pathways to those reported previously in constitutively expressing cells,10-13 suggesting an underlying difference in these cells. Evidence indicates that BCR/ABL may be involved in genomic instability44 that could contribute to secondary genetic changes, facilitating transformation. Based on our evidence and that previously reported, we propose that TEL/PDGFβR may act via a similar mechanism.
It is not clear how the levels of TEL/PDGFβR we have expressed in BaF/3 correspond to levels in cells of patients with CMML and it will be important to compare the effects of regulated expression of TEL/PDGFβR in other cell models and experimental systems to examine if effects similar to those we report here are observed. However, our results have important potential implications for how we view disease progression. The hematopoietic environment in the bone marrow exposes cells to many different soluble and cell-bound cytokines; thus, cells will always be in contact with a variety of growth factors. Cells expressing high levels of TEL/PDGFβR may sense a stress signal when receiving signals from both TEL/PDGFβR and cytokines, which results in apoptosis, removing the “damaged” cells. In this situation, the oncogenic fusion proteins would have to down-regulate responses to cytokines to enable leukemic transformation. Alternatively, lower levels of TEL/PDGFβR expression may be tolerated and enable cells to survive for longer periods of time and gradually increase in number, which would resemble the chronic phase of CMML. Over time, these longer-lived cells could acquire additional genetic alterations, giving them a significant growth advantage, leading to full transformation and eventually an acute leukemia. Our findings that the functional outcome of TEL/PDGFβR expression can be modulated by the presence of cytokines has important implications for future studies investigating leukemic transformation.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-09-2974.
Supported by a Medical Research Council research training fellowship (H.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr M. Carroll for the pcDNA3 containing the TEL/PDGFβR cDNA.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal