Abstract
The CX3C chemokine fractalkine (CX3CL1) exists as a membrane-expressed protein promoting cell-cell adhesion and as a soluble molecule inducing chemotaxis. Transmembrane CX3CL1 is converted into its soluble form by defined proteolytic cleavage (shedding), which can be enhanced by stimulation with phorbol-12-myristate-13-acetate (PMA). PMA-induced CX3CL1 shedding has been shown to involve the tumor necrosis factor-α–converting enzyme (TACE), whereas the constitutive cleavage in unstimulated cells remains elusive. Here we demonstrate a role of the closely related disintegrin-like metalloproteinase 10 (ADAM10) in the constitutive CX3CL1 cleavage. The hydroxamate GW280264X, capable of blocking TACE as well as ADAM10, proved to be an effective inhibitor of the constitutive and the PMA-inducible CX3CL1 cleavage in CX3CL1-expressing ECV-304 cells (CX3CL1–ECV-304), whereas GI254023X, preferentially blocking ADAM10 but not TACE, reduced the constitutive cleavage only. Overexpression of ADAM10 in COS-7 cells enhanced constitutive cleavage of CX3CL1 and, more importantly, in murine fibroblasts deficient of ADAM10 constitutive CX3CL1 cleavage was markedly reduced. Thus, ADAM10 contributes to the constitutive shedding of CX3CL1 in unstimulated cells. Addressing the functional role of CX3CL1 shedding for the adhesion of monocytic cells via membrane-expressed CX3CL1, we found that THP-1 cells adhere to CX3CL1–ECV-304 cells but detach in the course of vigorous washing. Inhibition of ADAM10-mediated CX3CL1 shedding not only increased adhesive properties of CX3CL1–ECV-304 cells but also prevented de-adhesion of bound THP-1 cells. Our data demonstrate that ADAM10 is involved in the constitutive cleavage of CX3CL1 and thereby may regulate the recruitment of monocytic cells to CX3CL1-expressing cell layers.
Introduction
Leukocyte recruitment to inflammatory sites involves a sequence of adhesive events that are mediated by different classes of adhesion molecules expressed on the endothelium and the leukocytes.1 Whereas adhesion molecules of the selectin family usually contribute to the rolling of leukocytes under flow, members of the integrin family are involved in establishing a stable shear-resistant cell adhesion. Chemokines are thought to play a role in modulating cell adhesion by inducing shedding of L-selectin and by increasing functional integrins on the leukocyte surface. Thus, besides acting as chemoattractants in the tissue, chemokines can promote the transition from an early to a late adhesion type in the course of leukocyte recruitment.
Within the chemokine family a transmembrane molecule termed CX3C chemokine ligand 1 (CX3CL1), or fractalkine, has been identified that by itself induces adhesion.2 CX3CL1 is encoded as a 95-kDa multidomain molecule consisting of a chemokine domain linked to a transmembrane domain via a mucin-rich stalk. The chemokine is expressed on endothelial cells,2 epithelial cells,3,4 smooth muscle cells,5,6 dendritic cells,7,8 neurons,9,10 and macrophages.11 In vitro, CX3CL1 induces cell adhesion by interaction with its receptor CX3CR1 expressed on monocytes, T cells, mast cells, and natural killer cells.2,12-14 This adhesion does not require signaling of the receptor, is resistant to physiologic shear flow, and is independent of extracellular calcium.2,15,16 Besides its activity as an adhesion molecule, CX3CL1 can be cleaved from the cell membrane to produce a soluble 80-kDa molecule that induces chemotaxis of CX3CR1-expressing leukocytes.2 In vivo, upregulation of CX3CL1 has been found in atherosclerotic blood vessels,6,11 rejected transplants,17 inflamed rheumatoid joints,18,19 psoriatic skin,20,21 injured liver,22 inflamed kidney,23 and ischemia reperfusion–injured brain.24 Neutralization of CX3CL1 in animal models revealed that the chemokine is important for promoting transplant rejection17 and glomerulonephritis.25 No obvious phenotype was found in mice with targeted disruption of the Cxcl1orthe Cx3cr1 gene,26,27 but in models of cardiac allograft rejection and cerebral ischemia reperfusion injury CX3CR1-deficient animals were less susceptible to the disease.28,29 Evidence for a role of CX3CL1 in atherosclerosis emerges from 2 linked single-nucleotide polymorphisms found in the Cx3cr1 gene resulting in a valine to isoleucine and a threonine to methionine shift at positions 249 and 280, respectively. The latter polymorphism is associated with a significant reduction of CX3CR1-dependent cell-cell adhesion under physiologic flow,30 and both correlate with a reduced risk of atherosclerosis in heterozygote individuals.31 Together these reports reveal a role of CX3CL1 and its receptor in promoting vascular inflammation where the chemokine may either act as a chemoattractant or adhesion molecule depending on its proteolytic processing.
Proteolytic release from the cell surface, also termed ectodomain shedding, has been observed for a number of cytokines, growth factors, adhesion molecules, and their receptors (reviewed by Herren,32 Mullberg et al,33 Schlondorff and Blobel,34 and Black35 ). Shedding is believed to constitute an important regulatory mechanism for cellular signaling by either reducing the amount of distinct receptor proteins present on the cell surface leading to reduced cellular responsiveness to selected stimuli or by releasing soluble ectodomains of growth factors and cytokines capable of stimulating other cells. The ectodomain shedding may occur constitutively in unstimulated cells, but in many cases it is enhanced by stimulation with the protein kinase C agonist, phorbol-12-myristate-13-acetate (PMA). The 2 types of shedding are therefore referred to as constitutive and PMA-inducible shedding, respectively. Disintegrin-like metalloproteinases (ADAMs) have been implicated in the constitutive as well as in the PMA-inducible ectodomain shedding of several surface-expressed molecules. TNF-α–converting enzyme (TACE or ADAM17) was the first member of this protease family for which a role in ectodomain shedding has been found.36,37 As shown with fibroblast cell lines generated from Tace–/– mice, this enzyme mediates PMA-stimulated shedding of tumor necrosis factor-α (TNF-α), TNF-α receptors I and II, transforming growth factor-α, and interleukin-6 (IL-6) receptor.35 Of the other more than 30 ADAMs that have been identified, ADAM10/Kuzbanian has highly significant sequence homology with TACE. ADAM10 as well as TACE have been implicated in the shedding of the amyloid precursor protein (APP)38 and cellular prion protein (PrP).39,40 Furthermore, ADAM10 was found to be involved in the constitutive ectodomain release of L1 adhesion molecule.41 There is evidence that metalloproteinases of the ADAM family also play a role in the shedding of CX3CL1. Both the constitutive and the PMA-inducible shedding of CX3CL1 can be blocked by broad-spectrum metalloproteinase inhibitors such as batimastat.42 Using Tace–/– and Adam9–/– fibroblast cell lines, the PMA-inducible shedding of CX3CL1 has been shown to involve TACE43,44 but not ADAM9.43 However, deletion of either of the 2 ADAMs did not affect the constitutive cleavage. Hence, the metalloproteinase responsible for constitutive CX3CL1 cleavage remains unknown.
The aim of this study was to investigate the mechanism of constitutive CX3CL1 shedding and to elucidate its role for CX3CL1-mediated leukocyte adhesion. We here provide multiple lines of evidence that the constitutive shedding involves the activity of the disintegrin-like metalloproteinase ADAM10 by inhibition experiments with specific metalloproteinase inhibitors, by overexpression of the enzyme, and by using ADAM10-deficient fibroblasts. We show that this cleavage of the chemokine plays a functional role by regulating adhesion and detachment of monocytic cells to CX3CL1-expressing cell layers.
Materials and methods
Cytokines, antibodies, enzymes, inhibitors, and vectors
Recombinant human extracellular domain CX3CL1; unconjugated, biotinylated, or phycoerythrin-conjugated monoclonal antibodies to human CX3CL1 (clones 81506, 51637, and 51637.11, respectively) as well as phycoerythrin-conjugated mouse immunoglobulin G1 (IgG1) isotype control (clone 11711.11) were obtained from R&D Systems (Wiesbaden, Germany). The antiserum against CX3CL1 was raised in rabbits and characterized previously.45 The rabbit antiserum B42.1 was generated by immunizing rabbits with a peptide corresponding to the carboxy-terminal amino acid residues 1 to 17 of murine ADAM10.38
CX3CL1 was cloned into the expression vector pcDNA3.1 (Invitrogen, Karlsruhe, Germany) as previously described. The cloning and functional expression of bovine carboxy-terminally hemagglutinin (HA)–tagged ADAM10 and bovine carboxy-terminally Flag-tagged dominant-negative ADAM10 mutant with a single amino acid substitution from Glu to Ala at position 384 (Glu384Ala) was reported previously.39 Both constructs had been inserted into pcDNA3.
Metalloproteinase inhibitors
GW280264 ((2R,3S)-3-(formyl-hydroxyamino)-2-(2-methyl-1-propyl) hexanoic acid [(1S)-5-benzyloxycarbamoylamino-1-(1,3-thiazol-2-ylcarbamoyl)-1-pentyl] amide) and GI254023 ((2R,3S)-3-(formyl-hydroxyamino)-2-(3-phenyl-1-propyl) butanoic acid [(1S)-2,2-dimethyl-1-methylcarbamoyl-1-propyl] amide) were synthesized as described in US patents US 6 172 064, US 6 191 150, and US 6 329 400.
The compounds were assayed for inhibition of recombinant human TACE and ADAM10 ectodomains (R&D Systems). TACE activity was measured using streptavidin-coated scintillation proximity assay (SPA) beads and a biotinylated peptide corresponding to the cleavage site in pro–TNF-α as described previously.46,47 Assays were performed at room temperature with 200 nM substrate (biotin-SPLAQAVRSSSRTP(3H)S-NH2) in 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, pH 7.5, containing 0.0015% Brij-35. Reactions were stopped by addition of streptavidin-SPA beads (Amersham, Freiburg, Germany) in EDTA (ethylenediaminetetraacetic acid) and uncleaved substrate measured by scintillation counting. ADAM10 activity was assayed under essentially identical conditions except that the substrate used was DNP-SPLAQAVRSSSR-NH2. After quenching with 1% heptafluorobutyric acid, the reaction products and uncleaved substrate were separated and quantified by reverse-phase high-performance liquid chromatography (HPLC) on a C18 column with absorbance measured at 350 nm. Inhibitor dose-response curves were generated using an 11-point, 3-fold serial dilution series of the inhibitors. Percentage inhibition versus log of inhibitor concentration was plotted to determine the inhibitor concentration leading to half maximal inhibition (IC50).
Recombinant human tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1 and TIMP-2) were obtained from R&D Systems and controlled for inhibition of gelatinase activity mediated by matrix metalloproteinase-2 (MMP-2).
Cell culture and transfection
The human monocytic leukemia cell line THP-1 was cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and antibiotics at a density between 2 × 105 and 1 × 106 cells per milliliter (all media and reagents were from Invitrogen if not otherwise stated). The adherent human cell line ECV-304 was stably transfected with CX3CL1 (CX3CL1–ECV-304) and characterized previously.42 CX3CL1–ECV-304 cells were cultured in M199 medium supplemented with 10% FCS, antibiotics, and 500 μg/mL geneticin up to 90% confluence before subculture. Cryopreserved human aortic smooth muscle cells (SMCs) were obtained from BioWhittaker (Berkshire, United Kingdom), cultured, and costimulated with interferon-γ (IFN-γ) and TNF-α (both 20 ng/mL) for 24 hours to express CX3CL1 as described.5
The primate fibroblast cell line COS-7 was cultured in Dulbecco modified Eagle medium (DMEM) containing 10% FCS and antibiotics. For cotransfection of COS-7 cells with CX3CL1 and either ADAM10, dominant-negative ADAM10 (Glu384Ala), or empty vector, cells were grown to 70% confluence in 60-cm2 dishes (Greiner, Frickenhausen, Germany). The medium was replaced with 5 mL fresh medium containing 75 μM chloroquin; 5 μg of either vector construct was mixed with 260 μg 2-diethyl-aminoethyl-dextran in 500 μL medium and then added to each dish. Cells were incubated for 5 hours and then exposed to fresh medium containing 10% dimethylsulfoxide (DMSO) for 6 minutes. Cells were washed with phosphate-buffered saline (PBS) and cultured in full medium for 48 hours, before expression and cleavage of CX3CL1 were investigated.
Simian virus large T antigen (SV-T)–immortalized mouse embryonic fibroblast (MEF) cell lines from Adam10–/– mice and respective wild-type animals were generated and characterized as described elsewhere.38,40 Cells were cultured in DMEM containing 10% FCS. For transfection with CX3CL1, cells were seeded at 1 × 104/cm2 in 6-well dishes (Costar, Koolhaven, the Netherlands) and incubated for 24 hours to 70% confluence. The medium was replaced with 1 mL fresh medium. Each well received 2 μg CX3CL1 pcDNA3 preincubated with 3 μL liposomal transfection reagent (FuGENE; Roche, Mannheim, Germany) in 100 μL serum-free medium for 15 minutes. Cells were cultured for 48 hours and subsequently assayed for their capacity to cleave membrane-bound CX3CL1. All transient transfections were performed in triplicates for each stimulatory condition. In all experiments uptake of the vector was controlled by transfection with green fluorescent protein (GFP) in pcDNA3.1 in parallel and subsequent detection of expressed GPF by fluorescence microscopy.
CX3CL1 cleavage assays
CX3CL1-expressing cells were grown to 70% to 90% confluence in complete medium in 6-well dishes (Costar) or 60-cm2 dishes (Greiner) for 48 hours before stimulation. The cells were washed with PBS, and FCS-free medium containing metalloproteinase inhibitors was added. After 10 minutes, the cells were stimulated with PMA (200 ng/mL) for various times. The conditioned media were harvested, and a protease inhibitor cocktail (Complete; Roche) was added according to the instructions of the manufacturer. The supernatants were centrifuged and, if necessary, concentrated 10-fold using 10-kDa cutoff filtration units (Vivaspin; Vivascience, Hannover, Germany). The presence of released CX3CL1 in the conditioned media was demonstrated by Western blotting and quantified by enzyme-linked immunosorbent assay (ELISA). The cells were washed with 2 mL PBS and removed from the vessel by scraping in 1 mL ice-cold PBS. CX3CL1 surface expression on intact cells was determined by flow cytometry. To quantify cell-associated CX3CL1 by ELISA, the cells were centrifuged and resuspended in 500 μL PBS containing 0.1% Triton X-100 and a protease inhibitor cocktail (Complete; Roche). After 30 minutes of incubation on ice under agitation, lysates were centrifuged at 12 000g for 10 minutes.
CX3CL1-specific enzyme-linked immunosorbent assay
A 96-well plate (Microlon; Greiner) was coated overnight with 2 mg/mL mouse anti-CX3CL1 (clone 81506) in 50 mM Na2CO3 (pH 9.3), subsequently washed 3 times with PBS with 0.05% Tween (PBS-T), and blocked with PBS-T containing 2% BSA for 2 hours. Samples (50 μL per well) were added and the plate incubated at room temperature (RT) for 2 hours. A standard prepared as 8 serial 1:2 dilutions of 3.9 nM full-size CX3CL1 in either medium or cell lysis buffer was run in parallel. Following washing, 300 ng/mL biotinylated anti-CX3CL1 monoclonal antibody (clone 51637) in PBS-T containing 1% BSA was added to each well and the plate incubated at RT for 1 hour. After washing, 100 mU/mL streptavidinperoxidase (POD) conjugate (Roche) in PBS-T containing 1% BSA was added followed by 1 hour of incubation at RT. After washing, chromogenic POD substrate (BM Blue; Roche) was added. The reaction was stopped after 20 minutes of incubation at RT by addition of 1.8 M H2SO4 before the optical density was determined at 490 nm. The detection limit of the ELISA was 60 pM extracellular domain CX3CL1.
SDS-PAGE and Western blotting
Concentrated media samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (7.5% Tris [tris(hydroxymethyl)-aminomethane]–glycine gel) and transferred onto polyvinylidene difluoride membranes (Hybond-P; Amersham). The membranes were incubated in blocking buffer (PBS containing 5% milk powder) at room temperature for 1 hour and probed for 1 hour with dilutions of rabbit anti–HA-tag IgG (1:500, 0.4 μg/mL) or rabbit antiserum to human CX3CL1 (1:1000) or murine ADAM10 (1:10 000) in blocking buffer. After 3 washes with PBS-T, the membranes were incubated with a horseradish peroxidase–linked goat serum against rabbit Ig (Amersham) diluted 1:10 000 in PBS-T for 1 hour. After 3 washes, detection of bound antirabbit Ig was carried out using enhanced chemiluminescence substrate (ECLplus, Amersham). Signals were recorded by exposure to x-ray film (BioMax MS; Kodak, Rochester, NY) or by using a luminescent image analyzer (Fujifilm Image reader, LAS1000; Tokyo, Japan) and image analyzer software (AIDA 3.2.1; Raytest, Staubenhardt, Germany).
Flow cytometric analysis
Adherent cells were harvested from culture flasks by treatment with ice-cold PBS for 10 minutes, subsequent scraping, and centrifugation. The cells were fixed in 0.5 mL of 4% paraformaldehyde (PFA; Sigma) in PBS for 10 minutes on ice, washed with PBS/0.01%NaN3, and incubated at 2 × 106 cells per milliliter with a phycoerythrin (PE)–conjugated monoclonal antibody to CX3CL1 or a PE-conjugated IgG1 isotype control (both at 1.25 μg/mL in PBS with 0.1% BSA and 0.01% NaN3) for 1 hour on ice. Following 2-fold washing, cells were suspended in PBS containing 2% PFA. The fluorescence signal of the labeled cells was then analyzed by flow cytometry (FACScan; Becton Dickinson, Heidelberg, Germany) and calculated as median fluorescence intensity (MFI) of the cell population.
Cell adhesion assay
Wild-type and CX3CL1-expressing ECV-304 cells were seeded at 6 × 104 cells per well into 24-well dishes (Microlon; Greiner). The cells were cultured to full confluence in M199 medium containing 10% FBS. To investigate the effect of CX3CL1 shedding on the adhesive properties of the cells, shedding was induced by stimulation with PMA (200 ng/mL) for 1 hour in the absence or presence of metalloproteinase inhibitors for 1 hour. Subsequently, the cells were assayed for adhesion of fluorescently labeled THP-1 cells. For fluorescent labeling, cultured THP-1 cells were suspended at 2 × 106/mL in PBS/0.1% BSA and incubated with 2.5 μM fluorescent dye (calcein am; Molecular Probes, Leiden, the Netherlands) at 37°C for 30 minutes. Excess dye was removed by washing with 50 mL PBS. THP-1 cells were resuspended in a mixture of 50% RPMI 1640 medium and 50% M199 medium containing 0.1% BSA and added to the ECV-304 cells at 3 × 105 cells per well. The THP-1 cells were rapidly seeded onto the ECV-304 cell layer by centrifugation for 3 minutes at 100g. The plate was incubated at 37°C for 20 minutes and then washed repeatedly by inversion and by addition of PBS (1 mL per well), followed by inversion. After each wash step, the fluorescence signal from the adherent cells was measured using a fluorescence plate reader (Lambda Fluoro 230; MWG Biotech, Munich, Germany) at excitation wavelength of 480 nm and emission wavelength of 530 nm.
To investigate de-adhesion, THP-1 cells were loaded onto a CX3CL1-expressing ECV-304 cell layer. The adherent cells were gently washed by rinsing with 1 mL PBS followed by inversion of the plate, and subsequently fluorescence was determined. The cells were then incubated in 0.5 mL RPMI/0.1% BSA in the presence and absence of metalloproteinase inhibitors under constant agitation. After 15 and 30 minutes, those cells that detached from the ECV-304 cell layer were removed by washing with 1 mL PBS and inversion of the plate. The remaining adherent cells were quantified by fluorescence measurement.
Statistical analysis
Data were statistically analyzed using the unpaired 2-tailed t test. Two populations of data were considered significantly different at a P value less than .05.
Results
Constitutive as well as inducible cleavage mechanisms contribute to shedding of CX3CL1 in ECV-304 cells
In our first experiments we studied constitutive and inducible cleavage of CX3CL1 in ECV-304 cells that were stably transfected with CX3CL1. These cells (CX3CL1–ECV-304) have been used in several studies since the discovery of CX3CL1 to investigate its cleavage and function in cell adhesion.2,42,43,45 As shown in Figure 1A, unstimulated CX3CL1–ECV-304 cells constitutively release soluble CX3CL1 into the culture medium detectable as a single protein band in Western blot analysis using a CX3CL1-specific antiserum. The apparent molecular size of 80 kDa matched well with the size of cleaved CX3CL1 that we have found in other cell types such as endothelial cells and smooth muscle cells.5,45 Cell stimulation with PMA resulted in an enhanced release of CX3CL1 (Figure 1A). The increase in cleavage is defined as inducible shedding. One hour of stimulation was sufficient to detect a considerable increase in CX3CL1 shedding, and this increase was not notably different after 3 hours of stimulation. In untreated as well as in PMA-treated cells, the release of soluble CX3CL1 was clearly suppressed by the broad-spectrum metalloproteinase inhibitor batimastat (Figure 1B). The appearance of shed CX3CL1 was associated with a reduced expression of the transmembrane variant on the cell surface as demonstrated by immunofluorescent staining of surface-expressed CX3CL1 and subsequent flow cytometry (Figure 1C, upper histogram). One hour of treatment with PMA induced a considerable reduction in CX3CL1 surface expression (55% reduction of MFI compared with untreated control). In the presence of batimastat, constitutive and inducible shedding were blocked, leading to increased surface expression of CX3CL1, irrespectively of whether the cells were left unstimulated or stimulated with PMA (102% and 98% increase of MFI compared with untreated control receiving no batimastat; Figure 1C). In other terms, two thirds of the total shedding occurs constitutively and one third is inducible by PMA, suggesting that constitutive shedding may play a significant role.
Constitutive and inducible CX3CL1 cleavage in CX3CL1–ECV-304 cells. (A) PMA enhances the release of soluble CX3CL1 from CX3CL1–ECV-304 cells: cells were stimulated with 200 ng/μL PMA or left unstimulated for different lengths of time. Conditioned media were harvested, concentrated 10-fold, and analyzed by Western blotting using a specific antiserum against CX3CL1. (B) The broad-spectrum metalloproteinase (MP) inhibitor batimastat blocks constitutive and inducible CX3CL1 cleavage: CX3CL1–ECV-304 cells were treated with 20 μM batimastat or vehicle control (dimethylsulfoxide [DMSO]) and subsequently stimulated with 200 ng/mL PMA or left unstimulated. After 3 hours, conditioned media were harvested and analyzed for the presence of soluble CX3CL1. (C) Effect of PMA and batimastat on CX3CL1 surface expression: CX3CL1–ECV-304 cells were treated with inhibitor and stimulated with PMA as described in panel B and subsequently investigated for the surface expression of CX3CL1 by flow cytometry using a fluorescently labeled monoclonal antibody to CX3CL1. For each condition the median fluorescence intensity was calculated and given as mean and SD of triplicate determinations (values in parentheses). Unspecific antibody binding was evaluated with a fluorescently labeled IgG1 control and was not modulated by treatment with PMA or batimastat (MFI = 6 ± 1; not shown). Results shown in panels A-C are representative for 3 independent experiments.
Constitutive and inducible CX3CL1 cleavage in CX3CL1–ECV-304 cells. (A) PMA enhances the release of soluble CX3CL1 from CX3CL1–ECV-304 cells: cells were stimulated with 200 ng/μL PMA or left unstimulated for different lengths of time. Conditioned media were harvested, concentrated 10-fold, and analyzed by Western blotting using a specific antiserum against CX3CL1. (B) The broad-spectrum metalloproteinase (MP) inhibitor batimastat blocks constitutive and inducible CX3CL1 cleavage: CX3CL1–ECV-304 cells were treated with 20 μM batimastat or vehicle control (dimethylsulfoxide [DMSO]) and subsequently stimulated with 200 ng/mL PMA or left unstimulated. After 3 hours, conditioned media were harvested and analyzed for the presence of soluble CX3CL1. (C) Effect of PMA and batimastat on CX3CL1 surface expression: CX3CL1–ECV-304 cells were treated with inhibitor and stimulated with PMA as described in panel B and subsequently investigated for the surface expression of CX3CL1 by flow cytometry using a fluorescently labeled monoclonal antibody to CX3CL1. For each condition the median fluorescence intensity was calculated and given as mean and SD of triplicate determinations (values in parentheses). Unspecific antibody binding was evaluated with a fluorescently labeled IgG1 control and was not modulated by treatment with PMA or batimastat (MFI = 6 ± 1; not shown). Results shown in panels A-C are representative for 3 independent experiments.
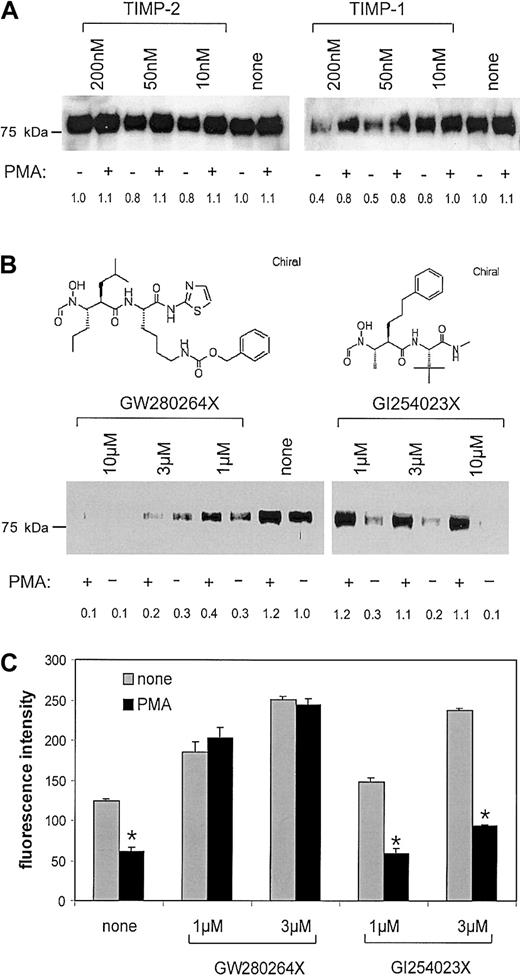
Metalloproteinase inhibitors that discriminate between ADAM10 and TACE differentially block constitutive and inducible shedding of CX3CL1
Knowing that the generation of soluble CX3CL1 is mediated by defined proteolysis that is sensitive to batimastat, we tested the ability of the natural metalloproteinase inhibitors TIMP-1 and TIMP-2 to block CX3CL1 cleavage. As seen by Western blotting of the conditioned media, TIMP-2 did not block the release of CX3CL1 from CX3CL1–ECV-304 cells. TIMP-1, however, blocked the generation of soluble CX3CL1 to a considerable degree in a concentration-dependent manner (Figure 2A). Densitometric quantification of the effect revealed that TIMP-1 had a more pronounced effect on the constitutive cleavage in the unstimulated cells than on the total cleavage in the PMA-stimulated cells (60% inhibition versus 36% inhibition). Interestingly, the relative contribution of PMA-mediated shedding to the total shedding activity was found to be even higher in the presence than in the absence of TIMP-1 (10% versus 50%). This observation suggests that the constitutive cleavage is more sensitive to TIMP-1 inhibition than that induced by PMA. Both TIMP-1 and TIMP-2 are known to block a number of membrane-type and soluble MMPs. TIMP-1, however, also blocks ADAM10, which is not affected by TIMP-2.48 No other member of the ADAM family is affected by any of the 2 TIMPs. We therefore reasoned that ADAM10 could be a candidate of constitutive CX3CL1 cleavage.
Effect of MP inhibitors on CX3CL1 cleavage in CX3CL1–ECV-304. (A-B) MP inhibitors differentially block constitutive and PMA-inducible release of the CX3CL1 ectodomain: CX3CL1–ECV-304 cells were treated with various concentrations of the natural metalloproteinase inhibitors TIMP-1 or TIMP-2 (A) or hydroxamate inhibitors (mixed ADAM10/TACE inhibitor GW280264X or selective ADAM10 inhibitor GI254023X) (B). Subsequently, cells were stimulated with 200 ng/mL PMA or left unstimulated. Generation of soluble CX3CL1 was analyzed by Western blotting of the conditioned media using a specific antiserum against CX3CL1. One representative of 3 independent experiments is shown. The CX3CL1-specific signal intensity for each lane was quantified by densitometry and expressed in relation to the control representing CX3CL1 released from unstimulated cells in the absence of inhibitor. (C) MP inhibitors differentially restore CX3CL1 surface expression: CX3CL1–ECV-304 cells were left unstimulated or stimulated with 200 ng/mL PMA in the presence and absence of mixed ADAM10/TACE inhibitor GW280264X or selective ADAM10 inhibitor GI254023X (both 1 and 3 μM). After 1 hour CX3CL1 surface expression was assayed by flow cytometry, and the CX3CL1-specific fluorescence signal was recorded as median fluorescence intensity (MFI) and calculated as mean and SD of triplicates performed in a representative of 3 independent experiments.
Effect of MP inhibitors on CX3CL1 cleavage in CX3CL1–ECV-304. (A-B) MP inhibitors differentially block constitutive and PMA-inducible release of the CX3CL1 ectodomain: CX3CL1–ECV-304 cells were treated with various concentrations of the natural metalloproteinase inhibitors TIMP-1 or TIMP-2 (A) or hydroxamate inhibitors (mixed ADAM10/TACE inhibitor GW280264X or selective ADAM10 inhibitor GI254023X) (B). Subsequently, cells were stimulated with 200 ng/mL PMA or left unstimulated. Generation of soluble CX3CL1 was analyzed by Western blotting of the conditioned media using a specific antiserum against CX3CL1. One representative of 3 independent experiments is shown. The CX3CL1-specific signal intensity for each lane was quantified by densitometry and expressed in relation to the control representing CX3CL1 released from unstimulated cells in the absence of inhibitor. (C) MP inhibitors differentially restore CX3CL1 surface expression: CX3CL1–ECV-304 cells were left unstimulated or stimulated with 200 ng/mL PMA in the presence and absence of mixed ADAM10/TACE inhibitor GW280264X or selective ADAM10 inhibitor GI254023X (both 1 and 3 μM). After 1 hour CX3CL1 surface expression was assayed by flow cytometry, and the CX3CL1-specific fluorescence signal was recorded as median fluorescence intensity (MFI) and calculated as mean and SD of triplicates performed in a representative of 3 independent experiments.
Screening of a panel of hydroxamate-based metalloproteinase inhibitors for their ability to inhibit CX3CL1 shedding further suggested the involvement of ADAM10 in the constitutive cleavage (data not shown). From this panel of different hydroxamate-based metalloproteinase inhibitors, 2 compounds that differed in their capacity to block the activities of the 2 disintegrin-like metalloproteinases TACE (ADAM17) and ADAM10 were selected to further characterize CX3CL1 cleavage. As shown in Table 1, compound GW280264X potently blocked both recombinant enzymes. Compound GI254023X, however, possessed comparable inhibitory potency for ADAM10 only and blocked TACE with more than 100-fold reduced potency. In the following text, the 2 compounds are referred to as mixed TACE/ADAM10 inhibitor and selective ADAM10 inhibitor, respectively. The 2 inhibitors were tested for their ability to block CX3CL1 shedding in CX3CL1–ECV-304 cells. Expecting less inhibition in the cell-based assay compared with the activity measured with the recombinant enzymes, we tested concentrations from 1 to 10 μM. As shown in Figure 2B, the mixed TACE/ADAM10 inhibitor blocked the CX3CL1 cleavage in unstimulated as well as in PMA-stimulated cells in a concentration-dependent manner. The selective ADAM10 inhibitor blocked the constitutive cleavage in unstimulated cells with a similar potency as the mixed TACE/ADAM10 inhibitor but hardly affected the cleavage in PMA-treated cells. Thus, the constitutive shedding of CX3CL1 was potently blocked by both compounds, whereas the PMA-inducible cleavage was prevented by the mixed TACE/ADAM10 inhibitor only.
Potency of metalloproteinase inhibitors used in the current study for inhibition of recombinant TACE and ADAM10 ectodomain
. | IC50, nM* . | . | Selectivity . | |
|---|---|---|---|---|
| Compound . | TACE . | ADAM10 . | ADAM10 potency/TACE potency† . | |
| GI254023X | 541.0 | 5.3 | 102.0 | |
| GW280264X | 8.0 | 11.5 | 1.4 | |
. | IC50, nM* . | . | Selectivity . | |
|---|---|---|---|---|
| Compound . | TACE . | ADAM10 . | ADAM10 potency/TACE potency† . | |
| GI254023X | 541.0 | 5.3 | 102.0 | |
| GW280264X | 8.0 | 11.5 | 1.4 | |
The inhibitor concentration leading to half maximal inhibition (IC50) of recombinant TACE or ADAM10 ectodomain was determined as described in “Materials and methods.”
Potency is defined as 1/IC50.
This was further confirmed by analyzing the effect of the inhibitors on the surface expression of CX3CL1 (Figure 2C). As detected by flow cytometry, 1 hour of treatment with either of the 2 inhibitors increased CX3CL1 expression on the surface of CX3CL1–ECV-304 cells in a concentration-dependent manner. In the absence of inhibitors, PMA stimulation decreased the level of CX3CL1 molecules on the cell surface (53% reduced MFI). This effect of PMA was completely blocked by the mixed TACE/ ADAM10 inhibitor but not by the selective ADAM10 inhibitor. To investigate whether the inhibitors would have a similar effect on the cleavage of endogenous CX3CL1 in primary cells, human aortic smooth muscle cells were costimulated with IFN-γ and TNF-α (20 ng/mL) for 24 hours, resulting in surface expression of CX3CL1.5 Subsequently, cells were treated with or without PMA for 1 hour in the presence and absence of inhibitors (3 and 10 μM). Flow cytometric analysis revealed that the selective ADAM10 inhibitor preferentially blocked the constitutive cleavage in primary SMCs in a similar concentration range as in CX3CL1–ECV-304 cells (data not shown). In summary, the differential inhibition of constitutive and PMA-inducible CX3CL1 shedding in cell lines as well as in primary cells implies 2 separate mechanisms of CX3CL1 shedding that differ in the metalloproteinase(s) involved. The inhibition data suggest that TACE is responsible for the inducible but not for the constitutive cleavage. The latter type of cleavage seems to involve ADAM10.
Overexpression of ADAM10 enhances constitutive CX3CL1 cleavage
To further examine the role of ADAM10 in CX3CL1 cleavage we decided to overexpress ADAM10 together with CX3CL1 in COS-7 cells. To control whether the shedding process in COS-7 cells was similar to that seen in ECV-304 cells, we first investigated the effect of the 2 inhibitors on the shedding in COS-7 cells transfected with CX3CL1 only (Figure 3A). As expected, the selective ADAM10 inhibitor GI254023X preferentially blocked constitutive (IC50 approximately 1 μM) but not PMA-inducible shedding, whereas the mixed TACE/ADAM10 inhibitor GW280264X blocked both types of cleavage in the CX3CL1-transfected COS-7 cells (IC50 approximately 1 μM for both). Notably, both inhibitors did not completely abrogate constitutive CX3CL1 cleavage. Even the broad-spectrum inhibitor of metalloproteinases batimastat (20 μM) blocked the cleavage by only 84% (data not shown), and therefore a minor involvement of a shedding mechanism not mediated by metalloproteinases cannot be excluded. Nevertheless, these data demonstrate that the major proportion of the constitutive cleavage in COS-7 cells is indeed due to metalloproteinase-mediated proteolysis and that ADAM10 seems to be involved. To explore this, HA-tagged ADAM10 was coexpressed with CX3CL1 in COS-7 cells. The presence of the tagged ADAM10 in its precursor as well as in its mature form with apparent molecular sizes of 90 kDa and 65 kDa, respectively, was demonstrated by Western blotting using an antibody against the HA-tag (Figure 3B). As shown in Figure 3C, overexpression of ADAM10 in COS-7 cells enhanced constitutive cleavage of CX3CL1 by 77% compared with the empty vector control. Soluble CX3CL1 released from ADAM10-overexpressing cells was detectable by Western blotting as a single protein band of about 80 kDa that corresponded well to the size of soluble CX3CL1 generated in the presence of endogenous proteases only (data not shown). Conversely, overexpression of the dominant-negative ADAM10 (Glu384Ala) reduced constitutive cleavage of CX3CL1. The effect of the dominant-negative mutant was only moderate (26% reduction compared with the vehicle control) but significant. The increase in CX3CL1 cleavage upon PMA stimulation was not affected by transfection of ADAM10 or dominant-negative ADAM10 (Glu384Ala). Taken together, these data indicated that ADAM10 is capable of cleaving CX3CL1 and may be relevant for the constitutive but not for the PMA-inducible cleavage of the chemokine.
Constitutive CX3CL1 cleavage is enhanced in COS-7 cells overexpressing ADAM10. (A) MP inhibitors differentially restore constitutive and PMA-inducible CX3CL1 shedding in COS-7 cells: COS-7 cells transiently transfected with CX3CL1 were treated with various concentrations of the mixed ADAM10/TACE inhibitor GW280264X or the selective ADAM10 inhibitor GI254023X and subsequently stimulated with 200 ng/mL PMA or left unstimulated. After 1 hour of incubation conditioned media were harvested and the concentration of soluble CX3CL1 was determined by ELISA (mean and SD, n = 3). (B) Overexpression of ADAM10 protein in COS-7 cells. Cells were transiently transfected with bovine HA-tagged ADAM10 or empty vector (vehicle). Subsequently, cells were lysed in SDS sample buffer and analyzed by Western blotting using a specific antiserum against the HA-tag. (C) CX3CL1 cleavage in COS-7 cells overexpressing wild-type (WT) ADAM10 or dominant-negative (DN) ADAM10 (Glu384Ala): COS-7 cells were cotransfected with CX3CL1 and either HA-tagged WT ADAM10, DN ADAM10, or empty vector. After 1 hour of incubation in the presence or absence of 200 ng/mL PMA, the concentration of released CX3CL1 in the conditioned media was determined by ELISA. Results are presented as mean and SD of triplicates performed in one experiment. Compared with the vehicle control, transfection of WT ADAM10 significantly increased CX3CL1 shedding, whereas transfection of DN ADAM10 significantly reduced the cleavage of the chemokine (P < .05 for both effects, indicated by the asterisks). The increase in shedding due to PMA stimulation (determined as difference in shedding between unstimulated and PMA-stimulated cells) was not significantly altered by transfection with either construct (P < .05, not indicated). The results shown in panels A-C are representative for 3 independent experiments.
Constitutive CX3CL1 cleavage is enhanced in COS-7 cells overexpressing ADAM10. (A) MP inhibitors differentially restore constitutive and PMA-inducible CX3CL1 shedding in COS-7 cells: COS-7 cells transiently transfected with CX3CL1 were treated with various concentrations of the mixed ADAM10/TACE inhibitor GW280264X or the selective ADAM10 inhibitor GI254023X and subsequently stimulated with 200 ng/mL PMA or left unstimulated. After 1 hour of incubation conditioned media were harvested and the concentration of soluble CX3CL1 was determined by ELISA (mean and SD, n = 3). (B) Overexpression of ADAM10 protein in COS-7 cells. Cells were transiently transfected with bovine HA-tagged ADAM10 or empty vector (vehicle). Subsequently, cells were lysed in SDS sample buffer and analyzed by Western blotting using a specific antiserum against the HA-tag. (C) CX3CL1 cleavage in COS-7 cells overexpressing wild-type (WT) ADAM10 or dominant-negative (DN) ADAM10 (Glu384Ala): COS-7 cells were cotransfected with CX3CL1 and either HA-tagged WT ADAM10, DN ADAM10, or empty vector. After 1 hour of incubation in the presence or absence of 200 ng/mL PMA, the concentration of released CX3CL1 in the conditioned media was determined by ELISA. Results are presented as mean and SD of triplicates performed in one experiment. Compared with the vehicle control, transfection of WT ADAM10 significantly increased CX3CL1 shedding, whereas transfection of DN ADAM10 significantly reduced the cleavage of the chemokine (P < .05 for both effects, indicated by the asterisks). The increase in shedding due to PMA stimulation (determined as difference in shedding between unstimulated and PMA-stimulated cells) was not significantly altered by transfection with either construct (P < .05, not indicated). The results shown in panels A-C are representative for 3 independent experiments.
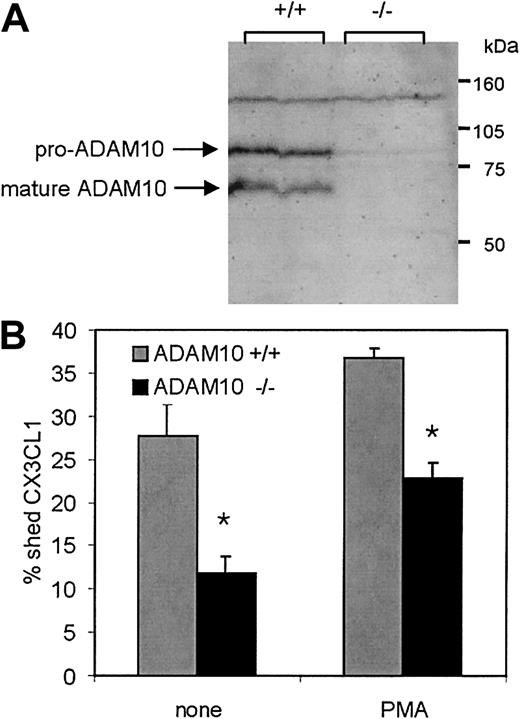
Constitutive CX3CL1 cleavage is reduced in murine fibroblasts lacking ADAM10
More evidence for a role of ADAM10 in constitutive CX3CL1 cleavage was obtained by using murine embryonic fibroblasts (MEFs) that were generated from mouse embryos with a targeted deletion of Adam10 as described previously.38 The absence of ADAM10 in these cells was confirmed by Western blotting with an antiserum raised against murine ADAM10. In Adam10+/+ MEFs that were generated from wild-type animals, the antibody recognized a protein band at 65 kDa that matched the molecular size of mature ADAM10 protein (Figure 4A, compare Figure 3B). This protein band was not present in the Adam10–/– MEF line. Adam10–/– as well as Adam10+/+ MEFs were transiently transfected with CX3CL1 and analyzed for their ability to shed the chemokine constitutively or upon PMA treatment. To normalize variations in transfection efficiency for each single transfection of the 2 cell lines, CX3CL1 levels in the conditioned media as well as in the lysates were determined and shedding was expressed as the percentage of soluble CX3CL1 in relation to total CX3CL1 including soluble and cell-associated molecules. Shedding of CX3CL1 in Adam10–/– MEFs was reduced by 61% as compared with that of Adam10+/+ cells (Figure 4B). By contrast, PMA stimulation led to a similar increase of shedding in both Adam10–/– and Adam10+/+ cells, indicating that the deletion of ADAM10 had no effect on the inducible cleavage of the chemokine. Similar results were obtained with an independent Adam10–/– MEF cell line (64% reduction of constitutive cleavage compared with that in wild-type cells, data not shown), giving further confirmation that the defect in constitutive CX3CL1 shedding was indeed due to the absence of ADAM10. In agreement with our findings in the previous experiments, the shedding was not completely abrogated in both Adam10–/– cell lines, and this residual cleavage activity could not be abolished by the selective ADAM10 inhibitor (data not shown), suggesting that other proteases might be implicated in some of the shedding as well. However, the major proportion of constitutive cleavage is due to ADAM10.
Constitutive CX3CL1 cleavage is reduced in murine embryonic fibroblasts (MEFs) lacking ADAM10. (A) Deficiency of ADAM10 protein in MEFs with targeted disruption of the Adam10 gene: SDS lysates of Adam10+/+ or Adam10–/– MEF cell lines were analyzed for the presence and absence of ADAM10 by Western blotting using an antiserum against murine ADAM10. (B) CX3CL1 shedding in Adam10+/+ and Adam10–/– MEF cell lines: CX3CL1 was transiently transfected into Adam10+/+ or Adam10–/– MEFs. Cells were stimulated with 200 ng/mL PMA for 1 hour, and subsequently conditioned media and cell lysates were analyzed for the presence of soluble and cell-associated CX3CL1, respectively, by ELISA. To take account of variations in the transfection efficiency, for each single transfection data were calculated as the percentage of soluble CX3CL1 released in the medium in relation to the total amount of soluble and cellbound CX3CL1 determined in the medium and the lysate. Results are shown as mean and SD of triplicate transfections of one experiment. In Adam10–/– MEFs, shedding was significantly reduced compared with that observed in Adam10+/+ MEFs (P < .05, indicated by asterisks). The increase in shedding due to PMA stimulation (determined as difference in shedding between unstimulated and PMA-stimulated cells) was similar in both cell lines (P < .05, not indicated). In panels A-C, 1 representative of 3 independent experiments is shown.
Constitutive CX3CL1 cleavage is reduced in murine embryonic fibroblasts (MEFs) lacking ADAM10. (A) Deficiency of ADAM10 protein in MEFs with targeted disruption of the Adam10 gene: SDS lysates of Adam10+/+ or Adam10–/– MEF cell lines were analyzed for the presence and absence of ADAM10 by Western blotting using an antiserum against murine ADAM10. (B) CX3CL1 shedding in Adam10+/+ and Adam10–/– MEF cell lines: CX3CL1 was transiently transfected into Adam10+/+ or Adam10–/– MEFs. Cells were stimulated with 200 ng/mL PMA for 1 hour, and subsequently conditioned media and cell lysates were analyzed for the presence of soluble and cell-associated CX3CL1, respectively, by ELISA. To take account of variations in the transfection efficiency, for each single transfection data were calculated as the percentage of soluble CX3CL1 released in the medium in relation to the total amount of soluble and cellbound CX3CL1 determined in the medium and the lysate. Results are shown as mean and SD of triplicate transfections of one experiment. In Adam10–/– MEFs, shedding was significantly reduced compared with that observed in Adam10+/+ MEFs (P < .05, indicated by asterisks). The increase in shedding due to PMA stimulation (determined as difference in shedding between unstimulated and PMA-stimulated cells) was similar in both cell lines (P < .05, not indicated). In panels A-C, 1 representative of 3 independent experiments is shown.
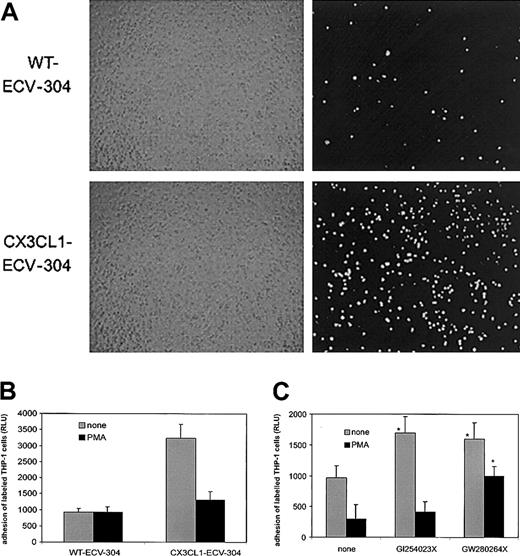
CX3CL1 shedding down-regulates the adhesive properties of CX3CL1-expressing cells
To elucidate the relevance of shedding for the biologic activity of CX3CL1, we performed static adhesion assays with the monocytic cell line THP-1 that is known to express functional CX3CR1.45 As shown in Figure 5A, fluorescently labeled THP-1 cells adhere in large numbers to CX3CL1–ECV-304 cells but not to wild-type cells, suggesting that to a large extent adhesion can be mediated by CX3CL1. To investigate whether PMA-induced shedding of CX3CL1 would affect cell adhesion, ECV-304 cells were pretreated with PMA for 1 hour and subsequently washed before addition of labeled THP-1 cells. Indeed, PMA pretreatment profoundly reduced THP-1 adhesion to CX3CL1–ECV-304 cells but did not further decrease adhesion to wild-type cells lacking endogenous CX3CL1 (Figure 5B). We next studied the effect of the metalloproteinase inhibitors on the adhesive properties of PMA-treated and untreated CX3CL1–ECV-304 cells. For this purpose, CX3CL1–ECV-304 cells were incubated with the inhibitors in the absence or presence of PMA for 1 hour prior to the adhesion assay. Consistent with the observation that both inhibitors enhance surface expression of CX3CL1 on ECV-304 cells, both hydroxamates increased THP-1 adhesion to unstimulated CX3CL1-ECV cells (Figure 5C). In further agreement with the differential ability of the 2 compounds to block the PMA-mediated shedding, only the mixed TACE/ADAM10 inhibitor GW280264X substantially restored adhesion to PMA-stimulated cells, whereas the selective ADAM10 inhibitor GI254023X had a minimal effect. These data indicate that inhibition of ADAM10 is sufficient to increase the adhesive properties of unstimulated CX3CL1–ECV-304–expressing cells and that additional inhibition of TACE is required to preserve adhesion to PMA-stimulated cells.
CX3CL1 shedding by PMA down-regulates adhesion of THP-1 cells to ECV-304 cells, and adhesion is restored by MP inhibitors. (A) CX3CL1 mediates adhesion of THP-1 cells: fluorescently labeled THP-1 cells were seeded onto confluent wild-type (WT) or CX3CL1-expressing ECV-304 cells. After 20 minutes of incubation and 2-fold washing, adherent cells were detected by fluorescence microscopy (right panel). The confluence of the ECV-304 cell layer was controlled by phase contrast microscopy (left panel). (B) PMA down-regulates THP-1 cell adhesion: WT and CX3CL1–ECV-304 cells were treated with 200 ng/mL PMA for 1 hour or left untreated. Subsequently, fluorescently labeled cells were added and, after 2-fold washing, adhesion was quantified as relative light units (RLU) of the fluorescence signal emitted from the adherent cells (mean and SD, n = 4). (C) MP inhibitors differentially restore THP-1 cell adhesion: CX3CL1–ECV-304 cells were treated with 3 μM of the mixed ADAM10/TACE inhibitor GW280264X or the selective ADAM10 inhibitor GI254023X and stimulated with 200 ng/mL PMA for 1 hour or left untreated. Fluorescently labeled cells were added and, after 2-fold washing, adhesion was quantified by reading the fluorescence (mean and SD, n = 4). While both inhibitors significantly increased THP-1 cell adhesion to unstimulated CX3CL1–ECV-304 cells, only GW280264X could significantly increase adhesion to the PMA-stimulated cells (P < .05, indicated by asterisk). In panels A-C, 1 representative of 3 independent experiments is shown.
CX3CL1 shedding by PMA down-regulates adhesion of THP-1 cells to ECV-304 cells, and adhesion is restored by MP inhibitors. (A) CX3CL1 mediates adhesion of THP-1 cells: fluorescently labeled THP-1 cells were seeded onto confluent wild-type (WT) or CX3CL1-expressing ECV-304 cells. After 20 minutes of incubation and 2-fold washing, adherent cells were detected by fluorescence microscopy (right panel). The confluence of the ECV-304 cell layer was controlled by phase contrast microscopy (left panel). (B) PMA down-regulates THP-1 cell adhesion: WT and CX3CL1–ECV-304 cells were treated with 200 ng/mL PMA for 1 hour or left untreated. Subsequently, fluorescently labeled cells were added and, after 2-fold washing, adhesion was quantified as relative light units (RLU) of the fluorescence signal emitted from the adherent cells (mean and SD, n = 4). (C) MP inhibitors differentially restore THP-1 cell adhesion: CX3CL1–ECV-304 cells were treated with 3 μM of the mixed ADAM10/TACE inhibitor GW280264X or the selective ADAM10 inhibitor GI254023X and stimulated with 200 ng/mL PMA for 1 hour or left untreated. Fluorescently labeled cells were added and, after 2-fold washing, adhesion was quantified by reading the fluorescence (mean and SD, n = 4). While both inhibitors significantly increased THP-1 cell adhesion to unstimulated CX3CL1–ECV-304 cells, only GW280264X could significantly increase adhesion to the PMA-stimulated cells (P < .05, indicated by asterisk). In panels A-C, 1 representative of 3 independent experiments is shown.
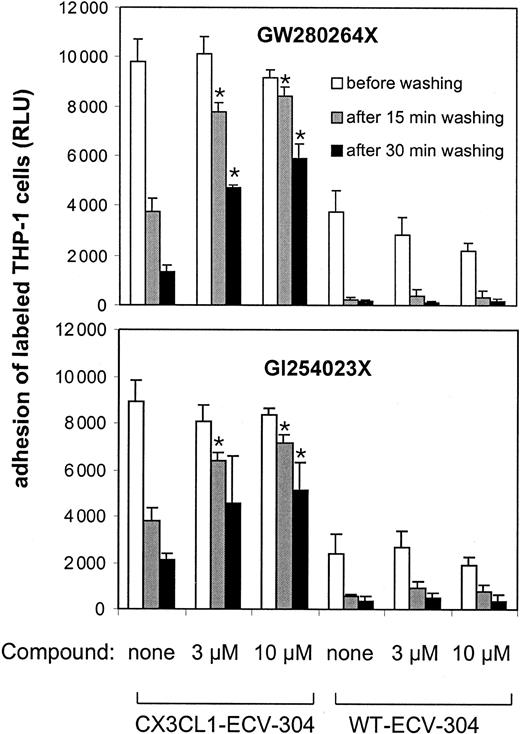
Inhibition of CX3CL1 shedding prevents de-adhesion
We next addressed the question of whether shedding of CX3CL1 would influence the cellular interaction mediated by CX3CL1 and its receptor in a way that could lead to the detachment of the adherent cells. For this purpose de-adhesion assays were performed by loading THP-1 cells onto CX3CL1–ECV-304 cells and subsequent intensive washing leading to the detachment of bound cells. Figure 6 shows that adherent THP-1 cells bound to the surface of CX3CL1–ECV-304 cells de-adhere in the course of vigorous washing. However, most cells remained adherent when they were washed in the presence of metalloproteinase inhibitors. By contrast, the adhesion of THP-1 cells that adhered to wild-type ECV-304 cells was not preserved in the presence of metalloproteinase inhibitors. This indicated that CX3CL1-mediated adhesion can be preserved by the inhibition of CX3CL1 cleavage. The effect of PMA stimulation on the detachment of adherent cells could not be investigated, because PMA by itself induces enhanced adhesiveness of THP-1 cells (data not shown), probably due to the up-regulation of other adhesion molecules. Taken together, our finding that the mixed TACE/ADAM10 inhibitor GW280264X blocked de-adhesion as potently as the selective ADAM10 inhibitor GI254023X suggests that ADAM10 rather than TACE is relevant for promoting de-adhesion from CX3CL1–ECV-304 cells.
MP inhibitors block de-adhesion of THP-1 cells bound to CX3CL1–ECV-304 cells. WT and CX3CL1–ECV-304 cells were loaded with fluorescently labeled THP-1 cells. After removal of nonadherent cells by gentle washing, the fluorescence signal of the adherent cells was determined. Subsequently, cells were washed under agitation in the presence or absence of different concentrations of the mixed ADAM10/TACE inhibitor GW280264X or the selective ADAM10 inhibitor GI254023X. After 15 minutes, the detached cells were washed off and the fluorescence signal of the cells that remained adherent was quantified. Thereafter, an additional wash was performed for 15 minutes and fluorescence was read (mean and SD, n = 4). Statistically significant preservation of cell adhesion in the presence of inhibitors compared with the control receiving no inhibitors is indicated by asterisks (P < .05). Results are presented as 1 representative of 3 independent experiments.
MP inhibitors block de-adhesion of THP-1 cells bound to CX3CL1–ECV-304 cells. WT and CX3CL1–ECV-304 cells were loaded with fluorescently labeled THP-1 cells. After removal of nonadherent cells by gentle washing, the fluorescence signal of the adherent cells was determined. Subsequently, cells were washed under agitation in the presence or absence of different concentrations of the mixed ADAM10/TACE inhibitor GW280264X or the selective ADAM10 inhibitor GI254023X. After 15 minutes, the detached cells were washed off and the fluorescence signal of the cells that remained adherent was quantified. Thereafter, an additional wash was performed for 15 minutes and fluorescence was read (mean and SD, n = 4). Statistically significant preservation of cell adhesion in the presence of inhibitors compared with the control receiving no inhibitors is indicated by asterisks (P < .05). Results are presented as 1 representative of 3 independent experiments.
Discussion
Several in vivo studies have revealed a role of CX3CL1 and its receptor CX3CR1 for leukocyte recruitment in inflammed vascular tissue,28,30,31,49 but the contribution of cell membrane–expressed CX3CL1 or its soluble variant that is generated by proteolytic shedding of the transmembrane molecule is not yet clear. Because the proteolytic shedding of CX3CL1 is likely to have an influence on the chemokine's biologic activity, it is of interest to characterize the involved proteases. Recently, the disintegrin-like metalloproteinase TACE could be identified to mediate PMA-inducible shedding of the chemokine.43,44 In this report we extend the current knowledge of CX3CL1 shedding by providing multiple lines of evidence that the related metalloproteinase ADAM10 is involved in the constitutive cleavage of CX3CL1. We find that both constitutive and PMA-inducible shedding play a role in regulating CX3CL1-mediated adhesion. We also present the first evidence that shedding is relevant for disrupting CX3CL1-mediated adhesion and therefore contributes to the detachment of adherent cells.
Initial evidence that ADAM10 is a candidate for constitutive CX3CL1 cleavage was obtained in inhibition studies with the natural metalloproteinase inhibitors TIMP-1 and TIMP-2, the latter of which neither affected constitutive nor inducible CX3CL1 cleavage, whereas the former preferentially inhibited the constitutive shedding. This excludes the involvement of matrix-type MMPs (MT MMPs type 1, 2, and 3) in CX3CL1 shedding, because these proteases are blocked by TIMP-2.50-52 Interestingly, among the disintegrin-like metalloproteinases that have been tested for inhibition by TIMPs, ADAM10 is blocked by TIMP-1, but TACE, ADAM8, ADAM9, ADAM12, and ADAM28 are not. None of the 6 ADAMs is affected by TIMP-2.48,53-56 Our conclusion that ADAM10 is a likely candidate for the constitutive CX3CL1 cleavage was further supported by inhibition experiments with a novel hydroxamate compound with 102-fold higher potency to inhibit ADAM10 than TACE. Similar to TIMP-1, this compound selectively blocked the constitutive cleavage but had no effect on the PMA-induced shedding. The activity of the compound to selectively block CX3CL1 cleavage was the same in 3 different cell lines used in this study, suggesting that the underlying mechanism of cleavage is very similar. The inhibition profile of the compound on other ADAMs is not known, because the recombinant enzymes are not yet available for testing. However, when one also considers the TIMP inhibition profile of the shedding event, the number of ADAMs that could potentially mediate constitutive CX3CL1 shedding is very small. We here provide further strong evidence that ADAM10 is indeed responsible for most constitutive CX3CL1 cleavage and exclude possible reservations that the effect of the ADAM10 inhibitor on CX3CL1 cleavage is not due to the inhibition of ADAM10. First, ADAM10 is able to cleave CX3CL1, because overexpression of the enzyme resulted in enhanced constitutive CX3CL1 cleavage. Second, inhibiting endogenous ADAM10 activity by overexpressing a dominant-negative mutant of ADAM10 decreased constitutive shedding of the chemokine. Third and most importantly, deletion of the Adam10 gene abolished a major proportion of constitutive CX3CL1 shedding in murine embryonic fibroblasts. Notably, the residual constitutive generation of soluble CX3CL1 in Adam10–/– MEFs could not be further reduced by the selective ADAM10 inhibitor. Taken together, these findings demonstrate that the major proportion of constitutive CX3CL1 shedding is mediated by ADAM10 and can be blocked by the ADAM10 inhibitor, although there remains a minor cleavage activity that is not due to ADAM10 and is not affected by the inhibitor.
The current understanding of CX3CL1 shedding is based on 2 recent studies employing TACE-deficient fibroblast cell lines to demonstrate that TACE is involved in the PMA-inducible but not in the constitutive shedding of CX3CL1.43,44 In agreement with both studies we found that TACE inhibition but not ADAM10 inhibition blocked CX3CL1 shedding in different types of PMA-stimulated cell lines. By contrast, ADAM10 is not involved in this effect of PMA, because the PMA-induced increase in CX3CL1 cleavage was not affected by the selective ADAM10 inhibitor, by overexpression of ADAM10, or by deletion of the Adam10 gene. Taken together, both ADAM10 and TACE cleave CX3CL1, but ADAM10 exclusively contributes to the constitutive cleavage, whereas TACE exclusively mediates inducible shedding. Interestingly, ADAM10 and TACE have been reported to share other substrates as well, of which the cellular prion protein (PrP) and the amyloid precursor protein (APP) are shed constitutively by ADAM10 and in a PMA-inducible manner predominantly by TACE.39,40,57 Although these studies and our present work imply that ADAM10-mediated shedding activity is not modulated by PMA, it could still be subject to regulation as demonstrated for cleavage of APP that is markedly increased in ADAM10-overexpressing HEK293 cells upon treatment with the cholesterol-extracting agent methyl-β-cyclodextrin.58
Our study demonstrates that constitutive as well as inducible shedding of CX3CL1 are 2 regulatory elements in CX3CL1-mediated cell adhesion. On the one hand, increased surface expression of CX3CL1 upon inhibition of constitutive shedding clearly up-regulates the adhesiveness for CX3CR1-expressing cells. Having demonstrated that most constitutive CX3CL1 shedding is due to ADAM10, we conclude that ADAM10-mediated CX3CL1 shedding is involved in down-regulating adhesive properties of CX3CL1-expressing cells. On the other hand, decreased surface expression of CX3CL1 upon PMA treatment was associated with profoundly reduced cell adhesion, which was abrogated by TACE but not by ADAM10 inhibition, implying a role of TACE rather than ADAM10 in down-regulating cell adhesion to PMA-treated cells. Notably, PMA stimulation reduced CX3CL1 surface expression by not more than one half, whereas CX3CL1-mediated adhesion is almost completely abrogated. This may suggest that small changes in CX3CL1 expression are sufficient to trigger dramatic effects in cell adhesion.
We here addressed the question of how CX3CL1-bound cells can be released from their cellular substrate. It has been shown that the interaction of immobilized CX3CL1 with its receptor on leukocytes under physiologic flow conditions is extremely tight due to the slow receptor dissociation rate of the chemokine.59 Thus, receptor dissociation does not seem to play a major role for allowing cell detachment. We here propose that the protolytic cleavage of CX3CL1 could constitute a mechanism for the resolution of CX3CL1-mediated cell binding. This idea is supported by our adhesion studies demonstrating that CX3CR1-expressing cells detach from CX3CL1-expressing cell layers under stringent washing conditions and that this detachment is prevented by metalloproteinase inhibitors. Interestingly, the mixed ADAM10/TACE inhibitor as well as the selective ADAM10 inhibitor prevented the detachment of CX3CL1-bound cells to a large extent. These data suggest that ADAM10 rather than TACE may be physiologically relevant for promoting the detachment of cells bound to CX3CL1.
Although there is accumulating in vivo evidence for the involvement of CX3CL1 and its receptor in promoting monocyte recruitment and vascular inflammation,28,30,31,49 there is little knowledge of how CX3CL1 shedding can influence this activity in vivo. Within inflamed vascular tissue CX3CL1 is up-regulated in microvascular endothelial cells, endothelial cells of large blood vessels, smooth muscle cells, and infiltrating macrophages.3,11,60,61 ADAM10 and TACE are likely candidates for CX3CL1 shedding in vivo because they are expressed in the inflamed vascular tissue62-64 and most probably coexpressed with CX3CL1 in the same cell. Thus, CX3CL1-mediated leukocyte adhesion would be controlled by the balance between gene expression of CX3CL1 and its removal from the vascular cell surface. It can be imagined that, under severe inflammatory conditions, the gene expression of CX3CL1 overrides the constitutive shedding of the chemokine, resulting in enhanced surface presentation of CX3CL1 on the blood vessel wall. Following selectin-mediated rolling, more firm adhesion could be mediated by up-regulated adhesion molecules including CX3CL1. Activation of further adhesion molecules including integrins is then required to promote further transmigration through the vascular wall. This is possibly mediated via CX3CL1 signaling through CX3CR1 resulting in integrin activation.65 CX3CL1 cleavage would then disrupt the interaction between CX3CL1 and its receptor and thereby allow the recruited leukocytes to proceed in diapedesis. Thus, whether CX3CR1–expressing leukocytes are arrested to CX3CL1-expressing vascular wall or finally transmigrate into the underlying tissue could depend on the activity of CX3CL1-cleaving metalloproteinases such as TACE and ADAM10.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-12-3775.
Supported by Deutsche Forschungsgemeinschaft grant LU 869/1-2.
Two of the authors (T.A.B. and N.B.) are employed by a company (GlaxoSmithKline) whose potential product was studied in the present work.
C.H., D.M., and T.A.B. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Barbara Mütze for expert technical assistance.

![Figure 1. Constitutive and inducible CX3CL1 cleavage in CX3CL1–ECV-304 cells. (A) PMA enhances the release of soluble CX3CL1 from CX3CL1–ECV-304 cells: cells were stimulated with 200 ng/μL PMA or left unstimulated for different lengths of time. Conditioned media were harvested, concentrated 10-fold, and analyzed by Western blotting using a specific antiserum against CX3CL1. (B) The broad-spectrum metalloproteinase (MP) inhibitor batimastat blocks constitutive and inducible CX3CL1 cleavage: CX3CL1–ECV-304 cells were treated with 20 μM batimastat or vehicle control (dimethylsulfoxide [DMSO]) and subsequently stimulated with 200 ng/mL PMA or left unstimulated. After 3 hours, conditioned media were harvested and analyzed for the presence of soluble CX3CL1. (C) Effect of PMA and batimastat on CX3CL1 surface expression: CX3CL1–ECV-304 cells were treated with inhibitor and stimulated with PMA as described in panel B and subsequently investigated for the surface expression of CX3CL1 by flow cytometry using a fluorescently labeled monoclonal antibody to CX3CL1. For each condition the median fluorescence intensity was calculated and given as mean and SD of triplicate determinations (values in parentheses). Unspecific antibody binding was evaluated with a fluorescently labeled IgG1 control and was not modulated by treatment with PMA or batimastat (MFI = 6 ± 1; not shown). Results shown in panels A-C are representative for 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2002-12-3775/6/m_h81634759001.jpeg?Expires=1769088868&Signature=I5q3g7Nv0XumSln9yUGFE77jokRlMkvyYf~jFsiGhJzLUvxbBWnmHtWDNnwx68aMMuewjHjJCd5tXs-qKXLDtkKAl0dzCL~JDuzemvFTGs0TbxEvXZPoq8B-6gld-ECZZIn~xTrnKAOt-z5bNh8Aa2Vi4HTxYA6XK8ZAZEgk4QEIeURABkDz3SuVwY4rcz83fpn-mItRRkjngiuxaOvNsTrNlO6BuwOUuuMCcU3TMXDn~TChRQGnT7oH~KykqH~~B2EySufOdnbWFXF~ONUMESRkTiLlPuzb0Ngad7YMlOtA1gdjpZEGKycKLgzo0rcMc3piMxiHekAcuBT-hACYpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal