Abstract
Chronic myeloid leukemia (CML) is characterized by formation of a BCR-ABL fusion gene, usually as a consequence of the Philadelphia (Ph) translocation between chromosomes 9 and 22. Recently the development of new fluorescence insitu hybridization (FISH) techniques has allowed identification of unexpected deletions of the reciprocal translocation product, the derivative chromosome 9, in 10% to 15% of patients with CML. These deletions are large, span the translocation breakpoint, and occur at the same time as the Ph translocation. Such deletions therefore give rise to previously unsuspected molecular heterogeneity from the very beginning of this disease, and there is mounting evidence for similar deletions associated with other translocations. Several studies have demonstrated that CML patients who carry derivative chromosome 9 deletions exhibit a more rapid progression to blast crisis and a shorter survival. Deletion status is independent of, and more powerful than, the Sokal and Hasford/European prognostic scoring systems. The poor prognosis associated with deletions is seen in patients treated with hydroxyurea or interferon, and preliminary evidence suggests that patients with deletions may also have a worse outcome than nondeleted patients following stem cell transplantation or treatment with imatinib. Poor outcome cannot be attributed to loss of the reciprocal ABL-BCR fusion gene expression alone, and is likely to reflect loss of one or more critical genes within the deleted region. The molecular heterogeneity associated with the Philadelphia translocation provides a new paradigm with potential relevance to all malignancies associated with reciprocal chromosomal translocations and/or fusion gene formation.
Introduction
Chronic myeloid leukemia (CML) is a clonal hematologic malignancy that arises in the stem cell compartment.1-3 Its molecular hallmark is the BCR-ABL fusion gene,4,5 which usually occurs as the result of the Philadelphia (Ph) translocation involving the long arms of chromosomes 9 and 22.6 The chimeric BCR-ABL gene encodes a constitutively activated protein tyrosine kinase, which leads to the activation of multiple signaling pathways, with profound effects on cell cycle, adhesion, and apoptosis.2,3 In murine transgenic and retroviral transduction models, expression of BCR-ABL has been shown to be both sufficient for initiation and necessary for maintenance of a leukemic phenotype.7-14
The natural history of CML follows a biphasic pattern with an initial chronic phase, which is often asymptomatic. This is inevitably followed by progression of the disease through an ill-defined stage termed accelerated phase to the terminal blast crisis. During chronic phase, the myeloid compartment is expanded but the cells retain their capacity to differentiate and function normally, and drug treatment is usually effective. By contrast, blast crisis is characterized by loss of differentiation capacity together with refractoriness to therapy and is associated with the acquisition of new cytogenetic abnormalities in approximately 80% of patients.15 In some cases, additional molecular abnormalities have been identified including mutations or deletions of p53,16 p16INKA,17 or the RB1 protein,18,19 and mutation or overexpression of RAS20,21 or EVI-1.22,23 However in most cases the additional molecular lesions responsible for progression to blast crisis remain obscure.
Until recently first-line treatment for CML consisted of either allogeneic stem cell transplantation or an α-interferon–based regimen. However, both options are associated with considerable drawbacks. Although potentially curative, stem cell transplantation is associated with considerable morbidity and mortality,24 while α-interferon–based regimens adequately control chronic-phase disease25-27 but result in few long-term survivors.28 Recently, treatment with the protein tyrosine kinase inhibitor imatinib mesylate (Gleevec/Glivec, formerly known as STI 571; Novartis, Basel, Switzerland) has resulted in excellent hematologic and cytogenetic responses in all phases of CML.29-32 Comparison with historical controls shows improved survival in the later stages of the disease31-36 for patients treated with imatinib, and it is hoped that the excellent response rates obtained in chronic-phase patients will also translate into improved survival.
There are 2 prognostic scoring systems currently in use for patients with CML. The Sokal and Hasford/European scores37,38 are mathematical calculations derived from similar clinical and laboratory parameters measured at diagnosis. Both have limitations: the Sokal score is nearly 20 years old and was generated using patients treated with busulphan or hydroxyurea, while the Hasford score was derived and validated using patients treated only with α-interferon. Neither scoring system is powerful enough to play a major role in guiding individual patient management decisions, a process that has become increasingly complex in the post-imatinib era. Robust prognostic indicators are therefore badly needed to help clinicians and patients make informed management decisions.
Recently a number of groups have described deletions of the reciprocal product of the Ph translocation, the derivative chromosome 9, in a subset of patients with CML and have demonstrated that deletion status is a powerful prognostic indicator. This paper will review the discovery and clinical significance of detecting such deletions, their role in the pathogenesis of CML, the methods available for their detection, and the significance of this paradigm for other malignancies associated with balanced translocations.
The anatomy of derivative chromosome 9 deletions in CML
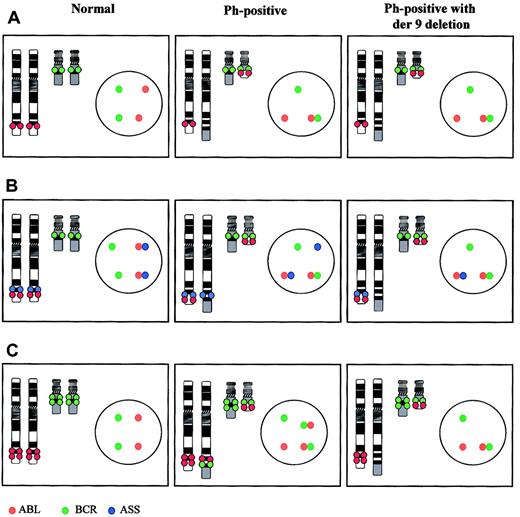
The discovery of derivative chromosome 9 deletions provides a good example of scientific serendipity. In the mid 1990s a number of groups were exploring the possibility of using fluorescence in situ hybridization (FISH) to detect minimal residual disease in patients with CML by monitoring interphase nuclei in peripheral blood. The early probe systems relied solely on detecting colocalization of BCR and ABL probes (Figure 1A), but this approach was of little use for measuring residual disease since coincidental colocalization occurs in approximately 5% of normal interphase nuclei. To circumvent this problem, a number of new probe systems were designed that allowed identification of the derivative chromosome 9 as well as the Ph chromosome39-41 (Figure 1B-C), and this strategy did indeed greatly reduce the number of false-positive results.39-41
FISH systems for detection of the Ph translocation and derivative chromosome 9 deletions. In each case an ideogram and an interphase nucleus are shown for a normal cell, a cell carrying the Ph translocation alone (Ph positive), and a Ph-positive cell carrying a derivative chromosome 9 deletion (Ph positive with der9 deletion). (A) Early probe system. The ABL (red) and BCR (green) loci are labeled with different colored fluorochromes. No difference in the metaphase or interphase patterns is seen between patients who carry a deletion and those who do not. (B) Triple-probe system. In addition to the ABL and BCR loci a third probe containing the ASS gene is labeled in blue. With this system, the translocation again produces a fusion of the BCR and ABL probes on the Ph chromosome but also marks the derivative chromosome 9 with a single blue signal. This signal is missing in patients with deletions. (C) Dual-fusion probe system. The ABL and BCR loci are again labeled with different colored fluorochromes. However in these probe systems the probe size is larger and spans the translocation breakpoints. Both of these probes hybridize to the Ph chromosome and also to the derivative chromosome 9 creating 2 fusion signals in a Ph-positive patient who lacks a deletion but only one fusion signal in a Ph-positive patient with a derivative chromosome 9 deletion. In addition this system can differentiate between loss of chromosome 9 sequences only, chromosome 22 sequences only, or both from the derivative chromosome 9.
FISH systems for detection of the Ph translocation and derivative chromosome 9 deletions. In each case an ideogram and an interphase nucleus are shown for a normal cell, a cell carrying the Ph translocation alone (Ph positive), and a Ph-positive cell carrying a derivative chromosome 9 deletion (Ph positive with der9 deletion). (A) Early probe system. The ABL (red) and BCR (green) loci are labeled with different colored fluorochromes. No difference in the metaphase or interphase patterns is seen between patients who carry a deletion and those who do not. (B) Triple-probe system. In addition to the ABL and BCR loci a third probe containing the ASS gene is labeled in blue. With this system, the translocation again produces a fusion of the BCR and ABL probes on the Ph chromosome but also marks the derivative chromosome 9 with a single blue signal. This signal is missing in patients with deletions. (C) Dual-fusion probe system. The ABL and BCR loci are again labeled with different colored fluorochromes. However in these probe systems the probe size is larger and spans the translocation breakpoints. Both of these probes hybridize to the Ph chromosome and also to the derivative chromosome 9 creating 2 fusion signals in a Ph-positive patient who lacks a deletion but only one fusion signal in a Ph-positive patient with a derivative chromosome 9 deletion. In addition this system can differentiate between loss of chromosome 9 sequences only, chromosome 22 sequences only, or both from the derivative chromosome 9.
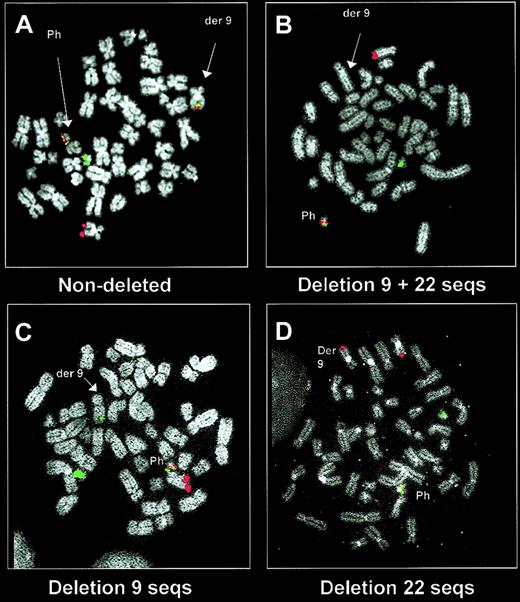
However, the use of these new probe systems also resulted in an unexpected observation. Samples from some patients exhibited an abnormal signal pattern in which the BCR-ABL fusion signal on the Ph chromosome was accompanied by loss of the signals, which should have marked the derivative chromosome 942-44 (Figures 1, 2). The presence of a deletion was confirmed by microsatellite polymerase chain reaction (PCR) and by using further locus-specific probes for chromosome 9 and 22 sequences adjacent to the translocation breakpoints42,44 (Figure 3). Although small deletions (approximately 10 kb) of chromosome 22 sequences next to the translocation breakpoint had previously been demonstrated by Southern blotting, these were thought to be of no pathophysiologic importance.45-47 By contrast the FISH data suggested the existence of substantial deletions spanning at least several hundred kilobases (Figure 3).
Demonstration of derivative chromosome 9 deletions by a dual-fusion probe system. Metaphase images are shown using a dual-fusion system (dual color, dual fusion probe system; Vysis) in a patient without a derivative chromosome 9 deletion (A) and in patients with a deletion (B-D). The ABL (red) and BCR (green) signals mark the normal 9 and 22 chromosomes, respectively, and the Ph chromosome is marked by a fusion signal. In patients without a deletion, the dual-color/dual-fusion system also labels the derivative chromosome 9 with a fusion signal (A). This fusion signal is missing from the derivative chromosome 9 in patients who carry a deletion of both chromosome 9 and 22 sequences (B). This probe system is also able to detect those patients in whom only chromosome 9 sequences (C) or chromosome 22 sequences (D) are deleted.
Demonstration of derivative chromosome 9 deletions by a dual-fusion probe system. Metaphase images are shown using a dual-fusion system (dual color, dual fusion probe system; Vysis) in a patient without a derivative chromosome 9 deletion (A) and in patients with a deletion (B-D). The ABL (red) and BCR (green) signals mark the normal 9 and 22 chromosomes, respectively, and the Ph chromosome is marked by a fusion signal. In patients without a deletion, the dual-color/dual-fusion system also labels the derivative chromosome 9 with a fusion signal (A). This fusion signal is missing from the derivative chromosome 9 in patients who carry a deletion of both chromosome 9 and 22 sequences (B). This probe system is also able to detect those patients in whom only chromosome 9 sequences (C) or chromosome 22 sequences (D) are deleted.
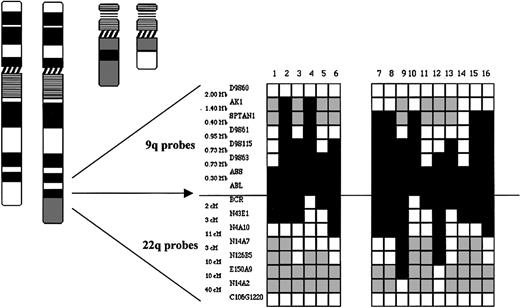
Derivative chromosme 9 deletions are large, span the translocation breakpoint, and demonstrate heterogeneous deletion breakpoints. A summary of FISH mapping of 16 patients with deletions is shown. Locus-specific probes from 9q34 and 22q12 together with corresponding physical and genomic map data are shown. White boxes indicate the retention of a locus; black boxes indicate the loss of a locus; and gray boxes indicate not performed. (Adapted from Sinclair et al.39 )
Derivative chromosme 9 deletions are large, span the translocation breakpoint, and demonstrate heterogeneous deletion breakpoints. A summary of FISH mapping of 16 patients with deletions is shown. Locus-specific probes from 9q34 and 22q12 together with corresponding physical and genomic map data are shown. White boxes indicate the retention of a locus; black boxes indicate the loss of a locus; and gray boxes indicate not performed. (Adapted from Sinclair et al.39 )
In order to delineate the size of the deletions FISH mapping was performed in the 16 patients with deletions originally described by Sinclair et al.44 The results highlighted 3 main features of the deletions. First, the deletions were adjacent to and usually spanned the translocation breakpoint of the derivative chromosome 9. Second, the deletions were very large and in some instances involved several megabases of both chromosome 9 and 22 sequences. Third, the size of the deletions varied considerably and there was no obvious clustering of centromeric or telomeric deletion breakpoints (Figure 3). Large deletions of chromosome 22 sequences were identified independently by Grand et al42 who demonstrated that a cosmid containing the hSNF/INI1 gene, over a megabase telomeric to the chromosome 22 breakpoint, was deleted in 9 of 25 cases of CML in blast crisis and 5 of 21 cases in chronic phase. More recently, Storlazzi et al have mapped deletions in a further 10 patients using an extensive panel of probes covering the chromosome 9 and 22 regions adjacent to the translocation breakpoints.48 Their results identified deletions ranging from a few hundred kilobases to 8 megabases with variable centromeric and telomeric breakpoints, although the latter appeared to cluster in 2 regions in 7 of 10 patients.
Deletions occur at the time of the Ph translocation in CML and therefore produce genetic heterogeneity ab initio
Initial results raised the possibility that deletions were a relatively late event and reflected karyotypic instability associated with disease progression.42 However, several lines of evidence have subsequently demonstrated that deletions occur at the time of the Ph translocation. First, distinct cohorts of patients analyzed in different phases of the disease were found to exhibit virtually identical frequencies of deletions.49,50 Sequential paired samples have also been analyzed, with the first sample at diagnosis and the second sample taken following disease progression to blast crisis. No individual was found to acquire a deletion following disease progression despite the analysis of large numbers of metaphases.49,50
Second, deletions were around 3 times more common in patients with variant Ph translocations when compared with patients with classical Ph translocations.50,51 More recently a study of 8 patients confirmed the relative frequency of deletions in patients with variant translocations and demonstrated loss of sequences from the third partner chromosome in those patients with deletion of chromosome 9 and/or 22 sequences.52 Since the formation of variant translocations is thought to involve multiple double-stranded DNA breaks,53 these observations are consistent with a model in which each recombination event has a finite probability of inaccurate repair resulting in a deletion adjacent to the breakpoint.
Third, if deletions occurred during disease progression it should be possible to identify cells carrying the Ph translocation but no deletion. However, several groups have analyzed large numbers of metaphases from patients who carried deletions and have reported that every metaphase contained both a Ph translocation and a derivative chromosome 9 deletion.48-51
Taken together these data demonstrate that, in a subset of patients, the recombination event that generates an apparently reciprocal translocation can also produce large genomic deletions. Since the Ph translocation is thought to initiate chronic-phase CML1-3 these results point to the existence of previously unsuspected genetic heterogeneity in a proportion of patients with CML from the very beginning of their disease, an observation with potential relevance for other malignancies associated with balanced translocations and/or fusion genes.
Prognostic significance of derivative chromosome 9 deletions in CML
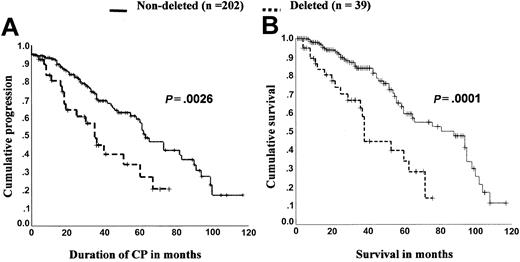
Sinclair et al first noted that deletions of the derivative chromosome 9 were associated with a worse survival.44 Although deletion status did not appear to correlate with clinical features or laboratory results at diagnosis, 16 patients with deletions had a significantly shorter survival than 39 patients lacking deletions (P = .006). There have been 2 subsequent large studies that confirmed the poor prognostic value of deletion status.50,51 In a study of 241 patients (39 with deletions and 202 without), Huntly et al found the median survival of patients with deletions to be roughly half that of patients who did not carry deletions (median survival 38 months versus 88 months, respectively, P = .0001) (Figure 4).50 This finding was corroborated by Kolomietz et al, who compared the survival of 186 patients (23 with deletions and 163 without) and found a similar median survival difference (36 vs 84 months, respectively, P = .005).51 In both of these patient cohorts51 (and B.J.P.H., unpublished data, June 2002) (Figure 4) and in another 2 smaller studies48,49 shorter survival reflected a shorter duration of chronic phase, with earlier disease progression.
The presence of derivative chromosome 9 deletions shortens length of chronic phase and survival. Shown are 2 Kaplan-Meier graphs for a cohort of 241 patients with duration of chronic phase and survival compared according to deletion status. Log-rank analysis demonstrates significantly shorter duration of chronic phase and overall survival for those patients who carry deletions (median follow-up was similar for both groups, at 31 months for patients who carried deletions and 34 months for those without a deletion). Data shown are from Huntly et al50 with permission and B.J.P.H., unpublished observations, June 2002.
The presence of derivative chromosome 9 deletions shortens length of chronic phase and survival. Shown are 2 Kaplan-Meier graphs for a cohort of 241 patients with duration of chronic phase and survival compared according to deletion status. Log-rank analysis demonstrates significantly shorter duration of chronic phase and overall survival for those patients who carry deletions (median follow-up was similar for both groups, at 31 months for patients who carried deletions and 34 months for those without a deletion). Data shown are from Huntly et al50 with permission and B.J.P.H., unpublished observations, June 2002.
The higher incidence of deletions in patients with variant Ph translocations may also provide an explanation for previous conflicting reports of the prognostic significance of variant Ph translocations. In some series, patients with a variant Ph translocation have been reported to have a worse prognosis compared with patients with a classical Ph translocation,54,55 while in other studies no difference has been found.56,57 However, the relative survival of patients with variant or classical Ph translocations will depend on the proportions of patients with a deletion in the 2 groups. Consistent with this concept, there was no difference in the survival of patients with variant or classical Ph translocations when patients with deletions were removed from the analysis.50
It is important to know whether deletion status remains a useful prognostic indicator for patients receiving different treatment modalities. Early studies of the clinical significance of derivative chromosome 9 deletions included a mixture of patients treated with hydroxyurea or interferon-based regimens.44,50,51 Deletion status remained a powerful predictor of response rate, duration of chronic phase, and overall survival when analysis was limited to patients treated with interferon alone.48-50 The significance of deletion status in the context of an allograft is largely unknown. In one relatively small series (12 patients with deletions and 58 without) there was an increased rate of relapse in patients with deletions following allogeneic transplantation.51 These results suggest that allogeneic transplantation may be less effective in achieving disease eradication in patients with deletions of the derivative chromosome 9.
Data concerning deletion status in patients receiving imatinib are also preliminary. In a series of 397 patients (275 chronic phase, 54 accelerated phase, and 68 blast crisis) who were treated with imatinib, survival of all patients was improved relative to historical controls and, with a median follow-up of 48 months, no significant survival difference was yet apparent.58 However, it should be noted that both hematologic and cytogenetic responses were uniformly lower in chronic-phase and more advanced-phase patients with deletions, with these differences reaching statistical significance for hematologic (89% vs 97%, patients with a deletion vs those without, P = .04) and major cytogenetic responses (55% vs 75%, P = .008) in chronic phase, and for hematologic response (46% vs 82%, patients with a deletion vs those without, P = .007) in more advanced phases. Progression-free survival following initiation of imatinib was also significantly shorter for patients with deletions, treated either in chronic phase (P = .02) or advanced phases of the disease (P = .02). These data indicate that deletion status may retain prognostic significance in patients treated with imatinib, but confirmation will require longer follow-up.
A number of novel therapies are currently showing promise in preclinical models and phase 1 clinical trials. These include inhibitors of signal transduction pathways further downstream of BCR-ABL, such as farnesyl transferase inhibitors (which inhibit ras)59,60 and phosphatidylinositol 3–kinase (PI3-kinase) inhibitors;61,62 agents that decrease intracellular levels of BCR-ABL, such as the BCR-ABL molecular chaperone, heat shock protein 90, and inhibitor AAG (17-allylaminogeldanamycin); and other agents such as homoharringtonine,63,64 decitabine,65,66 and troxatyl.67 Should the initial promise of these therapeutic agents be borne out, the design of randomized trials should include stratification for deletion status.
Deletion status appears to be both more powerful than, and independent of, the Sokal and Hasford scoring systems. A direct comparison of prognostic significance between deletion status and Sokal and Hasford scores was possible in 210 patients in the series of Huntly et al.50 As shown in Table 1 deletion status was a stronger prognostic indicator than either Sokal or Hasford score. Interestingly, patients with deletions are not merely a subset of those deemed high risk by the Sokal and Hasford scoring systems, since similar numbers of patients with deletions were found in the Sokal and Hasford low-, intermediate-, and high-risk groups (Table 2). Moreover, the Sokal and Hasford scoring systems retained prognostic significance if analysis was restricted to patients without a deletion. Taken together, these observations suggest that deletion status and the 2 clinical scoring systems represent independent prognostic variables. The relative prognostic power of deletion status may reflect the fact that it directly detects a molecular event with a critical role in the progression of CML.
Deletion status is a more powerful prognostic indicator than either the Sokal or Hasford clinical scores
. | High-risk . | . | Non—high-risk . | . | . | ||
|---|---|---|---|---|---|---|---|
| Prognostic factor . | Patients, % . | Median survival (95% CI) . | Patients, % . | Median survival (95% CI) . | Survival difference, P . | ||
| Deletion status | 15 | 37 (25-49) | 85 | 81 (51-110) | .0001 | ||
| Sokal | 37 | 56 (40-72) | 63 | 72 (51-93) | .057 | ||
| Hasford | 22 | 55 (34-76) | 78 | 76 (49-103) | .034 | ||
. | High-risk . | . | Non—high-risk . | . | . | ||
|---|---|---|---|---|---|---|---|
| Prognostic factor . | Patients, % . | Median survival (95% CI) . | Patients, % . | Median survival (95% CI) . | Survival difference, P . | ||
| Deletion status | 15 | 37 (25-49) | 85 | 81 (51-110) | .0001 | ||
| Sokal | 37 | 56 (40-72) | 63 | 72 (51-93) | .057 | ||
| Hasford | 22 | 55 (34-76) | 78 | 76 (49-103) | .034 | ||
Non—high-risk refers to patients lacking a derivative chromosome 9 deletion or, in the Sokal and Hasford systems, the combined low- and intermediate-risk groups. High-risk refers to patients with a deletion or to Sokal and Hasford high-risk groups. Comparison of survival was made by log-rank analysis with the P values shown. Data are reprinted from Huntly et al50 with permission.
Deletion status is independent of Sokal or Hasford score
. | Patients with deletions, % . | . | |
|---|---|---|---|
| Risk category . | Sokal . | Hasford . | |
| Low | 14 | 13 | |
| Intermediate | 13 | 18 | |
| High | 18 | 15 | |
. | Patients with deletions, % . | . | |
|---|---|---|---|
| Risk category . | Sokal . | Hasford . | |
| Low | 14 | 13 | |
| Intermediate | 13 | 18 | |
| High | 18 | 15 | |
Numbers represent the percentage of patients with a derivative chromosome 9 deletion in each risk category. Data are reprinted from Huntly et al50 with permission.
A reduction in telomere length has been described in a number of human cancers, including CML,68,69 and recent reports have suggested that telomere length may provide a prognostic indicator in patients with chronic phase CML. Iwama et al70 studied 32 patients treated with interferon and reported that patients with longer telomeres had improved cytogenetic responses, progression free-suvival, and overall survival. However when analysis was extended to include a further 12 patients receiving other treatments no significant differences were obtained for any of these outcomes. More recently, Brummendorf et al71 reported that samples taken more than 2 years before disease evolution displayed longer telomere length than those taken less than 2 years before disease evolution. However, this study was able to assess a total of only 22 patients. Last, Boultwood et al72 studied 59 patients and showed that telomere length was correlated with reduced time from diagnosis to accelerated phase but not with time to blast crisis or overall survival. These are small studies and require corroboration using larger cohorts of patients, but taken together they raise the possibility that assessment of telomere length may provide a useful additional prognostic marker. If this concept is confirmed it will be important to see whether there is any relationship between deletion status and telomere length.
Derivative chromosome 9 deletions in acute lymphoblastic leukemia (ALL)
The BCR-ABL gene rearrangement accompanies not only all cases of CML, but occurs also in around 25% of adult and around 5% of ALL.73 However, these 2 diseases are clinically distinct and are associated with different patterns of BCR-ABL rearrangement. Ph-positive ALL also lacks the chronic phase of CML and is a clinically aggressive disease with a poor prognosis, particularly in adults. Almost all cases of CML, but only one third of patients with ALL have an M-bcr breakpoint that results in fusion of the majority of the c-ABL oncogene to the first 13 or 14 exons of the BCR gene and gives rise to the p210 BCR-ABL protein.74 By contrast, in approximately two thirds of ALL patients, the breakpoint within BCR is more proximal, between exons 1 and 2 of the BCR gene (m-bcr breakpoint), giving rise to a smaller p190 BCR-ABL protein.
Reid et al investigated the possibility that the poor prognosis of Ph-positive ALL may correlate with deletion status.75 However, of 67 patients with Ph-positive ALL studied, only a single case was found to carry a deletion detectable by FISH. Interestingly this patient had an m-bcr rearrangement, demonstrating that deletions are not restricted to patients with an M-bcr breakpoint. These results show that deletions occur less frequently in Ph-positive ALL than in CML, and there are a number of possible explanations for this difference. First, deletions are approximately 3 times more common in variant Ph translocations than in classic Ph translocations, and the former are rare in ALL (1%-3% in ALL compared with 5%-10% in CML).15,76-78 A second and related possibility is that some features of the M-bcr region may render it inherently more likely to be repaired inaccurately following rearrangement. The lower incidence of deletions in ALL would then reflect the relative infrequency of M-bcr rearrangements in this disease. Third, deletion frequency may vary depending upon the target cell in which the Ph translocation occurs. CML results from the transformation of a multipotent hematopoietic stem cell,1-3 whereas ALL is thought to result from transformation of a committed B-cell progenitor.79 Lymphoid cells undergo antigen receptor rearrangements that require accurate joining of double-stranded DNA breaks and may therefore use more stringent mechanisms to minimize the occurrence of inaccurate repair.
Molecular basis for the poor prognosis associated with deletions
A number of molecular mechanisms could conceivably be responsible for the poor prognosis associated with derivative chromosome 9 deletions. Deletions can result in formation of a fusion gene,80 but this mechanism seems implausible given the considerable breakpoint heterogeneity on both centromeric and telomeric sides of the derivative chromosome 9 deletions44,48 (Figure 3). However 4 other mechanisms warrant close inspection.
Loss of ABL-BCR expression
Several groups have now shown that all patients with a deletion detectable by FISH lack expression of the ABL-BCR transcript.81-83 However 65% of patients lacking ABL-BCR expression do not have a deletion detectable by FISH (Table 3).81 This observation suggests the existence of other mechanisms by which ABL-BCR transcription can be abolished. It is likely that small deletions occur that would abolish ABL-BCR transcription but that would be below the threshold of detection for the dual-fusion FISH and other similar FISH-based techniques that use large probes. This would be consistent with previous evidence for small deletions adjacent to the translocation breakpoints in CML and other leukemias.45,46,84-87 In addition, approximately 10% of patients have a breakpoint on the derivative chromosome 9 that is upstream of ABL exon 1b, and that therefore removes both sites at which ABL transcription is normally initiated.84,88 Finally some patients with a variant Ph translocation have 5′ ABL and 3′ BCR sequences present on separate chromosomes, suggesting that an ABL-BCR transcript would not be formed.89
The presence of a deletion and ABL-BCR expression are mutually exclusive
. | ABL-BCR expression, % . | . | |
|---|---|---|---|
| Deletion status . | Negative . | Positive . | |
| Deleted | 35 | 0 | |
| Nondeleted | 65 | 100 | |
. | ABL-BCR expression, % . | . | |
|---|---|---|---|
| Deletion status . | Negative . | Positive . | |
| Deleted | 35 | 0 | |
| Nondeleted | 65 | 100 | |
Numbers represent percentages of deleted and nondeleted patients. All patients with a deletion lack ABL-BCR expression, and also all patients who express ABL-BCR are nondeleted. Data are reprinted from Huntly et al81 with permission.
Direct comparison of ABL-BCR expression with clinical outcome has shown that loss of ABL-BCR transcription is not associated with reduced survival or reduced length of chronic phase.81,83 This demonstrates that lack of ABL-BCR expression is not sufficient for the poor prognosis associated with an overt chromosome 9 deletion. However, these results do not exclude the possibility that lack of ABL-BCR expression may be necessary for deletions to confer a poor outcome. It is conceivable that the ABL-BCR protein could directly or indirectly modulate activity of the BCR-ABL protein, a situation described for the reciprocal fusion protein in murine models of acute promyelocytic leukemia (APML).90,91 However, existence of a stable ABL-BCR protein product has not yet been demonstrated.92,93
BCR-ABL transcript levels
Aberrant translocations that generate deletions on the derivative chromosome 9 may also result in the formation of small intronic deletions on the Ph chromosome. Such deletions would need to be small enough to permit formation of a BCR-ABL transcript but could nonetheless remove regulatory elements and thereby modulate BCR-ABL transcription. Several lines of evidence suggest that the level of BCR-ABL tyrosine kinase activity is important in determining the phenotype of BCR-ABL–positive leukemias. An extra Ph chromosome is the most common secondary change seen with development of blast crisis in CML15 ; the p190 BCR-ABL tyrosine kinase associated with ALL has increased kinase activity when compared with the standard CML p210 protein94 ; in murine cell lines BCR-ABL mediates cytokine independence and protection against apoptosis in a dose-dependent manner95 ; and an increase in p210 BCR-ABL expression precedes progression to accelerated phase or blast crisis in CML patients.96
To investigate this potential mechanism, BCR-ABL transcript levels were measured by real-time quantitative reverse transcriptase (RT)–PCR in patients with and without deletions.81 BCR-ABL transcript levels were not discernibly different between the 2 groups. Although the number of patients studied was small, these results argue that deletions are not associated with poor outcome as a consequence of altered BCR-ABL expression.
Deletions may represent a consequence of underlying genetic instability
Deletions may be associated with poor outcome because they represent a consequence of pre-existing genetic instability present within the target cell at the time of the Ph translocation. In this scenario, poor prognosis would not be caused by the deletions but instead would reflect an underlying predisposition to accumulate additional genetic alterations. The fact that deletions occur in a subset of patients could represent heterogeneity within the stem cell compartment and/or differences among individuals.
This is a difficult mechanism to exclude with confidence. Patients with chronic-phase CML do not exhibit genomic instability as assessed by microsatellite analysis,97,98 but it is not clear whether patients with deletions were included in these studies and in any case these studies do not exclude other levels of genetic instability. Since deletions involve double-stranded breaks, any predisposition to such events might be predicted to result in increased chromosomal rearrangements, and yet patients with and without deletions exhibit no difference in the number or type of chromosome rearrangements at diagnosis44 or at blast crisis.81 However, blast crisis may be associated with reaching a threshold of genomic damage, and the number of chromosomal rearrangements present at blast crisis may be similar whatever route is taken to reach that threshold level. Current data therefore do not rigorously exclude the possibility that pre-existing genomic instability gives rise to both an increased probability of derivative chromosome 9 deletions and more rapid disease progression. Interestingly, the presence of genomic instability in a minority of patients prior to the Ph translocation would be consistent with recent reports of chromosome abnormalities in Ph-negative cells in a small number of patients treated with imatinib.99,100
Loss of a tumor suppressor gene
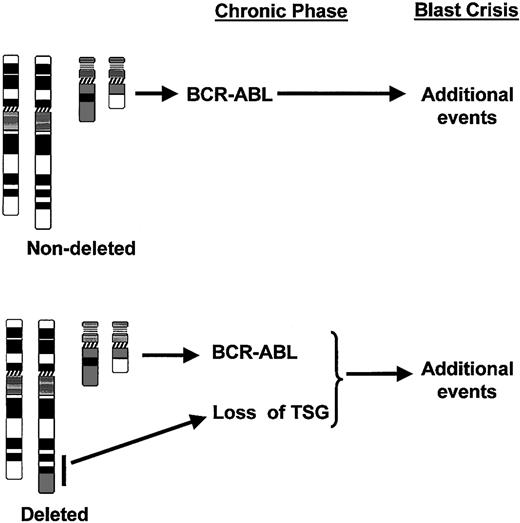
Notwithstanding the caveats already discussed in this section, loss of one or more genes important for disease evolution represents the most likely mechanism to explain the poor prognosis associated with deletions of the derivative chromosome 9. A model for the role of deletions in the progression of CML is shown in Figure 5. It is assumed that CML patients without a deletion develop blast crisis once the malignant clone has accumulated sufficient additional mutations. The model proposes that patients with a deletion have a head start in this process as a consequence of losing a critical gene or genes at the time of the Ph translocation. The molecular lesion associated with deletions may also cooperate with the BCR-ABL oncoprotein to accelerate genomic instability in patients carrying deletions, therefore progressing more rapidly to blast crisis.
Model for the role of deletions of the derivative chromosome 9 in the progression of CML. In patients without deletions, the BCR-ABL gene rearrangement and resultant expression of BCR-ABL protein initiates the chronic phase of the disease. Blast crisis develops with the accumulation of further mutations. However, in the subset of patients with deletions, the recombination event produces not only the translocation but also a deletion (black bar) with the resultant loss of one or more tumor suppressor genes (TSG) from the derivative chromosome 9. The time to blast crisis is therefore reduced since fewer additional mutations are required.
Model for the role of deletions of the derivative chromosome 9 in the progression of CML. In patients without deletions, the BCR-ABL gene rearrangement and resultant expression of BCR-ABL protein initiates the chronic phase of the disease. Blast crisis develops with the accumulation of further mutations. However, in the subset of patients with deletions, the recombination event produces not only the translocation but also a deletion (black bar) with the resultant loss of one or more tumor suppressor genes (TSG) from the derivative chromosome 9. The time to blast crisis is therefore reduced since fewer additional mutations are required.
The biologic consequences of deletion could be a direct effect of haploinsufficiency or a consequence of one or more “second hits” affecting the remaining normal alleles. The deletions are large, extending up to 8 megabases on the chromosome 9 side of the translocation breakpoint48 and up to 17 megabases on the chromosome 22 side.44,81 Existing data do not allow us to ascertain whether the critical area involves chromosome 9 sequences, chromosome 22 sequences, or both. Each of these regions are gene rich and between them contain at least 300 genes.
Mapping the likely location of critical genes will be more difficult than in the case of other deletions that are not associated with a translocation. In these other situations the presence of a clone of cells carrying a deletion implies that there is selection for such cells, and that in each patient their deletion is exerting a biologic effect, presumably as a result of gene loss. By contrast, cells carrying the derivative chromosome 9 deletions will have a growth and/or survival advantage as a consequence of the concomitant Ph translocation. As a result it cannot therefore be assumed that all patients with a deletion will have lost a critical target gene(s). It will be important to take this issue into account when designing strategies for identification of critical target genes.
Methods for detecting deletions
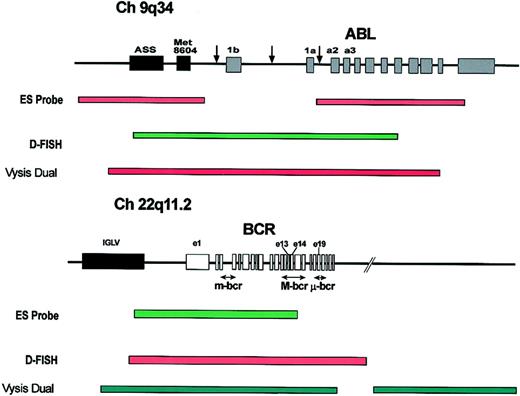
There are 3 commercial FISH probe systems currently available (Figure 6). The major difference between the 3 systems is the 3′ extent of the BCR probe. The ES probe (Vysis, Downers Grove, IL) does not extend beyond the M-bcr region and therefore will not detect loss of chromosome 22 sequences from the derivative chromosome 9. By contrast loss of such sequences will be detected by the D-FISH (Q-biogene, Carlsbad, CA) and dual-color/dual-fusion probes (Vysis) (Figure 6).
Commercially available FISH probe systems for the detection of derivative chromosome 9 deletions. Structure of the ABL and BCR loci showing the common breakpoints (arrows) and the probes used in the commercially available probe systems: the extra signal (ES), D-FISH, and dual color, dual fusion detection system (“Methods for detecting deletions”). ASS indicates arginine succinate synthetase; Met 8604, Met 8604 gene; and IGLV, immunoglobulin lambda light chain locus.
Commercially available FISH probe systems for the detection of derivative chromosome 9 deletions. Structure of the ABL and BCR loci showing the common breakpoints (arrows) and the probes used in the commercially available probe systems: the extra signal (ES), D-FISH, and dual color, dual fusion detection system (“Methods for detecting deletions”). ASS indicates arginine succinate synthetase; Met 8604, Met 8604 gene; and IGLV, immunoglobulin lambda light chain locus.
Different probe systems therefore detect distinct classes of deletion, and this is an important point to remember when using deletion status as a prognostic indicator. The ES probe system will not detect patients who have a deletion that involves only chromosome 22 sequences from the derivative chromosome 9, a situation thought to occur in approximately 5% of patients with deletions.50,81 Similarly by increasing the size of the BCR probe, the dual-color/dual-fusion system provides more robust detection of most such deletions. However, the BCR probe contig may now extend beyond the telomeric end of small deletions downstream of BCR, and such small deletions would no longer be detected. It is not yet known whether patients with small deletions have the same prognosis as those with larger deletions, and this is likely to depend upon the precise location of one or more critical genes. However, it is important to emphasize that different methodologies vary in their ability to detect distinct subtypes of deletion and that different sizes of deletion may have distinct prognostic implications.
In addition to FISH a number of other techniques can be helpful. Microsatellite PCR will detect deletions44 but requires a comparison between tumor DNA (usually granulocytes or bone marrow mononuclear cells) and constitutional DNA (usually T cells or buccal cells). RT-PCR for ABL-BCR can also be useful since the presence of a deletion and ABL-BCR expression are mutually exclusive (Table 3). It is therefore possible to restrict FISH analysis to the 30% to 40% of patients who are ABL-BCR negative, a strategy that significantly reduces the number of patients in whom FISH is required.
A paradigm for other tumors associated with reciprocal chromosomal translocations?
Deletions of a few kilobases at translocation breakpoints have been demonstrated previously in other hematologic malignancies, but, as with Ph-associated deletions, these were thought unlikely to be of any pathologic significance.85-87,101-107 The demonstration of unexpected large deletions in CML has prompted a number of FISH studies of other chromosomal translocations51,108,109 (Table 4). Deletions have been described in association with a number of these translocations, with an incidence of between 2% and 16%. No detailed mapping is available, but as the probes used are comparable in size with the BCR and ABL probes, these deletions must be minimally hundreds of kilobases in size. These deletions further demonstrate previously unsuspected genetic heterogeneity in association with a number of chromsomal translocations. Little data currently exist about the prognostic significance of deletions associated with other translocations. However, in one small series of 20 AML patients with inv(16), who would normally have been considered to have good-risk disease, both patients with associated 3′ CBFB deletions exhibited refractory disease.51
Other hematologic malignancies associated with chromsomal translocations in which large deletions have been demonstrated by FISH
Translocation . | Fusion partners . | Associated hematologic malignancy . | Incidence (%) . | Reference no. . |
|---|---|---|---|---|
| t(9;22) | BCR-ABL | ALL | 2/80 (2) | 51, 74 |
| inv(16) | CBFβ-MYH11 | AML | 2/20 (10) | 51 |
| t(8;21) | AML1-ETO | AML | 6/79 (8) | 51,108 |
| 11q23 | MLL + multiple partners | AL | 9/58 (16) | 51,107 |
Translocation . | Fusion partners . | Associated hematologic malignancy . | Incidence (%) . | Reference no. . |
|---|---|---|---|---|
| t(9;22) | BCR-ABL | ALL | 2/80 (2) | 51, 74 |
| inv(16) | CBFβ-MYH11 | AML | 2/20 (10) | 51 |
| t(8;21) | AML1-ETO | AML | 6/79 (8) | 51,108 |
| 11q23 | MLL + multiple partners | AL | 9/58 (16) | 51,107 |
The published incidence of deletions from FISH studies is shown. ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; and AL, acute leukemia.
Several lines of evidence suggest that balanced translocations may be genetically complex. Differences in the precise genomic breakpoint can produce biologically distinct protein products as seen with the p190, p210, and p230 BCR-ABL proteins.74,110,111 Alternative splicing can result in a single fusion producing more than one protein.74 Moreover, the product(s) of the reciprocal fusion gene may also contribute to the biology of some leukemias.90,91 The story of derivative chromosome 9 deletions in CML further emphasizes that the consequence of an apparently simple translocation may be both varied and complex.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2003-01-0123.
B.J.P.H. is currently a Leukaemia Research Fund (United Kingdom) Senior Clinical Fellow and was previously funded by a Medical Research Council (United Kingdom) Clinical Training Fellowship. Work in the authors' laboratories is funded by the Leukemia Research Fund and the Kay Kendall Leukemia Fund.
We are grateful to past and present members of our labs for helpful discussions, particularly Cath Andrews, Soheila Swanton, Paul Sinclair, Alistair Reid, and Ellie Nacheva for their cytogenetic expertise, and to all the clinicians and cytogenetists who have generously provided us with samples and clinical data.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal