Abstract
There is increasing evidence that serpin conformational alteration caused by single point mutations can be responsible for protein deficiency associated with human diseases. A typical example is the α1-antitrypsin deficiency caused by the Z variant carrying a Glu342Lys substitution. Only a few cases of “conformational disease” involving other serpins have been described so far. We investigated a severe antithrombin deficiency in a 13-month-old child with fever and cerebral venous thrombosis. The infant was found to be homozygous for a new antithrombin gene mutation (7396T>C, predicting a Phe229Leu antithrombin variant), and heterozygous for the factor V Leiden mutation. Mild atypical antithrombin deficiency was found in both parents, who were first cousins, asymptomatic, and heterozygous for the same antithrombin gene mutation. The Phe229Leu variant, which does not readily fit into the current classification of antithrombin deficiency, was shown to be a thermolabile antithrombin that spontaneously polymerized in the proband's circulation. This points to a key role for the conserved Phe at position 229, which is near the reactive site loop in a region critical for serpin function and stability. Molecular modeling suggested how the mutation might destabilize this region of the protein and thereby favor reactive site loop insertion and polymerization. This study provides the first direct evidence of antithrombin polymerization in vivo causing antithrombin deficiency and severe thrombotic disease.
Introduction
Antithrombin (AT) is a 58-kDa plasma protein that regulates hemostasis by inhibiting procoagulant serine proteases such as thrombin and factor Xa. Heparan sulfate glycosaminoglycans lining the vascular endothelium, as well as heparin administration, strongly accelerate these inhibitory reactions. Inherited AT deficiency is associated with a 25- to 50-fold increase in the risk of venous thomboembolism.1,2 It is found in 0.5% to 1% of unselected patients presenting with thrombosis.3,4 Depending on the phenotype, which is defined by plasma AT antigen and activity levels, AT deficiencies have been classified into several types and subtypes.5 This classification is clinically relevant, as the thrombotic risk in heterozygous individuals with type II heparin binding site (HBS) deficiency, a qualitative deficiency affecting the heparin-binding domain, is much lower than that in subjects with other inherited AT deficiencies. AT deficiency is inherited as an autosomal dominant trait, and most deficient individuals are heterozygotes. Homozygosity is very rare and is associated with severe thrombotic disease.6,7 Most homozygous individuals have type II HBS deficiency caused by 2 recurrent mutations, although more than 150 “private” mutations can be found in the AT gene mutation database.
AT belongs to the serpin family, whose members are structurally characterized by the presence of 3 β-sheets (A, B, and C) and 9 α-helices (A-I) connected by loop segments.8,9 Inhibitory serpins neutralize their target proteases by forming a stoichiometric 1:1 stable complex involving the serpin reactive center loop (RCL) and the protease active site serine. The RCL is crucial for the function of inhibitory serpins and supports a variety of conformations.10 Indeed, native serpins are unstable proteins that can rearrange to form several distinct stable conformations, such as the cleaved and latent forms elucidated from their crystal structure. In native inhibitory serpins the RCL is exposed to the solvent and is available for interaction with the target proteinase. The inhibition reaction cleaves the scissile bond and inserts the RCL into the A β-sheet.11 This molecular form of the serpin is more stable than the native inhibitory molecule. The latent form is an uncleaved stable form in which the RCL is also inserted into the A β-sheet, a rearrangement that requires displacement of strand 1C.12,13 Finally, in some circumstances, insertion of the RCL from one molecule into the A β-sheet of another molecule, or juxtaposition of the RCL from one molecule and the C β-sheet of another molecule can lead to serpin polymerization, which has been associated with human diseases.14 A well-known example is the Z α1-antitrypsin variant that bears a destabilizing substitution (Glu342Lys) at the base of the RCL. When present in the homozygous state, the Z mutation induces α-antitrypsin polymerization and aggregation in hepatocytes, causing cirrhosis.15,16 Likewise, neuroserpin polymerization in neurons causes neurologic disorders and familial dementia.17 In the same line, a few AT variants are known to have an increased tendency to polymerize in vitro, but in vivo polymerization of AT variants has not yet been described.18-20
We investigated a major AT deficiency in a 13-month-old child with cerebral thromboembolism. The child was found to be homozygous for a new AT variant, AT Phe229Leu, while both parents were heterozygous for the same mutation. Phe229Leu AT appears to be an “unstable” antithrombin variant that spontaneously polymerizes in the circulation.
Patients and methods
Patients
The patients were a 13-month-old boy of Tunisian origin; his parents, who were found to be first cousins; 3 of the mother's sisters; one of the father's brothers; and 7 distant relatives. The boy was admitted to the Hôpital Necker-Enfants Malades for fever and seizures.
AT assay
Heparin cofactor activity in citrated venous blood was measured with a chromogenic assay (Stachrom AT) on an STA analyzer or an ST888 analyzer for microassays (both from Diagnostica Stago, Asnières, France). Progressive AT activity was measured using bovine thrombin and S2238 (Biogenic, Mauguio, France) as substrate, as previously described.21 Immunoreactive AT was measured by means of Laurell immunoelectrophoresis using polyclonal antihuman-AT immunoglobulin G (IgG) antibodies (The Binding Site, Birmingham, United Kingdom). Crossed immunoelectrophoresis was performed with heparin in the first dimension, as described by Sas et al.22
DNA studies
The patients gave their informed consent to genetic analysis. DNA was isolated by the Miller method23 and stored at 4°C. The 7 AT exons and intron-exon junctions were amplified by polymerase chain reaction (PCR) as previously described24 (primer sequences and amplification program available on request). PCR products (8 μL) were submitted to digestion with exonuclease I (10 U) and shrimp alkaline phosphatase (2 U) for 15 minutes at 37°C, using a PCR product presequencing kit (USB; Amersham Biosciences, Freiburg, Germany). After enzyme inactivation, cycle sequencing was performed in 96-well plates using the same set of primers and the Big Dye Terminator Cycle v.2 sequencing kit (PE Applied Biosystems, Warrington, United Kingdom) for 25 cycles (95°C, 30 seconds; 58°C, 15 seconds; 60°C, 4 minutes). After purification by gel exclusion (Sephadex G50; Amersham Biosciences) on 96-well plates (Multiscreen; Millipore, Saint-Quentin en Yvelines, France), the reaction products were analyzed on an ABI prism 3700 DNA analyzer (PE Applied Biosystems). In addition, AluI restriction was used to confirm the presence of the mutation in the homozygous or heterozygous state. An aliquot (10 μL) of a separately amplified exon-4 PCR fragment was incubated overnight with AluI (1 U), and the digestion product was loaded on 6% acrylamide gel and analyzed after ethidium bromide staining.
AT purification, electrophoretic analysis, and heat stability assays
Human AT was purified from citrated frozen plasma essentially according to McKay.25 Latent AT was prepared as previously described.12 Briefly, AT (1 g/L) was incubated for 24 hours at 60°C in 0.35 M citrate, and latent AT was recovered by heparin sepharose chromatography (eluting at about 0.3 M NaCl) and concentrated on a Resource Q column (Amersham Biosciences). The AT concentration was estimated by measuring absorbance at 280 nm (extinction coefficient [E%] = 6.5 cm-1). AT polymers were obtained by heating purified AT (1 g/L) for 1 hour at 55°C.
Because little plasma was available from the proband, small-scale “analytic” AT purification was performed as follows. Citrated plasma was diluted 2-fold in phosphate-buffered saline and applied to a 1-mL heparin column (Hitrap heparin; Amersham Biosciences). Bound material was eluted by a 0.15- to 2.5-M NaCl gradient. Eluted fractions were then analyzed for AT by native polyacrylamide gel electrophoresis (PAGE) followed by immunoblotting. The same procedure was applied to a control plasma.
Nondenaturing PAGE was performed using 8% gel as previously described.26 Proteins were then transferred to nitrocellulose membranes. The membranes were saturated with 5% nonfat dried milk and incubated with polyclonal anti-AT sheep IgGs diluted 1:5000 in Tris (tris(hydroxymethyl)aminomethane)–buffered saline/Tween 0.5% for 2 hours at room temperature, then washed and incubated with a peroxidase-labeled donkey antisheep IgG antibody diluted 1:10 000 in Tris-buffered saline/Tween 0.5% (both antibodies from The Binding Site). AT was revealed by chemoluminescence (ECL; Amersham Biosciences).
The heat stability of Phe229Leu AT in plasma was tested by incubating 4-fold–diluted plasma in phosphate-buffered saline for 2 hours at 30°C, 35°C, 40°C, 45°C, 50°C, 55°C, and 60°C. AT cofactor activity was then measured as described in this section.
Structural analysis
X-ray structures of different forms of AT were obtained from the Protein Data Bank.27 The structure of the native AT-heparin complex (file 1azx)28 and of native and latent AT (files 1ant and 2ant)29,30 were analyzed with the Accelrys (San Diego, CA) computer package running on a Silicon Graphics Fuel or O2 workstation (Mountain View, CA). The InsightII, Biopolymer, Discover, and Homology modules were used to analyze the Phe229Leu mutation. Side-chain conformational searches were performed for F229 and L229 by using the rotamer library of Ponder and Richards.31 Steric clashes (van der Waals overlap) and nonbonded interaction energies (coulombic and Lennard-Jones) were evaluated for the different side-chain conformations. Three different x-ray structures were superimposed and the area around residue 229 was investigated. Solvent accessibility was computed according to Lee and Richards.32 Multiple sequence alignment information was obtained from the data reported by Irving et al.33
Results
Case report
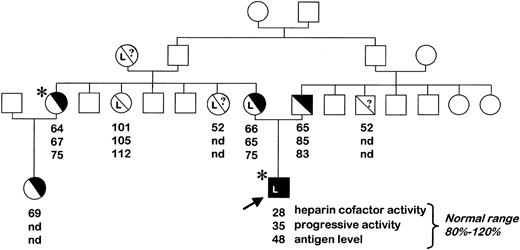
The proband was a 13-month-old boy, the only son of a family of Tunisian origin, who was admitted for fever and seizures. He had suffered a minor head injury few days previously, suggesting a subdural hematoma. However, magnetic resonance imaging showed major venous thrombosis of the longitudinal sinus. Otitis was also diagnosed and was treated with antibiotics. On admission the boy had very low AT heparin cofactor activity (11%; normal range, 80%-120%). The prothrombin, factor V, and factor X levels were 69%, 37%, and 102%, respectively (normal range, 70%-100%), while the activated partial thromboplastin time (APTT) level was normal. The amidolytic protein C level was low (41%), and the total and free protein S levels were normal. Intravenous heparin and AT concentrates (Aclotine; LFB, Saint-Quentin en Yvelines, France) were used as initial anticoagulant therapy in order to maintain the AT level at about 80%. This treatment was switched to oral warfarin anticoagulant therapy after one month. The treatment was difficult to equilibrate because of ongoing phenobarbital therapy. Follow-up magnetic resonance imaging and angiography 18 months later showed that the thrombosis had completely disappeared. Despite slightly delayed acquisition of language and motor milestones, the child, who is now 3 years old, is well on long-term oral anticoagulant therapy.
The parents, who were found to be first cousins, had borderline AT levels consistent with AT deficiency (Figure 1). The father is asymptomatic. The mother's first pregnancy ended in fetal loss at 7 months, and she received heparin prophylaxis during her second pregnancy. Her elder sister had a pulmonary embolism at the age of 38 years, while on oral contraception, and has since been on lifelong anticoagulant treatment.
Pedigree of the AT-deficient family. Plasma AT levels are indicated (nd indicates not determined). The proband, indicated by the arrow, had a severe type II AT deficiency and was homozygous for the 7396T>C mutation. Individuals heterozygous for the factor V Leiden mutation are noted (L), as are symptomatic subjects who had ascertained thrombosis (*). White squares/circles refer to individuals who were not investigated.
Pedigree of the AT-deficient family. Plasma AT levels are indicated (nd indicates not determined). The proband, indicated by the arrow, had a severe type II AT deficiency and was homozygous for the 7396T>C mutation. Individuals heterozygous for the factor V Leiden mutation are noted (L), as are symptomatic subjects who had ascertained thrombosis (*). White squares/circles refer to individuals who were not investigated.
Identification of a new homozygous AT mutation
The proband's prothrombin, factor V and X, and protein C levels were normal 2 weeks after the thrombotic event, indicating that the initial decrease in protein C and factor V was incidental and most likely due to a consumption process. However, in the absence of heparin and AT therapy, AT heparin cofactor activity subsequently remained very low (28%), with progressive AT activity and antigen levels of 35% and 48%, respectively (normal range, 80%-120%), which remained stable over time. Family investigations confirmed a modest decrease in AT heparin cofactor activity and borderline antigen levels in both parents, and also in the mother's elder sister, in keeping with type II AT deficiency (Figure 1). AT gene analysis revealed a new mutation (7396T>C) in exon 4, changing codon 229 (TTC) to CTC and resulting in a new AT variant (Phe229Leu). The proband was homozygous for the mutation, while both parents and the mother's elder sister were heterozygous. Since the mutation introduced a new AluI restriction site at nucleotide (nt) 7394, the homozygous state of the proband and the heterozygosity of both parents could be confirmed. Indeed, the wild-type exon-4 PCR product (492 base pair [bp]) was cleaved by AluI into 2 fragments of 378 and 114 bp, whereas an additional band of 318 bp appeared in the heterozygotes. Only the 318-bp and 114-bp cleavage fragments were present in the proband. Screening for the mutation in 7 nondeficient family members confirmed its cosegregation with the deficiency (not shown). Screening for other genetic risk factors for thrombosis indicated that the proband and his mother were both heterozygous for the factor V Arg506Gln mutation. The prothrombin gene 20210G>A mutation was not found in any of the family members tested.
Phe229Leu AT polymerizes in plasma
To characterize the AT variant Phe229Leu, we first compared homozygous plasma with heterozygous and normal plasma. Crossed immunoelectrophoresis was performed with heparin in the first dimension (not shown). Homozygous plasma yielded an unusual migration pattern, with 2 peaks (one normal and one with low heparin affinity) and a “high background” area between the peaks. This profile was repeatedly obtained with distinct plasma samples and was stable over time, suggesting that the homozygous proband's plasma Phe229Leu AT was a heterogeneous protein. This suggested either that Phe229Leu AT was partially bound to one or more plasma proteins or that distinct Phe229Leu AT species were present in the circulation. No such heterogeneity was found in the heterozygotes' plasma, in which AT migrated as a single main peak (like normal AT) plus a very minor peak with low heparin affinity.
Native PAGE analysis confirmed that distinct Phe229Leu AT species were indeed present in the proband's circulation (Figure 2A). As expected, normal plasma AT migrated as a single band, and the heterozygotes' plasma AT yielded basically the same profile. In contrast, 3 other, less mobile bands were obtained with the proband's plasma. This pattern was similar to that obtained with heat-polymerized AT, strongly suggesting that heterogeneity of Phe229Leu AT in plasma was due to AT polymers rather than to binding to other plasma proteins. Further tests for polymerized AT in the heterozygotes' plasma, using larger loads and longer revelation times, showed a weak band with low mobility that did not appear in normal plasma (not shown). Interestingly, analysis of a proband plasma sample obtained during the sepsis-associated thromboembolic event showed a striking decrease in the monomeric species and a predominance of AT polymers (Figure 2B).
Native PAGE analysis followed by immunoblotting indicated that Phe229Leu AT spontaneously polymerized in the proband's plasma. (A) AT migration profile with a control plasma (lane 1), a heterozygous parent (lane 2), and the homozygous proband (lane 3) compared with purified human latent AT (lane 4), native AT (lane 5), and heat-polymerized AT (lane 6). (B) Analysis of the proband's plasma sampled after (lane 1) and during (lane 2) the sepsis-associated thromboembolic event; note the fall in the monomeric AT species during the acute phase.
Native PAGE analysis followed by immunoblotting indicated that Phe229Leu AT spontaneously polymerized in the proband's plasma. (A) AT migration profile with a control plasma (lane 1), a heterozygous parent (lane 2), and the homozygous proband (lane 3) compared with purified human latent AT (lane 4), native AT (lane 5), and heat-polymerized AT (lane 6). (B) Analysis of the proband's plasma sampled after (lane 1) and during (lane 2) the sepsis-associated thromboembolic event; note the fall in the monomeric AT species during the acute phase.
The presence of AT polymers was confirmed by small-scale analytic purification of AT from the proband's plasma. Whereas AT from normal plasma eluted as a minor peak around 0.3 M NaCl and a major peak around 0.8 M NaCl, more than 80% of Phe229Leu AT eluted as monomers and polymers before 0.4 M NaCl. This also indicated that a monomeric AT species with low heparin affinity was present in the proband's plasma. Overall, the results of crossed immunoelectrophoresis, native PAGE, and heparin-affinity chromatography converged to suggest that most of the Phe229Leu AT in the proband's circulation was not in the native conformation but was present as low-heparin-affinity monomers and polymers.
Phe229Leu AT is an unstable variant
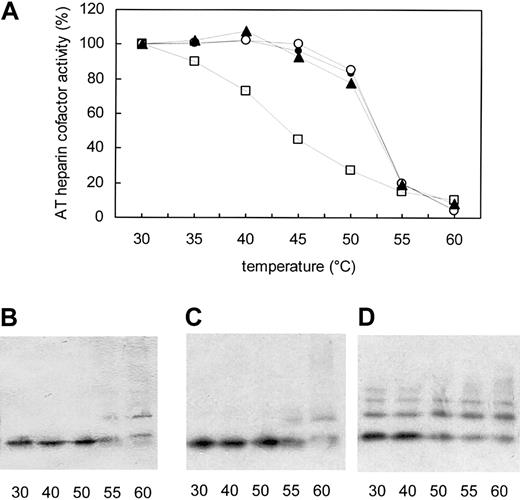
The thermostability of Phe229Leu AT was then tested to determine whether the variant was an unstable serpin. Heat-denaturation assays were performed with homozygous, heterozygous, and normal plasma (Figure 3A). Normal plasma yielded the expected AT denaturation profile, with 50% denaturation (T1/2) occurring at 52°C. Phe229Leu AT was much less stable than the normal protein, with T1/2 occurring at 45°C; 27% of the initial activity was lost after 2 hours at 40°C, whereas no decrease was observed with heterozygous plasma at the same temperature. The heterozygotes' plasma AT showed the same denaturation profile as normal plasma AT. Consistent with these results, PAGE analysis of the heated samples showed a decrease in monomeric AT between 50°C and 55°C in normal and heterozygous plasma, but between 40°C and 50°C in the proband's plasma (Figure 3B-D). These results demonstrated that Phe229Leu AT was a thermolabile variant, with deleterious consequences for protein stability and function when present in the homozygous state but rather minor effects in heterozygotes.
Phe229Leu AT is less thermostable than wild-type AT. (A) Plasma samples from the homozygous proband (□), a heterozygote (▴), and a nondeficient family member (○) and a control plasma (•) were heated for 2 hours at the indicated temperatures before measuring heparin cofactor activity. Results are expressed as a percentage of initial activity. Native PAGE analysis followed by immunoblotting was performed on control (B), heterozygous (C), and homozygous proband (D) samples heated at 30°C, 40°C, 50°C, 55°C, and 60°C. Increased AT polymerization was observed beyond 40°C in the proband's plasma, while the heterozygote's profile was essentially the same as that of normal plasma, AT being stable up to 50°C.
Phe229Leu AT is less thermostable than wild-type AT. (A) Plasma samples from the homozygous proband (□), a heterozygote (▴), and a nondeficient family member (○) and a control plasma (•) were heated for 2 hours at the indicated temperatures before measuring heparin cofactor activity. Results are expressed as a percentage of initial activity. Native PAGE analysis followed by immunoblotting was performed on control (B), heterozygous (C), and homozygous proband (D) samples heated at 30°C, 40°C, 50°C, 55°C, and 60°C. Increased AT polymerization was observed beyond 40°C in the proband's plasma, while the heterozygote's profile was essentially the same as that of normal plasma, AT being stable up to 50°C.
Structural analysis
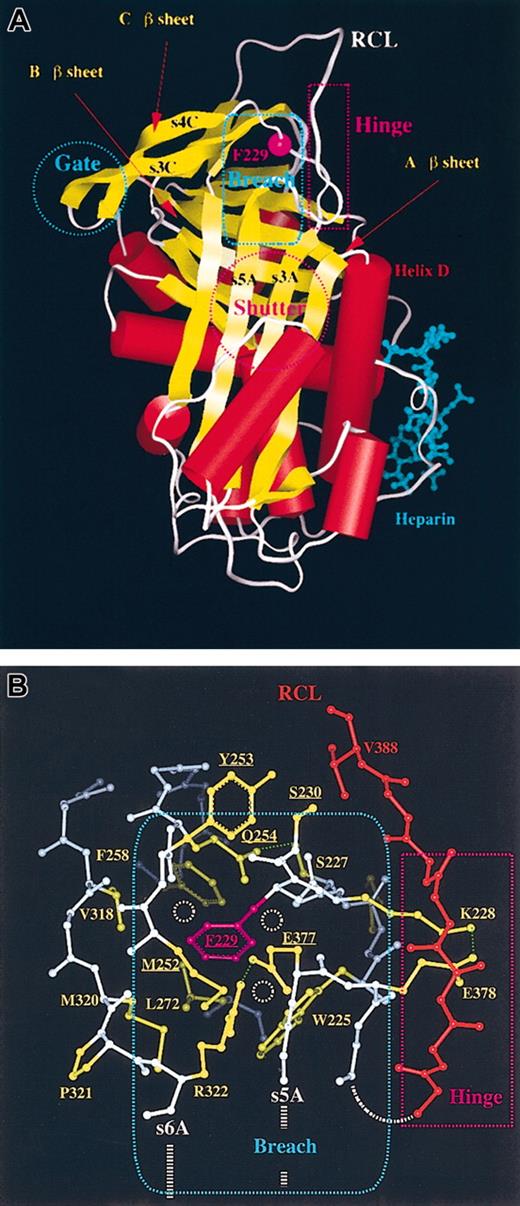
Residue Phe229 is fully buried, highly conserved, and located on a loop connecting strand 3 of β-sheet A with strand 4 of β-sheet C. It is next to the so-called hinge region, part of the breach area, and distant from the heparin-binding region (Figure 4A). The hinge region is located at the top of the A β-sheet and provides mobility for RCL conformational changes, while the breach area is essential for normal RCL insertion into the A β-sheet. The hinge region is also expected to make contact with the C β-sheet of another serpin molecule during some types of polymerization. AT residue 229 has essentially the same orientation and environment in the native, latent, and heparin-complexed forms, and these 3 structures were subjected to structural analysis. Residue Phe229 has hydrophobic and aromatic contacts with the side chains of numerous residues (eg, Trp225, Phe258, Met252, and Leu272; Figure 4B) as well as with some backbone atoms. Computation of nonbonded energy for Phe229, as present in x-ray–deduced AT structures, indicated that it has a rather low apparent energy level. Energy calculations suggested that only one overall conformation for Phe229 could be tolerated without inducing structural changes of surrounding side chains or backbone atoms. Visualization of van der Waals contacts and potential steric overlaps for Phe229, on the basis of the x-ray structure, showed that some empty space was available around this residue. Such small hydrophobic cavities could be slightly destabilizing but should be critical for the conformational changes that take place during RCL insertion. Residue 229 was then replaced on the computer by a Leu, which is shorter than Phe but is bulkier near the Cα atom. All the side-chain orientations (the original position followed the one of Phe229 while the others came from the rotamer library) gave very high relative nonbonded energy values and showed steric clashes with residues Ser230, Met252, Tyr253, Gln254, and Glu377 (side-chain and/or main-chain atoms). This indicated that, in order to tolerate such clashes, small conformational changes would have to occur at and around residue Leu229 (side chain and/or main chain). The shorter Leu side chain should also create an internal cavity that could destabilize this region of AT more markedly than the longer Phe. Such local destabilization is likely to propagate to surrounding residues and could, for instance, disturb normal hydrogen bonding between Gln254 and Ser227 or alter ionic interaction between Glu377 and Arg322 or Lys228 and Glu378. Together with lower packing density around mutated residue 229 (as around Ser230, which is close to RCL Val388), this could locally destabilize the molecule and/or favor conformational changes at the level of the RCL.
AT 3-dimensional structure indicating the location of residue 229. (A) Ribbon overview of native AT complexed with heparin, showing the location of residue 229 (magenta) and key serpin areas (see “Structural analysis”). (B) Close-up of the AT Phe229 area (ball-and-stick rendering). The residues surrounding Phe229 are shown in yellow. Part of the reactive center loop (RCL) is shown in red. Phe229 (magenta) is shown in the same orientation as in panel A. The free space available around Phe229 is symbolized by small dashed circles (white). The Phe229Leu substitution increased the amount of free space that might be filled by local structural reorganizations; alternatively, a destabilizing cavity may remain in the structure. Reorganization and/or cavity formation could induce structural changes that propagate toward the RCL and the breach area itself. In the absence of structural adjustment, a number of residues (mainly Glu377, Met252, Gln254, Tyr253, and Ser230, underlined) could clash with the Leu side chain.
AT 3-dimensional structure indicating the location of residue 229. (A) Ribbon overview of native AT complexed with heparin, showing the location of residue 229 (magenta) and key serpin areas (see “Structural analysis”). (B) Close-up of the AT Phe229 area (ball-and-stick rendering). The residues surrounding Phe229 are shown in yellow. Part of the reactive center loop (RCL) is shown in red. Phe229 (magenta) is shown in the same orientation as in panel A. The free space available around Phe229 is symbolized by small dashed circles (white). The Phe229Leu substitution increased the amount of free space that might be filled by local structural reorganizations; alternatively, a destabilizing cavity may remain in the structure. Reorganization and/or cavity formation could induce structural changes that propagate toward the RCL and the breach area itself. In the absence of structural adjustment, a number of residues (mainly Glu377, Met252, Gln254, Tyr253, and Ser230, underlined) could clash with the Leu side chain.
Discussion
Symptomatic thrombosis is rare during infancy and is usually associated with the presence of serious clinical situations or environmental risk factors for thrombosis.34 However, clotting abnormalities (ie, hereditary deficiencies of protein C and AT, and the presence of the factor V and factor II Leiden mutations) can also favor thrombosis occurrence in childhood.35 We report the case of a 13-month-old boy who suffered from cerebral venous thrombosis that occurred in the absence of severe triggering risk factors (only mild head trauma and otitis). Coagulation studies revealed a severe plasma AT deficiency explained by homozygosity for a new AT gene mutation (7396T>C). The patient was also heterozygous for the factor V Leiden mutation. The AT gene mutation was present at the heterozygous state in both parents (who were first cousins) and in the mother's elder sister. Heterozygotes had borderline AT levels and only one of 3 heterozygous adult relatives investigated—the mother's elder sister—had a relevant thrombosis history (pulmonary embolism at age 38 years while on oral contraception); the father, who was 42 years old, and the mother, who was 40 years old and was also heterozygous for the factor V Leiden mutation, were asymptomatic. That gene-gene interaction increases the risk of thrombosis in families that carry several genetic risk factors for thrombosis is now well established.36,37 However, in this family with hereditary AT deficiency, there is no clear evidence of any influence of factor V Leiden on thrombosis occurrence in heterozygotes, since the only symptomatic adult had a wild-type factor V. The early and severe clinical manifestations in the homozygous proband may be explained by the severity of the AT deficiency; however, a role of the additional genetic risk factor, heterozygosity for factor V Leiden, cannot be ruled out. Fewer than 20 cases of homozygous AT deficiency have so far been reported.5-7 It is often associated with severe early-onset thromboembolic disease, and there is often no family history of thrombosis.7 The case described here supports the current view that only AT deficiencies associated with a low thrombotic risk can occur in the homozygous state; otherwise, homozygosity is probably lethal. No homozygous quantitative (type I) AT deficiencies have been reported, and most homozygotes have type II HBS deficiency, which is associated with a very low or normal thrombotic risk in heterozygous individuals.5,7 Twelve of the 13 well-documented homozygous cases corresponded to type II HBS deficiency, while the last corresponded to type II atypical pleiotropic deficiency caused by the unique “Budapest” variant.38,39 Homozygous type II HBS deficiencies are caused by 2 recurrent AT variants, AT Leu99Phe (8 subjects, 6 unrelated) and AT Arg47Cys (4 unrelated subjects).5 Both substitutions affect the heparin-binding domain and are typically associated with strongly decreased heparin cofactor activity but normal or subnormal antigen and progressive activity levels. Our proband's phenotype, and location of Phe229 outside of the heparin binding area, were not compatible with a type II HBS deficiency but rather with a “pleiotropic” deficiency. Pleiotropic AT variants are characterized both by functional defects and a reduced circulating concentration of abnormal molecules. Indeed, we observed functional defects, since both AT heparin cofactor and progressive activity were decreased, together with the AT antigen level. However, typically, pleiotropic substitutions are located in strand 1C, which was not the case for the Phe229Leu variant.
There is increasing evidence that serpin conformational alterations are responsible for several human diseases. This is the case with the unstable Z α1-antitrypsin variant Glu342Lys, where intrahepatocytic polymerization and aggregation causes cirrhosis and plasma deficiency leads to emphysema.15,16 Neuroserpin is another striking example, as substitutions in the shutter area induce its aggregation in neurons, leading to dementia.17 Three unstable AT variants, namely AT Asn187Asp (Rouen VI) and AT Thr85Met and Thr85Lys (Wibble and Wobble), have been associated with severe thrombosis.18,19 In each case the affected individuals were heterozygous and the purified AT variant showed reduced thermostability and an increased tendency toward latent transition and polymerization in vitro. These conformational changes are expected to play a key role in pathogenesis, but their precise effect in vivo remains elusive. We found that the new Phe229Leu AT was a thermolabile variant that polymerized in circulation. The Phe229Leu AT instability and polymerization readily explained the observed phenotype, including the decreased AT heparin cofactor and progressive activity, since AT polymers are inactive and have low heparin affinity. In addition, the presence of low-heparin affinity AT monomers also contribute the strongly decreased AT heparin cofactor activity in the proband plasma. The low AT antigen level could be explained by increased plasma turnover and/or altered hepatic synthesis. Indeed, we hypothesize that the instability of the Phe229Leu AT is associated with misfolding and polymerization or aggregation inside the hepatocyte, accounting for a decreased rate of secretion.40,41 This mechanism has been demonstrated for the Z α1-antitrypsin variant: folding of the Z variant is slower than that of the wild-type protein, leading to accumulation of a folding intermediate that has a propensity to aggregate in the hepatocyte.41 In fact, the Z α1-antitrypsin variant, but also the rare Siiyama and Mmalton α1-antitrypsin variants, accumulate inside the hepatocyte endoplasmic reticulum. Interestingly, these 3 proteins, unlike wild-type α1-antitrypsin, also circulate under a polymeric form in the homozygotes' plasma.42-45
We found a high level of Phe229Leu AT polymers in the homozygous proband's plasma at normal body temperature. This is consistent with Phe229Leu AT being a thermolabile variant, as wild-type AT polymerizes in vitro at temperatures above 50°C. Very small amounts of AT polymers were detected in the heterozygous relatives' plasma, pointing to an equilibrium between monomeric and polymeric AT species. Polymerization is concentration-dependent, and we hypothesize that a lower level of the variant AT in the heterozygotes does not permit noteworthy polymerization. Alternately, the presence of wild-type native AT might interfere with Phe229Leu polymerization. The mechanism of AT polymer formation is unclear but likely requires initial transition to an intermediate monomeric form, which might be prelatent AT, latent AT, or yet another AT conformation.46 Interestingly, low levels of latent AT were detected in normal plasma, while higher levels have been found in plasma containing unstable variant ATs. We could not unequivocally demonstrate the presence of latent AT in the proband plasma, maybe because, in vitro or in vivo, latent AT can associate with native AT to form a native-latent dimer.18,47,48 This dimer cannot be readily identified in nondenaturing-PAGE since it has nearly the same migration as monomeric native AT. The high level of low-heparin-affinity nonpolymeric AT species present in the proband's plasma would be consistent with the presence of latent AT, which is inactive and binds heparin with low affinity. In addition, formation of an inactive monomer could also explain the small decrease in AT activity observed in the heterozygous relatives, which could not be explained by the very low level of polymers. However, an alternate explanation is that the Phe229Leu AT, even in its native state, has low heparin affinity and is not fully active.
Most known destabilizing serpin mutations affect a domain located at the center of the molecule called the shutter domain, but other regions have also been implicated, including a distant part of the RCL in Z α-antitrypsin and the F helix in AT Rouen VI.19 To our knowledge, no serpin variant affecting Phe229 (Phe198 in the α1-antitrypsin numbering system) has previously been reported. We noted that Phe229 is highly conserved in the serpin family, suggesting that this residue has a role in serpin architecture. A detailed structural study was performed to determine how this mutation may alter AT stability and lead to polymer formation. Phe229 is located within the “breach” area, which is the point of initial RCL insertion into the A β-sheet. This residue is buried and has many stabilizing contacts with surrounding residues (eg, Trp225, Phe258, Met252, and Leu272). However, the packing in this region is not very tight, suggesting that some local structural changes could occur (eg, slight reorganization of side chains and/or backbone atoms) consistent with the molecular function of the breach area. The Phe229Leu mutation is particularly interesting, as it is generally accepted that such a conservative substitution should not cause significant structural changes, severely destabilize a molecule, or significantly alter molecular functions. We found that a Leu residue at position 229 would create several steric clashes with surrounding side chains and/or backbone atoms (mainly Glu377, Met252, Gln254, Tyr253, and Ser230). Thus, local structural changes would be required to accommodate Leu229 at this position, and they would likely disturb some favorable contacts. Minor structural changes could directly propagate toward the RCL and breach area. Moreover, because the Leu side chain is shorter than the Phe side chain, a destabilizing cavity might be created. This could favor spontaneous RCL insertion and transition toward partially or fully RCL-inserted AT molecules prone to polymerization, in keeping with experimental data. In the Z α-antitrypsin variant, substitution of the solvent-exposed breach area Glu342 (Glu377 in AT) by a lysine eliminated an important salt bridge and caused polymerization.14,49 While the Glu342Lys substitution is expected to modify the breach region and, more specifically, an important nonbonded interaction between strand 6A and strand 5A, it is very likely that Phe229Leu disturbs this area in similar fashion. Supporting the role of the Phe229 region in serpin structure and function, it has been previously found that a thyroxine-binding globulin (TBG) variant bearing an Ala191Thr substitution located 3 residues before AT Phe229 was also an unstable serpin, with increased susceptibility to denaturation by heat and acid. TBG Ala191Thr (residue 195 in the α-antitrypsin numbering system) is a variant found in about 40% of healthy Australian Aborigines.50,51 Interestingly, this unstable serpin variant is also associated with plasma deficiency and altered function, as revealed by impaired hormone binding.
Unusually severe sepsis-associated thrombosis has been reported in several heterozygous individuals carrying an unstable AT variant.18-20 In our proband the thrombotic episode occurred during a febrile state related to otitis. Bruce et al19 suggested that fever could further destabilize unstable variant AT, causing a transient decrease in AT activity and resulting in thrombosis. However, this is hard to confirm unless plasma AT levels are measured during a febrile episode.19,20,41 This was the case with our proband, whose heparin cofactor activity measured before treatment was only 11%, compared with 28% after the episode. In addition, PAGE analysis showed a major decrease in monomeric AT, with plasma AT occurring essentially as polymers during the acute phase. These results might be consistent with the fever hypothesis, in which a small increase in body temperature displaces the equilibrium toward the inactive polymeric species. However, clotting assays in our patient pointed to a consumptive process that has likely contributed to the decrease in AT active monomers.
Finally, these results may shed light on the only other unique homozygous AT deficiency, AT Budapest (Pro429Leu AT).35,36 Homozygous plasma Pro429Leu AT was also detected as a heterogeneous protein migrating as a normal and a low-heparin-affinity peak in counterimmunoelectrophoresis. It was purified as 2 distinct species, one retaining its heparin binding activity and some thrombin inhibitory activity, and the other showing weak heparin affinity and no antiproteinase activity. It was then concluded that the decreased heparin affinity was due to an unidentified change in conformation.39 We hypothesize that AT Budapest may also be an unstable AT able to convert to an inactive, low-heparin-affinity conformation that may be monomeric or polymeric. The polymerization hypothesis would be in agreement with gel filtration experiments performed with the proband's plasma that identified 2 AT peaks, one with a molecular weight of 62 000 Da and the other with a molecular weight of 120 000 Da, when only the expected 62 000-Da species was present in normal plasma.52
In conclusion, this report provides the first direct evidence that spontaneous antithrombin polymerization in vivo can cause a plasma deficiency associated with severe venous thrombosis. In addition to this major AT deficiency, heterozygozity for the factor V Leiden mutation and sepsis were identified as additional thrombosis risk factors in the proband. However, the major clinical consequences of homozygosity for this mutation contrasted with the mild effect of heterozygosity, suggesting that other unstable variants remain to be identified in heterozygous individuals.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-11-3391.
Supported in part by grants from Fondation pour la Recherche Médicale (FRM, INE20001117044) (V.P.) and Association pour la Recherche contre le Cancer (ARC4341) (V.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Aurore Devocelle and Nadege Ochat for expert technical support and José Bon-Deguingand and Gisèle Blavy for excellent secretarial assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal