Abstract
As the zinc-finger transcription factor specificity protein 3 (Sp3) has been implicated in the regulation of many hematopoietic-specific genes, we analyzed the role of Sp3 in hematopoiesis. At embryonic day 18.5 (E18.5), Sp3-/- mice exhibit a partial arrest of T-cell development in the thymus and B-cell numbers are reduced in liver and spleen. However, pre–B-cell proliferation and differentiation into immunoglobulin M–positive (IgM+) B cells in vitro are not affected. At E14.5 and E16.5, Sp3-/- mice exhibit a significant delay in the appearance of definitive erythrocytes in the blood, paralleled by a defect in the progression of differentiation of definitive erythroid cells in vitro. Perinatal death of the null mutants precludes the analysis of adult hematopoiesis in Sp3-/- mice. We therefore investigated the ability of E12.5 Sp3-/- liver cells to contribute to the hematopoietic compartment in an in vivo transplantation assay. Sp3-/- cells were able to repopulate the B- and T-lymphoid compartment, albeit with reduced efficiency. In contrast, Sp3-/- cells showed no significant engraftment in the erythroid and myeloid lineages. Thus, the absence of Sp3 results in cell-autonomous hematopoietic defects, affecting in particular the erythroid and myeloid cell lineages.
Introduction
The transcription factor specificity protein 3 (Sp3) belongs to the Sp/X Krüppel-like factor (XKLF) family of nuclear proteins that share a zinc-finger domain containing 3 C2H2-type zinc fingers related to those found in the Drosophila melanogaster regulator protein Krüppel.1 Sp1, Sp3, and Sp4 bind with equal affinity to the classical GC box and the related GT/CACC boxes present in many housekeeping and tissue-specific genes. Sp1 and Sp3 are widely expressed, whereas Sp4 shows a complex expression pattern but is most abundant in neuronal tissues. Sp factors not only are able to stimulate transcription from proximal promoters or from distal enhancers,2 but can also physically interact with other transcription factors, such as E2F-I, nuclear factor–κB (NF-κB), and GATA-1.3-5 Sp1 and Sp3 can have additive or synergistic effects on gene activation, but Sp3 is also able to repress transcription driven by Sp1 or other transcription factors.6,7
The characterization of mutant mice has provided important information about the biologic function of the individual Sp/XKLF proteins. Yet only a limited number of target genes for the Sp/XKLF family members have been identified to date. In particular, the G-rich sequences in the locus control region (LCR) of the β-globin cluster were identified as direct targets of Sp/XKLF transcription factors. Sp1, Sp3, basic KLF (BKLF), and erythroid KLF (EKLF) are all present in erythroid cells, but only EKLF appears to be essential for β-globin expression and activation of the LCR.8-10 EKLF-deficient mice die in utero owing to a severe anemia caused by severely reduced β-globin expression.8,9 BKLF has a role in myeloid proliferation, as BKLF-/- mice have increased numbers of myeloid cells, which also show increased proliferation rates in vitro.11 Lung KLF (LKLF) is required for blood vessel development and has been implicated in the maintenance of mature T cells.12,13 Sp1-null embryos are severely retarded in growth and die after day 10 of embryonic development (E10). As Sp1-/- cells did not contribute to any tissue of newborn chimeric animals, the Sp1 deficiency causes a cell-autonomous defect.14 Sp3-/- embryos are also growth retarded, resulting in prenatal lethality or death at birth, apparently owing to respiratory failure.15 The cause for the observed breathing defect remains obscure, as only minor morphologic alterations were observed in the lung and surfactant protein expression was normal. Furthermore, Sp3-/- mice show a pronounced defect in late tooth formation associated with a deficiency of ameloblast-specific transcripts,15 and impaired skeletal ossification, reflected by significantly diminished expression of the osteoblast-specific marker gene osteocalcin.16 The phenotype of Sp4-/- mice differs from those described for Sp1-/- and Sp3-/- mice, as they develop until birth without obvious abnormalities. After birth, two thirds of the knockout mice die within 4 weeks as a consequence of cardiac arythmia.17 Surviving mice are growth retarded, and male Sp4-/- mice do not breed.18
Sp3 was originally cloned in a search for Sp1-related factors that bind to a GT box required for the transactivation of a T-cell receptor (TCR) Vα gene segment.19 Since then, numerous functional binding sites for Sp factors have been identified that implicate Sp factors in the regulation of T- and B-cell development, for example, in TCR and immunoglobulin-enhancer regions, the promoter that directs germ line transcription of DβJβ gene segments in precursor T lymphocytes, the interleukin-2 receptor (IL-2R) β-chain, and promoter regions of genes encoding the B-cell receptor signaling molecules immunoglobulin-α (Ig-α), Ig-β, and Bruton tyrosine kinase (Btk).2,19-25 In addition, Sp1/Sp3 sites have been shown to be involved in the regulation of macrophage colony-stimulating factor (M-CSF), which is essential for myeloid development.26
Therefore, the presence of Sp-binding sites in various regulatory regions of lymphoid-, erythroid-, and myeloid-specific genes prompted us to investigate hematopoietic development in Sp3-deficient mice. As the Sp3 gene was disrupted by targeted insertion of a lacZ reporter gene,15 we could quantify the Sp3 expression profile in the individual hematopoietic compartments by analysis of β-galactosidase activity. When we compared Sp3-/- embryos with wild-type littermates, we identified a significant delay in the formation of T and B lymphocytes at E18.5, and erythrocytes at E14.5 and E16.5. Moreover, Sp3-deficient E12.5 liver cells did not significantly repopulate the erythroid or myeloid cell lineages in an in vivo transplantation assay. Our findings indicate that the lack of Sp3 intrinsically affects hematopoietic development, especially of the erythroid and myeloid lineages.
Materials and methods
Mice
Embryos were derived from timed matings of Sp3+/- × Sp3+/- mice.15 Genotyping was performed by polymerase chain reaction (PCR), with the use of the following 3 primers: a sense primer in the Sp3 gene amplifying the wild-type allele (5′-GCGTGCAAGCCAGTGGTC-3′); a sense primer in the Neo gene amplifying the knockout allele (5′-AGCGCATCGCCTTCTATCG-3′); and a common antisense primer in the Sp3 gene (5′GGACGATTCTATGCCTCC-3′). PCR conditions were 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute for 30 cycles.
Flow cytometric analysis
The preparation of single-cell suspensions, determination of β-galactosidase activity by means of fluorescein-di-β-D-galactopyranoside (FDG), antibody incubations, and 3- or 4-color cytometry have been described previously.27 The following monoclonal antibodies were obtained from Pharmingen (San Diego, CA): fluorescein isothiocyanate (FITC)–conjugated anti-B220/RA3-6B2 and anti-CD3; phycoerythrin (PE)–conjugated anti-CD4, anti-CD5, anti-CD11b/macrophage 1 (Mac-1), anti-CD19, anti-C23, anti-CD25/IL-2R (clone 3C), anti-CD43/Ser7, anti-CD45RB, anti-CD69, anti–natural killer cell 1.1 (anti-NK1.1), and anti-Ter119; biotinylated anti-IgM, anti-CD4, and anti-CD8; CyChrome–conjugated anti-CD8, anti-CD44, and anti-B220/RA-6B2; peridinin chlorophyll protein (Per-CP)–conjugated anti-CD11b/Mac1; and allophycocyanin (APC)–conjugated anti-CD3, anti-CD5, and anti-CD4. Southern Biotechnology Associates (Birmingham, AL) supplied PE-conjugated anti-IgD. Anti-CD8/53-6.7, anti-CD21, anti–Gr-1/RB6-8C5, and ER-MP20/Ly6-C28 were purified monoclonal antibodies conjugated to biotin according to standard procedures. Secondary antibodies used were Tricolor- or PE-conjugated streptavidin (Caltag Laboratories, Burlingame, CA) or CyChrome- or APC-conjugated streptavidin (Pharmingen).
In vitro culture of fetal liver–derived B and erythroid cells
Primary pre–B-cell cultures were essentially performed as described previously.29,30 In brief, fetal liver cells were cultured in Iscove modified Dulbecco medium (IMDM) medium, supplemented with 10% heat-inactivated fetal calf serum (FCS) at 2 to 3 × 106 cells per well in 24-well plates in the presence of 100 U/mL recombinant IL-7 (R&D Systems, Minneapolis, MN). After 5 days of culture, cells were harvested and recultured on S17 stromal cells with or without 100 U/mL IL-7 for 48 hours.
For suspension culture assays, fetal livers were collected from embryos and disaggregated to single-cell suspensions; then, 5 × 104 cells were placed in 20-μL drops suspended on the lid of a Petri dish (Lindeboom et al, manuscript in preparation).
Colony assays
Colony assays were performed essentially as described.31,32 Fetal livers were disaggregated into single cells by pippeting and were plated out in methyl cellulose containing 1 U/mL erythropoietin (Epo) at a cell density of 15 × 105 and 3 × 105/mL for erythroid colony-forming units (CFU-Es) and erythroid burst-forming units (BFU-Es), respectively. The appearance of colonies was scored after 3 (CFU-Es) and 9 (BFU-Es) days.
Analysis of peripheral blood
E12.5, E14.5, and E16.5 embryos were dissected and bled on a dish to collect the fetal blood in 1 × phosphate-buffered saline (PBS) containing 0.5 mM EDTA (ethylenediaminetetraacetic acid). Cell size distributions were determined on a Casy1 instrument (Schärfe System, Reutlingen, Germany). Aliquots of the blood were loaded on a cytofunnel and spun at 1000 rpm for 2 minutes on microscope slides. The preparations were left to air dry and stained with a combined histologic and neutral benzidine-staining procedure.33
In vivo transplantation assay
Fetal liver cell suspensions, prepared by treatment with 0.125% wt/vol collagenase for 1 hour at 37°C in PBS with 10% FCS, were transferred intravenously into 8- to 10-week-old C57BL/6 female mice, which were exposed to a split dose of 9 Gy at a 3-hour interval by a 137Cs source. To provide short-term survival, 2 × 105 C57BL/6 female splenocytes were coinjected. Mice were maintained in filter-top cages and received 0.16% neomycin–supplemented water.34,35 Donor tissues were examined for Sp3 genotype and sex (Y chromosome) by DNA PCR of somite tissue or body remnants from dissected embryos. Donor-derived engraftment was examined by semiquantitative PCR of DNA from peripheral blood with the use of Sp3 wild-type and mutant-specific primers, or primers for the male-specific YMT2 gene.34,35
Results
Expression of the lacZ knock-in reporter gene during T-cell development in the thymus
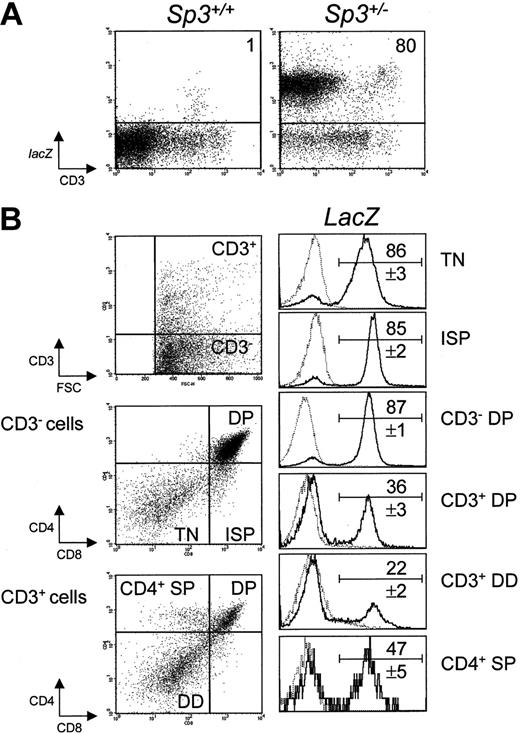
As a role for Sp3 in particular hematopoietic cell lineages may be indicated by modulations of the Sp3 gene expression, we took advantage of the presence of a lacZ reporter gene in the targeted allele of Sp3+/- heterozygous mice, which was placed under direct Sp3 transcriptional control15 (Table 1; Figure 1). The expression of Sp3-directed lacZ was analyzed in flow cytometry experiments, with the use of FDG as a fluorogenic β-galactosidase substrate in conjunction with antibodies specific for the individual hematopoietic cell lineages.
Expression of lacZ in hematopoietic lineages in Sp3+/- E18.5 embyros
Organ and cell population . | Fraction of lacZ cells, % . |
|---|---|
| Liver | |
| B220+ B-lineage cells | 92 ± 1.4 |
| Ter119+ erythroid, large FSC | 75 ± 2.4 |
| Ter119+ erythroid, small FSC | 45 ± 3.4 |
| Spleen | |
| IgM-B220+ pre-B cells | 93 ± 0.7 |
| IgM+B220+ B cells | 92 ± 3.7 |
| Ter119+ erythroid, large FSC | 90 ± 2.6 |
| Ter119+ erythroid, small FSC | 38 ± 3.7 |
| Bone marrow | |
| Ly-6Cmed granulocyte precursors* | 72 ± 3.8 |
| Ly-6Chigh monocyte precursors | 89 ± 1.1 |
Organ and cell population . | Fraction of lacZ cells, % . |
|---|---|
| Liver | |
| B220+ B-lineage cells | 92 ± 1.4 |
| Ter119+ erythroid, large FSC | 75 ± 2.4 |
| Ter119+ erythroid, small FSC | 45 ± 3.4 |
| Spleen | |
| IgM-B220+ pre-B cells | 93 ± 0.7 |
| IgM+B220+ B cells | 92 ± 3.7 |
| Ter119+ erythroid, large FSC | 90 ± 2.6 |
| Ter119+ erythroid, small FSC | 38 ± 3.7 |
| Bone marrow | |
| Ly-6Cmed granulocyte precursors* | 72 ± 3.8 |
| Ly-6Chigh monocyte precursors | 89 ± 1.1 |
Data are presented as mean values ± standard deviation (SD); n = 4
FSC indicates forward scatter
See de Bruijn et al.28
Analysis of lacZ expression in thymocytes from Sp3+/- embryos by 4-color cytometry. Thymus cell suspensions were loaded with FDG substrate and subseqently stained for CD3, CD4, and CD8. (A) Total thymocytes were analyzed for CD3 expression and lacZ activity. (B) Cells were analyzed for CD3 expression; CD3- and CD3+ fractions were analyzed for CD4 and CD8; and the indicated subpopulations were gated and analyzed for lacZ activity. The results are displayed as histograms of Sp3+/- embryos (bold lines), together with those of wild-type embryos (dashed lines). The numbers are the proportions of lacZ-expressing cells (mean values ± SD, n = 6). Background values in wild-type mice (n = 4) were below 1% in all fractions, except for the CD3+ double-dull (DD) fraction, in which 3% ± 1% of the cells were lacZ+.
Analysis of lacZ expression in thymocytes from Sp3+/- embryos by 4-color cytometry. Thymus cell suspensions were loaded with FDG substrate and subseqently stained for CD3, CD4, and CD8. (A) Total thymocytes were analyzed for CD3 expression and lacZ activity. (B) Cells were analyzed for CD3 expression; CD3- and CD3+ fractions were analyzed for CD4 and CD8; and the indicated subpopulations were gated and analyzed for lacZ activity. The results are displayed as histograms of Sp3+/- embryos (bold lines), together with those of wild-type embryos (dashed lines). The numbers are the proportions of lacZ-expressing cells (mean values ± SD, n = 6). Background values in wild-type mice (n = 4) were below 1% in all fractions, except for the CD3+ double-dull (DD) fraction, in which 3% ± 1% of the cells were lacZ+.
In E18.5 Sp3+/- embryos, we found high levels of lacZ activity in the B-lymphoid and myeloid compartments. The differentiation of large into small Ter119+ erythroid cells was associated with a decrease of lacZ activity (Table 1). In the thymus, we identified a modulated expression profile, characterized by a significant downregulation of lacZ expression when differentiating T cells started to express the TCR/CD3 complex (Figure 1A). In the most immature population of CD3-CD4-CD8- triple-negative (TN) thymocytes, lacZ expression was detected in more than 80% of the cells. Also the 4 subpopulations of these TN cells, as defined by differential CD44 and CD25 expression invariably showed greater than 80% lacZ+ cells (data not shown). High levels of lacZ activity were maintained in the next stages of T-cell development, that is, the CD3-CD8+ immature single-positive (ISP) cells, which have successfully rearranged their TCRβ locus, and the CD3-CD4+CD8+ double-positive (DP) cells, which are in the process of TCRα gene rearrangement (Figure 1B). After successful TCRα rearrangement, a complete αβ TCR is expressed on the cell surface, and subsequently a relatively small number of TCRαβ-bearing DP cells are selected for major histocompatibility complex (MHC) recognition and up-regulate surface expression of the TCRαβ/CD3 complex during the process of positive selection.36 At this stage, CD3+ DP cells down-regulate surface CD4/CD8 coreceptor expression, to become CD4lowCD8low double-dull (DD) cells.37 Remarkably, at the CD3+CD4+CD8+ DP and DD cell stages, the proportion of lacZ-expressing cells was significantly down-regulated to approximately 20%. When cells further differentiated into mature CD4 single-positive (SP) cells, the proportion of lacZ+ cells increased to approximately 50%. At E18.5, CD8 single-positive cells were not yet detectable in the thymus.
In summary, these analyses indicate that Sp3 is abundantly expressed in the various hematopoietic lineages. In contrast, during T-cell development, lacZ expression is high in early CD3- precursor T-cell stages, specifically down-regulated during positive selection of TCRαβ+ DP T cells, and finally up-regulated in CD4 SP cells.
Partial arrest of T-cell development at the DP stage in Sp3-deficient embryos
At E18.5, Sp3-/- embryos are smaller (approximately 75%) than their wild-type littermates.15 With this taken into consideration, the development of the thymus appeared to be relatively more affected, as Sp3-/- E18.5 embryos had a considerably smaller thymus, containing only approximately 30% of the cell numbers of wild-type littermates (Figure 2A). To analyze the effect of Sp3 expression on T-cell development in more detail, we determined the sizes of the individual T-cell precursor subpopulations in the thymus of Sp3-/-, Sp3+/-, and Sp3+/+ mice at E18.5 by flow cytometry (Figure 2B). These analyses revealed that in Sp3+/- embryos, the various T-cell precursor populations were present in near-normal abundance, except for the CD4 SP population, which was approximately 60% of normal size. In contrast, the absence of Sp3 resulted in a partial arrest of T-cell development, as the numbers of DP and CD4 SP cells in the Sp3-/- mice were reduced by a factor greater than 3 and greater than 7, respectively, when compared with wild-type littermates.
Impaired T-cell development in embryos lacking Sp3. (A) Thymi of E18.5 embryos of one litter (left) and total numbers of thymocytes (right) of the indicated genotypes. (B) Absolute numbers of thymocytes within the indicated thymic subpopulations, as determined by flow cytometry. Tn = CD3-CD4-CD8-; ISP = CD3-CD8+;DP = CD4+CD8+; and CD4 SP = CD3+CD4+CD8-. (C) FSC characteristics of the indicated thymus subpopulations. Cells were analyzed for the expression of CD3, CD4, and CD8, and FSC characteristics are displayed as histogram overlays of Sp3-/- (bold lines) and Sp3+/+ (dashed lines) embyros. (D) CD3 and CD69 expression in DP (CD4+CD8+) and DD (CD4lowCD8low) thymocytes. Thymus cell suspensions were gated for CD4 and CD8, and results are displayed as histogram overlays for CD3 and CD69 of Sp3-/- (bold lines) and Sp3+/+ (dashed lines) embyros. Data shown are from 2 to 26 embryos in each group.
Impaired T-cell development in embryos lacking Sp3. (A) Thymi of E18.5 embryos of one litter (left) and total numbers of thymocytes (right) of the indicated genotypes. (B) Absolute numbers of thymocytes within the indicated thymic subpopulations, as determined by flow cytometry. Tn = CD3-CD4-CD8-; ISP = CD3-CD8+;DP = CD4+CD8+; and CD4 SP = CD3+CD4+CD8-. (C) FSC characteristics of the indicated thymus subpopulations. Cells were analyzed for the expression of CD3, CD4, and CD8, and FSC characteristics are displayed as histogram overlays of Sp3-/- (bold lines) and Sp3+/+ (dashed lines) embyros. (D) CD3 and CD69 expression in DP (CD4+CD8+) and DD (CD4lowCD8low) thymocytes. Thymus cell suspensions were gated for CD4 and CD8, and results are displayed as histogram overlays for CD3 and CD69 of Sp3-/- (bold lines) and Sp3+/+ (dashed lines) embyros. Data shown are from 2 to 26 embryos in each group.
As these findings indicate that Sp3 deficiency affects T-cell development, particularly at the DP stage, we investigated Sp3-/- and Sp3+/+ DP thymocytes in more detail. We observed that Sp3-/- CD3- DP cells had increased average forward scatter values, suggesting that a large proportion of the Sp3-deficient DP cells still have morphologic characteristics of the actively cycling ISP cells (Figure 2C). However, an increased proliferation rate of Sp3-/- cells at the DP cells seems unlikely, as the total size of the DP compartment in Sp3-/- mice is decreased. Rather, it suggests that the Sp3-deficient DP cells still bear hallmarks of the previous stage owing to a defect in developmental progression. It has been shown that upon MHC/TCRαβ interaction, DP develop into DD cells, up-regulate expression of the TCR/CD3 complex, and start to express the very early activation antigen CD69 on the membrane.37 When we specifically analyzed DP and CD4lowCD8low DD cells, we found that in the absence of Sp3 the induction of CD3 and CD69 expression on the surface of DD cells was significantly reduced (Figure 2D).
In summary, we conclude that the Sp3+/- and Sp3-/- embryos manifest a defect in thymocyte maturation at the DP cell stage. This arrest in differentiation coincides with the observed downregulation of lacZ expression during positive selection of TCRαβ+ DP T cells.
Impaired B-cell development in Sp3-deficient embryos
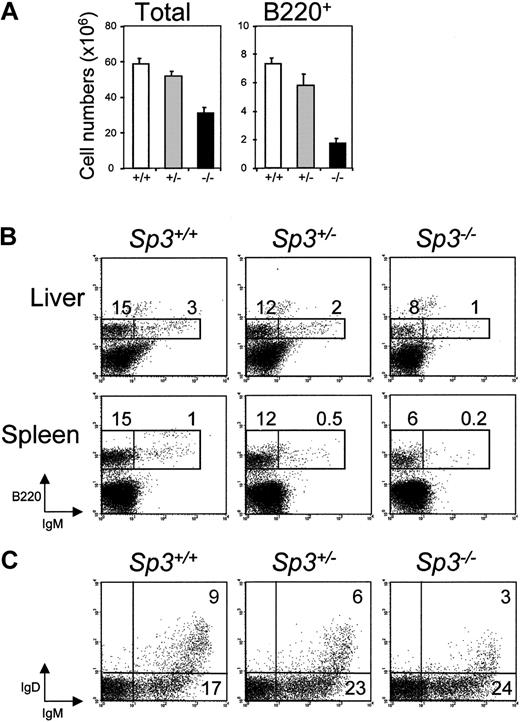
The absence of Sp3 also affected the B-cell system. E18.5 Sp3-/- livers were smaller in size and manifested a reduction of both total cellularity and the absolute numbers of B220+ B-lineage cells, when compared with livers from wild-type littermates (Figure 3A). Moreover, detailed flow cytometric analysis of liver and spleen demonstrated an approximately 2-fold lower percentage of surface IgM- B-cell precursors and an approximately 5-fold lower percentage of IgM+ B cells than normally found at this age (Figure 3B). Intermediate values were found for Sp3+/- mice, indicating an Sp3 gene dosage effect.
Impaired B-cell development in embryos lacking Sp3. (A) Numbers of total cells and B220+ cells in E18.5 liver of the indicated embryos, as determined by flow cytometry. (B) Flow cytometric analyses of the liver and spleen of the individual embryos. Single-cell supsensions were stained with B220 and IgM. Results are displayed as dot plots of lymphocyte gate cells; percentages of cells within the indicated gates are given. (C) Developmental progression of B-lineage cells in vitro. Total BM cells were cultured in the presence of IL-7 for 5 days, and recultured on S17 stromal cells in the absence of IL-7 to induce the formation of mature IgM+IgD+ B cells. Cultured cells were stained for B220, IgM, and IgD. Results are displayed as B220 versus IgD dot plots. Data shown are from 4 to 15 embryos examined in each group.
Impaired B-cell development in embryos lacking Sp3. (A) Numbers of total cells and B220+ cells in E18.5 liver of the indicated embryos, as determined by flow cytometry. (B) Flow cytometric analyses of the liver and spleen of the individual embryos. Single-cell supsensions were stained with B220 and IgM. Results are displayed as dot plots of lymphocyte gate cells; percentages of cells within the indicated gates are given. (C) Developmental progression of B-lineage cells in vitro. Total BM cells were cultured in the presence of IL-7 for 5 days, and recultured on S17 stromal cells in the absence of IL-7 to induce the formation of mature IgM+IgD+ B cells. Cultured cells were stained for B220, IgM, and IgD. Results are displayed as B220 versus IgD dot plots. Data shown are from 4 to 15 embryos examined in each group.
To investigate whether Sp3-deficient pre-B cells have an intrinsic defect in proliferative expansion or differentiation into Ig+ B cells, we performed in vitro IL-7–driven fetal liver pre–B-cell cultures. E18.5 fetal liver cells were cultured in the presence of 100 U/mL IL-7, thereby specifically inducing proliferation of cytoplasmic Igμ heavy-chain-positive pre-B cells.29 After 5 days of culturing, comparable numbers of B220+IgM- pre-B cells were generated in Sp3+/+, Sp3+/-, and Sp3-/- fetal liver cultures. Subsequently, IL-7 was removed from the medium, and the cells were placed on S17 stromal cells for 48 hours to allow further differentiation. Flow cytometric analysis demonstrated that Sp3-/- pre-B cells differentiated normally into surface IgM+ immature B cells upon IL-7 withdrawal. Furthermore, Sp3-/- B cells were able to differentiate into mature IgM+IgD+ B cells, although apparently with a reduced efficiency, as lower levels of surface IgD expression were observed in Sp3-/- cultures (Figure 3C).
Taken together, these results indicate that in the absence of Sp3, B-cell development was impaired, as the numbers of (pre-)B cells in the liver and spleen of Sp3-/- embryos did not reach the levels of normal embryos. The IL-7–driven fetal liver culture experiments show that the proliferative capacity of Sp3-/- pre-B cells and their developmental progression into IgM+ immature B cells in vitro are normal.
The formation of definitive erythrocytes is delayed in Sp3-deficient embryos
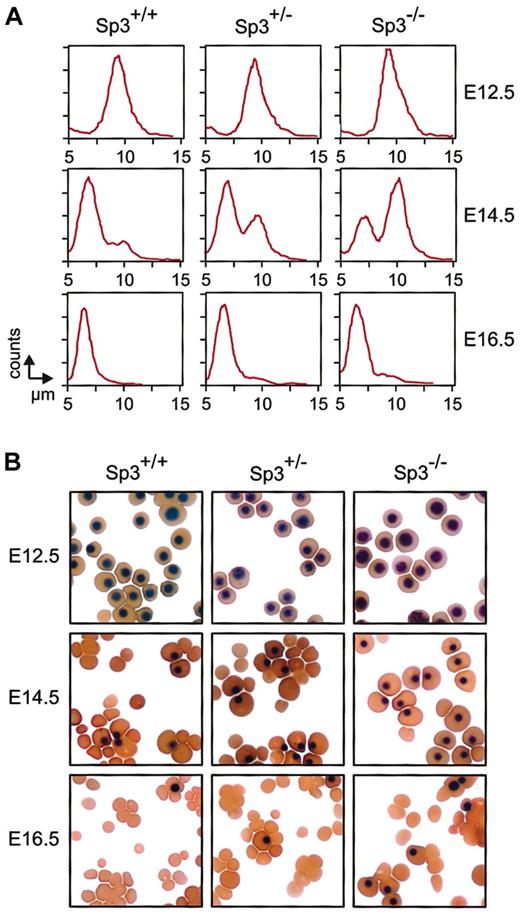
To study the effect of the absence of Sp3 on erythropoiesis, the number and the size of erythrocytes were analyzed in E12.5, E14.5, and E16.5 embryos (Figure 4A). Blood of wild-type embryos contained predominantly large primitive erythroid cells at E12.5, mainly small definitive erythrocytes at E14.5, and almost exclusively small definitive erythrocytes at E16.5, as described previously.38 In contrast, in E14.5 Sp3-/- blood, the majority of red cells were large primitive nucleated erythrocytes. A significant fraction of these primitive erythrocytes was still present at E16.5. As shown in Figure 4A, a delay in the formation of small definitive erythrocytes was also found in Sp3+/- blood, but the phenotype was much less striking. These findings were confirmed in cytospin analyses, where, particularly at E14.5, increased proportions of nucleated primitive erythroid cells were detected in the blood from Sp3-/- embryos and, to a lesser extent, also in Sp3+/- embryos (Figure 4B).
Delayed definitive erythropoiesis in Sp3-deficient embryos. (A) Analysis of the size distribution of circulating erythrocytes at different days of gestation. Yolk sac–derived erythrocytes (primitive) remain nucleated and have a diameter of approximately 10 μm. Fetal liver–derived erythrocytes (definitive) are enucleated and have a diameter of approximately 7 μm. Appearance of definitive erythroytes in the circulation is delayed in Sp3-/- embryos. (B) Cytospins were prepared from blood isolated from the indicated embryos and were stained with a combination of neutral benzidine and histologic dyes.33 Nucleated primitive erythroytes are clearly distinguished from enucleated definitive erythrocytes; both cell types have a normal morphologic appearance in Sp3-/- embryos.
Delayed definitive erythropoiesis in Sp3-deficient embryos. (A) Analysis of the size distribution of circulating erythrocytes at different days of gestation. Yolk sac–derived erythrocytes (primitive) remain nucleated and have a diameter of approximately 10 μm. Fetal liver–derived erythrocytes (definitive) are enucleated and have a diameter of approximately 7 μm. Appearance of definitive erythroytes in the circulation is delayed in Sp3-/- embryos. (B) Cytospins were prepared from blood isolated from the indicated embryos and were stained with a combination of neutral benzidine and histologic dyes.33 Nucleated primitive erythroytes are clearly distinguished from enucleated definitive erythrocytes; both cell types have a normal morphologic appearance in Sp3-/- embryos.
Sp3-deficient erythroid cells display a cell-autonomous differentiation defect
The observed delay in the appearance of circulating definitive erythrocytes could originate either from a delay in the colonization of the fetal liver by hematopoietic stem cells (HSCs) from the intraembryonic aorta-mesonephros-gonad (AGM) region35 and the yolk sac or from an intrinsic defect in the developmental progression of definitive erythrocytes. To distinguish between these 2 possibilities, the numbers of the erythroid progenitors erythroid BFU-Es and CFU-Es were determined in the liver of Sp3+/+, Sp3+/-, and Sp3-/- embryos at E12.5 and E14.5. The numbers of BFU-Es and CFU-Es per fetal liver were reduced approximately 3-fold in Sp3-/- fetal livers (Figure 5A). However, when the values were corrected for the cellularity of the fetal livers, the ratios of BFU-Es and CFU-Es were comparable in the 3 groups of embryos (Figure 5B). These findings indicate that, despite the reduced cellularity, the architecture of the hematopoietic compartment of the Sp3-/- fetal livers is normal, excluding a critical role for Sp3 in the colonization of the liver with HSCs. We therefore conclude that the observed delay in the formation of definitive erythrocytes most likely results from an intrinsic developmental defect of definitive erythroid cells.
Sp3-deficient erythroid cells display a cell-autonomous differentiation defect. (A) The number of BFU-Es and CFU-Es per E12.5 and E14.5 fetal liver was determined for embryos with the indicated genotypes and gestational ages. The values in wild-type embryos were set at 100%. (B) Abundance of BFU-Es and CFU-Es in E12.5 and E14.5 Sp3-deficient embryos corrected for the cellularity of fetal livers. Wild-type values were set at 100%. (C) Fluorescence-activated cell sorter (FACS) analysis of fetal liver cells that were allowed to differentiate in vitro during 0, 2, and 3 days, using the suspension culture system. As the cells progress through maturation, they become smaller. Enucleated cells with small FSC values (lower than 360, as indicated) appear during culture at days 2 and 3.
Sp3-deficient erythroid cells display a cell-autonomous differentiation defect. (A) The number of BFU-Es and CFU-Es per E12.5 and E14.5 fetal liver was determined for embryos with the indicated genotypes and gestational ages. The values in wild-type embryos were set at 100%. (B) Abundance of BFU-Es and CFU-Es in E12.5 and E14.5 Sp3-deficient embryos corrected for the cellularity of fetal livers. Wild-type values were set at 100%. (C) Fluorescence-activated cell sorter (FACS) analysis of fetal liver cells that were allowed to differentiate in vitro during 0, 2, and 3 days, using the suspension culture system. As the cells progress through maturation, they become smaller. Enucleated cells with small FSC values (lower than 360, as indicated) appear during culture at days 2 and 3.
To study the effect of Sp3 on the developmental progression of definitive erythroid cells in detail, E14.5 fetal liver cells were grown for 3 days in a suspension culture system. Since erythroid precursors are taken out of their embryonic microenvironment and cultured under specific conditions for further differentiation, cell-autonomous defects in erythroid differentiation can be identified in this assay.32 After 2 days of culture, 45% of the cells in the Sp3+/+ cultures were mature definitive erythrocytes, as compared with only 36% and 24% in Sp3+/- and Sp3-/- cultures, respectively (Figure 5C). On the third day of culturing, the differences between the 3 groups became less pronounced, but were still significant (Figure 5C).
In summary, these experiments show that Sp3-deficient erythroid cells display a cell-autonomous differentiation defect in vitro. This might explain the delay in the formation of circulating definitive erythrocytes observed in vivo in Sp3-/- embryos and, to a lesser extent, in Sp3+/- embryos.
Sp3-deficient fetal liver cells show severely reduced in vivo hematopoietic repopulation potential
The perinatal death of the Sp3-/- embryos precluded the analysis of adult hematopoiesis in the absence of Sp3. As we observed mild hematopoietic defects in Sp3-heterozygous embryos, we analyzed Sp3+/- mice at 8 weeks of age. However, when we compared the lymphoid, myeloid, and erythroid compartments of Sp3+/- and Sp3+/+ mice by flow cytometry, we did not observe any significant differences (data not shown).
To further define the role of Sp3 in the differentiation of the various hematopoietic cell lineages, we investigated the ability of Sp3-/- fetal liver hematopoietic stem cells and progenitors to contribute to the lymphoid, myeloid, and erythroid lineages in an in vivo transplantation assay.35 We transplanted E12.5 liver cells from Sp3+/+, Sp3+/-, and Sp3-/- embryos into irradiated adult female recipients. To correct for the differences in liver cellularity between the 3 genotypes, equal amounts of total liver cells were injected (approximately 2 × 105 cells). A limiting dose of syngeneic splenic cells was coinjected to aid the recipients in short-term survival following irradiation.
At 4 weeks after transplantation, recipient peripheral blood DNA was tested by PCR for the presence of the donor genetic marker, either the Sp3 mutant allele for the Sp3+/- and Sp3-/- embryos, or the Y chromosome-specific marker YMT2 for the Sp3+/+ embryos. The donor-derived genetic marker was detected to high levels (greater than 10%),35 in all mice that received Sp3+/+ cells (n = 4) or Sp3+/- cells (n = 11). The Sp3 mutant marker was also detected in 6 of 7 recipients receiving Sp3-/- fetal liver cells.
At 6 weeks after transplantation, we examined the extent of multilineage repopulation in mice receiving transplants of Sp3+/- and Sp3-/- fetal liver cells (n = 11 and n = 7, respectively) by flow cytometric analysis of peripheral blood leukocytes. The contribution of donor cells to individual hematopoietic cell lineages was quantified by calculating the percentage of lacZ-expressing cells within the following subpopulations: B220+IgM+/low IgD+/low B cells, CD4+ or CD8+ T cells, NK1.1+ NK cells, Mac-1+Gr-1+ granulocytes, and Mac-1+Gr-1- monocytes (Figure 6). Although the percentages of engraftment varied between individual mice and between cell lineages, Sp3+/- cells were able to repopulate both lymphoid (B, T, and NK cells) and myeloid lineages (granulocytes and monocytes) in 8 of 11 recipients. In contrast, repopulation by Sp3-/- cells appeared to be restricted to the B-cell lineage (5 of 7 recipients). In those mice that showed repopulation of the B-cell lineage with Sp3-/- cells, the proportion of lacZ+ cells was on average lower, when compared with the Sp3+/- group of mice. Only one mouse showed engraftment of Sp3-/- cells in the populations of CD4+ and CD8+ T cells, NK cells, granulocytes, and monocytes, but again the percentages of lacZ+ cells within these cell populations were low and did not exceed 20%.
E12.5 Sp3-deficient fetal liver cells show reduced in vivo hematopoietic reconstitution. Analysis of the peripheral blood for lacZ-expressing cells at 6 weeks after transplantation in the indicated cell lineages in peripheral blood from mice that received Sp3+/- (•) or Sp3-/- (○) fetal liver cells. The mean number of injected cells per recipient was 2.6 × 105 (0.41 ± 0.17 embryo equivalents) for Sp3+/- fetal liver cells and 2.1 × 105 (0.58 ± 0.13 embryo equivalents) for Sp3-/- fetal liver cells. Fetal liver cells from 10 of 11 Sp3+/- embryos and 4 of 7 Sp3+/- embryos were transplanted into 2 mice, and in these cases the symbols show the average values in the 2 mice. In the other cases, the symbols represent the values in individual mice. Only recipients with more than 10% lacZ+ cells are considered positive. For comparison, the proportions of lacZ expression in 3-month-old Sp3+/- heterozygous mice are indicated (approximately 70% in T and NK cells, approximately 80% in monocytes, and approximately 90% in B cells and granulocytes, values that would therefore reflect 100% engraftment).
E12.5 Sp3-deficient fetal liver cells show reduced in vivo hematopoietic reconstitution. Analysis of the peripheral blood for lacZ-expressing cells at 6 weeks after transplantation in the indicated cell lineages in peripheral blood from mice that received Sp3+/- (•) or Sp3-/- (○) fetal liver cells. The mean number of injected cells per recipient was 2.6 × 105 (0.41 ± 0.17 embryo equivalents) for Sp3+/- fetal liver cells and 2.1 × 105 (0.58 ± 0.13 embryo equivalents) for Sp3-/- fetal liver cells. Fetal liver cells from 10 of 11 Sp3+/- embryos and 4 of 7 Sp3+/- embryos were transplanted into 2 mice, and in these cases the symbols show the average values in the 2 mice. In the other cases, the symbols represent the values in individual mice. Only recipients with more than 10% lacZ+ cells are considered positive. For comparison, the proportions of lacZ expression in 3-month-old Sp3+/- heterozygous mice are indicated (approximately 70% in T and NK cells, approximately 80% in monocytes, and approximately 90% in B cells and granulocytes, values that would therefore reflect 100% engraftment).
In summary, from these analyses, we conclude that Sp3-/- fetal liver cells have a reproducible deficiency in short-term repopulation capacity of the hematopoietic system. In this respect, the presence of Sp3 appears to be less critical for reconstitution of the B-cell lineage.
Sp3-deficient fetal liver cells display a specific defect in the ability to repopulate the erythroid and myeloid lineages
The observed reduced repopulation capacity of Sp3-/- E12.5 fetal liver cells could either reflect a delayed development of mature lymphoid and myeloid cells from HSCs or multipotent progenitors or, alternatively, result from specific developmental arrests within these cell lineages.
To distinguish between these 2 possibilities, we analyzed, in more detail, the extent of hematopoietic repopulation in mice that received Sp3+/- (n = 3) and Sp3-/- (n = 3) fetal liver cells at 3 months after transplantation. The percentages of lacZ+ cells were quantified within specific cell lineages present in hematopoietic tissues, including thymus, spleen, blood, bone marrow, and peritoneal cavity. At 3 months after transplantation, repopulation by Sp3+/- cells appeared to be almost complete (Figure 7), as in all analyzed mice the percentages of lacZ+ cells within the lymphoid, myeloid, and erythroid cell lineages were close to the values found in control adult Sp3+/- mice. In contrast to our results at 6 weeks after transplantation, at 3 months after transplantation repopulation by Sp3-/- fetal liver cells was no longer confined to B cells, as the T-cell lineage also displayed significant repopulation by Sp3-/- donor cells: the fractions of lacZ+ cells were 30% ± 18% and 15% ± 5% for CD4+ and CD8+ peripheral blood T cells, respectively (not shown). Figure 7 shows the analyses of the animal receiving transplants of Sp3-/- cells in which the highest percentages of lacZ+ cells were observed. The thymocytes of the recipients grafted with Sp3+/- or Sp3-/- cells showed a similar pattern of modulated lacZ expression as was previously found in E18.5 thymus (Figures 1A,7A). Also in the spleen, Sp3-/- donor-derived cells contributed to the mature CD4+ and CD8+ T-cell populations. Moreover, Sp3-/- CD4+ T cells were not different from Sp3+/- CD4+ T cells in their ability to differentiate in vivo into antigen-experienced CD45RBlow T cells39 (Figure 7B). Sp3-/- cells were less capable of repopulation in the NK-cell lineage (the proportions of lacZ+ cells were 10% ± 5% (n = 3) versus 56% ± 19% in the group of mice with transplants of Sp3+/- cells). Sp3-/- cells contributed substantially to all peripheral B-cell subpopulations, including mature IgMlowIgDhigh follicular B cells and CD21high CD23- marginal zone cells in the spleen, and CD19highCD5+ B-1 peritoneal B cells (Figure 7B and data not shown), as well as to the B-cell compartment in the bone marrow (Figure 7C).
Absence of erythroid and myeloid repopulation by Sp3-/- fetal liver cells in an in vivo transplantation assay. (A) Analysis for CD3 expression and lacZ activity in total thymocytes from Sp3+/- and Sp3-/- transplant recipients. (B-E) Total cell suspensions from spleen (panel B), bone marrow (panel C), peripheral blood (panel D), and peritoneal cavity (panel E) were analyzed for lymphoid-, erythroid-, or myeloid-specific marker expression (left). In Sp3+/- and Sp3-/- transplant recipients, the indicated subpopulations were gated and analyzed for donor-derived lacZ expression (right). The results for transplanted Sp3-/- cells are from the animal in which the highest percentages of lacZ+ cells were observed. LacZ expression data are displayed as histograms; the numbers are the proportions of lacZ+ cells in which background values in wild-type mice were lower than 4%.
Absence of erythroid and myeloid repopulation by Sp3-/- fetal liver cells in an in vivo transplantation assay. (A) Analysis for CD3 expression and lacZ activity in total thymocytes from Sp3+/- and Sp3-/- transplant recipients. (B-E) Total cell suspensions from spleen (panel B), bone marrow (panel C), peripheral blood (panel D), and peritoneal cavity (panel E) were analyzed for lymphoid-, erythroid-, or myeloid-specific marker expression (left). In Sp3+/- and Sp3-/- transplant recipients, the indicated subpopulations were gated and analyzed for donor-derived lacZ expression (right). The results for transplanted Sp3-/- cells are from the animal in which the highest percentages of lacZ+ cells were observed. LacZ expression data are displayed as histograms; the numbers are the proportions of lacZ+ cells in which background values in wild-type mice were lower than 4%.
Even though the donor-derived Sp3-/- cells showed a substantial contribution to the lymphoid lineages, in the same recipient mice these cells did not repopulate the erythroid or myeloid lineages. Very low proportions of lacZ+ cells were found in the large FSC Ter119+ erythroid precursors or the Ly6-Cmed granulocyte and Ly6-Chigh monocyte precursors in the bone marrow (Figure 7C). Also mature granulocytes in the peripheral blood and mature macrophages in the peritoneum did not contain significant populations of Sp3-/-lacZ+ cells (Figure 7D-E).
From these findings, we conclude that at 3 months after transplantation, E12.5 Sp3-/- fetal liver cells can substantially repopulate the B- and T-cell lineages in lethally irradiated recipients. Sp3-/- cells were able to repopulate the NK-cell compartment, although the efficiency was very low. In strong contrast, the absence of Sp3 precluded significant repopulation of the erythroid- and myeloid-cell lineages in the in vivo transplantation assay.
Discussion
In this report, we have studied the impact of the absence of the widely expressed transcription factor Sp3 on the developing hematopoietic system in the mouse. We demonstrate that lymphopoiesis, erythropoiesis, and myelopoiesis are all affected in Sp3 knockout embryos. Since these embryos are retarded in development,15 our observations might merely reflect the general delay in developmental progression. However, several lines of evidence argue against this hypothesis: (1) The finding of phenotypic alterations in erythroid cells in our in vitro cell culture experiments point toward an intrinsic defect in erythropoiesis in Sp3-null cells. (2) Even if the general developmental retardation is taken into account, the appearance of definitive erythrocytes in the circulation of Sp3-null embryos is delayed, and the B- and T-cell precursor populations are reduced in size. (3) The in vivo transplantation assay revealed differential effects of the absence of Sp3 for the individual cell lineages: delayed but almost complete repopulation of the B- and T-lymphoid compartment, and no significant repopulation in the erythroid and myeloid lineages. (4) The observed intermediate phenotype of Sp3+/- embryos with regard to the appearance of definitive erythrocytes suggests a dosage effect. Effects of haploinsufficiency on hematopoiesis have been reported before, for instance, for EKLF40 and acute myeloid leukemia 1 (AML1),41 and are a strong indication for a direct role of the factor in the lineage affected. Therefore, our data collectively suggest that Sp3 deficiency has a specific impact on hematopoiesis.
Sp/XKLF family members and erythropoiesis
The hematopoietic defects of Sp3-null mice are subtle, and it appears unlikely that hampered hematopoiesis contributes significantly to the perinatal lethality. Severe anemia resulting from insufficient production of functional erythroid cells is the cause of embryonic lethality in the knockout of another Sp/XKLF family member, EKLF. In these mice, definitive erythropoiesis is defective owing to failure to activate expression of the adult β-globin genes.8,9 Surprisingly, the embryonic globins and the adult α-globins are expressed normally in the EKLF knockouts. Many, if not all, erythroid-specific genes contain functionally important Sp/XKLF–binding sites in their promoters and other regulatory elements. Yet the transcription factors by which these genes are activated in vivo are still largely unknown, owing to the fact that the Sp/XKLF family comprises more than 20 different transcription factors. At least 5 of these (Sp1, Sp3, BKLF, EKLF, and fetal KLF [FKLF])1 are expressed in erythroid cells. BKLF knockout mice do not appear to display an overt erythroid phenotype, but BKLF is known to be up-regulated in EKLF-null cells.8,11 This suggests that there might be overlapping functionality between BKLF and EKLF. Analysis of compound mutant mice would be required to clarify this issue. We have generated Sp1 knockout mice in our laboratory, but the early embryonic lethality precludes the analysis of definitive hematopoiesis in Sp1-deficient embryos.14 We have now created a conditional knockout allele of the Sp1 mutation. Preliminary data on erythroid-specific knockouts indicate that Sp1 is essential for primitive and definitive erythropoiesis (U. Jägle and S.P., unpublished results, December 2001). Our analysis of Sp4 knockout mice has failed to demonstrate any hematopoietic defect, apart from the reduced cellularity of the thymus and spleen.18 This is thought to reflect a defect in the hormonal pituitary-gonadal axis, since it is known that such defects correlate with a reduced size of the thymus and delayed sexual maturation, as observed in Sp4 knockout mice.18
Sp3 and embryonic/fetal erythropoiesis
The analysis of erythropoiesis in Sp3-null mice presented here demonstrates a role for Sp3 in the transition from primitive to definitive erythropoiesis, and in the progression of erythroid maturation. Moreover, the lack of detectable repopulation of the erythroid compartment in the bone marrow by Sp3-deficient fetal liver cells in the transplantation experiments in vivo, together with the observed cell-autonomous differentiation defect in vitro, indicates that Sp3 function is essential for normal erythropoiesis. However, we did not find evidence for a critical role of Sp3 in the developmental timing of fetal- to adult-globin switching in vivo. In crosses of Sp3-deficient mice with transgenic mice containing the human globin cluster,42 the switch from γ- to β-globin expression takes place between E11.5 and E13.5, irrespective of the Sp3 genotype (P.B. and S.P., unpublished results, September 2001).
Sp3 and lymphopoiesis
In the T-cell lineage, it is of interest to note that lacZ staining indicates down-regulation of Sp3 expression upon transition of thymocytes from the DP to the SP cell stage. Of course, this may imply that Sp3 expression directly regulates genes that promote apoptosis of DP cells. However, Sp3 differs biochemically from the other Sp factors in that it contains an inhibitory domain.43 Sp3 may therefore also function as a repressor of Sp-mediated transcription on some promoters.1,43 Thus, down-regulation of Sp3 at the DP stage may shift the balance of factors bound to GT boxes toward other Sp/XKLF family members. In this context, Sp3 could modulate the activity of the LKLF, which is induced at the progression from DP into SP and appears to play a critical role in programming the quiescent phenotype of SP thymocytes.13 However, Sp3 does not appear to be an essential regulator at this stage of T-cell development, as the observed partial arrest of T development at the DP stage in E18.5 Sp3-/- embryos was not paralleled by a defect at this stage in the mice in the transplantation group that received Sp3-/- fetal liver cells. The lacZ+ DP and DD subpopulations in the mice receiving transplants of Sp3+/- and Sp3-/- cells did not differ in FSC characteristics or the level of CD3 expression (data not shown). Also, the finding of similar contributions of Sp3+/- and Sp3-/- cells to the CD45RBlow population of antigen-experienced CD4+ T cells in the spleen indicates that Sp3-/- T cells can function normally and argues against a crucial role of Sp3 in positive selection in the thymus. Rather, the reduced size of the T-cell compartment in E18.5 embryos and the decreased capacity and kinetics to repopulate the T-cell lineage in the in vivo transplantation assay suggest a subtle defect in the expansion or generation of early T-cell progenitors. As the defects in the B-cell lineage are similar, it is attractive to speculate that Sp3 deficiency affects a common lymphoid progenitor. However, it is still unknown whether the common lymphoid progenitor that has been identified in adult mouse bone marrow also exists in fetal liver.44,45
Sp3 and adult erythropoiesis/myelopoiesis
The transplantation experiments demonstrate that Sp3-/- fetal liver cells have a severely impaired capacity to repopulate the erythroid and myeloid compartments. Our analysis reveals the absence of Sp3-/- myeloid cells at all peripheral sites investigated. In the bone marrow, we found that Sp3-/- cells fail to contribute to the erythroid/myeloid progenitor pool. Since these cells are contributing quite efficiently to the lymphoid system in the same animals, we conclude that Sp3 plays an important role in the development of erythroid/myeloid progenitors. Possibly, Sp3 acts at the early level of the common progenitors to the erythroid and myeloid lineages. Clearly, more experiments are needed to further define the function of Sp3 in myeloid development. In this regard, it is of interest to note that mice deficient for the Sp/XKLF family member BKLF develop a myeloproliferative disorder,46 raising the possibility of antagonistic functions of Sp3 and BKLF in myelopoiesis.
Conclusions
Here, we describe the effect of the Sp3-null mutation on hematopoiesis. Our findings, in particular the in vivo transplantation assay, showed that the absence of Sp3 results in cell-autonomous differentiation defects in the erythroid and myeloid cell lineage. Sp/XKLF factors share 3 highly conserved DNA-binding zinc fingers, and most hematopoietic cell populations express multiple members of the Sp/XKLF family. In conjunction with the phenotypes of the Sp/XKLF knockouts for which hematopoietic defects have been reported in this paper and elsewhere,8,9,11,13,47 it can be anticipated that Sp/XKLF factors have overlapping functions in hematopoietic cells. We are currently generating compound mutants to address this issue.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-06-1848.
Supported by the Netherlands Organization for Scientific Research (NWO) (R.W.H., F.G., and S.P.) and the Deutsche Forschungsgemeinschaft (DFG) (G.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank H. Dronk from the Erasmus MC animal facility and G. Dingjan from Erasmus MC Department of Immunology, Rotterdam, The Netherlands, for their assistance.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal