Abstract
The incidence of invasive mold infections has increased during the 1990s among patients undergoing allogeneic hematopoietic stem cell transplantation (HCT) after myeloablative conditioning. In this study, we determined risk factors for invasive mold infection and mold infection-related death among 163 patients undergoing allogeneic HCT with nonmyeloablative conditioning. The cumulative incidence rates of proven or probable invasive fungal infections, invasive mold infections, invasive aspergillosis, and invasive candidiasis during the first year after allogeneic HCT with nonmyeloablative conditioning were 19%, 15%, 14%, and 5%, respectively, which were similar to those after conventional myeloablative HCT. Invasive mold infections occurred late after nonmyeloablative conditioning (median, day 107), with primary risk factors including severe acute graft-versus-host disease (GVHD), chronic extensive GVHD, and cytomegalovirus (CMV) disease. The 1-year survival after diagnosis of mold infections was 32%. High-dose corticosteroid therapy at diagnosis of mold infection was associated with an increased risk for mold infection–related death. Overall, nonrelapse mortality was estimated at 22% (36 patients) after nonmyeloablative conditioning, of which 39% (14 patients) were mold infection-related (9% of the overall mortality). More effective strategies are needed to prevent invasive mold infections, which currently account for a notable proportion of nonrelapse mortality after nonmyeloablative allogeneic HCT.
Introduction
The incidence of postengraftment invasive fungal infections, especially invasive aspergillosis, among patients undergoing allogeneic hematopoietic stem cell transplantation (HCT) increased during the 1990s.1 Infections caused by other molds, such as Zygomycetes and Fusarium species, also increased during the late 1990s.1 This increase in invasive mold infections has been attributed to multiple factors, including successful prevention of both candidiasis and cytomegalovirus (CMV) disease early after transplantation and corticosteroid-based treatment of severe graft-versus-host disease (GVHD).1-8
Over recent years, allogeneic HCT with nonmyeloablative or reduced-intensity conditioning regimens has been developed and explored for the treatment of patients who are considered ineligible for myeloablative HCT because of age or medical contraindications.9-13 The toxicity profiles of nonmyeloablative conditioning regimens are generally considered to be lower with fewer myelosuppression and gastrointestinal toxicities than those caused by conventional myeloablative HCT. However, acute and chronic GVHD and their therapies continue to be important risk factors for viral infections, particularly late after HCT.14 Results of several small studies suggest also that invasive fungal infections persist in the late time period after nonmyeloablative allogeneic HCT.15,16 Here, we describe risk factors for and outcomes after invasive fungal infections that occurred among 163 patients given allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning at the Fred Hutchinson Cancer Research Center (FHCRC).
Patients and methods
Study patients
This retrospective analysis was approved by the FHCRC Institutional Review Board. Medical records of 173 consecutive patients given allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning at the FHCRC between December 1997 and October 2001 were reviewed. Ten patients who had a known history of invasive fungal infections before HCT were excluded from the analysis. Characteristics of the 163 remaining patients whose data were retained for risk factor analysis are summarized in Table 1. Median age was 53 years. Most (92%) had hematologic malignancies. Fifty-two patients (32% of the total) had received previous autologous, syngeneic, or allogeneic stem cell transplants with myeloablative conditioning. Donors were HLA-matched relatives (n = 108) or unrelated volunteers (n = 55), and most patients (92%) received granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs).
Patient and transplantation characteristics of allogeneic transplant recipients after nonmyeloablative conditioning
. | . | Invasive mold infection . | . | |
|---|---|---|---|---|
| Factors . | Total . | Yes . | No . | |
| Median patient age, y (range) | 53 (0-72) | 54 (25-67) | 53 (0-72) | |
| Sex, M/F | 107/56 | 15/10 | 92/46 | |
| Underlying diagnosis, n (%) | ||||
| Acute leukemia | 25 | 4 (16) | 21 (84) | |
| CML | 15 | 2 (13) | 13 (87) | |
| MDS/MPD | 18 | 3 (17) | 15 (83) | |
| Lymphoma/CLL/myeloma | 90 | 14 (16) | 76 (84) | |
| Other malignancy | 8 | 2 (25) | 6 (75) | |
| Nonmalignancy | 7 | 0 (0) | 7 (100) | |
| Disease risk, n (%)* | ||||
| High | 82 | 12 (15) | 70 (85) | |
| Low | 81 | 13 (16) | 68 (84) | |
| Prior transplant, n (%) | ||||
| Yes | 52 | 8 (15) | 44 (85) | |
| No | 111 | 17 (15) | 94 (85) | |
| Conditioning, n (%) | ||||
| 2 Gy TBI | 51 | 9 (18) | 42 (82) | |
| 2 Gy TBI + fludarabine | 112 | 16 (14) | 96 (86) | |
| Donor, n (%) | ||||
| HLA-matched related | 108 | 20 (19) | 88 (82) | |
| HLA-matched unrelated | 55 | 5 (9) | 50 (91) | |
| Stem cell source, n (%) | ||||
| PBSC | 148 | 24 (16) | 124 (84) | |
| Bone marrow | 15 | 1 (7) | 14 (93) | |
| Season of transplant, n (%) | ||||
| Winter | 35 | 2 (6) | 33 (94) | |
| Spring | 45 | 8 (18) | 37 (82) | |
| Summer | 49 | 10 (20) | 39 (80) | |
| Fall | 34 | 5 (15) | 29 (85) | |
| CMV risk group, n (%)† | ||||
| Low | 46 | 11 (24) | 35 (76) | |
| Intermediate | 27 | 3 (11) | 24 (89) | |
| High | 90 | 11 (12) | 79 (88) | |
. | . | Invasive mold infection . | . | |
|---|---|---|---|---|
| Factors . | Total . | Yes . | No . | |
| Median patient age, y (range) | 53 (0-72) | 54 (25-67) | 53 (0-72) | |
| Sex, M/F | 107/56 | 15/10 | 92/46 | |
| Underlying diagnosis, n (%) | ||||
| Acute leukemia | 25 | 4 (16) | 21 (84) | |
| CML | 15 | 2 (13) | 13 (87) | |
| MDS/MPD | 18 | 3 (17) | 15 (83) | |
| Lymphoma/CLL/myeloma | 90 | 14 (16) | 76 (84) | |
| Other malignancy | 8 | 2 (25) | 6 (75) | |
| Nonmalignancy | 7 | 0 (0) | 7 (100) | |
| Disease risk, n (%)* | ||||
| High | 82 | 12 (15) | 70 (85) | |
| Low | 81 | 13 (16) | 68 (84) | |
| Prior transplant, n (%) | ||||
| Yes | 52 | 8 (15) | 44 (85) | |
| No | 111 | 17 (15) | 94 (85) | |
| Conditioning, n (%) | ||||
| 2 Gy TBI | 51 | 9 (18) | 42 (82) | |
| 2 Gy TBI + fludarabine | 112 | 16 (14) | 96 (86) | |
| Donor, n (%) | ||||
| HLA-matched related | 108 | 20 (19) | 88 (82) | |
| HLA-matched unrelated | 55 | 5 (9) | 50 (91) | |
| Stem cell source, n (%) | ||||
| PBSC | 148 | 24 (16) | 124 (84) | |
| Bone marrow | 15 | 1 (7) | 14 (93) | |
| Season of transplant, n (%) | ||||
| Winter | 35 | 2 (6) | 33 (94) | |
| Spring | 45 | 8 (18) | 37 (82) | |
| Summer | 49 | 10 (20) | 39 (80) | |
| Fall | 34 | 5 (15) | 29 (85) | |
| CMV risk group, n (%)† | ||||
| Low | 46 | 11 (24) | 35 (76) | |
| Intermediate | 27 | 3 (11) | 24 (89) | |
| High | 90 | 11 (12) | 79 (88) | |
N = 163; with invasive mold infection: n = 25 patients (15%); without invasive mold infection: n = 138 patients (85%)
Patients were stratified based on underlying disease, as described previously15 : high-risk was defined as active or de novo or relapsed acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) (refractory anemia with excess of blasts or excess blasts in transformation), myeloproliferative disorder (MPD), acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), Hodgkin disease (HD), multiple myeloma (MM) regardless of status, accelerated phase or blastic crisis of chronic myeloid leukemia (CML), or renal cell carcinoma; low-risk was defined as nonmalignant diseases including immune deficiency syndrome, any of the above diseases with unknown disease status or in remission except for MM, CML chronic phase, and MDS (refractory anemia with or without ringed sideroblasts)
Classification of the CMV risk group was based on pretransplantation CMV serostatus14 : CMV low-risk (donor and recipient serologically negative), CMV intermediate-risk (donor serologically positive and recipient negative), and CMV high-risk (recipient positive and donor negative or positive)
Conditioning regimens, prophylaxis, and treatment for GVHD
All patients received 2 Gy total body irradiation (TBI) on day 0 with (n = 112) or without (n = 51) fludarabine, 30 mg/m2 body surface area per day on days -4 to -2, followed by cyclosporine (CSP) and mycophenolate mofetil (MMF) after transplantation.9,17 Schedules of CSP were 6.25 mg/kg by mouth twice daily from day -3 to day 35 or 56 for HLA-matched related donors and from day -3 to day 100 for HLA-matched unrelated donors.17,18 In the absence of GVHD, the dose of CSP was then tapered, such that the last dose was scheduled to be given on days 56 to 180 (related donors) or 180 (unrelated donors). Tapering schedules were modified at the discretion of the attending physicians by disease status and activity of GVHD. MMF was given at a dose of 15 mg/kg orally twice daily from day 0 to day 27 for patients with related donors, and 2 or 3 times daily through day 40 with subsequent taper to day 96 for those with unrelated donors.18,19 G-CSF was not given routinely after transplantation. Diagnosis and clinical grading of acute and chronic GVHD were performed according to established criteria.20-22 GVHD was treated with 1 to 2 mg/kg/d prednisolone equivalents, resumption of CSP administration if the dose had been tapered, or both. Initial dosage and tapering schedules of corticosteroids were modified at the discretion of the attending physicians for disease status and activity of GVHD. Guidelines recommend that patients with acute GVHD receive 1 to 2 mg/kg/d prednisolone equivalent for 7 to 14 days, and then taper by 0.2 mg/kg every 5 days. Patients with only upper gastrointestinal GVHD are treated with 1 mg/kg/d prednisone with or without oral beclomethasone. Patients with chronic extensive GVHD were treated according to the tapering schedule for corticosteroids described in Koc et al23 Briefly, 1 mg/kg/d prednisone was given for 2 weeks and gradually tapered on alternate days to reach an every-other-day regimen of administration after 6 weeks. The dosage was maintained at 1 mg/kg every other day until week 20 and tapered to 0.5 mg/kg by week 22. This dosage was maintained until week 40 and then tapered to discontinuation if there was no clinical evidence of chronic GVHD.
Infection prophylaxis, monitoring, and diagnosis
In a majority of patients, transplantations were done on an outpatient basis. Inpatients were housed in rooms equipped with high-efficiency particulate air filtration, but laminar airflow was not used. Fluconazole (400 mg/d) was given to all patients from the start of conditioning to day 75 after transplantation,24,25 or longer for some patients who received corticosteroids for treatment of GVHD. Patients were monitored through day 365 after transplantation with weekly CMV pp65 antigenemia testing for the development of CMV infection and disease.14 Positive antigenemia, defined as more than 1 positive cell per 150 000 cells (or 200 000 cells after January 2001), was treated with ganciclovir twice daily for 1 week or until clearance of antigenemia followed by daily administration of ganciclovir until day 100 or for 3 weeks.14 Patients who had positive serologic test results for herpes simplex virus received prophylactic low-dose acyclovir from day-5 to day 30 or until resolution of mucositis, whichever occurred earlier.26 Patients who had positive serologic test results for varicella-zoster virus received prophylactic low-dose acyclovir until 1 year after transplantation.27 Prophylaxis against Pneumocystis carinii infection was provided primarily with trimethoprim-sulfamethoxazole or alternatively with dapsone (50 mg twice daily).27 Patients receiving systemic immunosuppressive therapy for chronic GVHD received prophylaxis against encapsulated bacteria, which included daily trimethoprim-sulfamethoxazole or penicillin. All patients received prophylactic antibiotics (ceftazidime or ciprofloxacin) if absolute neutrophil counts (ANCs) were less than 500/mm3.27 Patients with persistent fever during administration of prophylactic antibiotics were treated with additional agents (vancomycin, aminoglycosides, and/or amphotericin formulation) as clinically indicated. Physical examination, chest x-ray or computed tomography (CT) scans or both, and blood cultures were performed to identify the source of infections. All specimens were submitted for bacterial, fungal, and viral cultures, including shell vial cultures, which were performed according to standard methods.
Definitions and statistical analyses
In this study, we identified patients who developed proven or probable invasive fungal infections within the first year of transplantation by examination of a computerized microbiology database, histopathology reports, and prospectively collected hospital epidemiology records. Proven or probable invasive fungal infections were defined according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria.28 Briefly, proven disease required histopathologic or microbiologic documentation of disease from biopsied tissues, and infection was considered probable if the fungus was identified from culture of bronchoalveolar lavage (BAL) fluid or sputum when consistent signs and symptoms were present. Patients with only possible invasive fungal infections according to the consensus criteria were not included in this analysis. The day of diagnosis of the fungal infection was the day on which the first positive diagnostic test was performed. For patients whose diagnosis was established after death, the date of death was considered to be the day of diagnosis.
Cumulative incidence curves up to 1 year after transplantation and 6 months after initiating corticosteroids for GVHD treatment were produced for invasive fungal infections. Cumulative incidence curves up to 1 year after onset of acute GVHD were produced for invasive mold infections. One-year survival after invasive mold infections was estimated with Kaplan-Meier curves. Probability of invasive aspergillosis was compared to that of a previously described cohort of myeloablative transplant recipients at the FHCRC.2 Risk factors associated with invasive mold infections and mold infection-related deaths were identified in univariate and multivariable Cox regression models. The end point was the time to diagnosis of fungal infections, with censoring at the date of last follow-up, with relapsed malignancy, graft rejection, subsequent HCT, or death as competing risks. Candidate host variables included patient age, patient sex, risk group of underlying disease, prior history of HCT, and CMV risk group. To identify invasive mold infection risks related to transplantation, we evaluated addition of fludarabine to 2 Gy TBI as conditioning, hematopoietic stem cell source (bone marrow or peripheral blood), donor type (HLA-matched related or unrelated), and season of transplantation. Neutropenia (ANC < 500/mm3), lymphopenia (absolute lymphocyte counts < 300/mm3), and monocytopenia (absolute monocyte counts < 300 /mm3) were included as time-dependent covariates. Other time-dependent covariates included acute and chronic extensive GVHD, administration of corticosteroids for treatment of GVHD (1-2 mg/kg/d prednisolone equivalents), CMV antigenemia, and CMV disease (CMV detected by direct fluorescent antibody or cultured by shell vial or conventional culture from BAL or tissue samples). These time-dependent variables were considered potential risk factors of invasive mold infection only if their onset occurred before the diagnosis of the first infection. All candidate variables were entered into the multivariable models and sequentially eliminated in a stepwise backward fashion until all remaining variables were statistically significant. The univariate and multivariable models containing chronic extensive GVHD also controlled for inapplicable or missing chronic GVHD.
Risk factors evaluated for mold infection-related death included proven diagnosis of invasive mold infections (versus probable), onset of mold infection (day < 100 versus day > 100), CMV as a copathogen, neutropenia, lymphopenia, and monocytopenia at mold infection. Doses of corticosteroid therapy were categorized as daily prednisolone equivalents administered on the day when the mold infection was first diagnosed. Categories were 1 mg/kg/d or less versus more than 1 mg/kg/d, or less than 2 mg/kg/d versus 2 mg/kg/d or more. Because there were low numbers of patients and events available for this analysis, pairwise bivariate models were run for those factors whose univariate models predicting mold infection-related death had P < .20.
Confidence limits for all models were calculated assuming normality of parameter estimates, and P values were calculated with the likelihood ratio test. Association in 2 × 2 tables was analyzed with χ2 and Fisher exact tests, when applicable. In this exploratory study, 2-sided P = .05 or less was considered significant.
Results
Clinical outcomes after allogeneic HCT with nonmyeloablative conditioning
Clinical outcomes after allogeneic HCT with nonmyeloablative conditioning among 163 patients are summarized in Table 2. The median follow-up period was 717 days (range, 73-1548 days) for the 79 surviving patients. The proportions of patients with acute GVHD grades II to IV, grades III to IV, and chronic extensive GVHD were 61%, 15%, and 58%, respectively. Median onsets of acute and chronic extensive GVHD were day 35 (range, days 5-107) and day 100 (range, days 76-394), respectively. A total of 122 patients (75% of the total) required corticosteroid therapy for treatment of acute or chronic GVHD; initial dose of corticosteroids was 1 mg/kg/d (n = 55) or 2 mg/kg/d (n = 67) prednisolone equivalents. The median day of corticosteroids initiation after HCT was day 43 (range, days 5-322). Positive CMV antigenemia was detected in 60% of CMV high-risk recipients, 33% of CMV intermediate-risk recipients, and 2% of CMV low-risk recipients. The median onset of any positive CMV antigenemia was day 41 (range, days 4-238). Fourteen patients (9%) developed CMV disease at a median onset of day 109 (range, days 13-188). Thirty-six patients (22% of the total) died of non–relapse-related causes.
Clinical outcomes after allogeneic HCT with nonmyeloablative conditioning
. | . | Invasive mold infection . | . | |
|---|---|---|---|---|
. | Total . | Yes . | No . | |
| Acute GVHD grades II-IV, n (%) | ||||
| Yes | 99 | 21 (21) | 78 (79) | |
| No | 64 | 4 (6) | 60 (94) | |
| Acute GVHD grades III-IV, n (%) | ||||
| Yes | 25 | 10 (40) | 15 (60) | |
| No | 138 | 15 (11) | 123 (89) | |
| Chronic extensive GVHD, n (%) | ||||
| Yes | 82 | 15 (18) | 67 (82) | |
| No | 60 | 5 (8) | 55 (92) | |
| Corticosteroids for GVHD, n (%) | ||||
| None | 41 | 1 (2) | 40 (98) | |
| 1 mg/kg | 55 | 7 (13) | 48 (87) | |
| 2 mg/kg | 67 | 17 (25) | 50 (75) | |
| CMV antigenemia, n (%) | ||||
| Yes | 64 | 14 (22) | 50 (78) | |
| No | 99 | 11 (11) | 88 (89) | |
| CMV disease, n (%) | ||||
| Yes | 14 | 9 (64) | 5 (36) | |
| No | 149 | 16 (11) | 133 (89) | |
| Nonrelapse mortality, n (%) | ||||
| Yes | 36 | 16 (44) | 20 (56) | |
| No | 127 | 9 (7) | 118 (93) | |
. | . | Invasive mold infection . | . | |
|---|---|---|---|---|
. | Total . | Yes . | No . | |
| Acute GVHD grades II-IV, n (%) | ||||
| Yes | 99 | 21 (21) | 78 (79) | |
| No | 64 | 4 (6) | 60 (94) | |
| Acute GVHD grades III-IV, n (%) | ||||
| Yes | 25 | 10 (40) | 15 (60) | |
| No | 138 | 15 (11) | 123 (89) | |
| Chronic extensive GVHD, n (%) | ||||
| Yes | 82 | 15 (18) | 67 (82) | |
| No | 60 | 5 (8) | 55 (92) | |
| Corticosteroids for GVHD, n (%) | ||||
| None | 41 | 1 (2) | 40 (98) | |
| 1 mg/kg | 55 | 7 (13) | 48 (87) | |
| 2 mg/kg | 67 | 17 (25) | 50 (75) | |
| CMV antigenemia, n (%) | ||||
| Yes | 64 | 14 (22) | 50 (78) | |
| No | 99 | 11 (11) | 88 (89) | |
| CMV disease, n (%) | ||||
| Yes | 14 | 9 (64) | 5 (36) | |
| No | 149 | 16 (11) | 133 (89) | |
| Nonrelapse mortality, n (%) | ||||
| Yes | 36 | 16 (44) | 20 (56) | |
| No | 127 | 9 (7) | 118 (93) | |
N = 163; with invasive mold infection: n = 25 patients (15%); without invasive mold infection: n = 138 patients (85%)
Incidence and timing of invasive fungal infections
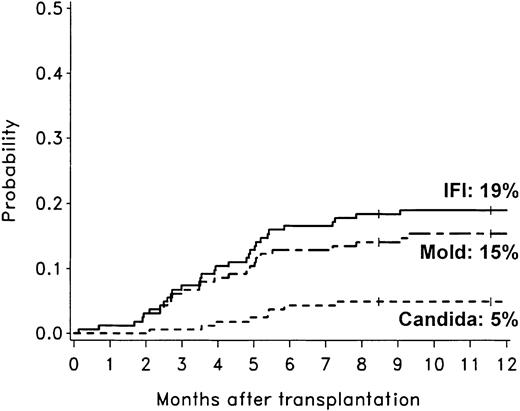
The cumulative incidence rates of proven or probable invasive fungal infections, invasive mold infections, invasive aspergillosis, and invasive candidiasis during the first year after allogeneic HCT with nonmyeloablative conditioning were 19% (31 patients), 15% (25 patients), 14% (23 patients), and 5% (8 patients), respectively (Figure 1). Two patients developed both invasive aspergillosis and candidiasis.
Cumulative incidence rates of invasive fungal infections among 163 patients after allogeneic HCT with nonmyeloablative conditioning. The 1-year cumulative incidence rates of invasive fungal infections (IFI; solid line), invasive mold infections (dashed line), and invasive candidiasis (dotted line) were 19%, 15%, and 5%, respectively. Ticks on probability lines indicate dates of censoring at last follow-up.
Cumulative incidence rates of invasive fungal infections among 163 patients after allogeneic HCT with nonmyeloablative conditioning. The 1-year cumulative incidence rates of invasive fungal infections (IFI; solid line), invasive mold infections (dashed line), and invasive candidiasis (dotted line) were 19%, 15%, and 5%, respectively. Ticks on probability lines indicate dates of censoring at last follow-up.
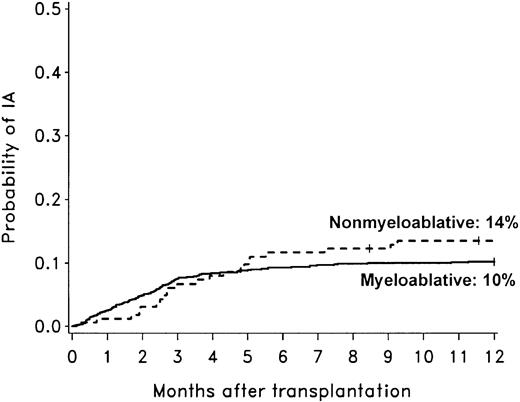
The cumulative incidence rate of invasive aspergillosis after nonmyeloablative conditioning was not different than in a cohort of patients who underwent allogeneic HCT after myeloablative conditioning (Figure 2).2 A survival bias caused by differences in risk of early mortality between nonmyeloablative and myeloablative transplant recipients could have influenced the evaluation of late-onset mold infections. Therefore, we attempted to address this issue by comparing the probability of invasive aspergillosis only among patients who were alive at day 40 in the 2 cohorts shown in Figure 2. The probability of invasive aspergillosis during the first year of transplant was comparable for the 2 cohorts, although there was a trend suggesting a higher risk of invasive aspergillosis among nonmyeloablative transplant recipients (hazard ratio [HR] = 1.54; 95% CI, 0.96-2.47; P = .09).
Comparison of invasive aspergillosis after nonmyeloablative and myeloablative HCT. Cumulative incidence rates of invasive aspergillosis among 163 patients undergoing nonmyeloablative HCT compared to 1673 patients undergoing conventional myeloablative HCT (1993-1998).2 The 1-year cumulative incidence rates of invasive aspergillosis (IA) were 14% after nonmyeloablative conditioning (dotted line) and 10% after conventional myeloablative conditioning (solid line). The diagnostic tests, the methods used to identify cases of invasive aspergillosis, and definitions of infection were similar in the 2 studies.2 Ticks on probability lines indicate dates of censoring at last follow-up.
Comparison of invasive aspergillosis after nonmyeloablative and myeloablative HCT. Cumulative incidence rates of invasive aspergillosis among 163 patients undergoing nonmyeloablative HCT compared to 1673 patients undergoing conventional myeloablative HCT (1993-1998).2 The 1-year cumulative incidence rates of invasive aspergillosis (IA) were 14% after nonmyeloablative conditioning (dotted line) and 10% after conventional myeloablative conditioning (solid line). The diagnostic tests, the methods used to identify cases of invasive aspergillosis, and definitions of infection were similar in the 2 studies.2 Ticks on probability lines indicate dates of censoring at last follow-up.
The median onset of invasive mold infections was day 107 (range, days 4-282) after nonmyeloablative conditioning. Only 2 cases (8% of the total) were diagnosed early (< 40 days) after HCT; 19 cases (76%) were diagnosed between days 40 and 180; and an additional 4 cases (16%) developed later than 6 months after HCT. Most invasive mold infections were identified within lungs (n = 23). Eight patients developed disseminated invasive mold infections. Other involved sites included brain (n = 4), sinus (n = 2), and kidney, heart, spleen, gastrointestinal tract, thigh, and skin (n = 1 each; more than one site involved in 8 patients). Four cases were diagnosed postmortem as proven disease. Organisms that caused invasive mold infections in 25 patients (15 proven, 10 probable) were Aspergillus (n = 22: Aspergillus fumigatus [n = 17], Aspergillus niger [n = 3; 2 of those were coinfections with A. fumigatus], Aspergillus, not specified [n = 4]); zygomycetes (n = 4); and Fusarium (n = 1). Two patients with Zygomycetes infection also had Aspergillus or Fusarium infections.
The median onset of invasive candidiasis was day 156 (range, days 64-221) after nonmyeloablative conditioning. All 8 patients had candidemia, 3 of whom also had proven deep tissue candida infections (lung, n = 2; gastrointestinal tract, n = 2; kidney, n = 1). Species that caused candidemia were Candida glabrata (n = 5), Candida parapsilosis (n = 3), and Candida albicans (n = 1; coinfection with C glabrata). Four patients developed candidemia (C glabrata [n = 3], C parapsilosis [n = 1]) while they were receiving fluconazole prophylaxis.
Risk factors for invasive mold infections
Univariate analysis revealed that acute GVHD grades III to IV, acute GVHD grades II to IV, chronic extensive GVHD, corticosteroid therapy (≥ 2 mg/kg/d prednisolone equivalents), positive CMV antigenemia, CMV disease and lymphopenia (< 300 /mm3) were associated with increased risks of invasive mold infections (Table 3). The following variables were not significant: patient age, patient sex, underlying disease risk, donor, stem cell source, conditioning including fludarabine, prior history of HCT, pretransplant CMV serostatus, season of transplantation, neutropenia, and monocytopenia. Severe acute GVHD grades III to IV (HR = 2.8; 95% CI, 1.1-6.9; P = .04), chronic extensive GVHD (HR = 3.7; 95% CI, 1.0-13.9; P = .04), and CMV disease (HR = 13.3; 95% CI, 4.7-37.7; P < .0001) remained significant in a multivariable model (Table 3). CMV serostatus and CMV antigenemia were omitted from the multivariable model because they were closely associated with CMV disease. When CMV disease was replaced by CMV antigenemia or CMV serostatus in a separate multivariable model, they were not significant.
Univariate and multivariable analysis of risk factors for invasive mold infections after allogeneic HCT with nonmyeloablative conditioning
Variables . | Univariate analysis HR (95% CI) . | Univariate analysis P . | Multivariable analysis HR (95% CI) . | Multivariable analysis P . |
|---|---|---|---|---|
| Acute GVHD grades III-IV | 7.1 (3.1-16.1) | <.0001 | 2.8 (1.1-6.9) | .04 |
| Acute GVHD grades II-IV | 4.7 (1.6-14.3) | .001 | — | — |
| Chronic extensive GVHD | 5.7 (1.7-19.1) | <.002 | 3.7 (1.0-13.9) | .04 |
| Corticosteroids (2 mg/kg or more) | 4.0 (1.7-9.6) | .0009 | — | — |
| CMV antigenemia | 2.9 (1.3-6.7) | .01 | — | — |
| CMV disease | 32.9 (12.6-85.8) | <.0001 | 13.3 (4.7-37.7) | <.0001 |
| Lymphopenia (less than 300/mm3) | 2.2 (1.0-5.0) | 0.05 | — | — |
Variables . | Univariate analysis HR (95% CI) . | Univariate analysis P . | Multivariable analysis HR (95% CI) . | Multivariable analysis P . |
|---|---|---|---|---|
| Acute GVHD grades III-IV | 7.1 (3.1-16.1) | <.0001 | 2.8 (1.1-6.9) | .04 |
| Acute GVHD grades II-IV | 4.7 (1.6-14.3) | .001 | — | — |
| Chronic extensive GVHD | 5.7 (1.7-19.1) | <.002 | 3.7 (1.0-13.9) | .04 |
| Corticosteroids (2 mg/kg or more) | 4.0 (1.7-9.6) | .0009 | — | — |
| CMV antigenemia | 2.9 (1.3-6.7) | .01 | — | — |
| CMV disease | 32.9 (12.6-85.8) | <.0001 | 13.3 (4.7-37.7) | <.0001 |
| Lymphopenia (less than 300/mm3) | 2.2 (1.0-5.0) | 0.05 | — | — |
Other variables examined included patient age, patient sex, underlying disease risk, donor, stem cell source, conditioning including fludarabine, prior history of HCT, CMV risk group, season of transplantation, neutropenia, and monocytopenia
— indicates not significant in the multivariate model
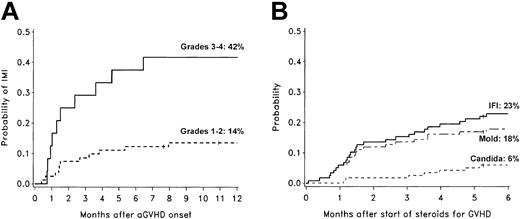
Of 25 invasive mold infections, 21 occurred after the onset of acute GVHD. There was a 42% probability of invasive mold infection for patients with grades III to IV acute GVHD, compared to a 14% probability for patients with grades I to II acute GVHD (Figure 3A). Only 6% of patients with grade 0 acute GVHD developed invasive mold infections. Half of the invasive mold infections occurred within 46 days, and 84% occurred within 6 months after beginning corticosteroid therapy (≥ 1 mg/kg/d prednisolone equivalents) for acute or chronic GVHD (Figure 3B).
Invasive fungal infections and GVHD. Cumulative incidence rates of invasive mold infections among 105 nonmyeloablative transplant recipients who developed acute GVHD (A) and cumulative incidence rates of invasive fungal infections among 118 nonmyeloablative transplant recipients who were treated with corticosteroids for GVHD (B). Patients who developed invasive fungal infections before the onset of acute GVHD or before beginning corticosteroid therapy were not included in these graphs. The 1-year cumulative incidence rates of invasive mold infection (IMI) were 42% in patients with grades III to IV GVHD (n = 24; solid line) and 14% in patients with grades I to II GVHD (n = 81; dotted line). The 6-month cumulative incidence rates of invasive fungal infections (IFI; solid line), invasive mold infections (dashed line), and invasive candidiasis (dotted line) after beginning corticosteroid therapy (≥ 1 mg/kg/d prednisolone equivalents) were 23%, 18%, and 6%, respectively. Ticks on probability lines indicate dates of censoring at last follow-up.
Invasive fungal infections and GVHD. Cumulative incidence rates of invasive mold infections among 105 nonmyeloablative transplant recipients who developed acute GVHD (A) and cumulative incidence rates of invasive fungal infections among 118 nonmyeloablative transplant recipients who were treated with corticosteroids for GVHD (B). Patients who developed invasive fungal infections before the onset of acute GVHD or before beginning corticosteroid therapy were not included in these graphs. The 1-year cumulative incidence rates of invasive mold infection (IMI) were 42% in patients with grades III to IV GVHD (n = 24; solid line) and 14% in patients with grades I to II GVHD (n = 81; dotted line). The 6-month cumulative incidence rates of invasive fungal infections (IFI; solid line), invasive mold infections (dashed line), and invasive candidiasis (dotted line) after beginning corticosteroid therapy (≥ 1 mg/kg/d prednisolone equivalents) were 23%, 18%, and 6%, respectively. Ticks on probability lines indicate dates of censoring at last follow-up.
Outcomes after invasive fungal infections
Initial antifungal treatments for patients with invasive mold infections included amphotericin formulation (n = 21) and itraconazole (n = 3). One patient did not receive treatment because of terminal liver failure. Five patients also received voriconazole with or without caspofungin as secondary therapies.
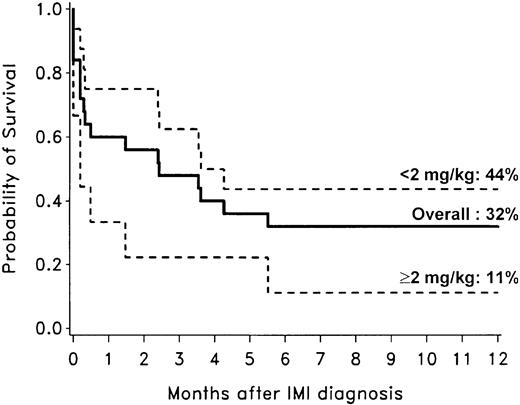
Among 25 patients with invasive mold infections, 20 died, and 14 of the deaths were related to the mold infection. Overall nonrelapse mortality was estimated at 22% (36 patients) after nonmyeloablative conditioning, of which 39% (14 patients) were related to the mold infection. Thus, mold infections contributed to 9% of overall mortality. The Kaplan-Meier probability of survival among patients with invasive mold infections 1 year after diagnosis was 32% (Figure 4). Outcomes were dependent on GVHD treatment requirements. The 1-year overall survival of patients with invasive mold infections whose corticosteroid doses were less than 2 mg/kg/d at the time of diagnosis was 44%. In contrast, survival among 9 patients whose corticosteroid doses were 2 mg/kg/d or more (3 patients received additional immunosuppression including rapamycin [sirolimus] and anti–T-cell antibodies) was only 11% (Figure 4).
Overall survival after diagnosis of invasive mold infections among 25 allogeneic transplant recipients with nonmyeloablative conditioning. The 1-year overall survival for all 25 patients with mold infection (solid line) was 32%. Among 9 patients whose corticosteroid doses were 2 mg/kg/d or higher at the time of diagnosis (dotted line), the 1-year overall survival was 11%, but survival was 44% in 16 patients whose corticosteroid doses were less than 2 mg/kg/d (dotted line).
Overall survival after diagnosis of invasive mold infections among 25 allogeneic transplant recipients with nonmyeloablative conditioning. The 1-year overall survival for all 25 patients with mold infection (solid line) was 32%. Among 9 patients whose corticosteroid doses were 2 mg/kg/d or higher at the time of diagnosis (dotted line), the 1-year overall survival was 11%, but survival was 44% in 16 patients whose corticosteroid doses were less than 2 mg/kg/d (dotted line).
All 8 patients who had developed invasive candidiasis died from causes other than candidiasis within 6 months after diagnosis. Therefore, the risk factor analysis focused on mold infection-related deaths. The bivariate model with both factors having the greatest statistical significance showed that corticosteroid therapy (≥ 2 mg/kg/d prednisolone equivalents; HR = 6.7; 95% CI, 1.8-25.1; P = .005) and proven diagnosis of invasive mold infections (HR = 7.1; 95% CI, 1.6-31.7; P = .004) were associated with statistically significantly increased risks of mold infection-related death.
Discussion
This study demonstrated that invasive fungal infections accounted for a notable portion (39%) of nonrelapse mortality among patients undergoing allogeneic HCT after nonmyeloablative conditioning. In the largest cohort of patients receiving nonmyeloablative conditioning examined to date, we have demonstrated that invasive mold infections typically occurred late, with primary risk factors including severe acute GVHD, chronic extensive GVHD, and CMV disease. The corticosteroid dose administered for GVHD treatment was an important predictor of deaths related to mold infection.
Recent reports have indicated that the incidence rates of invasive mold infections (8%-16%) have increased among recipients of allogeneic hematopoietic cell transplants after myeloablative conditioning, particularly after engraftment.1,3-5,7,29,30 The cumulative incidence rates of invasive aspergillosis (14%) or invasive mold infections (15%) after nonmyeloablative conditioning in the current study was similar to the incidence after conventional myeloablative HCT. The diagnostic tests, the methods used to identify cases of invasive mold infections, and definitions of infection were similar to those used for our previous cohort of patients undergoing myeloablative HCT.1,2 Because we used a very conservative definition of invasive aspergillosis (“possible” cases were not included), cumulative incidence rates reported were likely to have underestimated the true infection burden.
Several small studies have reported variable incidence rates of invasive fungal infections among patients undergoing HCT after nonmyeloablative or reduced-intensity conditioning.15,16,31,32 Although there were no cases of invasive fungal infections after nonmyeloablative conditioning in 2 reports,31,32 Hagen et al16 reported a high rate of invasive fungal infections after nonmyeloablative HCT in a small cohort of 31 patients. In that study, the incidence of proven or probable invasive mold infections was 23% (7 patients).16 Direct comparison of incidence rates of invasive fungal infections with our results is difficult because of differences in donor selection, pretransplantation and posttransplantation immunosuppression, and treatment of GVHD.
Several investigators have described a shift from early- to late-onset invasive aspergillosis after conventional HCT with myeloablative conditioning, which may in part be associated with shorter periods of neutropenia after HCT with the use of PBSC grafts or hematopoietic growth factor administration.2-5,30 The results of the current study also emphasized the risk of late-onset invasive mold infections (median onset, day 107) after allogeneic HCT with nonmyeloablative conditioning. In this setting, the late onset was probably due to a combination of factors, including short periods of or no neutropenia, defense by residual host immunity (initial mixed chimerism), delayed onset of acute GVHD and corticosteroid therapy, and late infection with CMV.9,14,17,33
Nonmyeloablative conditioning reduced the risk of nonrelapse mortality because of lower toxicity, compared to myeloablative regimens, despite greater patient age and frequent comorbid medical conditions.9-13 However, invasive mold infections still accounted for a substantial proportion of nonrelapse mortality after nonmyeloablative conditioning. A survival bias caused by differences in risk of early mortality between nonmyeloablative and myeloablative transplant recipients did not influence the incidence of late-onset mold infections.
Since the introduction of fluconazole prophylaxis, the risks of invasive candidiasis and candidiasis-related mortality have decreased after allogeneic HCT with myeloablative conditioning.25,34 The cumulative incidence rate of invasive candidiasis (5%) after nonmyeloablative conditioning in the current study was similar to the incidence after conventional myeloablative HCT. Of note, 8 of 9 species isolated were C glabrata and C parapsilosis, suggesting an emergence of azole-resistant Candida species after fluconazole, as described before.34 These data suggest that fluconazole prophylaxis was effective for prevention of invasive candidiasis after nonmyeloablative conditioning. Due to the small number of patients with invasive candidiasis, it was not possible to analyze risk factors in this study.
Although neutropenia has been considered to be the primary risk for invasive aspergillosis, multiple studies indicated that invasive aspergillosis occurred in nonneutropenic hosts.2,7,8 Acute GVHD and its treatment with corticosteroids were perhaps the most important risks for the development of fungal infections after myeloablative allogeneic HCT.2,3,7,30 Chronic extensive GVHD was also reported to be a risk factor for invasive fungal infections.3,35 This was thought to be related both to neutrophil dysfunction and to the recently recognized importance of cellular immunity.36-38 A protective role for T lymphocytes was supported by increased risks of invasive aspergillosis associated with T-cell depletion or CD34+ cell selection in the setting of myeloablative HCT.2 More studies will be necessary to determine the relative contributions of cellular and neutrophil-mediated immunity in protection against invasive mold infections late after myeloablative and nonmyeloablative HCT.
An association between CMV disease and fungal infections has been noted not only in solid organ transplant recipients,39,40 but also in recipients of hematopoietic stem cell transplants.2,4,34 Mechanisms to explain the association between CMV infection (and disease) and fungal infections remain obscure. Neutropenia caused by treatment with ganciclovir was unlikely to be the sole explanation because CMV disease remained an independent risk factor even in models that controlled for neutropenia. Other potential mechanisms included the immune-modulating effects of CMV itself, because this virus is known to impair neutrophil activity and macrophage respiratory burst.41,42 Alternatively, clinical factors included in multivariable models might not adequately control for the immune variables that predicted both CMV and fungal infections.
Outcomes were poor among patients who developed invasive aspergillosis after myeloablative HCT, with survival rate estimates at 8% to 22%, regardless of therapy.2,8,43-46 The 1-year overall survival rates were equally poor (∼20%) in patients after diagnosis of aspergillosis, zygomycosis, and fusariosis in a large cohort of patients undergoing allogeneic HCT after myeloablative conditioning at our center.1 Results of a smaller case-control study suggested that patients who developed invasive aspergillosis after nonmyeloablative HCT might have better outcomes than those who developed aspergillosis after myeloablative HCT.15 These results were not verified in this large cohort. Results of this study revealed that the 1-year overall survival rate after diagnosis of invasive mold infections was 32%, similar to that reported among recipients of myeloablative HCT. It is possible that the improved survival in the case-control study was affected by the matching procedure, which may have adjusted for potentially confounding factors.15
High-dose corticosteroid therapy for GVHD was an important prognostic variable of outcomes after invasive mold infections in patients undergoing myeloablative and nonmyeloablative HCT.43,44 Also, proven diagnosis of invasive mold infections was associated with an increased risk of mold infection-related death. It may be possible that patients with proven infection may have a more advanced stage of disease.
Preventing invasive mold infections might significantly improve outcomes after allogeneic HCT with nonmyeloablative conditioning. Patients who develop CMV infection or start corticosteroid therapy for GVHD should be monitored closely for subsequent development of invasive mold infections. It is possible that one of the mold-active azole antifungals or echinocandins may be used to prevent infections in patients with recognized high risks.47-50 In addition, GVHD therapy regimens containing lower doses of corticosteroids could be explored. Further studies for the development of more effective prophylaxis or preemptive strategies against molds are warranted to decrease mold infection-related nonrelapse mortality after allogeneic HCT with nonmyeloablative conditioning.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2003-02-0456.
Supported by NIH grants K08-A1571, K23-CA92058, CA78902, CA18029, CA15704, and HL36444. T.F. was supported by a fellowship from the Kirin Brewery Company.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the medical, nursing, data processing, laboratory, pathology, and clinical staffs at the FHCRC for their important contributions to this study through dedicated care of the patients. The authors are indebted to Deborah Bassuk and Chris Davis for assistance with data collection, and Steve Minor, Mary Hinds, and Kathryn Keegan for their assistance with patient management. We also thank Helen Crawford and Bonnie Larson for manuscript preparation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal