Abstract
Killer immunoglobulin-like receptor (KIR) ligand incompatibility in the graft-versus-host direction was demonstrated to be associated with improved outcome in patients given haploidentical, T-cell–depleted hematopoietic stem cell transplants (HSCTs). The goal of this study was to evaluate whether that observation could be generalized for patients receiving unmanipulated HSCTs from unrelated donors (URD). One hundred thirty patients with hematologic malignancies entered the study. Graft-versus-host disease (GVHD) prophylaxis was uniform and consisted of cyclosporin, short-term methotrexate, and pretransplantation antithymocyte globulin (ATG). Patients were divided into those with (n = 20) and those without (n = 110) KIR ligand incompatibility with respect to their donors. At 4.5 years patients with KIR ligand incompatibility had higher probability of overall survival (87% versus 48%, P = .006) and disease-free survival (87% versus 39%, P = .0007) compared with those without KIR ligand incompatibility. Transplant-related mortality for the 2 groups equaled 6% and 40% (P = .01), respectively. Relapse rates for patients receiving transplants from a donor with or without KIR ligand incompatibility were 6% and 21%, respectively (P = .07). All patients with myeloid malignancies receiving transplants from KIR ligand–disparate donors (n = 13) are alive and disease free. These data indicate that natural killer (NK) cell alloreactivity is associated with better outcome after URD-HSC transplantation when ATG is used as part of GVHD prophylaxis.
Introduction
Natural killer (NK) cells constitute a part of the innate immune system that plays an important role in host response to infectious pathogens and tumor cells.1 The function of these cells is negatively regulated by inhibitory immunoglobulin-like receptors (KIRs) for major histocompatibility complex (MHC) class I molecules on target cells. Cells lacking specific KIR ligands, in the presence of additional activating signals, are exposed to NK cell action.2 In humans, KIRs are specific for epitopes shared by groups of class I alleles. KIR2DL1 recognizes HLA-Cw4–related alleles, KIR2DL2/3 HLA-Cw3–related alleles, KIR3DL1 HLA-B alleles sharing the Bw4 supertypic specificity, and KIR3DL2 recognizes HLA-A3 and -A11 loci.3
KIR receptors are clonally distributed on NK cells. Thus, in any given individual, KIR-bearing NK cells make up a discrete repertoire, which is tolerant to self-antigens, as it is blocked by the class I allele groups, but may result in reaction when confronted with allogeneic targets failing to express their own inhibiting class I alleles.4
A recently published study analyzed patients given haploidentical, T-cell–depleted hematopoietic stem cell transplants (HSCTs) from a relative.4 Those researchers demonstrated that KIR ligand incompatibility in the graft-versus-host direction was associated with the presence in the donor NK repertoire of alloreactive NK clones, which could not have been blocked by the recipient's target cells lacking the inhibiting HLA class I alleles.4 In patients with high-risk acute myeloid leukemia (AML), such an incompatibility resulted in lower rates of relapse, graft failure, and acute graft-versus-host disease (GVHD) that, altogether, translated into a significantly higher probability of overall survival (OS). In that study it was demonstrated, using murine models, that donor alloreactive NK cells eliminate not only residual leukemic cells but also host T cells and antigen-presenting cells, thereby favoring engraftment and preventing the development of acute GVHD.4
The goal of the present study was to determine whether a similar effect could be observed in patients receiving HSCTs from unrelated donors (URDs), some of them mismatched for HLA class I alleles. For this purpose, we analyzed retrospectively the outcome of patients with hematologic malignancies who received URD-HSCT, grouped according to KIR ligand compatibility. Patients were treated in 3 transplantation centers, in which the same regimen of immunosuppression (antithymocyte globulin [ATG], cyclosporin A, and short-term methotrexate) before and after HSC transplantation was used.
Patients and methods
Patients
The study was approved by the institutional review boards of the 3 participating centers, and patients or their legal guardians, as appropriate, gave written informed consent. One hundred thirty patients with hematologic malignancies receiving URD-HSCT between 1998 and 2002 were included in the study. The transplantations were performed in 3 centers: Paediatric Hematology and Oncology, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo, Pavia, Italy (n = 52); Department of Haematology and BMT, Silesian Medical Academy, Katowice, Poland (n = 43); and Division of Haematology, Hospital San Martino, Genova, Italy (n = 35). The donors were prospectively selected using high-resolution molecular typing for both HLA class I and class II loci (ie, locus A, B, C, DRB1, and DQB1). Patients were divided into those with (n = 20) and those without (n = 110) KIR ligand incompatibility, as described by Ruggeri et al.4 Within this latter group, patients were subdivided according to their HLA matching with the donor: 61 patients received transplants from a donor who was HLA fully matched according to high-resolution molecular typing of both HLA class I and class II alleles, whereas the remaining 49 patients received transplants from a donor with one or more HLA disparities not associated with KIR ligand incompatibility. KIR ligand incompatibility was defined by the absence of one donor KIR ligand class I allele in the recipient. The following receptors and their ligands were taken into consideration: KIR2DL1 with HLA-Cw4–related alleles, KIR2DL2/3 with HLA-Cw3–related alleles, and KIR3DL1 with HLA-Bw4 alleles. Within the 20 patients receiving transplants from a KIR-incompatible donor, the KIR incompatibility involved the HLA-C locus and HLA-B locus in 18 and 2 cases, respectively. The 2 groups did not differ in terms of both patient/donor and transplant characteristics (details in Table 1).
Patient and donor characteristics and details of procedures
. | Donor without KIR ligand incompatibility . | . | Donor with KIR ligand incompatibility . | |
|---|---|---|---|---|
. | HLA fully matched . | HLA mismatched, without KIR ligand disparity . | HLA mismatched, with KIR ligand disparity . | |
| N | 61 | 49 | 20 | |
| Median patient age, y (range) | 20 (0.5-40) | 18 (0.5-40) | 20.5 (0.5-40) | |
| Median donor age, y (range) | 36 (19.5-53) | 34 (19-56) | 33 (20-54) | |
| Patient/donor sex | ||||
| M/M, n (%) | 23 (38) | 24 (49) | 9 (45) | |
| M/F, n (%) | 11 (18) | 5 (10) | 4 (20) | |
| F/M, n (%) | 9 (15) | 7 (14) | 4 (20) | |
| F/F, n (%) | 18 (30) | 13 (27) | 3 (15) | |
| Patient/donor CMV status* | ||||
| Positive/positive, n (%) | 19 (31) | 22 (45) | 5 (25) | |
| Positive/negative, n (%) | 27 (44) | 17 (35) | 10 (50) | |
| Negative/positive, n (%) | 7 (11) | 4 (8) | 3 (15) | |
| Negative/negative, n (%) | 8 (13) | 6 (12) | 2 (10) | |
| Diagnosis | ||||
| ALL, n (%) | 19 (31) | 13 (27) | 6 (30) | |
| CR1, n | 3 | 5 | 1 | |
| CR2, n | 12 | 6 | 3 | |
| CR3, n | 1 | 1 | 1 | |
| Relapse, n | 3 | 3 | 1 | |
| AML, n (%) | 11 (18) | 6 (12) | 5 (30) | |
| CR1, n | 4 | 2 | 2 | |
| CR2, n | 5 | 2 | 3 | |
| Primary resistance, n | 1 | 0 | 0 | |
| Relapse, n | 1 | 2 | 0 | |
| MDS, n (%) | 9 (15) | 12 (24) | 2 (10) | |
| CR1, n | 2 | 1 | 0 | |
| Nonremission, n | 7 | 11 | 2 | |
| CML, n (%) | 19 (31) | 17 (35) | 6 (30) | |
| Chronic phase 1, n | 19 | 20 | 5 | |
| Blast crisis, n | 0 | 0 | 1 | |
| NHL, n (%) | 1 (2) | 1 (2) | 0 (0) | |
| CR2, n | 1 | 1 | 0 | |
| HD, n (%) | 0 (0) | 0 (0) | 1 (5) | |
| Relapse, n | 0 | 0 | 1 | |
| MM, n (%) | 2 (3) | 0 (0) | 0 (0) | |
| Primary resistance, n | 1 | 0 | 0 | |
| Relapse, n | 1 | 0 | 0 | |
| Disease phase at HSC transplantation† | ||||
| Early, n (%) | 46 (75) | 32 (65) | 14 (70) | |
| Advanced, n (%) | 15 (25) | 17 (35) | 6 (30) | |
| Patient/donor HLA compatibility | ||||
| Single mismatch, n (%) | 0 | 35 (71) | 12 (60) | |
| Multiple mismatch, n (%) | 0 | 14 (29) | 8 (40) | |
| Source of stem cells | ||||
| BM, n (%) | 59 (97) | 48 (98) | 18 (90) | |
| PB, n (%) | 2 (3) | 1 (2) | 2 (10) | |
| Preparative regimen | ||||
| TBI-based, n (%) | 29 (48) | 23 (47) | 11 (55) | |
| Busulfan-based, n (%) | 32 (52) | 26 (53) | 9 (45) | |
. | Donor without KIR ligand incompatibility . | . | Donor with KIR ligand incompatibility . | |
|---|---|---|---|---|
. | HLA fully matched . | HLA mismatched, without KIR ligand disparity . | HLA mismatched, with KIR ligand disparity . | |
| N | 61 | 49 | 20 | |
| Median patient age, y (range) | 20 (0.5-40) | 18 (0.5-40) | 20.5 (0.5-40) | |
| Median donor age, y (range) | 36 (19.5-53) | 34 (19-56) | 33 (20-54) | |
| Patient/donor sex | ||||
| M/M, n (%) | 23 (38) | 24 (49) | 9 (45) | |
| M/F, n (%) | 11 (18) | 5 (10) | 4 (20) | |
| F/M, n (%) | 9 (15) | 7 (14) | 4 (20) | |
| F/F, n (%) | 18 (30) | 13 (27) | 3 (15) | |
| Patient/donor CMV status* | ||||
| Positive/positive, n (%) | 19 (31) | 22 (45) | 5 (25) | |
| Positive/negative, n (%) | 27 (44) | 17 (35) | 10 (50) | |
| Negative/positive, n (%) | 7 (11) | 4 (8) | 3 (15) | |
| Negative/negative, n (%) | 8 (13) | 6 (12) | 2 (10) | |
| Diagnosis | ||||
| ALL, n (%) | 19 (31) | 13 (27) | 6 (30) | |
| CR1, n | 3 | 5 | 1 | |
| CR2, n | 12 | 6 | 3 | |
| CR3, n | 1 | 1 | 1 | |
| Relapse, n | 3 | 3 | 1 | |
| AML, n (%) | 11 (18) | 6 (12) | 5 (30) | |
| CR1, n | 4 | 2 | 2 | |
| CR2, n | 5 | 2 | 3 | |
| Primary resistance, n | 1 | 0 | 0 | |
| Relapse, n | 1 | 2 | 0 | |
| MDS, n (%) | 9 (15) | 12 (24) | 2 (10) | |
| CR1, n | 2 | 1 | 0 | |
| Nonremission, n | 7 | 11 | 2 | |
| CML, n (%) | 19 (31) | 17 (35) | 6 (30) | |
| Chronic phase 1, n | 19 | 20 | 5 | |
| Blast crisis, n | 0 | 0 | 1 | |
| NHL, n (%) | 1 (2) | 1 (2) | 0 (0) | |
| CR2, n | 1 | 1 | 0 | |
| HD, n (%) | 0 (0) | 0 (0) | 1 (5) | |
| Relapse, n | 0 | 0 | 1 | |
| MM, n (%) | 2 (3) | 0 (0) | 0 (0) | |
| Primary resistance, n | 1 | 0 | 0 | |
| Relapse, n | 1 | 0 | 0 | |
| Disease phase at HSC transplantation† | ||||
| Early, n (%) | 46 (75) | 32 (65) | 14 (70) | |
| Advanced, n (%) | 15 (25) | 17 (35) | 6 (30) | |
| Patient/donor HLA compatibility | ||||
| Single mismatch, n (%) | 0 | 35 (71) | 12 (60) | |
| Multiple mismatch, n (%) | 0 | 14 (29) | 8 (40) | |
| Source of stem cells | ||||
| BM, n (%) | 59 (97) | 48 (98) | 18 (90) | |
| PB, n (%) | 2 (3) | 1 (2) | 2 (10) | |
| Preparative regimen | ||||
| TBI-based, n (%) | 29 (48) | 23 (47) | 11 (55) | |
| Busulfan-based, n (%) | 32 (52) | 26 (53) | 9 (45) | |
All differences among the 3 groups were not statistically significant (P > .1). M indicates male; F, female; CMV, cytomegalovirus; CR, complete remission; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; MM, multiple myeloma; BM, bone marrow; PB, peripheral blood; TBI, total body irradiation
CMV status was tested by serologic methods
Patients with ALL or AML in CR1 and CR2 or CML-first chronic phase or with low-blast MDS were considered in early disease phase
URD-HSC transplantation procedure
All patients were given a myeloablative therapy, which included total body irradiation (TBI) in 63 cases. In case of lymphoid malignancies, most patients received TBI (12 Gy in 6 fractions) + cyclophosphamide (120 mg/kg) ± thiotepa (10 mg/kg), whereas most patients with myeloid malignancies were treated with busulphan (16 mg/kg) + cyclophosphamide (120 mg/kg) ± melphalan (140 mg/m2).
Bone marrow was used as a source of hematopoietic stem cells in 125 patients, whereas granulocyte colony-stimulating factor (G-CSF]–mobilized peripheral blood stem cells were transplanted in the remaining 5 cases. A minimum of 3 × 108 nucleated donor cells/kg recipient body weight was requested of the donor center. For patients given a transplant of bone marrow stem cells without major blood group mismatch, the median nucleated cell (NC) dose infused was 4.4 × 108/kg (range, 1.1-23.2 × 108/kg) with no difference among the 3 groups. Prophylaxis of GVHD was the same for all patients and consisted of cyclosporin, short-term methotrexate (MTX), and pretransplantation ATG (7-11 mg/kg from day -4 to day -2). Methylprednisolone was used as first-line treatment of acute GVHD. In case of steroid resistance, either extracorporeal phototherapy or ATG was introduced.5 No patient was given G-CSF after the allograft.
Definitions
For the purpose of this study, patients with acute lymphoblastic leukemia (ALL) or with AML in first or second complete remission (CR), chronic myeloid leukemia (CML) in first chronic phase, and myelodysplasia characterized by a low blast percentage (ie, < 5%) were considered to belong to the early phase group. Acute GVHD was diagnosed and graded according to previously reported criteria.6 All patients surviving more than 7 days after transplantation were considered at risk of developing acute GVHD. Patients alive 80 days after transplantation with sustained donor engraftment were considered to be evaluable for chronic GVHD, which was classified as previously described.7
Time of myeloid engraftment was defined as the first of 3 consecutive days with absolute neutrophil count (ANC) more than 0.5 × 109/L, and platelet engraftment as the first of 7 consecutive days with an unsupported platelet count (PLT) more than 50 × 109/L.
Graft failure was diagnosed if an ANC more than 0.5 × 109/L either was not achieved within 28 days after URD-HSC transplantation or the neutrophil count declined below 0.2 × 109/L for patients in remission after engraftment.
Transplant-related mortality (TRM) was defined as death from all causes in the absence of relapse. OS was calculated from the time between transplantation and death as a result of any cause, whereas disease-free survival (DFS) was calculated from the time interval from HSC transplantation to either relapse or death in remission, whichever occurred first.
Statistical analysis
Data were analyzed as of January 20, 2003. The primary end point of this study was the probability of OS. The rates of DFS, TRM, and relapse, as well as the incidence of acute and chronic GVHD and probability of both ANC and PLT recovery, represented secondary end points.
OS and DFS were estimated using the Kaplan-Meier method, whereas the probabilities of relapse, TRM, acute and chronic GVHD, and ANC and PLT recovery were calculated as cumulative incidence8,9 to adjust the analysis for competing risks. In detail, for the analysis on acute and chronic GVHD, both relapse and death in remission in the absence of GVHD were considered competing events; for the analysis on relapse incidence, death was the competing event, whereas relapse was the competing event for the analysis on TRM. Log-rank test statistics were used to evaluate the univariate effects of KIR ligand incompatibility on outcome. Additionally, other factors, namely presence of HLA disparity in the patient/donor pair, patient and donor age, patient and donor sex, CMV status, diagnosis, disease status at HSC transplantation, and type of conditioning were tested in univariate analysis. Median observation time of surviving patients is 13 months (range, 4-60 months) for patients with KIR ligand incompatibility and 15 months (range, 4-85 months) for those without KIR ligand incompatibility.
Results
Survival, disease-free survival, and relapse incidence
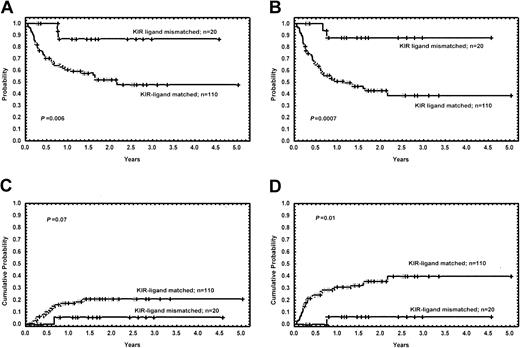
Eighteen of the 20 patients (90%) with KIR ligand incompatibility are alive and disease free. The Kaplan-Meier estimates for OS at 4.5 years are 87% and 48% for patients with and for those without KIR ligand incompatibility, respectively (P = .006). The probability of DFS for the 2 groups is 87% and 39%, respectively (P = .0007), and the cumulative incidence of relapse was 6% and 21% (P = .07) (Figure 1). Only one patient with ALL who was given HSCT from a KIR ligand–mismatched donor relapsed. None of the other factors regarding recipient, donor, or transplant-related characteristics were found to influence survival. For patients without KIR ligand mismatch, the OS rate did not depend on the degree of HLA compatibility, as it was 48% for fully matched patient/donor pairs, 58% for those with a single mismatch, and 39% for those with multiple disparities. For all the above subgroups, the probability of OS was significantly lower compared with KIR ligand–mismatched patients, with P = .01, .02, and .007, respectively.
Long-term outcome of patients with and without KIR ligand incompatibility. (A) Kaplan-Meier estimates for the probability of overall survival. (B) Kaplan-Meier estimates for the probability of disease-free survival. (C) Cumulative incidence estimates for the probability of relapse. (D) Cumulative incidence estimates for the probability of transplant-related mortality.
Long-term outcome of patients with and without KIR ligand incompatibility. (A) Kaplan-Meier estimates for the probability of overall survival. (B) Kaplan-Meier estimates for the probability of disease-free survival. (C) Cumulative incidence estimates for the probability of relapse. (D) Cumulative incidence estimates for the probability of transplant-related mortality.
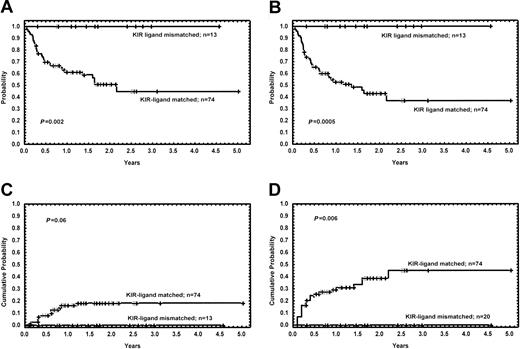
When analyzed separately, patients with myeloid malignancies and KIR ligand mismatch (n = 13) had better outcome compared with their KIR ligand–compatible counterparts (n = 74) (OS, 100% versus 45%, P = .002; DFS, 100% versus 37%, P = .0005; relapse rate, 0% versus 18%, P = .06) (Figure 2).
Long-term outcome of patients with myeloid malignancies (acute myeloid leukemia, chronic myeloid leukemia, myelodysplastic syndrome), with and without KIR ligand incompatibility. (A) Kaplan-Meier estimates for the probability of overall survival. (B) Kaplan-Meier estimates for the probability of disease-free survival. (C) Cumulative incidence estimates for the probability of relapse. (D) Cumulative incidence estimates for the probability of transplant-related mortality.
Long-term outcome of patients with myeloid malignancies (acute myeloid leukemia, chronic myeloid leukemia, myelodysplastic syndrome), with and without KIR ligand incompatibility. (A) Kaplan-Meier estimates for the probability of overall survival. (B) Kaplan-Meier estimates for the probability of disease-free survival. (C) Cumulative incidence estimates for the probability of relapse. (D) Cumulative incidence estimates for the probability of transplant-related mortality.
Transplant-related mortality
The cumulative probability of TRM was 6% and 40% for patients with and without KIR ligand incompatibility, respectively (P = .01) (Figure 1). The only patient receiving a transplant from a KIR-incompatible donor who died from a transplant-related event had ALL, and he died as a result of chronic GVHD at 7.5 months after the allograft. The causes of death in patients receiving transplants from a KIR ligand–compatible donor were as follows: acute GVHD (n = 10), chronic GVHD (n = 8), pulmonary toxicity (n = 7), infectious complications (n = 3), hemorrhage (n = 1), graft failure (n = 1), veno-occlusive disease (n = 1), hemorrhagic cystitis (n = 1), and thrombotic thrombocytopenic purpura (n = 1). The cumulative probability of TRM of patients with myeloid malignancies receiving transplants from a donor with or without KIR incompatibility was 0% and 45%, respectively (P = .006).
Engraftment
Four patients experienced primary graft failure; all of them had received transplants from a KIR ligand–compatible donor. The probability of ANC recovery at day 100 was 100% for KIR ligand–mismatched patients and 97% for KIR ligand–matched HSCT recipients, with the same median recovery time of 19 days for both groups. The probability of PLT engraftment at day 100 was 100% for patients with and 85% for those without KIR ligand incompatibility, respectively. The median time to PLT recovery in the 2 groups was 34 days and 28 days, respectively (P = NS).
Graft-versus-host disease
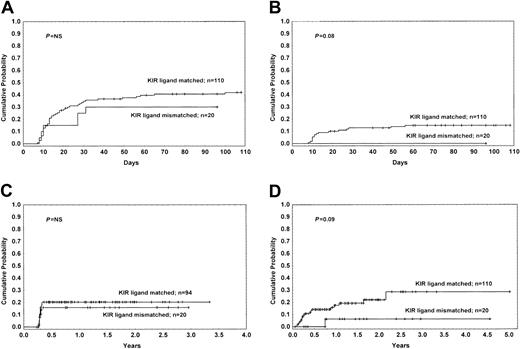
The cumulative probability of developing grade II-IV acute GVHD for patients with and without KIR ligand mismatch was 30% and 43%, respectively (P = NS). The cumulative probability of developing grade III-IV acute GVHD was 0% and 15% (P = .08), respectively (Figure 3).
The incidence of GVHD for patients with and without KIR ligand incompatibility. (A) Cumulative incidence estimates for the probability of acute GVHD grade II-IV. (B) Cumulative incidence estimates for the probability of acute GVHD grade III-IV. (C) Cumulative incidence estimates for the probability of extensive chronic GVHD. (D) Cumulative incidence estimates for the probability of GVHD-related mortality.
The incidence of GVHD for patients with and without KIR ligand incompatibility. (A) Cumulative incidence estimates for the probability of acute GVHD grade II-IV. (B) Cumulative incidence estimates for the probability of acute GVHD grade III-IV. (C) Cumulative incidence estimates for the probability of extensive chronic GVHD. (D) Cumulative incidence estimates for the probability of GVHD-related mortality.
No difference between patients receiving transplants from a donor with or without KIR ligand incompatibility was found with respect to overall incidence of chronic GVHD, as well as the incidence of extensive chronic GVHD (Figure 3). The cumulative incidence of GVHD-related mortality for patients with and without KIR ligand disparity was 6% and 29%, respectively (P = .09) (Figure 3).
Discussion
In a setting of haploidentical transplant recipients with AML, Ruggeri et al4 demonstrated that, in the presence of KIR ligand incompatibility in the GVHD direction, NK cells of donor origin showed alloreactivity that protected from leukemia relapse, rejection, and development of GVHD. In view of these findings, the long-term outcome of KIR ligand–mismatched patients was significantly better compared with control subjects.4 The reduced incidence of GVHD was thought to be due to the elimination of recipient antigen-presenting cells, which have been demonstrated to trigger the attack of donor lymphocytes toward recipient tissues.10
Because approximately 50% of URD-HSC transplantations are being performed in the presence of one or more HLA allele-level mismatches, even when donor selection is based on high-resolution molecular typing,11 KIR ligand incompatibility may occur also in this type of transplant. In fact, in our study, among 130 donor/recipient pairs, we identified 20 patients (29% of the 69 patients receiving transplants from an HLA-disparate donor) with KIR ligand incompatibility in the GVH direction. Likewise, in a recently published study by Davies et al12 approximately one third of donor/recipient HLA class I mismatches were associated with KIR ligand disparity. However, the analysis of outcome in that cohort of patients receiving transplants over 10 years did not show any advantage in terms of survival and relapse for patients with KIR ligand mismatches. Moreover, the presence of KIR ligand incompatibility tended to increase the risk of development of acute GVHD grade II-IV.
We found an OS probability at 4.5 years of 87% in the KIR incompatible group; this result is significantly better as compared with patients without KIR ligand incompatibility. Interestingly, patients with KIR ligand mismatch in the GVH direction had a better outcome when compared not only with other recipients of HLA mismatched transplants but also with recipients of HLA fully compatible HSCTs. In addition, KIR ligand incompatibility was associated with a reduced incidence of grade III-IV acute GVHD. Indeed, none of the patients in the KIR ligand–mismatched group developed grade III-IV acute GVHD. This lower incidence of severe GVHD may have also contributed to reduce the risk of regimen-related complications.
Differences in GVHD prophylaxis and graft cellularity may explain the differences in the results of our study and those of others. The study group reported by Davies et al12 receiving transplants over a wide time period was heterogeneous in terms of GVHD prophylaxis regimens and comprised partial T-cell depletion + posttransplantation cyclosporine A in one third of patients and posttransplantation cyclosporine A + MTX in the remaining patients.12 In the interpretation of their results, those researchers suggested that NK cell effects may have been obscured by donor T-cell effects or by the immune suppression necessary to prevent or control GVHD caused by donor T cells.
In our study, all patients received transplants from a donor prospectively selected with the use of high-resolution molecular typing for both class I and class II loci, and they received the same regimen of GVHD prophylaxis, which included pretransplantation ATG, a potent immunosuppressive agent, recently proven to reduce the incidence of both acute and chronic GVHD in a dose-dependent manner.13 Although the data on ATG are controversial, this serotherapy seems to have strong activity against CD4+ lymphocytes and lesser, if any, effect on NK cells.14 Moreover, ATG, because of its potent immune suppression of the host, may facilitate engraftment and, in consequence, faster recovery of donor NK cells. Administration of ATG prior to HSC transplantation results in efficient in vivo depletion of donor T cells. Because ATG persists in the patient circulation for weeks after the allograft,14 it may also modulate and hamper T-cell reconstitution. If no early T-cell priming occurs on a large scale, alloreactive NK cells could better proceed to kill host hematopoietic targets (ie, leukemia blasts, dendritic cells, and T cells) and to positively influence outcome. Thus, this kind of serotherapy may accentuate the beneficial effects of KIR ligand incompatibility.
In our study and in that of Davies et al12 patients were given bone marrow cells. However, the mean NC dose in our cohort was twice that received by patients receiving transplants in Minneapolis (4.3 × 108/kg versus 2 × 108/kg cells, respectively; S.D., oral communication, December 20, 2002). This larger number of transplanted cells might be an additional factor in promoting the beneficial effects of alloreactive NK cells on survival.
Finally, 13 of 20 patients with KIR ligand incompatibility included in our study had myeloid malignancies, which represent the disorders in which Ruggeri et al4 better demonstrated the role of NK cells. In our study, none of these patients relapsed or died of transplant-related complications, and their outcome was significantly better than those of patients with myeloid malignancies given HSCTs from a KIR ligand–compatible donor.
Although obtained in a limited number of patients with a relatively short follow-up, the results of our study suggest that the findings of Ruggeri et al4 in a haploidentical HSCT setting may be extended to URD transplants, provided that the type of immunosuppressive treatment used favors development and expansion of NK cells. For patients with KIR ligand incompatibility in the GVHD direction, a regimen including pretransplantation ATG and a high NC dose seems to favor the beneficial effects of NK cell alloreactivity.
Further prospective studies involving larger numbers of patients are necessary to confirm our observations. If confirmed, these findings might lead to important changes in strategies for selecting unrelated donors for HSC transplantation, particularly in cases with myeloid malignancies. Further studies should evaluate different transplantation protocols separately, as the treatment regimen likely influences the biologic effect of KIR ligand incompatibility. Such studies could provide valuable information to answer the question of whether an HLA fully compatible URD is better than a “perfectly mismatched” donor.15
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2003-01-0091.
Supported in part by grants from AIRC (Associazione Italiana Ricerca sul Cancro), CNR (Consiglio Nazionale delle Ricerche), MURST (Ministero dell'Università e della Ricerca Scientifica e Tecnologica), and IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) Policlinico San Matteo, Ricerca Finalizzata (F.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal