Abstract
Hairy cell leukemia (HCL) is an indolent B-cell neoplasm, strongly expressing CD20. Despite initial very high response rates following cladribine, many patients (pts) ultimately relapse. Having relapsed after prior treatment with cladribine, 24 HCL pts (21 male, 3 female) with a median age of 53.5 years were treated with rituximab at 375 mg/m2 intravenously weekly for 4 weeks. Of the pts, 3 (13%) achieved complete remissions and 3 (13%), partial responses. Thus, 6 (25%) of 24 pts achieved a response following rituximab. At a median follow-up of 14.6 months, 2 responders have relapsed; median time to relapse was not yet reached. The only grade III or IV toxicities demonstrated were culture-negative febrile neutropenia, transient and reversible disseminated intravascular coagulation related to rituximab administration, and a diverticular abscess, each in single patients. Of 18 nonresponders, 9 pts subsequently received other treatments; 5 pts were retreated with cladribine, 3 underwent splenectomy, and 1 received pentostatin. Follow-up data are available on 7 of these 9 patients; all 7 patients achieved improvements in hematologic parameters. Rituximab, administered at this dose and schedule, has only modest single-agent activity in cladribine-failed HCL patients when compared with other agents active in this disease.
Introduction
Hairy cell leukemia (HCL) is an uncommon chronic lymphoproliferative disorder whose defining features are the presence of B lymphocytes displaying prominent cytoplasmic projections, positive staining with tartrate-resistant acid phosphatase, and a typical pattern of infiltration in both the bone marrow and spleen.1 The disease has an indolent, but progressive course.2 Patients typically present with pancytopenia, splenomegaly, and recurrent infections. Cladribine has emerged as the treatment of choice since a single course of treatment results in complete responses in the vast majority of patients.3 However, within 7 years of follow-up one third of patients relapsed.4 Although patients who relapse after cladribine can be successfully retreated with cladribine, the optimal treatment for relapsing patients remains to be defined. Other therapeutic options for these cladribine-failed patients include pentostatin (2-deoxycoformycin), interferon, and splenectomy. Anti-CD22 antibody conjugated to the Pseudomonas exotoxin (BL22) has recently shown promise as a treatment option in relapsed patients,5 but is not yet widely available.
Hairy cells typically brightly express the B-cell antigen CD20.6 Rituximab is a chimeric humanized mouse anti-CD20 monoclonal antibody that recognizes the CD20 antigen expressed on normal B cells and most malignant B-cell lymphomas. There are several case reports of patients with HCL developing complete remissions after treatment with rituximab.7-9 To assess the efficacy of rituximab in this clinical situation, we studied 24 patients with HCL who had relapsed after prior cladribine.
Patients, materials, and methods
Eligibility criteria
Patients were included if they had HCL as defined by their peripheral blood and bone marrow morphology and flow cytometry, had received one or more prior courses of cladribine (Leustatin; Ortho Biotech, Raritan, NJ), and were more than 6 months from their last cladribine treatment. Patients had at least one of the following treatment indications: neutropenia (absolute neutrophil count, < 1.5 × 109/L), anemia (hemoglobin level, < 100g/L), thrombocytopenia (platelet count, < 100 × 109/L), symptomatic splenomegaly, or documented repeated infections. All subjects exhibited CD20 expression on their hairy cells in the peripheral blood and/or bone marrow.
Patients were excluded if they had significant renal dysfunction (serum creatinine level, > 190.6 μM) or hepatic dysfunction (total bilirubin level, > 42.75 μM, alkaline phosphatase level > 2 times normal, or aspartate transaminase level > 2 times normal). Patients with HIV, pregnancy, or concomitant malignancy (other than adequately treated basal or squamous cell carcinoma of the skin or carcinoma in situ of the cervix) were excluded.
Treatment
Patients were treated with 375 mg/m2 rituximab (Rituxan; IDEC Pharmaceuticals, San Diego, CA) intravenously each week for 4 consecutive weeks. The drug was administered in a standard manner with acetaminophen and diphenhydramine premedication. Initial infusions were given at 50 mg per hour for the first 30 minutes, and if no toxicity was observed, the infusion rate was escalated at 30-minute intervals to a peak infusion rate of 300 mg per hour. All patients were treated with 300 mg allopurinol per day orally for the first 7 days of treatment, as prophylaxis against tumor lysis syndrome. No growth factors were permitted.
The study protocol was approved by the Scripps Clinic Human Subjects Committee. Informed consent was obtained from all patients prior to enrollment.
Evaluations
All patients underwent a pretreatment evaluation that included a complete blood count (CBC) with differential, metabolic panel, peripheral blood tartrate-resistant acid phosphatase stain, bone marrow biopsy, peripheral blood or bone marrow flow cytometry, and computerized tomographic (CT) scans of the abdomen and pelvis. Patients were evaluated for response at 6 months because data in patients with indolent lymphoma indicated that patients may have delayed responses to rituximab.10 Response evaluation consisted of a CBC, bone marrow biopsy, as well as repeated CT scanning for patients who had significant lymphadenopathy or splenomegaly on initial CT scan.
Response criteria
Complete response (CR). CR was defined as the disappearance of all evidence of disease. The CBC demonstrated a neutrophil count higher than 1.5 × 109/L, a hemoglobin concentration higher than 120 g/L, platelet count higher than 100 × 109/L, and the absence of hairy cells in the bone marrow biopsy specimen and peripheral blood. Patients with baseline adenopathy had a reduction in lymph node size to less than 2 cm. Immunohistochemical staining was performed on all specimens but was not included in the definition of a complete response.
Partial response (PR). PR was defined as a more than 50% reduction in the percentage of bone marrow medullary space involvement by HCL, more than 50% improvement in abnormal lymphadenopathy and/or hepatosplenomegaly, and a 50% improvement in all cytopenias.
Nonresponse (NR). NR was defined as the absence of findings to satisfy the criteria for CR or PR.
Hematologic improvement (HI). HI was defined as the achievement of a hemoglobin level higher than 100 g/L, platelet count higher than 100 × 109/L, and neutrophil count higher than 1.5 × 109/L, without meeting the above criteria for a PR or CR. Patients with HI were assessed as nonresponders when overall response determination was analyzed. This category was not prospectively defined at the initiation of the study.
Toxicity. Toxicity was evaluated using standard toxicity criteria.11
Relapse criteria
Relapse after complete response was defined as the reappearance of hairy cells in the peripheral blood smear and/or bone marrow, development of peripheral blood cytopenias, and/or splenomegaly on physical examination. Relapse after partial response was defined as a more than 50% increase in residual hairy cells in the bone marrow, development of cytopenias, or development of lymphadenopathy or splenomegaly to a size no longer satisfying the criteria for a partial response.
Statistical considerations
Response rates were calculated using an intent-to-treat analysis. Changes in hematologic parameters before and after treatment were evaluated using a paired 1-sided Student t test. Differences between responders and nonresponders in baseline characteristics were evaluated using an unpaired, 2-sided Student t test.
Results
Patient characteristics
Between January 2000 and January 2002, 24 patients (21 male and 3 female) with relapsed HCL after prior cladribine treatment were enrolled. All patients who enrolled had achieved at least a PR to their last cycle of cladribine. No patients had refractory disease. Their median age was 53.5 years (range, 38-81 years). Patients had been diagnosed with HCL a median of 91 months prior to receiving rituximab (range, 24-157 months). All patients had received prior treatment with cladribine, and there was a median of 73 months since their last infusion of cladribine (range, 20-140 months). There were 8 patients (33%) who had received 2 prior courses of cladribine and 1 had received 3 prior courses; 7 patients (29%) had previously received interferon; 3 patients (13%) had previously undergone splenectomy. No patients had received prior pentostatin. When all forms of therapy are considered (ie, purine analogs, interferon, and splenectomy), patients had received a median of 2 prior treatment regimens (range, 1-4). All patients had achieved a response to their last course of cladribine, with two thirds of patients having achieved a complete remission. There were no significant differences between responders and nonresponders to rituximab with respect to baseline characteristics, including the quality of CD20 expression as determined by standard flow cytometric methods (Table 1). However, there was a trend toward less bone marrow disease burden of HCL in responders compared with nonresponders. Rituximab responders had a median of 17% of the bone marrow medullary space involved by HCL (range, 5%-50%), while nonresponders had a median of 39% of the medullary space involved (range, 5%-70%). This difference was not statistically significant (P = .054).
Baseline patient characteristics for responders and nonresponders to rituximab, N = 24
. | Responders (range) . | Nonresponders (range) . |
|---|---|---|
| Median age, y | 47 (38-68) | 54.5 (42-81) |
| Male, % | 83 | 87 |
| Median time from HCL diagnosis, mo | 87.5 (30-157) | 98 (24-154) |
| Average number of prior courses of cladribine | 1.4 (1-2) | 1.5 (1-3) |
| Time from last cladribine, mo | 35.9 (22-121) | 72.8 (20-140) |
| Complete remission after last cladribine, % | 66 | 75 |
| Previous splenectomy, % | 0 | 17 |
| Previous interferon, % | 33 | 27 |
| Median bone marrow cellularity consisting of hematopoesis, % | 40 (10-75) | 50 (20-75) |
| Median bone marrow medullary space involved with HCL, % | 17.5 (5-50) | 39 (5-70) |
| Median absolute neutrophil count, × 109/L | 0.9 (0.6-1.4) | 0.8 (0.2-3.3) |
| Median absolute lymphocyte count, × 109/L | 0.6 (0.4-1.0) | 0.8 (0.3-2.4) |
| Median hemoglobin level, g/L | 139 (123-148) | 130 (81-155) |
| Median platelet count, × 109/L | 115 (86-153) | 100 (27-238) |
. | Responders (range) . | Nonresponders (range) . |
|---|---|---|
| Median age, y | 47 (38-68) | 54.5 (42-81) |
| Male, % | 83 | 87 |
| Median time from HCL diagnosis, mo | 87.5 (30-157) | 98 (24-154) |
| Average number of prior courses of cladribine | 1.4 (1-2) | 1.5 (1-3) |
| Time from last cladribine, mo | 35.9 (22-121) | 72.8 (20-140) |
| Complete remission after last cladribine, % | 66 | 75 |
| Previous splenectomy, % | 0 | 17 |
| Previous interferon, % | 33 | 27 |
| Median bone marrow cellularity consisting of hematopoesis, % | 40 (10-75) | 50 (20-75) |
| Median bone marrow medullary space involved with HCL, % | 17.5 (5-50) | 39 (5-70) |
| Median absolute neutrophil count, × 109/L | 0.9 (0.6-1.4) | 0.8 (0.2-3.3) |
| Median absolute lymphocyte count, × 109/L | 0.6 (0.4-1.0) | 0.8 (0.3-2.4) |
| Median hemoglobin level, g/L | 139 (123-148) | 130 (81-155) |
| Median platelet count, × 109/L | 115 (86-153) | 100 (27-238) |
Response
All patients completed the 4 weeks of rituximab therapy without interruption. There was one patient death at day 66 related to complications of a ruptured diverticular abscess. This patient was scored as a nonresponder but was not evaluable for changes in hematologic parameters at 6 months. At 6 months, 23 patients underwent examination of blood and bone marrow and are evaluable for response to therapy, hematologic parameters, and toxicity.
Of 24 patients treated, 3 achieved a complete response (13%); 1 of these patients demonstrated evidence of minimal residual involvement of HCL in the bone marrow identified only by immunohistochemistry. An additional 3 patients (13%) met criteria for a partial response; each of these patients had less than 10% residual HCL involvement of their bone marrow at 6 months of follow-up. An additional 2 patients (8%) achieved transient hematologic improvements, occurring from 1 to 3 months following rituximab. These 2 patients experienced a return to baseline on their blood counts and bone marrow profile at the 6-month follow-up analysis and were therefore scored as nonresponders. Since the completion of rituximab, 2 of the 6 responders have relapsed at 14 and 16 months, after receiving rituximab. Of the 2 relapsed patients, 1 had achieved a complete response to rituximab. Neither of these patients has received additional treatment for HCL at this time, and they are both being observed.
Of the 18 nonresponders to rituximab, 9 received subsequent therapy for their HCL; 5 patients were re-treated with cladribine, 3 underwent splenectomy, and 1 received pentostatin. Of these 9 patients, 7 are evaluable for response; all 7 have achieved hematologic improvement with a more than 50% improvement in their peripheral blood cytopenias. At a median follow-up of 11 months from salvage treatment (range, 5-18 months), 5 of 7 patients maintained their hematologic improvement. Both patients who relapsed after salvage therapy underwent splenectomy. Both patients were subsequently treated with purine analogs with improvement in hematologic parameters. Of the 7 evaluable patients, 2 have undergone a subsequent bone marrow biopsy; both patients were in complete response after salvage treatment, with pentostatin in 1 patient and cladribine in the other.
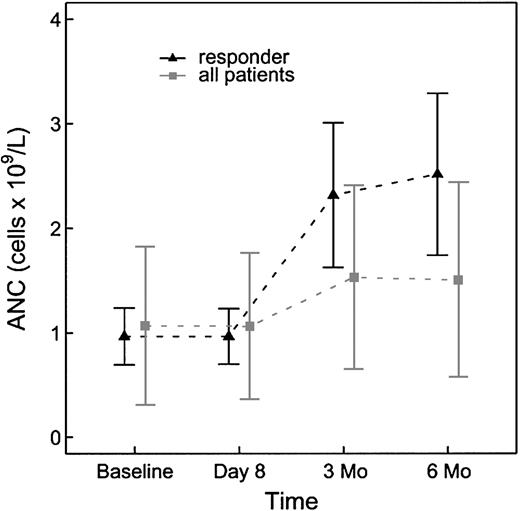
Hematologic parameters were assessed for changes in response to rituximab therapy at baseline and at 6 months after treatment (Table 2). The baseline neutrophil count in the study population was 0.8 × 109/L (range, 0.2-3.3 × 109/L). At 6 months of follow-up the absolute neutrophil count had increased to 1.3 × 109/L (range, 0.2-3.4 × 109/L). This change was significant with a P value of .021 (Figure 1). There was no discernable difference in hemoglobin concentration in the study population at baseline and at 6 months. The platelet count at baseline for the study population was 110 × 109/L (range, 27-283 × 109/L). The platelet count at 6 months had increased to 123 × 109/L (range, 16-246 × 109/L). This change, however, was not statistically significant with a P value of .60.
Changes in hematologic parameters at baseline and at 6 months after treatment, N = 23
Parameter . | Baseline (range) . | 6 months (range) . | P . |
|---|---|---|---|
| Neutrophil count, × 109/L | 0.8 (0.2-3.3) | 1.3 (0.2-3.3) | .021 |
| Hemoglobin level, g/L | 132 (81-157) | 133 (82-165) | .298 |
| Platelet count, × 109/L | 110 (27-283) | 123 (16-246) | .060 |
Parameter . | Baseline (range) . | 6 months (range) . | P . |
|---|---|---|---|
| Neutrophil count, × 109/L | 0.8 (0.2-3.3) | 1.3 (0.2-3.3) | .021 |
| Hemoglobin level, g/L | 132 (81-157) | 133 (82-165) | .298 |
| Platelet count, × 109/L | 110 (27-283) | 123 (16-246) | .060 |
Absolute neutrophil count changes from baseline to 6 months for all patients (n = 23) and for the subset of responders (n = 6).
Absolute neutrophil count changes from baseline to 6 months for all patients (n = 23) and for the subset of responders (n = 6).
Toxicity
Febrile infusion reactions were the most common toxicity, occurring in 13 (54%) of 24 patients. These were generally mild and responded to antipyretics and reductions in the rate of rituximab infusion. There were 3 patients who experienced unexpected grade III or higher toxicity. One patient death occurred on day 66, the result of a ruptured diverticular abscess that occurred within 60 days after the patient received rituximab. This patient was the oldest in the study (age, 81 years) and had previously been treated with 2 prior courses of cladribine as well as splenectomy. A single patient experienced transient and reversible disseminated intravascular coagulation, manifest as thrombocytopenia and a positive assay for fibrin monomer. There was no prolongation of the prothrombin or partial thromboplastin time, hypofibrinogenemia, neurologic changes, renal insufficiency, bleeding, or clotting complications, nor was there evidence of microangiopathic hemolytic anemia. This event occurred with both the first and second infusions of rituximab and did not recur when the patient was pretreated with corticosteroids for doses 3 and 4. A third patient with neutropenia prior to receiving rituximab developed culture-negative febrile neutropenia, which responded to empiric antibiotic therapy.
Discussion
Rituximab, administered at 4 weekly doses of 375 mg/m2 intravenously, induces only a modest response rate in HCL patients who have failed prior cladribine therapy. Somewhat higher response rates have been recorded in other studies. In one series of 11 patients by Hagberg and Lundholm,12 a response rate of 64% was achieved using the same dose and schedule of rituximab reported in this study. This difference in response rate may be due to patient selection since all patients in this series had relapsed disease, with many patients having suffered multiple relapses. The Scripps Clinic patients were receiving, on average, third-line therapy. Another study by Lauria et al13 demonstrated a response rate of 50% in 10 patients with relapsed disease. Another report of 10 patients presented in abstract form did report a response rate of 67% using a regimen of 8 weekly doses of rituximab at 375mg/m2. Most patients in that report did not experience severe neutropenia, and this higher response rate may have been related to the lower disease burden in their patients.14 Alternatively, this difference may be due to the longer duration of rituximab therapy and the higher cumulative doses administered.
Responders in our series had a trend toward having less bone marrow medullary space involvement with HCL, suggesting that patients with a lower burden of disease may have a higher probability of response to rituximab. Patients with advanced HCL have more impairment of antibody-dependent cell-mediated cytotoxicity than earlier stage patients,15 and thus may be less capable of mounting an effective immune response against rituximab-targeted HCL cells. This may explain why patients in this series had lower response rates to rituximab than did patients in other series with less advanced HCL.
Many patients who fail rituximab are still likely to benefit from subsequent purine analogs. In this series, 2 patients who failed rituximab achieved documented complete remissions using cladribine and pentostatin in the third- and fifth-line treatment setting. An additional 2 patients who received cladribine after a rituximab failure had significant improvement in their cytopenias, although bone marrow data for determination of response are not available.
Use of rituximab may be advantageous in selected cladribine-failed patients despite the modest response rate recorded here because of its favorable toxicity profile. Unlike purine analogs, rituximab does not induce long-term bone marrow hypoplasia, and thus may be an option for patients who have developed or are in danger of developing potentially irreversible bone marrow damage as the result of multiple prior regimens with purine analogs. Rituximab also induces less profound, and a shorter duration of, immunosuppression than purine analogs.16-18 It may therefore be a superior choice for patients in whom recurrent severe infections preclude readministration of purine analogs.
Other treatment options for cladribine-failed HCL patients include re-treatment with cladribine, pentostatin, splenectomy, interferon, and novel investigational approaches. Splenectomy is also an option for the treatment of HCL,19 however, there are no prospective studies defining the duration of response for splenectomy in this subset of HCL patients who progress after purine analog therapy. More recently, anti-CD22 recombinant immunotoxin (BL22) has been investigated in heavily pretreated patients with promising results. A recently reported series in cladribine-resistant patients with this antibody conjugate documented a response rate of 81%.20 This agent is, however, significantly more toxic than rituximab, with 2 cases of hemolytic-uremic syndrome developing in the first 16 patients reported, and it is not yet widely available.
For this rare and indolent disease, in the absence of prospective, randomized clinical trial results to guide therapeutic decision-making in HCL patients who have relapsed after purine analog therapy, we have adopted the following treatment algorithm at Scripps Clinic. Patients are retreated at relapse only when they demonstrate significant cytopenias, somewhat arbitrarily defined as an absolute neutrophil count lower than 1 × 109/L, or a hemoglobin concentration lower than 100 g/L, or platelet count less than 100 × 109/L. For patients who achieve a prior cladribine-induced response of longer than 18 months' duration and who demonstrate no significant marrow hypoplasia, a repeat course of cladribine is generally recommended. Re-treatment with cladribine in patients who relapsed after a single prior course of cladribine achieved a response rate of 88%.3 Cladribine re-treatment within a 12-month interval is avoided in order to prevent the potential development of cumulative myelotoxicity. Pentostatin also represents a therapeutic alternative in this clinical setting. Although no large series of patients receiving pentostatin at relapse after cladribine has been reported, evidence from data in the first-line setting and at relapse after primary interferon therapy makes this approach reasonable.21,22
For HCL patients who have relapsed after prior cladribine therapy, with a response duration of fewer than 18 months, who demonstrate a significantly hypoplastic bone marrow or a prior severe opportunistic infection, nonpurine analog treatment is instituted. This approach spares patients from the myelotoxicity and immunosuppression associated with successive courses of cladribine.23 In these patients splenectomy is generally recommended for symptomatic or palpable splenomegaly at relapse. Nonpurine analog systemic therapeutic options include interferon and rituximab.
Further investigations using rituximab in the treatment of cladribine-failed HCL patients should focus on combination therapy with other active agents, or alternate doses and schedules, as the single-agent response rate at this dose and schedule was only modest.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2003-01-0014.
Supported by the Hairy Cell Leukemia Research Foundation (Schaumburg, IL), Assure-a-Cure (Atascadero, CA), and a research grant from IDEC Pharmaceuticals (San Diego, CA). J.N. is a recipient of an Amgen Fellow Award.
Presented in part at the American Society of Hematology annual meeting on December 9, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Anne Feng, Academic Affairs, Scripps Clinic, for her assistance with the preparation of graphics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal