Abstract
t(11;18)(q21;q21) is a specific chromosomal translocation associated with mucosa-associated lymphoid tissue (MALT) lymphoma. It fuses the amino terminal of the API2 gene to the carboxyl terminal of the MALT1 gene and generates a chimeric fusion product. Although the translocation is frequently detected in gastric and pulmonary MALT lymphoma, its incidence in MALT lymphomas from other sites is largely unknown. It also remains unknown whether the occurrence of the translocation is influenced by the nature of preceding diseases associated with MALT lymphomas. We screened for t(11; 18)(q21;q21) in 417 cases of MALT lymphoma from 8 major sites by reverse transcription–polymerase chain reaction. t(11;18)(q21;q21) was found at highest frequencies in MALT lymphomas from the lung (38%) and stomach (24%), and at moderate frequencies in those from the conjunctiva (19%) and orbit (14%). However, the translocation was found only rarely in MALT lymphomas from the salivary gland (1%) and was absent in those from the thyroid, skin, liver, and other rare sites, and in immunoproliferative small intestinal disease (IPSID). In gastric MALT lymphoma, t(11;18)(q21;q21) was significantly associated with infection by CagA-positive strains of Helicobacter pylori. As CagA-positive strains of H pylori are much more potent in induction of host inflammatory responses, including activation of neutrophils, which release highly genotoxic oxygen reactive species, we therefore examined neutrophil infiltration in recognized precursors of MALT lymphoma including H pylori–associated gastritis, lymphoepithelial sialadenitis, and Hashimoto thyroiditis. Neutrophil infiltration was prominent in H pylori–associated gastritis but not in lymphoepithelial sialadenitis and Hashimoto thyroiditis. Our results demonstrate that t(11;18)(q21; q21) occurs at markedly variable frequencies in MALT lymphoma of different sites and suggest that the occurrence of the translocation is influenced by the nature of premalignant diseases associated with MALT lymphoma. Oxidative damage might play a role in development of t(11;18)(q21; q21).
Introduction
In 1983, Isaacson and Wright1 noted that primary low-grade gastric B-cell lymphoma and immunoproliferative small intestinal disease (IPSID) recapitulated the histology of mucosa-associated lymphoid tissue (MALT) exemplified by the Peyer patches, and coined the term “MALT lymphoma.” Subsequently, the MALT lymphoma concept was extended to include low-grade B-cell lymphomas from a number of extranodal sites, including both mucosal organs such as the salivary gland, lung, thyroid, conjunctiva, and liver and nonmucosal organs such as the orbit and skin.2 Interestingly, these organs are devoid of any native lymphoid tissue, and the lymphoma at these sites arises from the MALT acquired as a result of a chronic inflammatory or autoimmune disorder. Notably, gastric MALT lymphoma is invariably preceded by Helicobacter pylori–associated chronic gastritis, while salivary gland and thyroid MALT lymphomas are commonly associated with lymphoepithelial sialadenitis and Hashimoto thyroiditis, respectively.3 The antigenic and immunologic stimuli incurred in these preceding diseases play a critical role in the genesis and expansion of the lymphoma clone.
Genetically, MALT lymphoma is characterized by t(1;14)(p22; q32) and t(11;18)(q21;q21) that are specific for this lymphoma type. t(1;14)(p22;q32) occurs in approximately 5% of MALT lymphomas and causes deregulation of BCL10,4,5 which specifically links the antigen receptor signaling to the NFκB pathway.6 In MALT lymphoma with t(1;14)(p22;q32), BCL10 is highly expressed in both the nucleus and cytoplasm, in contrast to its weak expression in the cytoplasm of normal germinal center B cells.7 Moderate BCL10 nuclear expression also is found in MALT lymphomas without t(1;14)(p22;q32) and significantly correlates with both t(11;18)(q21;q21) and clinical stage in gastric MALT lymphoma.8
t(11;18)(q21;q21) fuses the amino terminal of the API2 gene to the carboxyl terminal of the MALT1 gene and generates a chimeric fusion product,9 which activates NFκB.10,11 The translocation can be detected by reverse transcription–polymerase chain reaction (RT-PCR) or fluorescence in situ hybridization (FISH), and its occurrence has been extensively examined in gastric MALT lymphoma.8,12-17 t(11;18)(q21;q21) occurs in 30%-50% of gastric MALT lymphomas and is significantly associated with cases of more advanced stage. Translocation-positive gastric MALT lymphomas do not respond to H pylori eradication.18-20 Intriguingly, the translocation is found only rarely in transformed MALT lymphoma.21
Several studies have examined t(11;18)(q21;q21) in nongastric MALT lymphomas.8,13,15,17,22,23 However, with the exception of pulmonary MALT lymphoma, only a few cases from other mucosal sites have been studied. The true incidence of the translocation in MALT lymphoma from nongastric sites and in IPSID is unknown. Since MALT lymphoma from different mucosal sites is associated with distinct prelymphomatous disorders, the nature of these diseases may contribute to the development of genetic abnormalities and therefore influence the occurrence of t(11;18)(q21;q21). To test this hypothesis, we screened t(11;18)(q21;q21) in a large series of MALT lymphoma from different sites including the stomach, lung, salivary gland, thyroid, conjunctiva, orbit, skin, and liver, as well as in IPSID. In gastric MALT lymphoma, we also correlated the translocation with CagA strains of H pylori, which are more virulent and pathogenic.24
Materials and methods
Materials
Tumor tissues from 417 cases of MALT lymphoma were randomly retrieved from the authors' institutions (Table 1). They included 173 cases from the gastrointestinal tract, 47 from the lung, 27 from the conjunctiva, 28 from the orbit, 72 from the salivary gland, 18 from the thyroid, 27 from the skin, 6 from the liver, and 19 from other rare sites. Fresh frozen tissue was available in 59 cases that were subjects of a previous study,8 while formalin-fixed and paraffin-embedded tissues were available in the remaining cases. In addition, paraffin-embedded tissues were retrieved from 30 cases of H pylori–associated gastritis, 22 cases of lymphoepithelial sialadenitis, and 22 cases of Hashimoto thyroiditis.
Frequency of t(11;18) and BCL 10 nuclear expression in various MALT lymphomas
. | . | . | BCL10 nuclear expression . | . | . | ||
|---|---|---|---|---|---|---|---|
| Site of MALT lymphoma . | No. of cases . | t(11;18) (%) . | Moderate with API2-MALT1 fusion (%) . | Strong like in t(1;14) (%) . | Moderate but lacking API2-MALT1 fusion (%) . | ||
| Gastrointestinal tract | |||||||
| Stomach | 138 | 33 (23.9) | 30 of 30 (100) | 6 of 123 (4.9) | 26 of 87 (30) | ||
| Small intestine | 8 | 5 (62.5) | 1 of 1 (100) | 0 of 4 (0) | 0 of 3 (0) | ||
| IPSID | 22 | 0 (0) | — | 0 of 5 (0) | 1 of 5 (20) | ||
| Large intestine | 5 | 1 (20) | 1 of 1 (100) | 0 of 3 (0) | 0 of 2 (0) | ||
| Lung | 47 | 18 (38.3) | 17 of 17 (100) | 5 of 41 (12) | 5 of 19 (26) | ||
| Conjunctiva | 27 | 5 (18.5) | 3 of 3 (100) | 0 of 19 (0) | 8 of 16 (50) | ||
| Orbit | 28 | 4 (14.3) | 3 of 3 (100) | 0 of 19 (0) | 1 of 15 (6.7) | ||
| Salivary gland | 72 | 1 (1.4) | 1 of 1 (100) | 0 of 70 (0) | 15 of 69 (21.7) | ||
| Thyroid | 18 | 0 (0) | — | 0 of 14 (0) | 0 of 14 (0) | ||
| Skin | 27 | 0 (0) | — | 1 of 13 (7.7) | 0 of 12 (0) | ||
| Liver | 6 | 0 (0) | — | 0 of 4 (0) | 1 of 4 (25) | ||
| Other* | 19 | 0 (0) | — | 0 of 16 (0) | 3 of 16 (18.7) | ||
| Total | 417 | 67 (16.2) | 56 of 56 (100) | 12 of 331 (3.6) | 60 of 263 (23) | ||
. | . | . | BCL10 nuclear expression . | . | . | ||
|---|---|---|---|---|---|---|---|
| Site of MALT lymphoma . | No. of cases . | t(11;18) (%) . | Moderate with API2-MALT1 fusion (%) . | Strong like in t(1;14) (%) . | Moderate but lacking API2-MALT1 fusion (%) . | ||
| Gastrointestinal tract | |||||||
| Stomach | 138 | 33 (23.9) | 30 of 30 (100) | 6 of 123 (4.9) | 26 of 87 (30) | ||
| Small intestine | 8 | 5 (62.5) | 1 of 1 (100) | 0 of 4 (0) | 0 of 3 (0) | ||
| IPSID | 22 | 0 (0) | — | 0 of 5 (0) | 1 of 5 (20) | ||
| Large intestine | 5 | 1 (20) | 1 of 1 (100) | 0 of 3 (0) | 0 of 2 (0) | ||
| Lung | 47 | 18 (38.3) | 17 of 17 (100) | 5 of 41 (12) | 5 of 19 (26) | ||
| Conjunctiva | 27 | 5 (18.5) | 3 of 3 (100) | 0 of 19 (0) | 8 of 16 (50) | ||
| Orbit | 28 | 4 (14.3) | 3 of 3 (100) | 0 of 19 (0) | 1 of 15 (6.7) | ||
| Salivary gland | 72 | 1 (1.4) | 1 of 1 (100) | 0 of 70 (0) | 15 of 69 (21.7) | ||
| Thyroid | 18 | 0 (0) | — | 0 of 14 (0) | 0 of 14 (0) | ||
| Skin | 27 | 0 (0) | — | 1 of 13 (7.7) | 0 of 12 (0) | ||
| Liver | 6 | 0 (0) | — | 0 of 4 (0) | 1 of 4 (25) | ||
| Other* | 19 | 0 (0) | — | 0 of 16 (0) | 3 of 16 (18.7) | ||
| Total | 417 | 67 (16.2) | 56 of 56 (100) | 12 of 331 (3.6) | 60 of 263 (23) | ||
— indicates not applicable
Includes brain (4), bladder (4), tonsil (2), thymus (2), lacrimal (1), eye lid (2), breast (1), gall bladder (1), lip (1), and ovary (1)
DNA extraction
DNA samples were prepared from whole sections of formalin-fixed and paraffin-embedded tissues as described previously.25
RNA extraction
For frozen tissues, total RNA was extracted from up to 10 mg tissue using the RNeasy Mini Kit (Qiagen, West Sussex, United Kingdom).8 For formalin-fixed and paraffin-embedded tissues, total RNA was extracted using an Ambion RNA isolation kit (Ambion Ltd Huntingdon, Cambridgeshire, United Kingdom).20 Briefly, 5-10 5-μm paraffin sections were deparaffinized in xylene. The tissue was digested with proteinase K (1 mg/mL) for 2 hours at 45°C and solubilized in a guanidinium-based buffer. RNA was extracted with acid phenol:chloroform and precipitated in isopropanol. The precipitated RNA was washed in 75% ethanol and redissolved in 20 μL RNA storage solution.
Detection of t(11;18)(q21;q21) by RT-PCR
The synthesis of cDNA from frozen tissues was performed as described previously.8 PCR detection of the API2-MALT1 fusion transcript was performed with a single set of primers (f-S: 5′-ACATTCTTTAACTGGCCCTC; f-AS: 5′-TAGTCAATTCGTACACATCC) that covered all the known breakpoints (Figure 1A).8 RT-PCR of the glucose-6-phosphate dehydrogenase (G6PD) gene was carried out in parallel as a control. PCR products were analyzed on agarose gels.
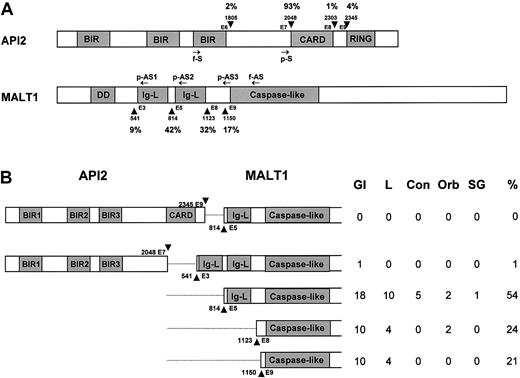
Schematic illustration of the API2 and MALT1 gene structure and the position of primers used. (A) Known breakpoints are indicated by arrow-head, and nucleic acids are numbered according to cDNA sequence of the API2 (GenBank, NM_001165) and MALT1 genes (AF316597). The frequency of known breakpoints was shown. Arrows indicate the position of primers used. f-S and f-AS indicate sense and antisense primer for PCR from frozen tissue; p-S and p-AS, sense and antisense primer for PCR from paraffin-embedded tissues. BIR indicates baculovirus IAP repeat; CARD, caspase recruitment domain; DD, death domain; and Ig-L, Ig-like domain. (B) Distribution of breakpoints of the API2 and MALT1 genes in MALT lymphomas of different sites. GI indicates gastrointestine; L, lung; Con, conjunctiva; Orb, orbit; and SG, salivary gland.
Schematic illustration of the API2 and MALT1 gene structure and the position of primers used. (A) Known breakpoints are indicated by arrow-head, and nucleic acids are numbered according to cDNA sequence of the API2 (GenBank, NM_001165) and MALT1 genes (AF316597). The frequency of known breakpoints was shown. Arrows indicate the position of primers used. f-S and f-AS indicate sense and antisense primer for PCR from frozen tissue; p-S and p-AS, sense and antisense primer for PCR from paraffin-embedded tissues. BIR indicates baculovirus IAP repeat; CARD, caspase recruitment domain; DD, death domain; and Ig-L, Ig-like domain. (B) Distribution of breakpoints of the API2 and MALT1 genes in MALT lymphomas of different sites. GI indicates gastrointestine; L, lung; Con, conjunctiva; Orb, orbit; and SG, salivary gland.
For paraffin-embedded samples, cDNA was synthesized using Super-Script Preamplification System (Invitrogen, Paisley, United Kingdom) with a mixture of gene-specific primers, including 1 primer for G6PD, that were specially designed for formalin-fixed and paraffin-embedded tissues.20 Three sets of PCR primers with a common sense primer covering 93% of the known breakpoints on the API2 gene (p-S: 5′-GGAAGAGGAGAGAGAAAGAGCA) and 3 antisense primers (p-AS1: 5′-CCAAGACTGCCTTTGACTCT; p-AS2: 5′-GGATTCAGAGACGCCATCAA and p-AS3: 5′-CAAAGGCTGGTCAGTTGTTT) targeting all 4 breakpoints on the MALT1 gene were used for PCR of the API2-MALT1 fusion transcript as described previously (Figure 1A).20 G6PD was amplified in parallel as a control. PCR was performed separately with each primer set in duplicate. PCR products were analyzed by electrophoresis on 10% polyacrylamide gels.
PCR of H pylori–associated urease and CagA gene
For H pylori–associated urease gene, a pair of primers (sense primer 5′-CATCTTGTTAGAGGGATTGG-3′; antisense primer 5′-TAACAAACCGATAATGGCGC-3′) were used, which yielded a 203–base pair (bp) product and was thus suitable for DNA samples prepared from formalin-fixed and paraffin-embedded tissues.26 Similarly, the CagA gene was amplified with a primer set (sense primer 5′-TCAGAAATTTGGGGATCAG-3′; antisense primer 5′-TCATCARGGATAGGGGGTT-3′), which gave rise to a 132-bp product.27 For both genes, PCR was carried out in a 25-mL reaction on a thermocycler (ThermoHybaid Px2, Franklin, MA) under the following conditions: 4 minutes at 94°C for initial denaturation, 40 cycles of 30 seconds at 94°C, 30 seconds at 53°C, 45 seconds at 72°C, and finally 10 minutes at 72°C to conclude the reaction. PCR products were analyzed on 10% polyacrylamide gels.
Sequencing of PCR products
Where indicated, PCR products were gel purified (QIAquick Gel Extraction Kit; Qiagen) and sequenced in both directions using dRhodamine dye terminators on an ABI Prism 377 sequencer (PE Applied Biosystems, Foster City, CA).
Immunohistochemistry
BCL10 was immunostained using a mouse monoclonal antibody (clone 151) on formalin-fixed and paraffin-embedded tissues as described previously.7 Neutrophils were detected with a mouse monoclonal antibody to the neutrophil elastase (Dako, Cambridgeshire, United Kingdom) on formalin-fixed and paraffin-embedded tissues preceded by antigen retrieval with chymotrypsin digestion. The extent of neutrophil infiltration was estimated in 10 randomly chosen fields and expressed as a mean of number of neutrophils per high-power field (× 40). Only neutrophils outside blood vessels were counted.
Interphase fluorescence in situ hybridization
Using bioinformatic resources available at the University of California at Santa Cruz (http://genome.ucsc.edu), BAC clones RP11-1080I1 and RP11-40K4 (telomeric) as well as RP11-1077C10 and RP11-36L4 (centromeric) flanking the BCL10 locus were selected as interphase FISH probes. The clones were differentially labeled with Spectrum Orange (SO, telomeric) and Spectrum Green (SG, centromeric) and pooled to obtain a break-apart assay. Bacteria culture, BAC DNA isolation and labeling, and probe preparation were performed as previously described.28 The diagnostic reliability of the newly developed BCL10 break-apart assay was recently proven in a series of controls including 4 cases of cytogenetically proven t(1;14)(p22;q32)5 (R.S. et al, unpublished data, March 2003). For detection of alterations affecting the MALT1 locus, the commercially available LSI-MALT1 probe was applied (Vysis, Downers Grove, IL).
Locus-specific interphase FISH was performed on paraffin-embedded tissue sections. Briefly, deparaffinized sections were pretreated by pressure cooking (between 3 minutes and 3 minutes and 30 seconds) in EDTA (ethylenediaminetetraacetic acid) buffer (1 mM, pH 8.0) and subsequent incubation in pepsin solution (5-20 minutes) at 37°C to increase DNA accessibility. Sections were then fixed in 1% paraformaldehyde for 2 minutes, dehydrated through increasing ethanol series, and air dried. The appropriate probe mix (1.5 μL) was applied to the tissue section and covered with a round 10 mm coverslip. Both probe and target DNA were simultaneously denatured at 70°C for 30-45 minutes and incubated up to 3 days at 37°C. Posthybridization washes were performed according to the “rapid-wash protocol” provided by Vysis. Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI) and mounted in Vectashield antifade solution (Vector Laboratories, Burlingame, CA). Image acquisition and processing was performed as previously described.28
Statistical analysis
Fisher exact test was used to analyze the correlation between the occurrence of t(11;18)(q21;q21) and CagA status. One-way analysis of variance (ANOVA) and Student t test were used to examine the difference in neutrophil infiltration among H pylori–associated gastritis, lymphoepithelial sialadenitis, and Hashimoto thyroiditis.
Results
t(11;18)(q21;q21) occurred at markedly variable frequencies in MALT lymphomas of different sites
All the cases included in Table 1 were successful for RT-PCR of the reference gene G6PD. RT-PCR products of the API2-MALT1 fusion transcript from frozen tissues were further confirmed by sequencing, while those from paraffin-embedded tissues were characteristic for each breakpoint on polyacrylamide gels, allowing confident detection of t(11;18)(q21;q21) without the need of sequencing confirmation in most cases. In 5% of cases, PCR products were ambiguous, and sequencing confirmation was carried out.
The cases included in this study were randomly selected and thus the frequency of t(11;18)(q21;q21) found in MALT lymphoma of different sites reflected their natural incidences (Table 1). Of the 9 major sites from which MALT lymphoma commonly arises, t(11;18)(q21;q21) was found at the highest frequencies in those derived from the lung (38.3%) and stomach (23.9%) and at moderate frequencies in those from the conjunctiva (18.5%) and orbit (14.3%). The translocation was seen in only 1 case of salivary gland MALT lymphoma (1.4%) but was absent in those from the thyroid, skin, liver, and other rare sites, as well as in IPSID.
t(11;18)(q21;q21) was not found in 22 cases of lymphoepithelial sialadenitis and 22 cases of Hashimoto thyroiditis, the preceding disease associated with salivary gland and thyroid MALT lymphomas, respectively. In our previous study, the translocation also was not detected in H pylori–associated gastritis.8
The distribution of breakpoints in both the API2 and MALT1 genes among MALT lymphomas of different sites was similar. All the breakpoints in the API2 gene were immediately downstream of exon 7, while the breakpoints in the MALT1 gene occurred variably immediately upstream of 4 exons,3,5,8,9 with upstream exon 5 showing the highest frequency (Figure 1B).
BCL10 expression pattern and its correlation with t(11;18)(q21;q21) in MALT lymphoma of different sites
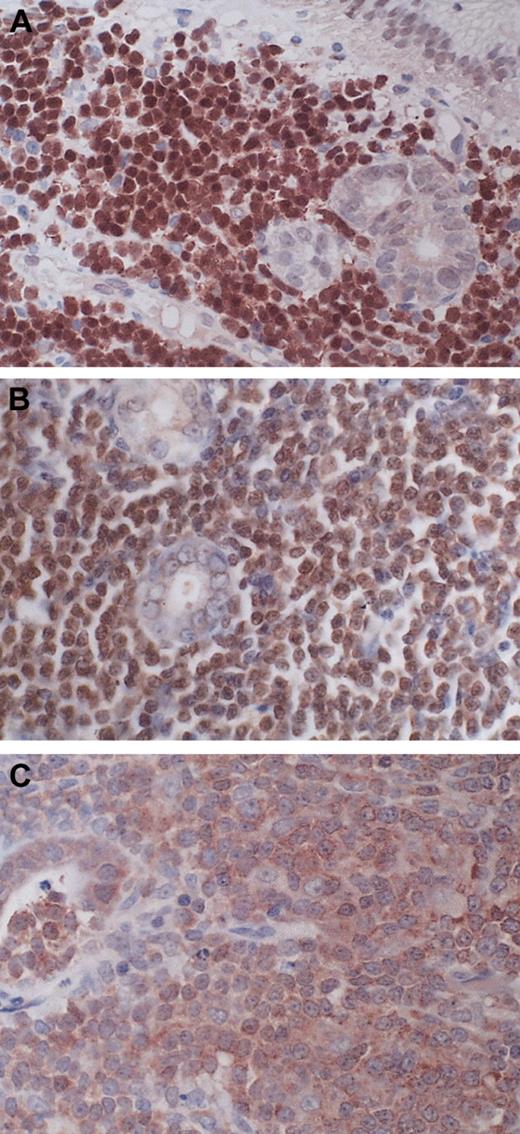
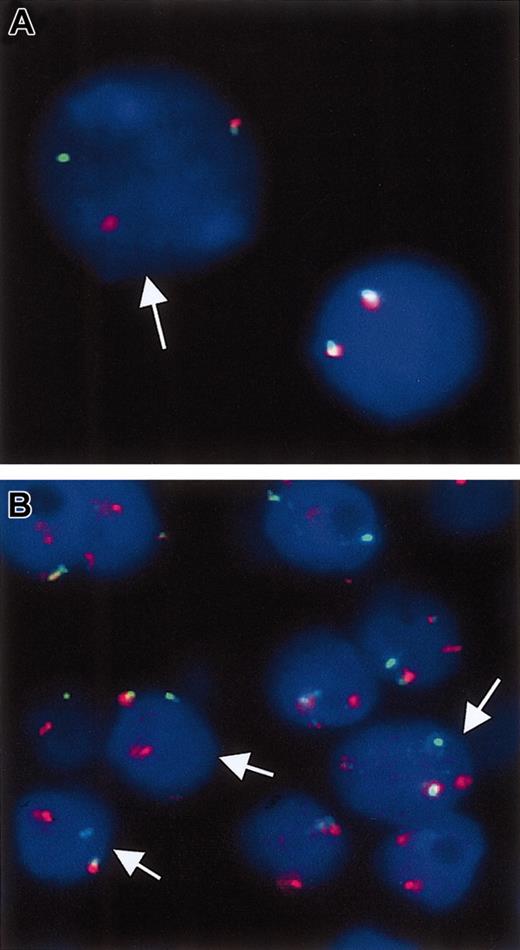
BCL10 expression pattern was investigated in 331 cases examined for t(11;18)(q21;q21). All 56 cases of t(11;18)(q21;q21)–positive lymphoma stained showed moderate BCL10 expression in the nuclei of most tumor cells (Figure 2). Among t(11;18)(q21;q21)–negative cases, 12 cases, including 6 from the stomach, 5 from the lung, and 1 from the skin, showed strong BCL10 nuclear staining, similar to that seen in MALT lymphoma with t(1;14)(p22;q32) (Table 1; Figure 2). To further ascertain whether these cases harbored a BCL10–involved chromosomal translocation, we performed interphase FISH for detection of chromosomal breakpoints affecting the BCL10 locus, like t(1;14)(p22;q32) or variants in 4 pulmonary and 3 gastric cases where sufficient tissue materials were available. Five cases showed a signal constellation, indicating a chromosomal translocation affecting the BCL10 locus (Figure 3). The remaining 2 cases lacked evidence for a breakpoint involving the BCL10 gene, and one case contained a heavy reactive component that might have compromised the FISH analysis. Interestingly, these 2 cases showed an increased signal number (3 and more signals) for the intact MALT1 locus.
BCL10 expression pattern in MALT lymphoma. (A) A gastric MALT lymphoma shows strong BCL10 nuclear expression similar to that found in those with t(1;14)(p22;q32). (B) A t(11;18)(q21;q21)–positive gastric MALT lymphoma displays moderate BCL10 nuclear expression. (C) A gastric MALT lymphoma lacking t(11; 18)(q21;q21) shows cytoplasmic BCL10 expression. Original magnification, × 400 for all panels.
BCL10 expression pattern in MALT lymphoma. (A) A gastric MALT lymphoma shows strong BCL10 nuclear expression similar to that found in those with t(1;14)(p22;q32). (B) A t(11;18)(q21;q21)–positive gastric MALT lymphoma displays moderate BCL10 nuclear expression. (C) A gastric MALT lymphoma lacking t(11; 18)(q21;q21) shows cytoplasmic BCL10 expression. Original magnification, × 400 for all panels.
Break-apart double-color interphase FISH for the detection of breakpoints in the BCL10 locus. (A) Case with cytogenetically proven t(1;14)(p22; q32). The arrow points to a tumor cell in which the dissociation of the red and green signals indicates the presence of a chromosomal breakpoint in the BCL10 locus. The nucleus on the right displays 2 colocalized signals pointing to 2 intact copies of the BCL10 locus. (B) A gastric MALT lymphoma with strong BCL10 nuclear expression. Interphase nuclei from a paraffin section shows that several cells (arrows) display a split of the red and green signals, suggestive of t(1;14)(p22;q32) or variants.
Break-apart double-color interphase FISH for the detection of breakpoints in the BCL10 locus. (A) Case with cytogenetically proven t(1;14)(p22; q32). The arrow points to a tumor cell in which the dissociation of the red and green signals indicates the presence of a chromosomal breakpoint in the BCL10 locus. The nucleus on the right displays 2 colocalized signals pointing to 2 intact copies of the BCL10 locus. (B) A gastric MALT lymphoma with strong BCL10 nuclear expression. Interphase nuclei from a paraffin section shows that several cells (arrows) display a split of the red and green signals, suggestive of t(1;14)(p22;q32) or variants.
Overall, cases suspicious of BCL10 chromosomal translocation accounted for 3.6% of the total cases examined for BCL10 expression pattern, with pulmonary MALT lymphoma showing the highest incidence (12%). BCL10 nuclear expression, similar to that seen in t(11;18)(q21;q21)–positive cases, also was found in 60 (23%) of the 263 cases that were negative for the translocation by RT-PCR.
Correlation of t(11;18)(q21;q21) with the CagA-positive strain of H pylori in gastric MALT lymphoma
Because CagA-positive strains of H pylori are more virulent and pathogenic, we correlated t(11;18)(q21;q21) with CagA status in gastric MALT lymphomas. DNA samples from all gastric MALT lymphomas first were screened for the presence of H pylori by amplification of the urease gene, and the positive samples were subsequently subjected to PCR of the CagA gene. Overall, CagA-positive strains of H pylori were found in 28 of the 42 cases (67%) of gastric MALT lymphoma in which PCR of the H pylori urease gene was positive. CagA-positive strains of H pylori were significantly higher in t(11;18)(q21;q21)–positive gastric MALT lymphoma (14 of 15 = 93.3%) than the translocation negative cases (14 of 27 = 51.9%) (P < .01).
Neutrophil infiltration in different premalignant diseases of MALT lymphoma
In view of the finding that t(11;18)(q21q;21) occurred at markedly variable frequencies in MALT lymphomas of different sites, it is highly likely that the occurrence of the translocation is influenced by the preceding disease associated with MALT lymphomas. In gastric MALT lymphoma, t(11;18)(q21;q21) is significantly associated with CagA-positive strains of H pylori that are strong inducers of interleukin-8, a potent chemokine for neutrophil activation.24 Activated neutrophils are known to release oxygen-reactive species, which can cause a wide range of DNA damage, including double-strand breaks.29 We therefore examined the extent of neutrophil infiltration in H pylori–associated gastritis, lymphoepithelial sialadenitis, and Hashimoto thyroiditis to further understand whether there is any difference in the exposure of potential genetic insults among these premalignant diseases. As expected, neutrophil infiltration was significantly higher in H pylori–associated gastritis (8.19 ± 0.92 neutrophils/high-power field) than in lymphoepithelial sialadenitis (0.09 ± 0.03 neutrophils/high-power field) and Hashimoto thyroiditis (0.38 ± 0.04 neutrophils/high-power field) (P < .01 for both).
Discussion
t(11;18)(q21;q21) is a specific chromosomal aberration of MALT lymphoma and has not been found in other lymphoma types, including nodal and splenic marginal zone B-cell lymphoma.8,12-17 Previous studies found the translocation in 35% to 50% of gastric MALT lymphomas and 55% to 75% of pulmonary MALT lymphomas, but the incidence of t(11;18)(q21;q21) in MALT lymphoma of other sites is largely unknown, as only limited cases have been examined.8,13-17 In the present study, we screened 417 cases of MALT lymphoma of 8 major sites for t(11;18)(q21;q21) and showed that the translocation occurred at the highest frequencies in those from the lung (38.3%) and stomach (23.9%) and at moderate frequencies in those from the conjunctiva (18.5%) and orbit (14.3%). The translocation was present in only a single case of salivary gland MALT lymphoma but was absent in those from the thyroid, skin, liver, and other rare sites, and in IPSID.
The incidence of t(11;18)(q21;q21) in both pulmonary and gastric MALT lymphoma in the present study is much lower than reported previously even after taking into account the fact that our RT-PCR approach for paraffin-embedded tissues would miss approximately 7% of rare breakpoints on the API2 gene. The number of cases studied in the previous reports was relatively small, and the investigations were commonly based on those treated by surgery and were therefore biased toward the advanced cases.8,12-17 At least in gastric MALT lymphoma, t(11;18)(q21;q21) has been shown to be significantly associated with cases of more advanced stage.8 Thus, the previous studies may have overestimated the incidence of t(11;18)(q21;q21) in pulmonary and gastric MALT lymphomas. In the present study, the cases included were randomly selected and a large cohort examined, and thus the frequency of t(11;18)(q21;q21) reported here should be much closer to its natural incidence.
The higher incidence of the translocation in pulmonary MALT lymphoma may be in part the result of the disease being diagnosed at relatively more advanced stages. Approximately 50% of patients with pulmonary MALT lymphoma are asymptomatic, and 25% to 47% of cases are at stage III or above at the time of diagnosis.30,31 In contrast, patients with gastric MALT lymphoma commonly present upper stomach discomfort, and only 13% of cases are at stage III or above at diagnosis.31
Unlike most chromosomal translocations associated with lymphoma, t(11;18)(q21;q21) does not involve the immunoglobulin (Ig) locus, and its occurrence is most likely not associated with the variable-diversity-joining (VDJ) recombination event. The finding of dramatically variable incidences of t(11;18)(q21;q21) in MALT lymphoma of various sites indicates that the occurrence of the translocation is influenced by different preceding diseases associated with MALT lymphoma. H pylori–associated gastritis is strongly related to the development of gastric MALT lymphoma, while lymphoepithelial sialadenitis and Hashimoto thyroiditis are closely associated with the genesis of salivary gland and thyroid MALT lymphoma, respectively. The mechanisms underlying the pathogenesis of these diseases are different. Lymphoepithelial sialadenitis and Hashimoto thyroiditis are principally due to generation of autoreactive B cells, while H pylori infection causes damage of gastric mucosa through bacterial toxins and host responses. CagA-positive strains of H pylori are known to be more virulent and pathogenic and are significantly associated with increased risk of development of gastric cancer and peptic ulcer.
The role of infection of CagA strains of H pylori in the development of gastric MALT lymphoma remains unclear since controversial results have been reported.27,32,33 Interestingly, our results showed that CagA-positive strains of H pylori were significantly associated with t(11;18)(q21q;21), suggesting that CagA-positive strains of H pylori may be highly potent in promoting the occurrence of t(11;18)(q21;q21). H pylori strains harboring the CagA island cause strong inflammatory responses, of which the key element is induction of interleukin-8, a potent chemokine for neutrophil activation.24 Activated neutrophils release oxygen-reactive species, which can cause a wide range of DNA damage, including double-strand breaks.29 It is possible that the occurrence of t(11;18)(q21;q21) is related to oxidative damage induced by H pylori infection. In line with this hypothesis, the genomic breakpoint of t(11;18)(q21;q21) on both derivative chromosomes was random and showed no association with sequence motifs known to be associated with chromosomal recombination34 (M.-Q.D. et al, unpublished results, November 2002). Furthermore, deletions and duplications ranging from a few to several kilobase pair are a common finding at the breakpoint for both the API2 and MALT1 loci14 (M.-Q.D. et al, unpublished results, November 2002).
To further examine whether there is any difference in the potential exposure of genetic insults among different premalignant diseases associated with MALT lymphoma, we examined the extent of neutrophil infiltration in H pylori–associated gastritis, lymphoepithelial sialadenitis, and Hashimoto thyroiditis. As expected, neutrophil infiltration was prominent in H pylori–associated gastritis but not in lymphoepithelial sialadenitis and Hashimoto thyroiditis. The difference in neutrophil infiltration among these premalignant diseases correlated well with the incidence of t(11;18)(q21;q21) detected in prospective MALT lymphoma. Thus, it is possible that the presence or absence of genotoxic factors such as activated neutrophils in the premalignant disease may determine the incidence of t(11;18)(q21;q21) in prospective MALT lymphoma.
The hypothesis described in the previous paragraph also may explain the finding of a high incidence of t(11;18)(q21;q21) in pulmonary MALT lymphoma. Acquired MALT in the lung is seen in the inflammatory disease known as follicular bronchiolitis.35 Although the etiology of follicular bronchiolitis is unknown, in at least 50% of cases, histological examination shows suppurative exudates in bronchiolar lumina and neutrophils in adjacent alveoli,35 suggesting that genotoxic factors could well be present.
In our previous studies, we showed that BCL10 was expressed strongly in both the nuclei and cytoplasm of tumor cells with t(1;14)(p22;q32) in 5 of 5 cases examined7 (M.-Q.D. et al, unpublished data, March 2003). We further confirm this finding in 5 of 7 cases by interphase FISH in the present study and show that this expression pattern is significantly associated with MALT lymphoma harboring t(1;14)(p22;q32) or variants. Based on BCL10 staining of 331 cases of MALT lymphoma from 8 major sites, our present study indicates that the overall incidence of t(1;14)(p22; q32) is less than 4%, with the highest frequency (12%) in pulmonary MALT lymphoma. Our present study also confirmed the significant association between t(11;18)(q21;q21) and nuclear BCL10 expression observed from previous studies based on smaller series of cases.8,36 Furthermore, the current study highlights the fact that a proportion of MALT lymphomas lacking both t(1;14)(p22;q32) and t(11;18)(q21;q21) showed nuclear BCL10 expression.
Interestingly, a novel t(14;18)(q32;q21) involving the MALT1 gene has been newly described in MALT lymphoma.37 This translocation appears to occur more often in MALT lymphomas from sites where t(11;18)(q21;q21) is uncommon. Based on interphase fluorescence in situ hybridization, additional API2 signals in the absence of t(11;18)(q21;q21) and trisomy 11 were frequently found in pulmonary MALT lymphoma, suggesting the presence of either novel API2-involved chromosomal translocation or API2 gene amplification.38 It remains to be investigated whether MALT lymphomas with such chromosomal aberrations express nuclear BCL10.
In summary, our results demonstrate that t(11;18)(q21;q21) occurs at markedly variable frequencies in MALT lymphoma of different sites and suggest that the occurrence of the translocation is influenced by the nature of premalignant diseases associated with MALT lymphoma. Oxidative damage might play a role in development of t(11;18)(q21;q21).
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-11-3502.
Supported by research grants from the Leukemia Research Fund, United Kingdom, the Cancer Research United Kingdom, the Deutsche Krebshilfe (grant no. 10-1641-De1), and the Interdisziplinäre Zentrum für klinische Krebsforschung (IZKF) Kiel.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Tim Diss for critical reading of the manuscript and Claudia Becher for technical assistance on the FISH assay.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal