Abstract
Gastric marginal zone lymphoma (GMZL) is strongly associated with Helicobacter pylori infection, which induces a chronic inflammatory response. Inflammation can result in DNA damage related to its severity, the cellular antioxidant capacity, and the integrity of DNA repair mechanisms. Interleukin-1 (IL-1) polymorphisms have been shown to be important mediators of inflammation, while glutathione S-transferase GST T1 and GST M1 polymorphisms are believed to affect cellular antioxidant capacity. We aimed to determine whether polymorphisms at the IL-1 and GST T1 and GST M1 loci modulate the risk of developing GMZL. Blood and biopsy samples were obtained for a historical series of 66 GMZL cases, whereas blood samples were available from 163 healthy controls. Genotypes were obtained for GST T1, GST M1, IL-1 RN, and IL-1B-31 using PCR-based techniques. H pylori infection was found in 86.0% of cases, whereas in the control population only 37.4% tested positive. The IL-1 RN 2/2 genotype was significantly associated with risk of GMZL (odds ratio [OR], 5.51; 95% confidence interval [CI] 2.16-14.07), but not the IL-1B-31 genotype. Likewise, the GST T1 null genotype was strongly associated with risk of GMZL (OR, 9.51; 95% CI 4.57-19.81), but not the GST M1 genotype. Evidence was found of effect modification between the IL-1 RN and GST T1 genotypes (P =.02). The combination of the IL-1 RN 2/2 and GST T1 null genotype was most strongly associated with risk of GMZL (OR, 32.29; 95% CI 6.92-150-63). These results support the hypothesis that the risk of developing GMZL is influenced by inter-individual variation in the cellular inflammatory immune responses to H pylori infection, and to antioxidative capacity.
Introduction
The association of gastric marginal zone lymphoma (GMZL) with prior infection by Helicobacter pylori1 has been reported in both epidemiologic2 and pathologic studies.3 The onset of GMZL is preceded by the development of mucosa-associated lymphoid tissue (MALT), acquired as a result of H pylori infection, which may undergo multistep progression through monoclonal proliferation to malignant lymphoma.3 Transition to GMZL is associated with an increasing level of DNA damage within the infiltrating lymphoid tissue,4 a putative cause of which is oxidative stress generated as a consequence of H pylori–induced inflammation. Reactive oxygen species (ROS) are important mediators of this effect, and a direct correlation between the level of ROS and the level of DNA damage to the gastric mucosa has been reported.5,6 The severity of DNA damage occurring in an infected individual will depend on a number of factors, including the strain and virulence of H pylori, the severity of the inflammatory response, and the individual's ability to detoxify ROS. Therefore, H pylori–associated GMZL provides an ideal system in which to study the effects of genetic variation on important modulators of the immune response and on mediators of oxidative stress.
The immune response resulting from H pylori infection is primarily via Th1, a proinflammatory response, mediated by a cascade of cytokines including interleukin 18 (IL-18), IL-12, IL-1, tumor necrosis factor–α and interferon-γ. These cytokines regulate their own levels of expression by autocrine effects, but they also influence downstream cytokine expression, thereby amplifying the inflammatory response. IL-1 is one of the principal proinflammatory cytokines that mediate the Th1 immune response following H pylori infection, with IL-1β production being up-regulated in the presence of H pylori.7,8 IL-1β is also one of the most powerful inhibitors of gastric acid secretion, the hypochlorhydria associated with high-producer variants of IL-1 governing the extent of H pylori infection and distribution of gastritis. As a consequence, polymorphic variants in IL-1 may affect not only the primary inflammatory response but also the level of response following amplification.
The IL-1 gene cluster is situated on chromosome 2q and comprises 3 related genes within a 430-kb region: IL-1A, IL-1B, and IL-1 RN, which encode the proinflammatory cytokines IL-1α and IL-1β and their endogenous receptor antagonist IL-1ra, respectively.9 Three biallelic polymorphisms in IL-1B have been described, all C>T base transitions found at positions -511, -31, and +3954 bp from the transcriptional start site.10,11 Strong linkage disequilibrium between the IL-1B-31 C and IL-1B-511 T variants has been reported, with the IL-1B-31, IL-1B-511 T C haplotype occurring at a frequency of 0.38 in the white population.10,11 The IL-1B-31 polymorphism is situated within a TATA sequence of the IL-1B promoter, and has been shown to markedly affect DNA-protein interaction in vitro.10,11 The homozygous genotype IL-1B-31 TT has been associated with increased IL-1β production,12 but this finding remains unconfirmed.13
For IL-1 RN a penta-allelic 86-bp variable number tandem repeat (VNTR) in intron 2 has been described, with the most common allele, IL-1 RN*1, corresponding to 4 repeats. The IL-1 RN*2 allele, corresponding to 2 repeats, has been reported at a frequency of 0.12 in the white population and has been associated with chronic inflammatory conditions such as ulcerative colitis and autoimmune conditions such as Sjögren syndrome.14,15 The IL-1 RN*2 allele has been associated with enhanced IL-1β production in vitro16 ; however, data regarding its effects on IL-1ra production are contradictory.17-19 It has been reported that IL-1B and IL-1 RN alleles are inherited as an extended haplotype, associated with inflammatory diseases.20,21
Major cellular defense mechanisms against DNA damage are the glutathione S-transferase (GST) enzymes. In addition to their role in the detoxification of potential carcinogens by catalyzing conjugation to glutathione, these enzymes also have a strong antioxidant function, neutralizing free radicals. There are 4 cytosolic GST families, including GST T1 and GST M1. Independent gene deletions exist at both the GST T1 and GST M1 loci, resulting in a lack of active protein,22 and the null genotypes of both loci have been associated with several malignancies.23-27 We hypothesize that lack of GST T1 or GST M1 activity may be associated with an increased risk of developing GMZL in H pylori–infected individuals, and that this risk may be enhanced by interaction with the proinflammatory genotypes of IL-1.
Materials and methods
Samples
Sixty-six cases of GMZL (aged 38-86 years) were identified from multiple sources throughout the Yorkshire region in the north of England. Archived samples, comprising paraffin blocks of biopsies, surgical specimens, and peripheral blood smears, were obtained, as were available. Pure populations of normal cells were extracted where possible from biopsy and surgical blocks, the presence of tumor cell contamination monitored by comparison with sections stained with hematoxylin and eosin. Normal cells were present in all samples extracted, although the presence of a small amount of tumor material in some samples cannot be excluded. All cases were histologically validated according to the criteria of the Revised European-American Classification of Lymphoid Neoplasms (REAL) classification,28 using the standard panel of markers utilized by the Hematological Malignancy Diagnostic Service at Leeds General Infirmary (CD20, 79, 10, 5, 23, 3, Ki67, and BCL-2). A reference series of 163 healthy population controls (aged 28-65 years) was collected as part of an ongoing lymphoma study carried out by the Leukemia Research Fund (LRF) Epidemiology and Genetics Unit; each control provided a blood sample. Ethical approval to establish a DNA repository for cases of hematologic malignancy and to carry out a case-control comparison of genotypes was obtained from the North and Yorkshire Multi-Centre Research Ethics Committee (MREC).
Determination of H pylori status
H pylori status of the reference population was determined using a commercial enzyme-linked immunosorbent assay (ELISA) system (Sigma Aldrich, Poole, United Kingdom) on frozen plasma. The H pylori status of the cases was determined using the above ELISA test where plasma samples were available, in conjunction with microscopic inspection of gastric biopsy sections stained with modified Giemsa by an expert pathologist to determine the presence or absence of the H pylori bacterium. Of the cases for which it was possible to determine H pylori status, 91% were determined by inspection of stained sections and 9% by ELISA. An overlap occurred for 1 case, with identical results determined by the 2 techniques.
Genotyping
Genomic DNA was extracted using the method of Jackson et al.29 In order to check the integrity of the DNA, all samples extracted from paraffin blocks were amplified using a primer set expected to amplify a product of 600 bp, ensuring that genotypic status was not misclassified owing to the absence of a polymerase chain reaction (PCR) product resulting from poor-quality DNA. IL-1B-31 genotypes were determined using TaqMan allelic discrimination PCR (Applied Biosystems, Foster City, CA).10,11 IL-1 RN alleles were determined using PCR product sizing,10,11 whereby products were sized relative to a 100-bp DNA ladder and were coded as follows: allele 1 = 4 repeats (442 bp), allele 2 = 2 repeats (270 bp), allele 3 = 5 repeats (528 bp), allele 4 = 3 repeats (356 bp), allele 5 = 6 repeats (614 bp). Because of their rarity, the 3, 4, and 5 alleles were grouped for statistical analysis. GST T1 and GST M1 genotypes were determined using PCR as previously described,27,30 with the modification that an annealing temperature of 56°C was used. These assays do not allow for discrimination between carriers of 1 and 2 intact alleles. Therefore, heterozygous and homozygous wild-type individuals were grouped for analysis.
Statistical analysis
Within the case series and within the population-based control series, associations between age and H pylori status were tested using a nonparametric test for trend.31 Associations between sex, genotype, and H pylori status were tested with the Pearson χ2 test, as were associations between age, sex, H pylori status, and polymorphism distribution. Hardy-Weinberg equilibrium was assessed for IL-1 RN and IL-IB-31 within the case and control series. Unconditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) associated with risk of GMZL, adjusted for age and sex. Statistical interactions were tested using the likelihood ratio test, by comparing the model with 2 genotype effects included as independent factors against the model where both independent effects plus an interaction term were included.32 All analyses were conducted using Stata version 7 (Stata, College Station, TX).
Results
The median age at diagnosis of the GMZL case series was 65 years (range, 38-86 years), with 27 (40.9%) being male. The median age of the reference population was 60 years (range, 28-65 years) with 99 (60.7%) being male. H pylori status was determined for 50 (75.8%) of the 66 GMZL cases, with 43 (86.0%) of the 50 cases confirmed as being positive for the presence of H pylori. H pylori status was determined for all individuals in the reference population, with 61 individuals (37.4%) being H pylori positive. Among controls, positive H pylori status was significantly associated with increasing age (P < .01), but there was no significant difference in H pylori distribution between the sexes.
Distribution of IL-1 genotypes
The distribution of IL-1B-31 was not significantly associated with age or sex in either the case series or the reference population (data not shown). Allele distributions at IL-1B-31 were in Hardy-Weinberg equilibrium for both the cases and reference samples (cases, P = .17; controls, P = .06). The frequency of the IL-1B-31 homozygous genotype (CC) in the reference population was 14.2%, in accordance with previously published data.10,11 Although there was an increased incidence of the IL-1B-31 CC genotype in the H pylori–negative controls (16.8%) compared with the H pylori–positive controls (9.8%), there was no statistically significant difference in IL-1B-31 genotype distribution between these 2 groups (P = .24). The IL-1B-31 CC genotype was observed in 19.0% of the GMZL cases, compared with 14.2% of the controls, with no associated risk of GMZL suggested (OR, 1.13; 95% CI, 0.47-2.73; Table 1). The IL-1B-31 CT genotype was observed in 34.9% of the GMZL cases, compared with 40.1% of the controls, also with no associated risk of GMZL suggested (OR, 0.76; 95% CI, 0.38-1.54).
Comparison of IL-1B-31, IL-1 RN, GST T1, and GST M1 gene frequencies in the GMZL case series and the reference population
. | Cases (n = 66) . | . | Reference population (n = 163) . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Allele . | No. . | % . | No. . | % . | OR* . | 95% CI . | ||
| IL-1B-31 | ||||||||
| Wt (TT) | 29 | 46.0 | 74 | 45.7 | 1.00 | — | ||
| Het (CT) | 22 | 34.9 | 65 | 40.1 | 0.76 | 0.38-1.54 | ||
| Hom (CC) | 12 | 19.0 | 23 | 14.2 | 1.13 | 0.47-2.73 | ||
| Not known | 3 | — | 1 | — | — | — | ||
| IL-1 RN | ||||||||
| 1/1 | 21 | 35.6 | 74 | 45.7 | 1.00 | — | ||
| 1/2 | 13 | 22.0 | 66 | 40.7 | 0.66 | 0.29-1.53 | ||
| 1/345 | 5 | 8.5 | 9 | 5.6 | 1.87 | 0.48-7.31 | ||
| 2/2 | 20 | 33.9 | 13 | 8.0 | 5.51 | 2.16-14.07 | ||
| Not known | 7 | — | 1 | — | — | — | ||
| GST T1 | ||||||||
| H/H | 28 | 42.4 | 141 | 86.5 | 1.00 | — | ||
| Null | 38 | 57.6 | 22 | 13.5 | 9.51 | 4.57-19.81 | ||
| GST M1 | ||||||||
| H/H | 25 | 37.9 | 74 | 45.4 | 1.00 | — | ||
| Null | 41 | 62.1 | 89 | 54.6 | 1.10 | 0.59-2.07 | ||
. | Cases (n = 66) . | . | Reference population (n = 163) . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Allele . | No. . | % . | No. . | % . | OR* . | 95% CI . | ||
| IL-1B-31 | ||||||||
| Wt (TT) | 29 | 46.0 | 74 | 45.7 | 1.00 | — | ||
| Het (CT) | 22 | 34.9 | 65 | 40.1 | 0.76 | 0.38-1.54 | ||
| Hom (CC) | 12 | 19.0 | 23 | 14.2 | 1.13 | 0.47-2.73 | ||
| Not known | 3 | — | 1 | — | — | — | ||
| IL-1 RN | ||||||||
| 1/1 | 21 | 35.6 | 74 | 45.7 | 1.00 | — | ||
| 1/2 | 13 | 22.0 | 66 | 40.7 | 0.66 | 0.29-1.53 | ||
| 1/345 | 5 | 8.5 | 9 | 5.6 | 1.87 | 0.48-7.31 | ||
| 2/2 | 20 | 33.9 | 13 | 8.0 | 5.51 | 2.16-14.07 | ||
| Not known | 7 | — | 1 | — | — | — | ||
| GST T1 | ||||||||
| H/H | 28 | 42.4 | 141 | 86.5 | 1.00 | — | ||
| Null | 38 | 57.6 | 22 | 13.5 | 9.51 | 4.57-19.81 | ||
| GST M1 | ||||||||
| H/H | 25 | 37.9 | 74 | 45.4 | 1.00 | — | ||
| Null | 41 | 62.1 | 89 | 54.6 | 1.10 | 0.59-2.07 | ||
H/H indicates heterozygous/homozygous wild-type;—, genotype not found
Adjusted for age and sex
The distribution of IL-1 RN was not significantly associated with age or sex in either the case series or the reference population (data not shown). Allele distributions for IL-1 RN were in Hardy-Weinberg equilibrium for the controls (P = .08), while the cases were strongly out of equilibrium (P = .0001). The frequency of the IL-1 RN 2/2 genotype was 8.0% (Table 1), in accordance with previously published data.10,11 The IL-1 RN 2/2 genotype was present at a similar frequency in H pylori–negative controls (7.9%) and H pylori–positive controls (8.2%). The IL-1 RN 2/2 genotype was observed in 33.9% of cases, compared with 8.0% in the control reference population, showing an association with an increased risk of GMZL (OR, 5.51; 95% CI, 2.16-14.07; Table 1). No associations were seen with GMZL risk for either the 1/2 genotype (OR, 0.66; 95% CI, 0.29-1.53) or the 1/3,4,5 genotype (OR, 1.87; 95% CI, 0.48-7.31). No evidence was found for statistical interaction between the IL-IB-31 and IL-1 RN genotypes (P = .25).
Distribution of GST genotypes
The frequency of the GST T1 null genotype in the reference population was 13.5% (Table 1), which was comparable to previously published data.33 The distribution of GST T1 was not significantly associated with age or sex in either the case series or the reference population (data not shown). The GST T1 null genotype was present in 16.7% of the H pylori–negative and 8.2% of the H pylori–positive controls; there was no statistically significant difference in GST T1 genotype distribution between these 2 groups (P = .13). The GST T1 genotype was significantly associated with risk of GMZL: 57.6% of GMZL cases had the GST T1 null genotype, compared with 13.5% of the reference population (OR, 9.51; 95% CI, 4.57-19.81).
The frequency of the GST M1 null genotype in the reference population was 54.6% (Table 1), comparable to previously published data.33 The distribution of GST M1 was not significantly associated with age or sex in either the case series or in the reference population (data not shown). The GST M1 null genotype was present in 57.8% of the H pylori–negative controls and 49.2% of the H pylori–positive controls (P = .28). The distribution of the GST M1 null genotype between cases (62.1%) and the reference population (54.6%) was similar (OR, 1.10; 95% CI, 0.59-2.07), suggesting no association with GMZL risk (Table 1). No evidence was found for statistical interaction between the GST T1 and GST M1 genotypes (P = .75).
Combination of GST T1 and IL-1 RN genotypes
IL-1 RN and GST T1 were both found to have significant associations with risk when they were analyzed together using a multiple variable logistic regression model. Evidence was found for a statistical interaction between IL-I RN and GST T1 genotypes (P = .02), suggesting that the effect of IL-1 RN may be modified depending on the GST T1 genotype and vice versa. Odds ratios, adjusted for age and sex, were calculated for each combination of the 2 genotypes (Table 1), using GST T1 H/H and IL-1 RN 1/1 as the baseline genotype in the comparison. The estimated effect of IL-1 RN 1/2 was nonsignificant in combination with the GST T1 H/H genotype (OR, 0.50; 95% CI, 0.18-1.36), but significant in combination with the GST T1 null genotype (OR, 4.82; 95% CI, 1.08-21.41). In combination with the GST T1 H/H genotype, no significant effect was found for the IL-1 RN 2/2 genotype (OR, 0.90; 95% CI, 0.17-4.78), whereas in combination with the GST T1 null genotype, the IL-1 RN 2/2 genotype was associated with a significant risk (OR, 32.29; 95% CI, 6.92-150.63). These results suggest that the risk associated with the IL-1 RN 2 allele may be dependent on the additional presence of the GST T1 null genotype.
Discussion
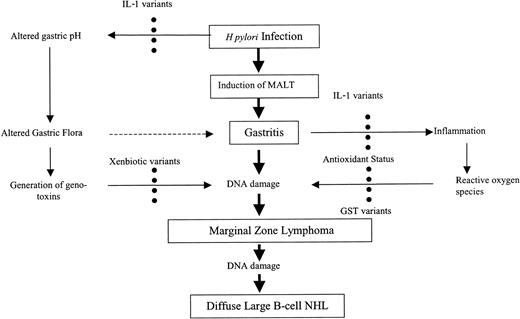
Our results suggest that the development of GMZL is modulated by genetic variants at both the IL-1 and GST loci. It is proposed that the genetic variants analyzed modulate the severity of the immune response to H pylori and determine the body's antioxidative capacity (Figure 1).
Model to assess genetic modification of risk for H pylori–induced GMZL. Black circles indicate modulated by; dashed arrows, possible link; solid arrows, established link; and bold arrows, disease progression.
Model to assess genetic modification of risk for H pylori–induced GMZL. Black circles indicate modulated by; dashed arrows, possible link; solid arrows, established link; and bold arrows, disease progression.
Two previous studies investigating the role of IL-1 RN and IL-1B genotypes in susceptibility to gastric cancer have been carried out.10,11,34 Both reported an increased associated risk of gastric carcinoma with inheritance of the IL-1B-31 (CC)/IL-1 RN 2/2 genotype. The authors suggest that the IL-1B-31 C allele in the context of H pylori infection is associated with a proinflammatory phenotype and therefore an increased risk of DNA damage and ultimately gastric carcinoma.10,11,34 However, reports on the functional effects of the IL-1B-31 polymorphism are conflicting,12,13 and functional effects at cytokine loci are often governed by extensive haplotypes.
Our data suggest a significant association between GMZL and inheritance of the IL-1 RN 2/2 genotype, consistent with that previously observed in gastric carcinoma. In contrast, we found no associated risk with IL-1B-31 genotype. An explanation for this may be that changes in IL-1 proinflammatory responses associated with IL-1B-31 may be mediated via an interaction with IL-1 RN; however, we found no evidence for this in our data.
IL-1 RN encodes the IL-1 receptor antagonist IL-1ra, and the IL-1 RN*2 allele has been associated with decreased IL-1ra levels17,19 and consequently increased IL-1β levels. We postulate that the association between the IL-1 RN*2 allele and GMZL functions via a mechanism similar to that seen in gastric carcinoma. The high IL-1β levels associated with the IL-1 RN*2 allele favor the proinflammatory response; at the same time, the concomitant inhibition of gastric acid facilitates widespread H pylori colonization of the gastric mucosa, promoting the development of MALT in response to infection. This decreased gastric acid secretion may also heighten DNA damage by permitting superinfection by enteric bacteria that enhance the production of carcinogenic N-nitroso compounds.35 H pylori–induced hypochlorhydria also significantly decreases levels of vitamin C in gastric juice,36 further facilitating the formation of N-nitroso compounds.
We describe a significant association between the null genotype of GST T1 and increased risk of GMZL. No effect was observed for the GST M1 null genotype. Associations between the GST T1 null genotype and increased risks of astrocytoma, meningioma, myelodysplasia,37 and acute myeloid leukemia25 have been reported. GST T1 has a strong antioxidant function in neutralizing free radicals. We propose that in GMZL, the associated risk seen with the GST T1 null genotype is due to compromised antioxidant capacity and not due to the metabolism of a specific xenobiotic substrate. Combinations of genetic variants that increase IL-1β levels, promoting the immune response to H pylori infection and the production of ROS, plus variants that decrease the levels of antioxidants would likely increase the risk of GMZL further (Figure 1). We found evidence of modulation of effect between the IL-1 RN and GST T1 genotypes and GMZL risk, showing that both play important roles in the pathway of disease development.
H pylori has been shown to be an infection strongly linked to the development of GMZL. Thus, in our hypothesis the effects of IL-1 variants would be through their modulation of the immune response to H pylori, and the effects of GST variants would be through the antioxidative capacity they provide, enabling the body to detoxify ROS produced in this response. Among cases for which H pylori status could be determined, 86.0% were positive for the presence of the bacterium; however, the frequency of both the GST T1 null and IL 1 RN 2/2 genotypes was similar in cases determined to be H pylori–positive and those with unknown or negative status. However, the absence of H pylori in the biopsy sections examined does not preclude the presence of H pylori elsewhere in the gastric mucosa. The demonstrated presence of H pylori in the majority of cases strongly supports the link between the presence of H pylori and development of GMZL in our case series. Within the reference population, no associations were found between the IL-1-31, IL-1 RN, GST T1, or GST M1 genotypes and H pylori status, providing no evidence that these genes are associated with the development of H pylori infection. The whole reference population serves well as a group with which to make comparisons of genotype frequencies in the cases, to determine which genotypes may modulate the development of GMZL subsequent to H pylori infection.
A small proportion of GMZL may not necessarily be associated with H pylori, for example in patients with autoimmune disease.38 Ideally, we would have been able to classify cases as to whether or not they were associated with H pylori and evaluate the genotype effects within the 2 subgroups. From the archived samples it could not be definitively demonstrated that H pylori had not been involved. H pylori serology and/or presence in tissue may change with increasing pathologic progression; thus, H pylori may not always be evident in samples of more advanced disease. Analysis restricted to cases and controls where H pylori status had been demonstrated estimated effects for GST T1 and IL-1 RN consistent with the full analysis. The IL-1 RN 2/2 genotype was seen in 30.0% of the cases, compared with 8.2% in the controls (OR, 3.93; 95% CI, 1.08-14.33). The GST T1 genotype was seen in 58.1% of the cases, compared with 8.2% of the controls (OR, 15.01; 95% CI, 4.67-48.23). Making this restriction substantially reduced the number of cases and controls, and the precision of the estimates was reduced, particularly for the more rare variants. Differential effects of the IL-1 RN 2/2 genotype in combination with GST T1 were not observed in this restricted analysis, as had been observed in the full analysis; however, this may have been because of limited statistical power due to small numbers. No evidence was found for a differential frequency of the GST T1 null and IL-1 RN 2/2 genotypes between cases determined to be H pylori–positive and those with unknown or negative status.
We were unable to obtain samples from population controls of comparable ages for all the cases. Population controls aged 28 to 65 years were used as a reference population for cases aged 38 to 86 years. In a study of metabolic gene polymorphism frequencies in control populations,33 it has been shown that the allele frequencies of metabolic genes including GST M1 and GST T1 do not differ significantly by age. Within our control series, age was not significantly associated with the allele frequencies of any of the genotypes used in our analyses. This would indicate that the controls could act as an appropriate reference group for the older cases. Adjusting for age and sex in our analyses of association between genotype and risk of GMZL did not alter the estimates to any substantial degree. Similarly, repeating the analyses using data restricted to cases and controls of comparable ages did not produce estimates substantially different from the estimates based on the full data (data not shown). This confirmed that the age differences between the case series and the population controls were not leading to bias in our estimates.
The limited statistical power of small studies to detect associations between polymorphic genotypes and disease predisposition is particularly important with regard to effect modification. To achieve adequate statistical power to estimate individual gene effects with good precision and further describe interaction between genes, large sample sizes may be required.39 This study, although small, suggests associations between genotypes and susceptibility to GMZL, which would be important to investigate further in larger studies.
This study provides support for the hypothesis that the risk of developing GMZL is influenced by interindividual variation in inflammatory response and antioxidative capacity, particularly through combination of the effects of the IL-1 RN genotype and the GST T1 genotype. H pylori infection, the anticipated exposure, was confirmed in the majority of the cases, and it is proposed that the genetic variants analyzed modulate the effects of this exposure.
The model we describe is common to the development of gastric carcinoma, and it would be valuable to determine whether there are also parallels, in genetically determined risk, with the development of other MALT lymphomas, such as the marginal zone lymphomas occurring at the site of inflammation in Hashimoto thyroiditis and Sjögren disease.40 Excesses in risk of the non-Hodgkin lymphomas have been described for patients with various conditions involving substantial immune dysfunction, particularly conditions where chronic antigenic stimulation is present.41 The consideration of polymorphisms involved in the immune response, in combination with those involved with the prevention of DNA damage (and further, those involved in DNA repair), could allow the mechanisms underlying these associations to be explored further.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-12-3803.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal