Abstract
Immunostimulatory sequences (ISS) are short oligonucleotides containing unmethylated cytosine-phosphate-guanine (CpG) dinucleotides that stimulate innate immune responses through Toll-like receptor-9 on B cells and plasmacytoid dendritic cell (PDC) precursors. The anti-inflammatory cytokine interleukin (IL)-10 is predicted to be a potent inhibitor of many of the activities described for ISS, and this may impact the use of ISS in disease states characterized by elevated IL-10. As the activities of ISS on PDCs are central to many clinical applications of ISS, we have studied the effects of IL-10 on PDC stimulation by 3 distinct classes of ISS. IL-10 inhibited cytokine production and survival of ISS-activated PDCs; however, IL-12 induction was much more sensitive to inhibition than interferon (IFN)-α induction. Within the PDC population are cells that respond to ISS by producing either IL-12 or IFN-α but not both cytokines. IL-12-producing PDCs require costimulation through CD40 and appear more mature than IFN-α-producing PDCs. The 3 distinct classes of ISS differed with respect to induction of PDC maturation and T-cell priming capacity. IL-10 regulated PDC activation but did not inhibit the subsequent T-cell-priming ability of PDCs already activated by ISS. (Blood. 2003;102:4487-4492)
Introduction
Immunostimulatory sequences (ISS) are short oligonucleotides (ODNs) that mimic the innate immune response to microbial DNA.1 ISS contain one or more cytosine-phosphate-guanine (CpG) dinucleotide-containing motifs with unmethylated cytosine residues and are recognized by Toll-like receptor-9 (TLR-9), one of a family of receptors prominent in innate responses to microbial pathogens.2,3 TLR-9 expression is not widely distributed, and ISS-responsive cells in human peripheral blood mononuclear cells (PBMCs) are limited to B cells and plasmacytoid dendritic cells (PDCs). ISS activate B cells to proliferate, secrete interleukin (IL)-6, and differentiate to plasma cells.4 PDCs respond to ISS by secreting type I interferons, tumor necrosis factor α (TNF-α), and, upon signaling through CD40, IL-12.2,5 In both cell types, ISS are potent enhancers of antigen-presenting cell (APC) function and induce key costimulatory molecules, such as CD40, CD80, and CD86.6 In mixed cell populations, such as human PBMCs, the direct response to ISS initiates a cascade of secondary responses, including activation of macrophages and natural killer (NK) cells and the induction of interferon γ (IFN-γ) and a wide range of other cytokines and chemokines characteristic of inflammatory responses.6-10
At least 3 classes of ISS can be distinguished on the basis of both structure and function. Uniformly modified phosphorothioate (PS) ISS, called CpG-B, strongly activate B cells but are weak stimulators of IFN-α from human PBMCs. Phosphodiester (PO)-linked sequences flanked by PS-linked poly-G ends, called CpG-A ISS, in contrast, are potent inducers of IFN-α and IFN-γ but are weak activators of B cells.11 Recently, a third class of ISS has been defined, called CpG-C. CpG-C ISS retain distinctive properties of both CpG-A and -B ISS with respect to IFN-α production and B-cell activation.6 Preliminary data have shown that ISS affect PDC survival, maturation, and cytokine production, but the differential properties of these ISS on PDCs are otherwise undefined.5,6,12 The efficient activation of APCs and induction of IL-12, IFN-α, and IFN-γ explains the potent ability of ISS to act as a strong T-helper cell 1 (Th)1-polarizing adjuvant.13 Besides inducing Th1 responses, administration of ISS mixed or covalently linked to antigens can inhibit Th2 responses14-17 and stimulate CD8 T-cell responses.7 These activities have stimulated much interest in the clinical use of ISS as a vaccine adjuvant and in the treatment of allergy, asthma, cancer, and infectious diseases, and clinical trials are currently being conducted in all of these disease areas.
IL-10 is a potent anti-inflammatory cytokine that can act as a feedback regulator of the inflammatory response to many microbial stimuli.18 IL-10 can be produced by a number of different cell types and can inhibit both Th1 and Th2 responses by affecting APC function and dendritic cell (DC) maturation.18-20 IL-10 inhibits activation and induces death of PDCs in vitro and can reduce PDC-derived IFN-α production in virus-activated PBMCs.21-23 Moreover, it was recently shown in mice that very low levels of IL-10 can inhibit the DC response to ISS activation both in vitro and in vivo.24 IL-10 is induced by ISS, but its overall role in regulating the specific activities of ISS on PDCs has not been studied. The varied effects of IL-10 are likely to have significance for the clinical application of ISS, as a number of the target disease states, especially cancer and chronic infection, are characterized by variable, often elevated IL-10 levels.18
In this paper, we show important differences in the response of PDCs to the 3 different classes of ISS and use these differences to explore the range of effects of IL-10 on ISS activity. Some activities of ISS appear to be much more sensitive to high levels of IL-10, and this may influence the choice of specific classes of ISS in disease states marked by elevations in IL-10.
Materials and methods
Medium and ISS oligonucleotides
Isolated cells were cultured in supplemented RPMI 1640 (Bio-Whitaker, Santa Rosa, CA) as previously described.6 All oligodeoxynucleotides were prepared as previously described.6 The prototypes for the ISS classes used were D19, 5′-GGtgcatcgatgcagGGGGG (CpG-A ISS); C393, 5′-GGtgcatgcatgcagGGGGG (CpG-A ISS control); 1018, 5′-TGACTGTGAACGTTCGAGATGA (CpG-B ISS); 1040, 5′-TGACTGTGAACCTTAGAGATGA (CpG-B control); C274, 5′-TCGTCGAACGTTCGAGATGAT (CpG-C ISS); and C661, 5′-TGCTTGCAAGCTTGCAAGCA (CpG-C control). Uppercase letters represent PS linkages, and lowercase letters represent PO linkages.
Cytokines and antibodies
Human recombinant IL-3 and IL-10 were purchased from R&D Systems (Minneapolis, MN). Blocking antibodies used in this study were anti-human IL-10 receptor (R) (Pharmingen, San Diego, CA) or a combination of polyclonal rabbit anti-IFN-α, anti-IFN-β, and monoclonal mouse anti-IFN-αβ receptor antibody (PBL Biomedical Laboratories, Piscataway, NJ). Monoclonal antibodies used for flow cytometry included anti-CD3, anti-CD4, anti-CD80, anti-CD86, anti-CD45R0, anti-CD45RA, anti-IL-12p40/p70 (Pharmingen), anti-CD123, anti-BDCA2, anti-BDCA4 (Miltenyi Biotech, Auburn, CA), and anti-IFN-α (Chromaprobe, Aptos, CA).
PBMC isolation and culture
Buffy coats were obtained from the Stanford Blood Center (Palo Alto, CA). PBMCs were isolated by centrifugation through a Ficoll (Pharmacia, Piscataway, NJ) density gradient. PBMCs were cultured for 24 hours at 0.4 × 106 cells per well in 96-well flat-bottom plates in duplicate with ODNs at 20 μg/mL, with human (h) IL-10 (10 ng/mL) or anti IL-10R (2.5 μg/mL).
Isolation and in vitro stimulation of blood PDCs
PDCs were isolated using BDCA-4 enrichment as previously described.6 Purity was routinely more than 97%. PDC experiments were conducted with 3 to 5 × 104 PDCs per well cultured in 96-well round-bottom plates. Irradiated CD40 ligand-transfected fibroblasts were used in 96-well flat-bottom plates at 104 cells per well. Stimulation was performed with 5 μg/mL ISS or IL-3 at 10 ng/mL for 24 to 72 hours with IL-10 at 0.01 to 10 ng/mL or a combination of polyclonal rabbit anti-IFN-α (5000 neutralizing U/mL), anti-IFN-β (2000 neutralizing U/mL), and mouse anti-IFN-α/β receptor monoclonal antibody (MAb) (20 μg/mL).
CFSE labeling and in vitro naive T-cell stimulation
Naive CD4+CD45RA+ T cells were prepared using a 2-step approach using magnetic microbead-labeled antibodies (Miltenyi Biotech). First, CD8-, CD11b-, CD16-, CD19-, CD36-, CD45R0-, and CD56-bearing cells were depleted, and nondepleted cells were then enriched for CD4. Purity was assessed by staining with CD4 and CD45RA antibodies and was routinely around 99%. Cells were then stained with 1 μM CFSE [5-(and-6-) carboxfluroescein diacetate succinmidyl ester] (Molecular Probes, Eugene, OR). For coculture experiments, 3 × 104 PDCs per well were stimulated with 5 μg/mL ISS and 104 irradiated CD40L-transfected cells for 24 hours. CFSE-labeled allogenic naive T cells were then added at a ratio of 1:5 (PDC/T cell) in the absence of IL-2. After 3 days the proliferation of CFSE-labeled CD4+ T cells was measured by flow cytometry.
ELISA and intracellular staining
IFN-α production was assayed by enzyme-linked immunosorbent assay (ELISA; PBL Biomedical Laboratories). IFN-γ and IL-12p40 were assayed with CytoSet antibody pairs (BioSource, Camarillo, CA). All kits and antibody pairs were used according to manufacturers' instructions. For intracellular flow cytometry analysis, PDCs were cultured as described in the presence of CD40L-transfected L cells for 10 hours followed by addition of Brefeldin A (10 ng/mL) for 2 hours. The cells were harvested, stained with anti-CD86, -BDCA2, -BDCA4, or -CD123 and then fixed with 2% paraformaldehyde (SIGMA, St Louis, MO), permeabilized with 0.5% saponin, and stained with the anti-IL-12p40/p70 and anti-IFN-α as described.25
Real-time quantitative PCR (TaqMan) analysis
Human PBMCs were stimulated with ISS for 24 hours. RNA was isolated and converted to cDNA, and polymerase chain reactions (PCRs) were performed as described.6 The human sequences for synthesized primers and the calculation method were as previously described.6 Briefly, threshold cycle (CT) values for each gene were normalized to the housekeeping gene HPRT by using the formula 1.8 × (HKG - GENE) × (1000), where HKG (housekeeping gene) is the mean CT of triplicate HPRT runs, GENE is the mean CT of duplicate runs of the gene of interest, and 1000 is arbitrarily chosen as a factor to bring all values above 0.
Results
Endogenous IL-10 differentially inhibits IFN-α and IFN-γ induction by ISS
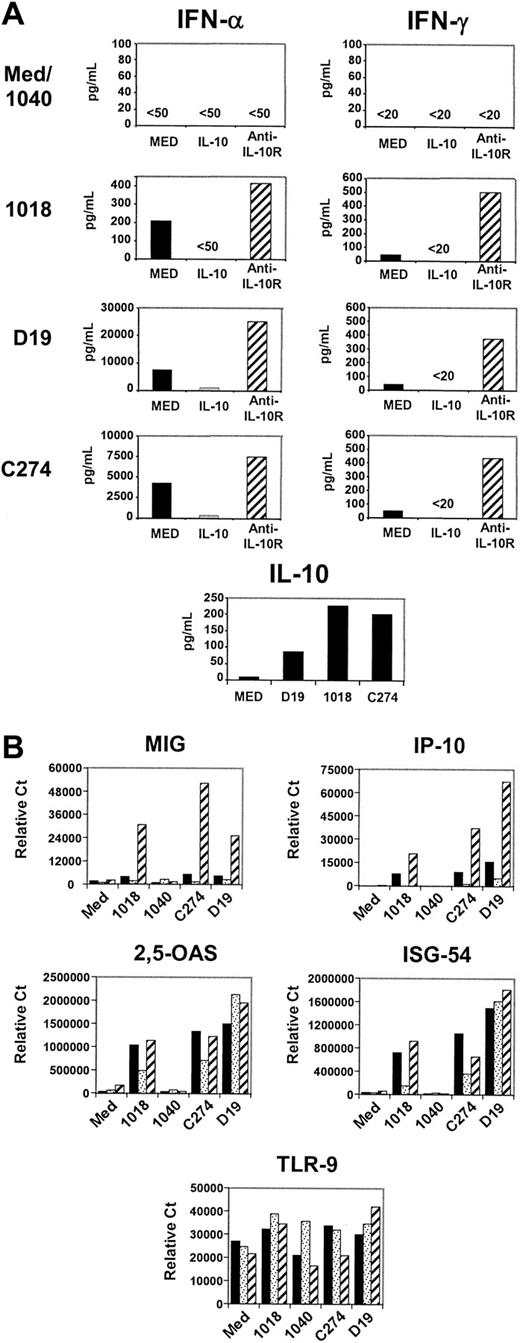
Three distinct classes of ISS can be distinguished on the basis of structure and biologic activity.6 Tested on human PBMC cultures, examples of each of these ISS classes induced similar low levels of IFN-γ but quite different levels of IFN-α (Figure 1A). The range of regulation of IFNs by IL-10 was evaluated by adding to parallel cultures either IL-10 or an antibody that blocks IL-10 binding to the IL-10R. Although only modest amounts of IL-10 were induced by ISS (Figure 1A), levels of both IFN-α and IFN-γ were significantly higher in cultures containing an anti-IL-10R MAb, reflecting the inhibitory activity of ISS-induced IL-10 within the culture. However, the increase in anti-IL-10R cultures was proportionately much greater for IFN-γ than for IFN-α (Figure 1A). Secretion of both IFN types could be reduced by adding saturating levels of rIL-10. This differential sensitivity of IFNs to IL-10 inhibition extended to IFN-α- and IFN-γ-inducible genes, as measured by real-time PCR (Figure 1B). The IFN-γ-inducible chemokines monokine induced by IFN-γ (MIG) and interferon-inducible protein 10 (IP-10) were dramatically enhanced when endogenous IL-10 was blocked, whereas levels of 2,5-OAS and ISG-54, genes inducible by IFN-α but not IFN-γ, were unaffected (Figure 1B). One mechanism of IL-10-mediated suppression could be reduction of TLR-9 expression; however, the level of TLR-9 mRNA in PBMCs was not significantly affected by either addition or blockade of IL-10 (Figure 1B).
IL-10 inhibits cytokines and gene induction by ISS-stimulated PBMCs. Freshly isolated PBMCs (2 × 106 cells/mL) were stimulated with ISS (20 μg/mL) for 24 hours alone (black bars) or with IL-10 (10 ng/mL) (dotted bars) or anti-human IL-10R (2.5 μg/mL) (hatched bars). (A) Cytokine production was evaluated with the use of immunoassay. (B) Level of gene expression was assessed by quantitative PCR analysis (Taqman). Results shown are representative of more than 20 donors for cytokine production and of 8 donors for gene expression analysis.
IL-10 inhibits cytokines and gene induction by ISS-stimulated PBMCs. Freshly isolated PBMCs (2 × 106 cells/mL) were stimulated with ISS (20 μg/mL) for 24 hours alone (black bars) or with IL-10 (10 ng/mL) (dotted bars) or anti-human IL-10R (2.5 μg/mL) (hatched bars). (A) Cytokine production was evaluated with the use of immunoassay. (B) Level of gene expression was assessed by quantitative PCR analysis (Taqman). Results shown are representative of more than 20 donors for cytokine production and of 8 donors for gene expression analysis.
IL-10 regulates the production of IL-12 by PDCs stimulated with CD40L and specific classes of ISS
PDCs are 1 of 2 ISS-responsive cell types in the blood and produce virtually all of the IFN-α induced by the CpG-A and CpG-C classes of ISS. In the course of presenting antigen to T cells, in vivo, PDCs would be expected to receive signals through CD40-CD40L interactions. Stimulation with a combination of ISS and CD40L enhanced IFN-α production by purified PDCs in response to CpG-A and CpG-C ISS and led to significant IL-12 induction with all 3 ISS classes (Figure 2A), whereas little to no cytokine was produced in response to control ODNs. The addition of a high concentration of IL-10 completely inhibited IL-12 production, but only inhibited IFN-α production with CpG-A and CpG-C ISS by 50% or less (Figure 2B). The strong inhibition of IL-12 production by PDCs may explain the strong IL-10 inhibition of IFN-γ in PBMCs stimulated with ISS.
Cytokines produced by ISS-activated PDCs are differentially inhibited by IL-10. (A) Purified PDCs (3-5 × 104) were stimulated with ISS (5 μg/mL) alone (i) or in combination with CD40L-transfected L cells (ii) for 24 to 48 hours and (B) in the presence of IL-10 (1 ng/mL). Representative results of more than 20 donors are shown.
Cytokines produced by ISS-activated PDCs are differentially inhibited by IL-10. (A) Purified PDCs (3-5 × 104) were stimulated with ISS (5 μg/mL) alone (i) or in combination with CD40L-transfected L cells (ii) for 24 to 48 hours and (B) in the presence of IL-10 (1 ng/mL). Representative results of more than 20 donors are shown.
IL-12 and IFN-α are differentially expressed by PDCs
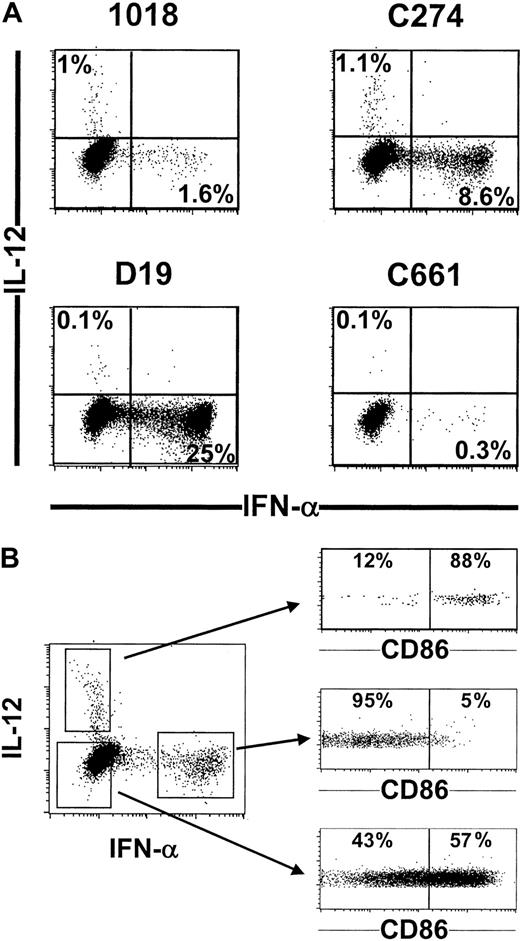
To explore the cellular basis for the differentially regulated expression of IL-12 and IFN-α, activated PDCs were costained for intracellular IL-12 and IFN-α. Substantial numbers of IFN-α-positive cells were detected in CD40L-stimulated PDCs cultured with D19 or C274, and fewer IFN-α-positive cells were found in cultures with 1018 (Figure 3A). This pattern correlated with secreted IFN-α levels (Figure 2A). In contrast, small numbers of IL-12-positive cells were reproducibly detected in the C274 and 1018 groups but not in the D19 or control group (Figure 3A). Most important, IFN-α and IL-12 were produced by different cells within the PDC population (Figure 3A), both subpopulations expressing BDCA-2 and CD123 (data not shown). This differential expression of cytokines may reflect different stages of maturation, as CD40 activation induces maturation and preincubation with IL-3 favors IL-12 production by DCs.5 To test this hypothesis, intracellular IFN-α and IL-12 staining was compared with CD86 expression after stimulation with CD40L and C274, conditions that stimulate both IL-12 and IFN-α (Figures 2A and 3A). Strikingly, IFN-α-producing cells were almost exclusively found in the CD86-negative population, whereas IL-12-positive cells were mostly CD86-positive (Figure 3B). These findings suggest that PDCs initially can produce IFN-α in response to ISS stimulation, but IFN-α is lost and IL-12 production acquired as the cells undergo maturation into DCs.
IFN-α and IL-12 are produced by different cells within the PDC population that are at a different stage of maturation. (A) Purified PDCs (105) were stimulated for 12 hours with CD40L-transfected L cells and various ISS 5 μg/mL) or (B) C274 only (5 μg/mL). Cells were then characterized for cytokine production by intracellular flow cytometry analysis. (B) In addition, cells were costained for CD86 expression. Representative results from 10 donors are shown.
IFN-α and IL-12 are produced by different cells within the PDC population that are at a different stage of maturation. (A) Purified PDCs (105) were stimulated for 12 hours with CD40L-transfected L cells and various ISS 5 μg/mL) or (B) C274 only (5 μg/mL). Cells were then characterized for cytokine production by intracellular flow cytometry analysis. (B) In addition, cells were costained for CD86 expression. Representative results from 10 donors are shown.
IL-10 is cytotoxic for ISS-activated PDCs and this effect is counterbalanced by IFN-α
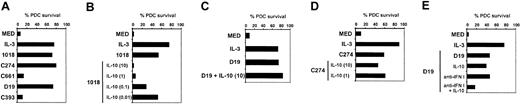
IL-10 has been described to induce PDC death, whereas ISS have been shown to induce PDC survival.12,21,22 Indeed, all ISS tested, as well as IL-3, strongly enhanced PDC survival. This survival activity was CpG specific, as PDCs cultured with control oligonucleotides died rapidly in vitro (Figure 4A). PDCs stimulated with the CpG-B-ISS 1018 were dramatically affected by IL-10 (Figure 4B), whereas PDCs stimulated with CpG-A ISS D19 and CpG-C ISS C274 were resistant to IL-10 (Figure 4C-D). Addition of anti-IL-10R did not increase survival of PDCs treated with ISS (data not shown). Both D19 and C274 can induce high levels of IFN-α, which is known to be a strong autocrine PDC survival factor.26 Blockage of IFN-α in the culture indeed rendered D19-stimulated PDCs sensitive to IL-10 (Figure 4E), which demonstrated that IFN-α is counteracting the cytotoxic effect of IL-10.
IL-10 has cytotoxic effects on ISS-activated PDCs that is counterbalanced by IFN-α (A) Purified PDCs (3-5 × 104) were stimulated with IL-3 (10 ng/mL) or ISS (5 μg/mL) for 60 hours alone or (B-E) in the presence of IL-10 (0.01-10 ng/mL) and (E) with a combination of anti-IFN-α (5000 neutralizing U/mL), anti-IFN-β (2000 neutralizing U/mL), and mouse anti-IFN-α/β receptor MAb (20 μg/mL). Viable PDCs were counted by using trypan blue exclusion criteria. Representative results of 10 donors are shown.
IL-10 has cytotoxic effects on ISS-activated PDCs that is counterbalanced by IFN-α (A) Purified PDCs (3-5 × 104) were stimulated with IL-3 (10 ng/mL) or ISS (5 μg/mL) for 60 hours alone or (B-E) in the presence of IL-10 (0.01-10 ng/mL) and (E) with a combination of anti-IFN-α (5000 neutralizing U/mL), anti-IFN-β (2000 neutralizing U/mL), and mouse anti-IFN-α/β receptor MAb (20 μg/mL). Viable PDCs were counted by using trypan blue exclusion criteria. Representative results of 10 donors are shown.
ISS-induced maturation of PDC is not inhibited by IL-10
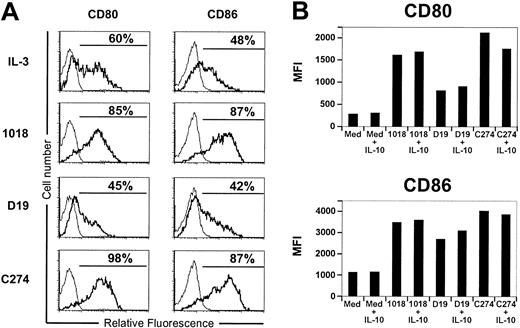
One of the best-described properties of IL-10 is the inhibition of costimulatory molecule expression on many types of antigen-presenting cells.18 Because many ISS have been shown to induce CD80 and CD86 expression on PDCs, the potential inhibition of that process by IL-10 was examined. All 3 classes of ISS induced CD80 and CD86 expression, although the CpG-A ISS (D19) was a much weaker inducer than CpG-B ISS (1018) and CpG-C ISS (C274) (Figure 5A). Costimulation with CD40L, which is likely to occur when PDCs encounter T cells, enhanced the overall stimulation but showed similar differences among sequences (data not shown and Figure 5B). Unexpectedly, IL-10 produced no effect on the induction of CD80 or CD86 with any of the ISS tested (Figure 5B). Similar observations were obtained when PDCs were stimulated in the absence of CD40L (data not shown), except that the 1018-stimulated group was not informative because of cell death induced by IL-10 (Figure 4A).
ISS-induced maturation of PDCs is not affected by IL-10. (A) Purified PDCs (3-5 × 104) were stimulated with ISS (5 μg/mL) alone or (B) in combination with CD40L-transfected L cells with or without IL-10 (1 ng/mL) for 48 hours. Cells were characterized for CD80 and CD86 by flow cytometry analysis. Representative results of 10 donors are shown.
ISS-induced maturation of PDCs is not affected by IL-10. (A) Purified PDCs (3-5 × 104) were stimulated with ISS (5 μg/mL) alone or (B) in combination with CD40L-transfected L cells with or without IL-10 (1 ng/mL) for 48 hours. Cells were characterized for CD80 and CD86 by flow cytometry analysis. Representative results of 10 donors are shown.
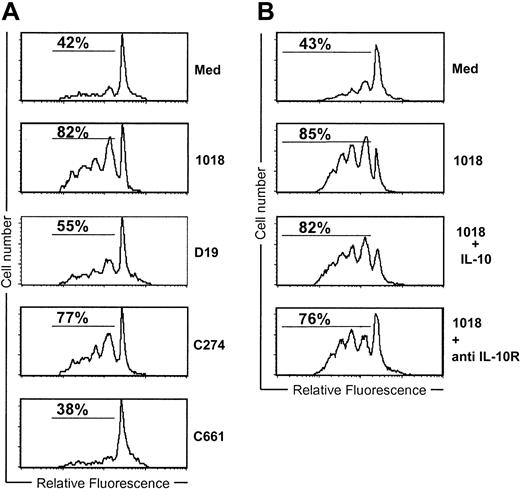
IL-10 does not regulate T-cell activation by ISS-activated PDCs
To address whether IL-10 can affect T-cell priming by ISS-stimulated PDCs, allogeneic T cells were labeled with CFSE and cultured with or without IL-10 for 3 days with PDCs previously activated with ISS and CD40L. PDCs cultured for 24 hours with CpG-B or CpG-C ISS very potently stimulated T cells (82% and 77% CFSE low T cells, respectively), whereas PDCs cultured with the CpG-A sequence, D19, were much less active (Figure 6A). This corresponds with the reduced activity of CpG-A ISS for the induction of IL-12, CD80, and CD86 (Figures 2 and 5). The addition or removal of IL-10 at the time of the coculture of the 1018-activated PDCs and allogeneic T cells did not affect the PDC-priming capacity (Figure 6B). Similar data were observed by using the other classes of ISS (data not shown). These data suggest that IL-10 regulates PDC-T-cell interaction by acting on the PDCs and not by impairing subsequent T-cell activation.
IL-10 does not regulate T-cell activation by ISS-activated PDCs. (A-B) Purified PDCs (3-5 × 104) were stimulated with ISS (5 μg/mL) in combination with CD40L-transfected L cells for 24 hours. Naive CFSE-labeled allogenic T cells were then added (1:5) and incubated for 3 additional days. (B) Either IL-10 (1 ng/mL) or anti-IL-10R MAb (2.5 μg/mL) was added at the time of T-cell addition. Cells were gated on CD3 and examined for the frequency of dividing cells on the basis of CFSE dilution by flow cytometry. Representative results of 10 donors are shown.
IL-10 does not regulate T-cell activation by ISS-activated PDCs. (A-B) Purified PDCs (3-5 × 104) were stimulated with ISS (5 μg/mL) in combination with CD40L-transfected L cells for 24 hours. Naive CFSE-labeled allogenic T cells were then added (1:5) and incubated for 3 additional days. (B) Either IL-10 (1 ng/mL) or anti-IL-10R MAb (2.5 μg/mL) was added at the time of T-cell addition. Cells were gated on CD3 and examined for the frequency of dividing cells on the basis of CFSE dilution by flow cytometry. Representative results of 10 donors are shown.
Discussion
IL-10 has been described as a key player in striking a balance between pathology and protection and is found elevated in numerous clinical situations, such as cancer and infectious disease. IL-10 is believed to be a major cause of ineffective immune response in these disease states, and high levels of IL-10 are often a negative prognostic factor for patient survival. We observed low IL-10 levels, 50 to 300 pg/mL, in supernatants of ISS-activated PBMCs (Figure 1A), but these levels were sufficient to inhibit IFN-γ- and IFN-γ-inducible gene expression. The preferential inhibition of IFN-γ versus IFN-α reflected the preferential inhibition of IL-12 versus IFN-α in ISS-stimulated PDCs by IL-10. This implies either distinct signaling requirements or the necessity that PDCs be at different stages of maturation to produce these cytokines. The latter interpretation was supported by the finding that IL-12 and IFN-α were made entirely by different cells within the PDC population. IL-12-producing PDCs expressed high levels of the important maturation marker CD86, whereas IFN-α-producing PDCs expressed comparatively low levels. Maturation of PDCs by ISS, however, was not sufficient for IL-12 production; an additional signal through CD40L-CD40 interaction was also required. This finding demonstrated that PDCs, as found in PBMCs, are not able to produce IL-12 without appropriate maturation and signaling. Conversely, the ability of PDCs to produce IFN-α was lost when the cells started to mature. IL-10 did not inhibit ISS-induced PDC maturation, thereby excluding blockade of maturation by IL-10 as a mechanism of IL-12 inhibition.
The PDC response to IL-10 appears quite different from the response to IL-10 of most other APCs. IL-10 had no effect on ISS-induced PDC maturation, whereas it strongly inhibited maturation of macrophages and other APCs.18 In PDCs, IL-10 promoted rapid cell death, whereas IFN-α enhanced survival, a distinct reversal of the activities of these 2 cytokines on most cell types. Interestingly, we noted that resistance to IL-10-mediated killing of PDCs activated with different classes of ISS strictly correlated with the amount of IFN-α induced. Neutralizing IFN-α in such cultures rendered PDCs sensitive to IL-10 cytotoxicity, showing clearly the opposing effects of the 2 cytokines.
The IL-10 regulation of the T-cell response by ISS-activated PDCs occurred upstream of the PDC-T-cell interaction since the addition of IL-10 to PDCs already stimulated by ISS had no effect on subsequent T-cell activation. In summary, IL-10 regulation of ISS activities occurs primarily on immature PDCs. Once activated, PDCs become progressively less sensitive to IL-10 for their ability to express costimulatory molecules or to prime naive T cells.
In addition, this work illustrates the important differences among the 3 structurally distinct ISS classes. For example, the cytokine response of ISS-activated PDCs was quite different, with CpG-A ISS inducing almost exclusively IFN-α, CpG-B ISS inducing almost exclusively IL-12, and CpG-C ISS inducing both IFN-α and IL-12. Sequences of the CpG-A class, although able to induce the highest levels of IFN-α, are very inefficient at inducing maturation of DCs into functional APCs.
The strong Th1-priming ability of ISS is the basis for current clinical trials in infectious disease, cancer, asthma, and allergic rhinitis. Elevations of IL-10 occur frequently in these diseases, especially in cancer and chronic infection, and have the potential to alter clinical responses to ISS. IL-10 inhibits T-cell responses primarily by acting on APCs, which include the primary targets of ISS activity. We show here that IL-10 is a potent modulator of ISS activity and have focused on the key responses of TLR-9-bearing PDCs to IL-10 and to 3 distinct classes of ISS. Both the activity differences among ISS classes and the differential effects of IL-10 on immature and mature PDC functions have important implications for the clinical application of these compounds. For example, the success of antiviral and antitumor therapies that require induction of IFN-α should be less affected by patient variations in IL-10 than actions that depend more on IL-12 and IFN-γ induction, such as control of allergy or intracellular pathogen infection. Different considerations would apply when ISS are used to enhance prophylactic or therapeutic vaccination. ISS of the CpG-B or CpG-C classes prime T cells in vitro much more efficiently than CpG-A sequences, and this has been experimentally confirmed for CpG-A versus CpG-B sequences in mice.27 This adjuvant activity is expected to be less sensitive to IL-10 variations than the initial burst of cytokine induction. In summary, the potential clinical application of different classes of ISS needs to take into account both the intrinsic activities of the particular sequence and structural class, as well as the likely interplay with pathways of immune regulation active in the specific disease state.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-07-2465.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christina Abbate for advice on performing cell purification; Josh Gregorio for ISS preparation; and Drs Edith Hessel, Vassili Soumelis, Gary Van Nest, and Anne O'Garra for their critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal