Abstract
Eph receptor tyrosine kinases and their ligands, the ephrins, have been primarily described in the nervous system for their roles in axon guidance, development, and cell intermingling. Here we address whether Eph receptors may also regulate dendritic cell (DC) trafficking. Reverse transcription-polymerase chain reaction (RT-PCR) analysis showed that DCs derived from CD34+ progenitors, but not from monocytes, expressed several receptors, in particular EphA2, EphA4, EphA7, EphB1, and EphB3 mRNA. EphB3 was specifically expressed by Langerhans cells, and EphA2 and EphA7 were expressed by both Langerhans- and interstitial-type DCs. EphA and EphB protein expression on DCs generated in vitro was confirmed by staining with ephrin-A3-Fc and ephrin-B3-Fc fusion proteins that bind to different Eph members, in particular EphA2 and EphB3. Immunostaining with anti-EphA2 antibodies demonstrated the expression of EphA2 by immature DCs and by skin Langerhans cells isolated ex vivo. Interestingly, ephrin expression was detected in epidermal keratinocytes and also in DCs. Adhesion of CD34+-derived DCs to fibronectin, but not to poly-l-lysine, was increased in the presence of ephrin-A3-Fc, a ligand of EphA2, through a β1 integrin activation pathway. As such, EphA2/ephrin-A3 interactions may play a role in the localization and network of Langerhans cells in the epithelium and in the regulation of their trafficking. (Blood. 2003;102:4431-4440)
Introduction
Dendritic cells (DCs) are antigen-presenting cells (APCs) that dwell in all lymphoid and nonlymphoid organs and have the unique capacity to activate naive T cells.1,2 DCs originate from the bone marrow and migrate as precursors through the bloodstream to nonlymphoid tissues. Tissue DCs (eg, the epidermal Langerhans cells) are at an immature stage and are able to capture antigens with high efficiency. Antigen-bearing cells migrate from the periphery toward lymphatic vessels to reach the lymphoid tissues and localize in T-cell-rich areas as mature interdigitating DC (IDCs).3-5 At this site, IDCs efficiently present the processed antigens to naive T cells and induce specific immune responses.1 Thus, migration constitutes an integral part of DC function. The recruitment of DCs to the tissue damage site and the subsequent migration of DCs into secondary lymphoid organs rely on a dynamic and complex series of events involving cell surface receptors, primarily selectins, and integrins, which mediate the initial engagement of rolling cells.6,7 Cell activation by signaling molecules, such as chemokines, leads to the activation of adhesion molecules, and the high-affinity binding of activated integrins to endothelial ligands, or extracellular matrix proteins, produces tight adhesion, shape change, and diapedesis to localize in extravascular foci. Although the role of chemokines in DC migration has been studied in depth,8 the roles of other molecules in regulating their tissue trafficking remain to be investigated.
Interactions of Eph receptor tyrosine kinases with their membrane-bound ligands, the ephrins, are implicated in important developmental processes, including tissue morphogenesis, control of angiogenesis, and axonal guidance.9,10 According to their structural features and their preference for different ephrins, Eph receptors have been divided into 2 groups: EphA (A1-A8) receptors bind preferentially glycosylphosphatidylinositol (GPI)-anchored ligands, the ephrin-A (A1-A5), whereas EphB (B1-B6) receptors bind transmembrane ligands, the ephrin-B (B1-B3)11,12 that are phosphorylated after Eph/ephrin interaction.13 An individual Eph receptor, however, has a wide variation in affinity for different ephrins.11 For example, ephrin-A3 binds to EphA3, EphA2, EphA7, EphA5, and EphA4 with decreasing affinities.11,12 Ephrins play important roles during axon guidance by providing a repulsive guidance signal to Eph receptor cells. Navigating growth cones or migrating cells expressing Eph receptors turn away from cells expressing the cognate ephrin ligand.14 Alternatively, ephrins can stimulate the assembly of endothelial cells to form blood vessels, strengthening the dual role of ephrins.15-17 A characteristic of the ephrin-Eph signaling system is the ability to elicit bidirectional signaling that leads to the restriction of cell migration and cell intermingling, as observed during hindbrain segmentation, and of vasculogenesis, possibly through the modulation of integrin function.15,18,19 Several studies, albeit with conflicting findings, have described a role for Eph molecules in the regulation of cell adhesion and the cytoskeleton.20-22
To date, a few Eph receptor members have been sporadically found in cells of the human hematopoietic system. EphB1 is abundantly detected in plasmacytoid DCs,23 EphB4 has a wide tissue distribution and includes several myeloid hematopoietic and human CD34+ cells24 ; EphB6 is expressed by CD4+CD8+ thymocytes and by peripheral T-lymphocytes25 ; EphA4 and EphA7 have been also described during B-cell differentiation26 ; EphA2 messenger RNA (mRNA) is found in skin and lymphoid tissues.27,28 Nevertheless, despite the expression of some Eph receptors in hematopoietic cells and the recent description of the EphB6 role in T-cell costimulation,29 their functions are largely unknown in the immune system.
In the present study, we report a comparative analysis of the expression of Eph members in cells from the hematopoietic system, and we focus our attention on their expression in human DCs. We found that DCs derived in vitro from cord blood CD34+ progenitor cells cultured with granulocyte macrophage-colony-stimulating factor (GM-CSF) and tumor necrosis factor-α (TNF-α) for 12 days express EphA2, EphA4, EphA7, EphB1, and EphB3 mRNA but not EphA1, EphA3, EphA5, EphB2, EphB4, and EphB6. Interestingly, EphA2 and EphA7 are preferentially expressed by DCs, and EphB3 is restricted to Langerhans cells. Using ephrin-A3-Fc and ephrin-B3-Fc fusion proteins, we confirmed the strong expression of EphA and EphB protein at the cell surface of CD34+-derived DCs and demonstrated the expression of EphA2 protein in immature DCs. Finally, we report functional evidence for the role of Eph/ephrin interactions in the regulation of integrin-mediated DC adhesion to fibronectin.
Materials and methods
Hematopoietic factors, reagents, cells, and cell lines
Recombinant human GM-CSF (rhGM-CSF) (specific activity, 2 × 106 U/mg; Schering-Plough Research Institute, Kenilworth, NJ), rhTNF-α (specific activity, 2 × 107 U/mg; Genzyme, Boston, MA), recombinant human stem cell factor (rhSCF) (specific activity, 4 × 105 U/mg; R&D Systems, Abington, United Kingdom), and recombinant human interleukin-4 (rhIL-4) (specific activity: 107 U/mg; Schering-Plough Research Institute) were used at 100, 2.5, 25, and 10 ng/mL, respectively.
Peripheral blood mononuclear cells (PBMCs), blood T cells, monocytes, and tonsil B cells were purified as previously described in detail.30 Granulocytes were generated in vitro from CD34+ progenitors in the presence of G-CSF and SCF for 12 days.30 T cells were activated with plastic-coated anti-CD3 and soluble anti-CD28 monoclonal antibodies (mAbs) for 3, 12, and 24 hours. Other cells were unactivated or activated by phorbol 12-myristate 13-acetate (PMA)-ionomycin for 1 hour and 6 hours (1 ng/mL PMA, Sigma, St Louis, MO; 1 μg/mL ionomycin, Calbiochem, La Jolla, CA) and pooled. Murine fibroblasts transfected with human CD40 ligand (CD40L cells) were produced in the laboratory.30 Langerhans cell-enriched epidermal cell suspensions were prepared from normal human skin as described.31 All cell types were cultured in RPMI 1640 (Gibco BRL, Gaithersburg, MD) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) (Flow Laboratories, Irving, United Kingdom), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM l-glutamine, 100 μg/mL gentamicin (Schering-Plough, Levallois-Perret, France) (hereafter referred to as complete medium).
Immunohistochemistry and adhesion assays were performed using chimera of human ephrin-A3 and ephrin-B3 fused to human immunoglobulin G (IgG) Fc (R&D Systems, Minneapolis, MN), or chimera of human CD152 (CTLA-4) or CD95 (Fas) fused to murine and human IgG Fc, respectively (Ancell, Bayport, MN; R&D Systems). Ephrin-A3-Fc can interact with multiple EphA receptors, in particular EphA3 and EphA2 with high affinity and EphA7, EphA5, and EphA4 with decreasing affinity, and ephrin-B3 can interact with EphB1, EphB2, EphB3, and EphA4 with widely varying affinity.11,12
Generation of DCs from CD34+ progenitors and monocytes
Umbilical cord blood samples were obtained according to institutional guidelines. CD34+ progenitors were isolated using Minimacs separation columns (Miltenyi Biotec, Bergish Gladbach, Germany) as described.32 In all experiments the isolated cells were 80% to 99% CD34+ as judged by staining with anti-CD34 mAb. Cultures of CD34+ cells were established in the presence of SCF, GM-CSF, TNF-α and 2.5% AB+ human serum as described.32,33 Cells collected after 6 days of culture were further cultured in the presence of GM-CSF and TNF-α until days 11 to 12, when 70% to 90% of cells were CD1a+ DC. In some experiments, cells were separated at day 6 according to CD1a and CD14 expression into CD14+CD1a- and CD14-CD1a+ using a FACStar+ cell sorter (Becton Dickinson, Mountain View, CA) and were cultured until day 12 with GM-CSF and TNF-α as described.32 In some instances, cells were activated with lipopolysaccharide (LPS) at 20 ng/mL (Sigma) in the presence of GM-CSF until day 14.
Monocytes were purified by immunomagnetic depletion (Dynal, Oslo, Norway) of low-density PBMCs isolated on a 52% Percoll gradient. The depletion was performed with anti-CD3, anti-CD19, and anti-CD8 mAbs produced in the laboratory and with purified anti-CD56 and anti-CD16 mAbs (Beckman Coulter, Miami, FL). Monocyte-derived DCs were produced by culturing purified monocytes for 6 days in the presence of GM-CSF and IL-4.34 Cells were activated with LPS at the concentration of 20 ng/mL for 1 hour to 72 hours or with CD40L-transfected cells (1 CD40L cell for 5 DCs).30
Reverse transcription-polymerase chain reaction analysis
Total RNA extracted from 1 to 10 × 106 cells30 were treated with DNase I for 30 minutes at 37°C (Promega Biosciences, San Luis Obispo, CA). The absence of genomic DNA was controlled by polymerase chain reaction (PCR) on RNA preparations using the following β actin primers: sense (exon 2), ATCTGGCACCACACCTTCTA; antisense (exon 3), AATGTCACGCACGATTTCCC. RNA samples were further reverse transcribed using random hexamer primers (Pharmacia, Uppsala, Sweden) and the Superscript RNase-H reverse transcriptase (Gibco BRL). PCR were performed in a 100-μL volume using 50 ng cDNA, 10 μL10 × PCR reaction buffer (Perkin Elmer Cetus, Norwalk, CT), 2.5 U Taq polymerase (Gene Amp PCR reagents kit: Perkin Elmer Cetus), and 200 mM dNTP and 500 nM of the 5′ and 3′ amplification primers. PCR reactions were made in a DNA thermal cycler (Perkin Elmer) for 35 cycles (1-minute denaturation at 94°C, 1-minute annealing at 60°C, and 2-minute elongation at 72°C). β-Actin reverse transcription-PCR (RT-PCR) was used as positive control for the efficiency of the reaction using sense and antisense primers (Stratagene, La Jolla, CA). All cDNA samples were normalized according to the results of β-actin PCR amplification of 21, 28, and 35 cycles (data not shown). RT-PCR of the different Eph mRNAs was performed with the primers listed in Table 1.
RT-PCR of Eph mRNA
mRNA . | Sense primer . | Antisense primer . | Size, bp . |
|---|---|---|---|
| EphA2 | 241-259 CTCACACACCCGTATGGCAA | 976-957 GGCTCTCAGATGCCTCAAAC | 736 |
| EphA4 | 54-73 CGCCCTATTTTCGTGTCTCT | 666-647 CAGATTGCGGACTGTGAGTG | 613 |
| EphA7 | 1027-1046 GGGTTCTACAAGTCTTCCTC | 1538-1519 TCCTTCATTACTCCGCTCAC | 512 |
| EphB1 | 577-597 GTCATTGCCACCAAGAAGTC | 1015-996 CCACGCTGTTCTCAGGCTCA | 439 |
| EphB2 | 471-491 CACCAAGACCTTCCCCAACT | 905-887 GACGGTGCCATTCTCAACGG | 436 |
| EphB3 | 670-690 ACCACCGCA GGCTTCGCACT | 1408-1389 GGCGTAGTGTGGGCACTTCA | 739 |
| EphB6 | 824-843 TCCTGGTGTCTTCAGTTCTG | 1475-1456 GAAGCAAAGGATCGGAGCAC | 652 |
mRNA . | Sense primer . | Antisense primer . | Size, bp . |
|---|---|---|---|
| EphA2 | 241-259 CTCACACACCCGTATGGCAA | 976-957 GGCTCTCAGATGCCTCAAAC | 736 |
| EphA4 | 54-73 CGCCCTATTTTCGTGTCTCT | 666-647 CAGATTGCGGACTGTGAGTG | 613 |
| EphA7 | 1027-1046 GGGTTCTACAAGTCTTCCTC | 1538-1519 TCCTTCATTACTCCGCTCAC | 512 |
| EphB1 | 577-597 GTCATTGCCACCAAGAAGTC | 1015-996 CCACGCTGTTCTCAGGCTCA | 439 |
| EphB2 | 471-491 CACCAAGACCTTCCCCAACT | 905-887 GACGGTGCCATTCTCAACGG | 436 |
| EphB3 | 670-690 ACCACCGCA GGCTTCGCACT | 1408-1389 GGCGTAGTGTGGGCACTTCA | 739 |
| EphB6 | 824-843 TCCTGGTGTCTTCAGTTCTG | 1475-1456 GAAGCAAAGGATCGGAGCAC | 652 |
All Eph primers except EphB1 and EphB2 allowed distinctions in cDNA and genomic DNA.
bp = base pair.
Flow cytometry analysis
For cell surface labeling, cells were stained with biotinylated CD152-Fc (CTLA-4-Fc) or CD95-Fc (Fas-Fc) (Ancell), biotinylated human ephrin-A3-Fc and ephrin-B3-Fc (R&D Systems), or biotinylated human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) (5 μg/mL). Then the cells were incubated with phycoerythrin (PE)-conjugated streptavidin (DAKO, Copenhagen, Denmark). For EphA2 cell surface labeling, cells were stained with an mAb specific for human EphA2 (20 μg/mL) (clone D7; Sigma) and were revealed with PE-conjugated goat F(ab')2 antimouse immunoglobulin (DAKO) according to standard techniques. Negative control was performed with an unrelated isotype-matched murine mAb (DAKO).
For intracellular staining of rabbit polyclonal antibodies against intracellular epitopes, cells were first permeabilized for 15 minutes with permeabilization medium (0.1% saponin, 1% FCS) and then incubated with 20 μg/mL each of the following antibodies: anti-EphA2 (C-20), anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), anti-ephrin-B2 (P20) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-CD4 (Immunodiagnostics, Woburn, MA) or unrelated rabbit IgG (Jackson ImmunoResearch Laboratories) for 30 minutes at 4°C in the presence of permeabilization medium. After 2 washes, cells were incubated with PE-antirabbit IgG (Sigma). For double-color immunofluorescence staining, after the first step, the cells were incubated for 30 minutes in 2% normal mouse serum (DAKO) in phosphate-buffered saline (PBS) and then were surface labeled with fluorescence isothiocyanate (FITC)-CD1a (BD Biosciences PharMingen, Erembodegen, Belgium), FITC-HLA-DR, or FITC-CD86 (Becton Dickinson) according to standard techniques. Negative controls were performed with FITC-conjugated unrelated isotype-matched murine mAbs (DAKO). Fluorescence was analyzed using a FACScan flow cytometer (Becton Dickinson).
Immunocytochemistry and immunohistochemistry
Cytospins of CD34+-derived DCs or frozen 6-μm tissue sections of normal human skin were fixed and permeabilized with acetone at -20°C for 30 minutes. To block endogenous nonspecific activities, cytospins or sections were pretreated with Power block universal blocking reagent, followed by avidin and biotin block solutions (InnoGenex, San Ramon, CA) for 15 minutes each step at room temperature (rt). After being washed in PBS, the coverslips were incubated for 30 minutes at rt in 2% normal goat serum (DAKO) (same species as secondary antibody). Then they were stained for 1 hour at rt in a humid atmosphere with 20 μg/mL each of the following rabbit polyclonal antibodies prepared in 2% normal goat serum in PBS: anti-EphA2 (C-20), anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), antiephrin-B2 (P20) (Santa Cruz Biotechnology), anti-CD4 (Immunodiagnostics), or unrelated rabbit IgG (Jackson ImmunoResearch Laboratories). After a wash in PBS, the coverslips were incubated for 30 minutes at rt with biotinylated goat antirabbit IgG (H+L) (Vector Laboratories, Burlingame, CA) and then with streptavidin alkaline phosphatase (Biosource International, Camarillo, CA). Alkaline phosphatase activity was revealed using alkaline phosphatase substrate 3 (Vector Laboratories) for 1 to 10 minutes at rt. For double staining, sections were simultaneously stained with anti-EphA2 polyclonal antibody and DCGM4 mAb (produced in the laboratory) and revealed sequentially with biotinylated goat antirabbit IgG (H+L) (Vector Laboratories) and with peroxidase-labeled antimouse IgG1 (Binding Sites, Birmingham, United Kingdom). Biotinylated goat antirabbit IgG was revealed with streptavidin alkaline phosphatase following by specific substrate as described above. Peroxidase was revealed using 3-amino-9-ethylcarbazole (AEC) substrate (Vector Laboratories) for 5 to 10 minutes at rt.
Adhesion assays
Adhesion assays were performed according to previously described protocols20,35 with modifications. Ninety-six-well flat-bottom plates (Nunc, Roskilde, Denmark) were coated with 5 μg/mL or were serially diluted with (1-20 μg/mL) fibronectin or poly-l-lysine (PLL; Sigma) overnight at 4°C. Nonspecific binding sites were blocked at rt for 1 hour using 1% bovine serum albumin (BSA)/PBS. Human ephrin-A3-Fc, ephrin-B3-Fc, ephrin-A4-Fc, Fas-Fc (R&D Systems) or human IgG1 (Jackson ImmunoResearch Laboratories) were deposited at a concentration of 5 μg/mL, or from 1 to 20 μg/mL, into the plates. Soluble arginyl-glycyl-aspartic acid peptide (RGD) (Calbiochem GmbH, Schwalbach, Germany) was added to the plates at 0.01 to 10 μM. Cultured cells were washed once and resuspended in serum-free RPMI medium. Then 1 × 105 cells in 100 μL were plated per well (in triplicate) and centrifuged at 1200 rpm for 3 minutes. The medium was discarded, and remaining cells were washed gently once with PBS (without Ca2+ and Mg2+) at rt. Subsequently, the cells were fixed with 0.5% paraformaldehyde/0.5% glutaraldehyde (Sigma) at rt for 30 minutes and were stained with 0.5% crystal violet (Merck Eurolab GmbH, Darmstadt, Germany) in 20% methanol at rt for 10 minutes. The cells were washed 3 times with water and were extracted with 50% ethanol/50 mM sodium citrate, pH 4.5 (50 μL/well). Optical density (OD) was measured at 570 nm.
Results
CD34+-derived DCs express high levels of mRNA for protein tyrosine kinase receptors of the Eph family
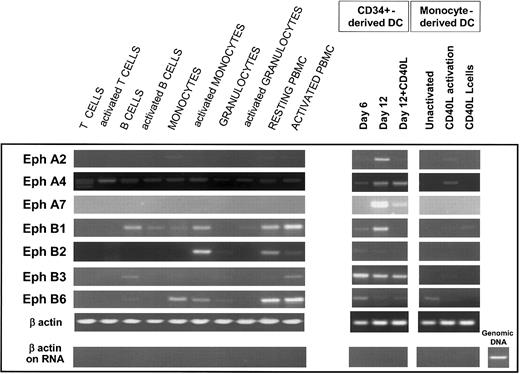
To determine whether Eph receptors were expressed by human DCs, we used RT-PCR to analyze the expression of EphA and EphB members in different cDNA samples from DCs and other hematopoietic cells that were previously normalized according to the results of β-actin PCR amplification and checked for the absence of genomic contamination (Figure 1, lower panels). We compared this expression in 2 types of DCs generated in vitro—those obtained from monocytes cultured in the presence of GM-CSF and IL-4 for 6 days and those obtained from CD34+ progenitors cultured with GM-CSF and TNF-α for 6 and 12 days. DCs were also further activated by hCD40L expressing L cells. Interestingly, cDNA encoding some Eph members—in particular EphA2, EphA4, EphA7, EphB1, EphB3, and, more weakly, EphB2 and EphB6—were amplified in day 12 CD34+-derived DCs; in contrast, in monocyte-derived DCs only EphA4, after activation of the cells by CD40L, and, to a weaker extent, EphB6, were detected (Figure 1). Messengers for EphA1, EphA3, EphA5, and EphB4 were not detected in DCs generated in vitro after 35 cycles of RT-PCR (data not shown). EphA2 expression seems to be restricted to DCs because only a significant level of messenger was detected in CD34+-derived DCs, and a very faint band was present in freshly isolated monocytes, in CD40L-activated monocyte-derived DCs, and in PBMCs but not in the other hematopoietic cells tested. Similarly, EphA7 was only detected by RT-PCR in day 12 CD34+-derived DCs. EphB3 was strongly expressed in DCs and weakly in B cells and in PBMCs. EphA4 and EphB1 mRNA, both present in DCs, were also detected in other hematopoietic cells. Furthermore, the expression of Eph members, particularly EphA2, EphA7, EphB1, and EphB6, was most abundant in relatively immature DCs recovered after 12 days of culture but were decreased in fully activated DCs recovered after 4 additional days of coculture with hCD40L-transfected fibroblasts. Taken together, these results reveal for the first time that DCs express receptor protein tyrosine kinases of the EphA and EphB families, originally described in the nervous system for their roles in axon guidance.
CD34+-derived DCs express high levels of mRNA for Eph receptors. (Left) RT-PCR analysis of Eph expression in hematopoietic cells. cDNA was prepared from unactivated and PMA-ionomycin-activated freshly isolated PBMCs, blood T cells, and monocytes, tonsil B cells, or granulocytes generated in vitro. Monocytes, granulocytes, and B cells were resting or activated with PMA-ionomycin for 1 hour and 6 hours and pooled. T cells and PBMCs were activated with PMA-ionomycin for 6 hours. (Right) RT-PCR analysis of Eph expression in CD34+-derived DCs and monocyte-derived DCs generated in vitro. cDNA was prepared from cord blood CD34+ progenitors cultured in the presence of GM-CSF and TNF-α for 6 and 12 days, and for an additional 4 days with CD40L L cells, from monocytes cultured in the presence of GM-CSF and IL-4 for 6 days (unactivated), after 24-hour activation with CD40L L cells, or as a control from CD40L L cells. RT-PCR was carried out under standard conditions using 50 ng cDNA for 35 cycles. All cDNA samples were normalized according to the results of β-actin PCR amplification of 21, 28, and 35 cycles (only the amplification of 28 cycles is shown). The absence of genomic contamination was controlled in all RNA samples (before the reverse transcription step) by PCR amplification of β-actin with primers designed to amplify genomic DNA, as shown in the lower panel. Results are representative of 3 independent RT-PCR samples.
CD34+-derived DCs express high levels of mRNA for Eph receptors. (Left) RT-PCR analysis of Eph expression in hematopoietic cells. cDNA was prepared from unactivated and PMA-ionomycin-activated freshly isolated PBMCs, blood T cells, and monocytes, tonsil B cells, or granulocytes generated in vitro. Monocytes, granulocytes, and B cells were resting or activated with PMA-ionomycin for 1 hour and 6 hours and pooled. T cells and PBMCs were activated with PMA-ionomycin for 6 hours. (Right) RT-PCR analysis of Eph expression in CD34+-derived DCs and monocyte-derived DCs generated in vitro. cDNA was prepared from cord blood CD34+ progenitors cultured in the presence of GM-CSF and TNF-α for 6 and 12 days, and for an additional 4 days with CD40L L cells, from monocytes cultured in the presence of GM-CSF and IL-4 for 6 days (unactivated), after 24-hour activation with CD40L L cells, or as a control from CD40L L cells. RT-PCR was carried out under standard conditions using 50 ng cDNA for 35 cycles. All cDNA samples were normalized according to the results of β-actin PCR amplification of 21, 28, and 35 cycles (only the amplification of 28 cycles is shown). The absence of genomic contamination was controlled in all RNA samples (before the reverse transcription step) by PCR amplification of β-actin with primers designed to amplify genomic DNA, as shown in the lower panel. Results are representative of 3 independent RT-PCR samples.
EphA2 and EphA7 mRNA are present in different types of DC, but EphB3 transcript is specifically present in Langerhans cells
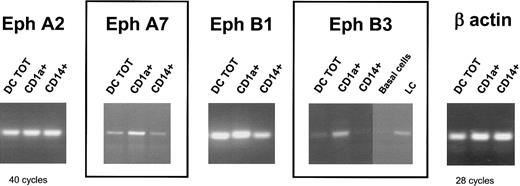
Given the expression of Eph messenger in in vitro CD34+-derived DCs, we next wondered whether Eph expression might correlate with a particular type of DC. Thus, we analyzed by RT-PCR the expression of the most DC-restricted Eph members in either CD1a+- or CD14+-derived DCs in GM-CSF and TNF-α culture conditions. Only the CD1a+ subset has been shown to differentiate into Langerhans cells, characterized by the presence of Birbeck granules and the expression of E cadherin, in the absence of exogenous transforming growth factor-β (TGF-β)36 ; the CD14+ subset was more closely related to interstitial DCs.32 CD1a+ and CD14+ precursors were fluorescence-activated cell sorter (FACS) sorted at day 6 and recultured with GM-CSF and TNF-α for 6 additional days. Eph transcripts in the 2 purified subsets were compared with total CD34+-derived DCs (corresponding to unsorted cells) using samples previously normalized according to the results of PCR amplification of β-actin (Figure 2) and were checked for the absence of genomic contamination (data not shown). RT-PCR analysis of EphA2 and EphB1 transcripts showed a strong signal in CD1a+ and CD14+-purified DCs (Figure 2). In contrast, EphA7 mRNA was more abundant in the CD1a+-derived DCs than in the CD14+ subset. Interestingly, EphB3 was only detected in CD1a+-derived DCs but not in CD14+-derived DCs. To confirm the restricted expression of EphB3, we performed RT-PCR in skin Langerhans cells isolated ex vivo. As shown in Figure 2, whereas basal keratinocytes did not express EphB3 mRNA, the transcript was amplified in fresh Langerhans cells isolated from epidermis. Therefore, although 2 other Eph members (EphA2 and EphA7) are also strongly expressed by CD34+-derived DCs, EphB3 represents the most restricted Langerhans cell Eph member.
Eph expression in different subsets of CD34+-derived DCs generated in vitro and of skin Langerhans cells isolated ex vivo. Cord blood CD34+ progenitors were cultured in the presence of GM-CSF, SCF, TNF-α, and 2.5% AB+ human serum. At day 6, CD1a+ and CD14+ precursors were sorted by flow cytometry and recultured with GM-CSF and TNF-α for 6 additional days. The presence of Eph transcripts in the 2 purified subsets was compared to total CD34+-derived DCs (DC TOT, corresponding to unsorted cells). cDNA was prepared from basal cells and from Langerhans cells, freshly isolated from normal skin. RT-PCR was carried out under standard conditions using 50 ng cDNA for 40 cycles. All cDNA samples were normalized according to the results of β-actin PCR amplification of 21, 28, and 35 cycles (only the amplification of 28 cycles is shown). Results are representative of 3 independent RT-PCR samples.
Eph expression in different subsets of CD34+-derived DCs generated in vitro and of skin Langerhans cells isolated ex vivo. Cord blood CD34+ progenitors were cultured in the presence of GM-CSF, SCF, TNF-α, and 2.5% AB+ human serum. At day 6, CD1a+ and CD14+ precursors were sorted by flow cytometry and recultured with GM-CSF and TNF-α for 6 additional days. The presence of Eph transcripts in the 2 purified subsets was compared to total CD34+-derived DCs (DC TOT, corresponding to unsorted cells). cDNA was prepared from basal cells and from Langerhans cells, freshly isolated from normal skin. RT-PCR was carried out under standard conditions using 50 ng cDNA for 40 cycles. All cDNA samples were normalized according to the results of β-actin PCR amplification of 21, 28, and 35 cycles (only the amplification of 28 cycles is shown). Results are representative of 3 independent RT-PCR samples.
Expression of EphA and EphB protein is regulated during DC differentiation
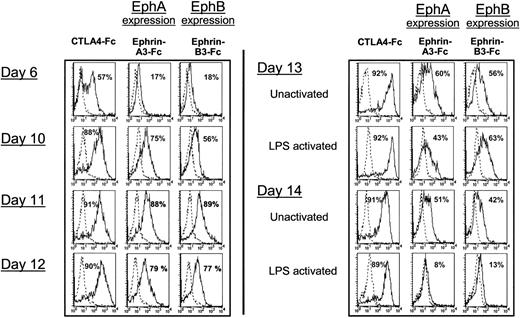
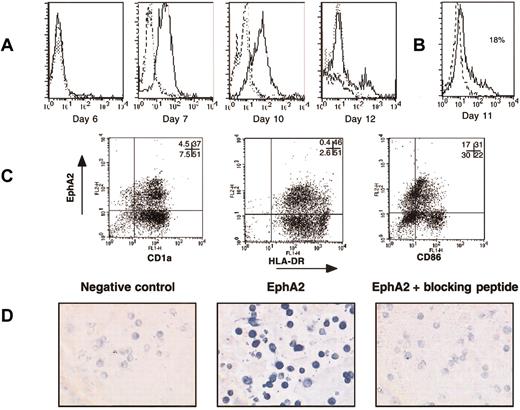
To study the overall expression of Eph proteins during the culture of human CD34+ cord blood progenitors with GM-CSF and TNF-α, we next used a biotinylated ephrin-A3-Fc fusion protein (composed of the extracellular domain of ephrin-A3 fused to the Fc fragment of a human IgG1) and a biotinylated ephrin-B3-Fc fusion protein. Indeed, because of the considerable promiscuity in interactions among ephrin ligands and Eph receptors, ephrin-A3 can interact with multiple EphA receptors, in particular EphA3 and EphA2, with high affinity and with EphA7, EphA5, and EphA4 with decreasing affinity. Ephrin-B3 can interact with EphB1, EphB2, EphB3, and EphA4 with a wide variation in affinity.11 In agreement with the RT-PCR data (Figure 1), flow cytometry analysis showed weak expression of EphA and EphB proteins (17%-18%) at the immature stage of DC differentiation (Figure 3, day 6). Expression of EphA and EphB further increased during DC differentiation until days 11 to 12. Indeed, the expression of EphA was optimal at days 11 to 12 of DC culture (88% and 79%, respectively), and the expression of EphB increased from day 10 to day 12 (56%-77%) (Figure 3). The expression of EphA and EphB was down-regulated by further activation of the cells by LPS (only 8%-13% of expression at day 14). Cell maturation was controlled by the increased binding of CTLA4-Fc to CD80/86 during DC differentiation (day-10 mean fluorescence intensity [MFI], 274; day-12 MFI, 605; day-13 unactivated MFI, 1297; day-13 activated MFI, 1608; values for the representative experiment in Figure 3). Based on the RT-PCR results, the binding of ephrin-A3-Fc is likely to be primarily mediated through the interaction with EphA2. Furthermore, in agreement with the RT-PCR results, we did not find a significant expression of EphA or EphB in monocyte-derived DCs obtained from culture of monocytes with GM-CSF and IL-4 for 6 days (data not shown). The specificity of Eph expression in cultured CD34+-derived DCs was also studied using FACS analysis or immunocytochemistry with a rabbit polyclonal antibody specific for an EphA2 intracellular epitope. EphA2, absent at the immature stage of DC differentiation (day 6), was induced during DC differentiation (days 10-12) (Figure 4A). EphA2 expression at the DC cell surface was additionally shown by staining nonpermeabilized DCs with an mAb specific for EphA2 (Figure 4B). Double staining showed that the EphA2+ cells expressed CD1a and major histocompatibility complex (MHC) class II, confirming that a significant proportion (46%) of DCs generated in vitro expressed EphA2 at day 12 of culture (Figure 4C). Moreover, EphA2+ cells expressed a low level of CD86 and HLA-DR, reflecting their relatively immature stage (Figure 4C). In addition, immunocytochemistry showed that day-12 CD34+-derived DCs were stained with the anti-EphA2 polyclonal antibody (Figure 4D). The specificity of anti-EphA2 polyclonal antibody was demonstrated by inhibition of staining by the peptide used for immunization. Because of the lack of specific reagents, we have been unable to confirm EphA7 and EphB3 expression by FACS analysis or immunocytochemistry. Consistent with the RT-PCR results, we demonstrated the presence of EphA and EphB at the surface of DCs generated in vitro and identified EphA2 as one of the molecules recognized by the ephrin-A3-Fc fusion protein in DCs.
Expression of EphA and EphB protein is regulated during DC differentiation. Time-kinetics of EphA and EphB expression during CD34+-derived DC culture from day 6 to day 14. Flow cytometric analysis was performed on cord blood CD34+ progenitors, cultured in the presence of GM-CSF and TNF-α until day 12, and after 24 hours and 48 hours of LPS activation. Cell surface EphA and EphB expression was followed by staining with biotinylated ephrin-A3-Fc and ephrin-B3-Fc chimera, respectively. As a positive control, DC maturation was followed by CD80/CD86 staining using biotinylated CTLA4-Fc chimera. Staining was revealed by PE-conjugated streptavidin. The percentages of CTLA4-Fc-, ephrin-A3-Fc-, and ephrin-B3-Fc-positive cells are indicated. Dotted-line overlay histograms show nonspecific staining with Fas-Fc chimera. Results are representative of FACS analysis of 3 independent samples.
Expression of EphA and EphB protein is regulated during DC differentiation. Time-kinetics of EphA and EphB expression during CD34+-derived DC culture from day 6 to day 14. Flow cytometric analysis was performed on cord blood CD34+ progenitors, cultured in the presence of GM-CSF and TNF-α until day 12, and after 24 hours and 48 hours of LPS activation. Cell surface EphA and EphB expression was followed by staining with biotinylated ephrin-A3-Fc and ephrin-B3-Fc chimera, respectively. As a positive control, DC maturation was followed by CD80/CD86 staining using biotinylated CTLA4-Fc chimera. Staining was revealed by PE-conjugated streptavidin. The percentages of CTLA4-Fc-, ephrin-A3-Fc-, and ephrin-B3-Fc-positive cells are indicated. Dotted-line overlay histograms show nonspecific staining with Fas-Fc chimera. Results are representative of FACS analysis of 3 independent samples.
Expression of EphA2 by CD34+-derived DCs. (A) EphA2 expression was analyzed by flow cytometry with anti-EphA2 polyclonal antibody directed against an intracytoplasmic epitope after DC permeabilization with saponin, as described in “Materials and methods.” Cord blood CD34+ progenitors were cultured in the presence of GM-CSF and TNF-α for 6 to 12 days. Dotted-line overlay histograms represent staining in the presence of the EphA2 peptide used for immunization, and bold dotted-line overlay histograms represent the negative control with an antibody of unrelated specificity. (B) EphA2 expression was analyzed by flow cytometry with an anti-EphA2 mAb on nonpermeabilized DCs (day 11 with GM-CSF and TNF-α. Bold dotted-line overlay histogram shows the staining of an isotype-matched control. (C) Double staining of day-11 CD34+-derived DCs cultured with GM-CSF and TNF-α with surface FITC-CD1a, FITC-HLA-DR, or FITC-CD86 antibodies, and intracellular anti-EphA2 polyclonal antibody revealed with PE-antirabbit IgG. Quadrant limits were set up on the isotype-matched control dot plot. The percentage value of control used to establish the crosshairs were 92% in the lower left quadrant for the double color-negative control. Numbers in dot plots indicate percentages of cells in the relevant quadrant. (D) Immunocytochemistry analysis of EphA2 on day-11 CD34+-derived DC cytospins. Staining was performed with anti-EphA2 polyclonal antibody or with anti-EphA2 polyclonal antibody plus the peptide used for immunization (blocking peptide), as described in “Materials and methods.” The negative control represents a polyclonal rabbit antibody of unrelated specificity. Staining was revealed using a biotinylated goat antirabbit IgG followed by alkaline phosphatase streptavidin. Original magnification × 200.
Expression of EphA2 by CD34+-derived DCs. (A) EphA2 expression was analyzed by flow cytometry with anti-EphA2 polyclonal antibody directed against an intracytoplasmic epitope after DC permeabilization with saponin, as described in “Materials and methods.” Cord blood CD34+ progenitors were cultured in the presence of GM-CSF and TNF-α for 6 to 12 days. Dotted-line overlay histograms represent staining in the presence of the EphA2 peptide used for immunization, and bold dotted-line overlay histograms represent the negative control with an antibody of unrelated specificity. (B) EphA2 expression was analyzed by flow cytometry with an anti-EphA2 mAb on nonpermeabilized DCs (day 11 with GM-CSF and TNF-α. Bold dotted-line overlay histogram shows the staining of an isotype-matched control. (C) Double staining of day-11 CD34+-derived DCs cultured with GM-CSF and TNF-α with surface FITC-CD1a, FITC-HLA-DR, or FITC-CD86 antibodies, and intracellular anti-EphA2 polyclonal antibody revealed with PE-antirabbit IgG. Quadrant limits were set up on the isotype-matched control dot plot. The percentage value of control used to establish the crosshairs were 92% in the lower left quadrant for the double color-negative control. Numbers in dot plots indicate percentages of cells in the relevant quadrant. (D) Immunocytochemistry analysis of EphA2 on day-11 CD34+-derived DC cytospins. Staining was performed with anti-EphA2 polyclonal antibody or with anti-EphA2 polyclonal antibody plus the peptide used for immunization (blocking peptide), as described in “Materials and methods.” The negative control represents a polyclonal rabbit antibody of unrelated specificity. Staining was revealed using a biotinylated goat antirabbit IgG followed by alkaline phosphatase streptavidin. Original magnification × 200.
Langerhans cells express EphA2
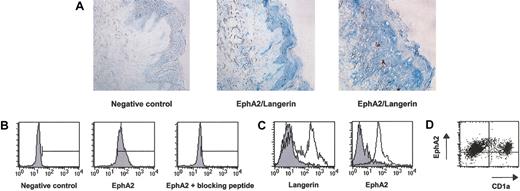
The EphA2 expression in CD34+-derived DCs generated in vitro prompted us to examine the expression of EphA2 in tissues. Because it has been previously reported that EphA2 was predominantly expressed in epithelial tissues, particularly by keratinocytes,27 we performed immunohistochemistry analysis on normal skin sections using the polyclonal antibody specific for EphA2 and an mAb specific for Langerin, a Langerhans-cell-specific marker.37 We observed that EphA2 was present in the suprabasal layer of the epidermis, corresponding certainly to keratinocytes (Figure 5A). However, double staining with Langerin did not allow us to formally demonstrate EphA2 expression by Langerhans cells. Next, we confirmed the expression of EphA2 by freshly purified keratinocytes from normal skin by FACS analysis (Figure 5B). Moreover, we detected the expression of EphA2 by a preparation of freshly purified Langerhans cells from skin whose purity was approximately 80%, as shown by the expression of Langerin (Figure 5C). In another preparation of skin cells, double staining with EphA2 and CD1a clearly demonstrated that all Langerhans cells (CD1a+) and keratinocytes (CD1a-) expressed EphA2, confirming the results of immunohistochemistry analysis (Figure 5D). Thus, these results establish the expression of EphA2 protein by Langerhans cells and keratinocytes in normal skin.
Keratinocytes and Langerhans cells from skin expression isolated ex vivo. (A) Immunohistochemistry analysis of EphA2 expression in vivo. Double staining was performed on normal serial skin sections with anti-EphA2 polyclonal antibody and the anti-DCGM4 mAb, specific for Langerin. The specificity of the staining was confirmed by the absence of detection in the presence of the immunization peptide (data not shown). The negative control represents a rabbit polyclonal antibody and an mAb of unrelated specificity. Original magnifications, × 200 (left and middle panels) and × 400 (right panel). (B) Expression of EphA2 by keratinocytes from normal skin isolated ex vivo. Flow cytometry staining was performed with anti-EphA2 polyclonal antibody or with anti-EphA2 polyclonal antibody plus the peptide used for immunization, after DC permeabilization with saponin, as described in “Materials and methods.” The negative control represents a rabbit polyclonal antibody of unrelated specificity. The bar corresponds to the region of the positive EphA2+ cells. Results are representative of 3 experiments. (C) Expression of EphA2 by Langerhans cells from skin isolated ex vivo. Langerin and EphA2 expression was analyzed by flow cytometry on enriched Langerhans cells with an anti-EphA2 polyclonal antibody or with mAb DCGM4, specific for Langerin (open histogram), and compared with species-specific unrelated antibodies (filled histogram). Results are representative of 3 experiments. (D) Double staining of another Langerhans cells preparation with surface FITC-CD1a and intracellular anti-EphA2 polyclonal antibody revealed with PE-antirabbit IgG.
Keratinocytes and Langerhans cells from skin expression isolated ex vivo. (A) Immunohistochemistry analysis of EphA2 expression in vivo. Double staining was performed on normal serial skin sections with anti-EphA2 polyclonal antibody and the anti-DCGM4 mAb, specific for Langerin. The specificity of the staining was confirmed by the absence of detection in the presence of the immunization peptide (data not shown). The negative control represents a rabbit polyclonal antibody and an mAb of unrelated specificity. Original magnifications, × 200 (left and middle panels) and × 400 (right panel). (B) Expression of EphA2 by keratinocytes from normal skin isolated ex vivo. Flow cytometry staining was performed with anti-EphA2 polyclonal antibody or with anti-EphA2 polyclonal antibody plus the peptide used for immunization, after DC permeabilization with saponin, as described in “Materials and methods.” The negative control represents a rabbit polyclonal antibody of unrelated specificity. The bar corresponds to the region of the positive EphA2+ cells. Results are representative of 3 experiments. (C) Expression of EphA2 by Langerhans cells from skin isolated ex vivo. Langerin and EphA2 expression was analyzed by flow cytometry on enriched Langerhans cells with an anti-EphA2 polyclonal antibody or with mAb DCGM4, specific for Langerin (open histogram), and compared with species-specific unrelated antibodies (filled histogram). Results are representative of 3 experiments. (D) Double staining of another Langerhans cells preparation with surface FITC-CD1a and intracellular anti-EphA2 polyclonal antibody revealed with PE-antirabbit IgG.
Expression of several Eph ligands, the ephrins, in skin
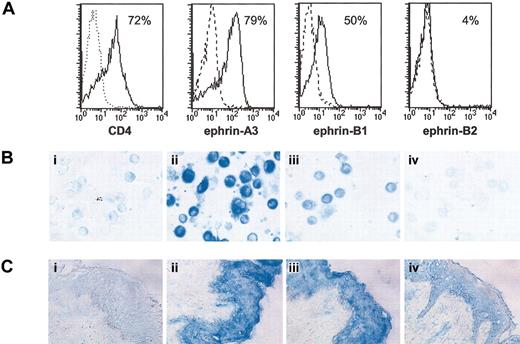
It has been previously reported that a receptor tyrosine kinase and its cognate ligand can be coexpressed in the same cell.38 Given that we observed the expression of EphA2 in CD34+-derived DCs, we extended the analysis of ephrin in these cells using polyclonal antibodies specific for ephrin-A3, ephrin-B1, and ephrin-B2 (Figure 6A). We found that ephrin-A3, like its cognate ligand EphA2, was significantly expressed by CD34+-derived DCs at day 11 (79% of expression). Ephrin-B1 was detected at a lower level (50% of expression) compared with the positive CD4 control and with ephrin-A3 staining; ephrin-B2 was not detected in these cells. The specificity of the FACS staining was confirmed by inhibiting detection in the presence of the immunization peptide. Furthermore, the ephrin-A3 polyclonal antibody and, to a weaker extent, the ephrin-B1 polyclonal antibody also stained cytospin preparations of day-11 CD34+-derived DCs cultured with GM-CSF and TNF-α (Figure 6B). Next, we performed immunohistochemistry analysis with the different polyclonal antibodies, on serial normal skin sections fixed with acetone. Ephrin-A3, ephrin-B1, and ephrin-B2 were also detected within epidermal keratinocytes, demonstrating that the expression of EphA2 ligand, ephrin-A3, and other ephrins of the B family match the expression patterns of EphA2 (Figure 6C).
Expression of several Eph ligands in normal skin (ephrin-A3, ephrin-B1, and ephrin-B2). (A) Expression of ephrin-A3, ephrin-B1, and ephrin-B2 by day-11 CD34+-derived DCs cultured in the presence of GM-CSF and TNF-α. Ephrin-A3, ephrin-B1, and ephrin-B2 expression was analyzed by cytofluorometry with anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), or anti-ephrin-B2 (P20) polyclonal antibodies directed against intracytoplasmic epitope, after DC permeabilization with saponin, as described in “Materials and methods.” Dotted-line overlay histograms show negative fluorescence control with an antibody of unrelated specificity, and bold dotted-line overlay histograms represent staining after addition of the blocking peptides. (B) Immunocytochemistry analysis of ephrin-A3 (ii), ephrin-B1 (iii), and ephrin-B2 (iv) on day-11 CD34+-derived DC cytospins. Staining was performed with anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), or anti-ephrin-B2 (P20) polyclonal antibodies. The specificity of the staining was confirmed by the absence of detection in the presence of the immunization peptide (data not shown). The negative control (i) represents a polyclonal antibody of unrelated specificity. (C) Immunohistochemistry analysis of ephrin-A3 (ii), ephrin-B1 (iii), and ephrin-B2 (iv) expression in vivo. Staining was performed on normal serial skin sections with anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), or anti-ephrin-B2 (P20) polyclonal antibodies. The negative control (i) represents a polyclonal antibody of unrelated specificity (original magnification × 200 for B and C).
Expression of several Eph ligands in normal skin (ephrin-A3, ephrin-B1, and ephrin-B2). (A) Expression of ephrin-A3, ephrin-B1, and ephrin-B2 by day-11 CD34+-derived DCs cultured in the presence of GM-CSF and TNF-α. Ephrin-A3, ephrin-B1, and ephrin-B2 expression was analyzed by cytofluorometry with anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), or anti-ephrin-B2 (P20) polyclonal antibodies directed against intracytoplasmic epitope, after DC permeabilization with saponin, as described in “Materials and methods.” Dotted-line overlay histograms show negative fluorescence control with an antibody of unrelated specificity, and bold dotted-line overlay histograms represent staining after addition of the blocking peptides. (B) Immunocytochemistry analysis of ephrin-A3 (ii), ephrin-B1 (iii), and ephrin-B2 (iv) on day-11 CD34+-derived DC cytospins. Staining was performed with anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), or anti-ephrin-B2 (P20) polyclonal antibodies. The specificity of the staining was confirmed by the absence of detection in the presence of the immunization peptide (data not shown). The negative control (i) represents a polyclonal antibody of unrelated specificity. (C) Immunohistochemistry analysis of ephrin-A3 (ii), ephrin-B1 (iii), and ephrin-B2 (iv) expression in vivo. Staining was performed on normal serial skin sections with anti-ephrin-A3 (K19), anti-ephrin-B1 (C18), or anti-ephrin-B2 (P20) polyclonal antibodies. The negative control (i) represents a polyclonal antibody of unrelated specificity (original magnification × 200 for B and C).
Taken together, these observations reveal the presence of receptors and ligands in keratinocytes and DCs, suggesting that the EphA2/ephrin-A3 interaction may play a role in the function, localization, or network of Langerhans cells in the epithelium of skin.
Engagement of Eph receptor by its ligand increases DC adhesion to fibronectin but not to PLL
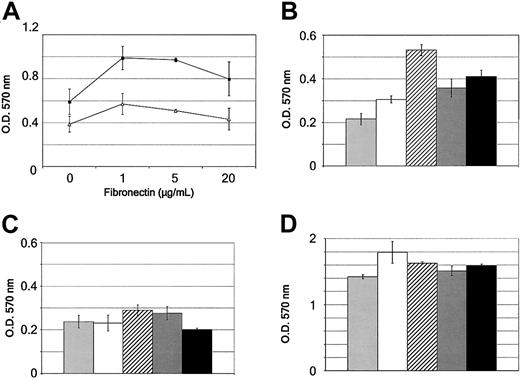
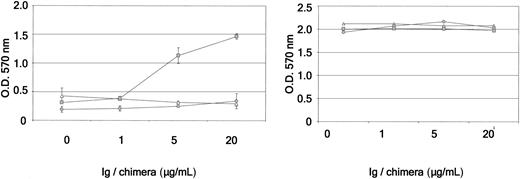
Eph receptors and their ligands have been implicated in integrin-mediated cell adhesion.39 To investigate the role of Eph receptors expressed by CD34+-derived DCs in the regulation of cell attachment, we developed an adhesion assay on various components of the extracellular matrix with ephrin-Fc chimera and IgG or Fas-Fc as negative controls. First, we performed an adhesion assay with day-11 CD34+-derived DCs stimulated with 10 μg/mL ephrin-A3-Fc or immunoglobulin on plates precoated with increasing concentrations of fibronectin (0-20 μg/mL). The plates were immediately centrifuged for 3 minutes at 1200g. After removing cells in suspension, attached cells were carefully fixed and stained by crystal violet, and the number of cells was quantified by OD reading. As shown in Figure 7A, no spontaneous adhesion of day-11 CD34+-derived DCs to fibronectin or PBS alone was observed when the cells were treated with immunoglobulin. However, we found that ephrin-A3-Fc induced increased adhesion to fibronectin, reaching its maximum at a concentration of 5 μg/mL. In the next experiments, we used the latter concentration of extracellular matrix to study the effects of other ephrin-Fc chimeras on DC adhesion. Day-11 CD34+-derived DCs were deposited on fibronectin-, poly-l-lysine- (5 μg/mL), or PBS-precoated plates, with or without increasing concentrations (1-20 μg/mL) of ephrin-A3-Fc, ephrin-B3-Fc, ephrin-A4-Fc, or, as a negative control, PBS or Fas-Fc. As shown in 1 representative experiment in Figure 7B, treatment of DCs with 5 μg/mL ephrin-A3-Fc led to a 2.2 ± 0.9 (mean ± SD, n = 13)-fold increase in adhesion assays to fibronectin compared with Fas-Fc control, which is statistically significant (P < .001; paired t test). Stimulation of the cells with ephrin-A4-Fc, another ligand for EphA2 but with a lower affinity, did not significantly increase CD34+-derived DC adhesion to fibronectin (1.1 ± 0.1-fold; n = 3), whereas ephrin-B3-Fc had generally no effect. In the absence of fibronectin, the stimulation of CD34+-derived DCs did not induce adhesion to plastic (Figure 7C), and no increase in the adhesive properties of the DCs on PLL-coated plates was observed (Figure 7D). The restricted effect on fibronectin suggested an activation of the integrin system. To assess the role of integrins in DC adhesion to fibronectin on ephrin-A3-Fc stimulation, we next used RGD peptide, the amino acid sequence within fibronectin that mediates cell binding to integrins.40 As shown in Figure 8, ephrin-A3-Fc inducing day-11 CD34+-derived DC attachment to fibronectin, but not to PLL, was sensitive to competition by the RGD peptide at the concentration of 10 μM. This RGD peptide inhibited the adhesion induced by 20 μg/mL ephrin-A3-Fc by 72% ± 25%, which is statistically significant (P = .003; n = 3). Competitive inhibition experiments using a function-blocking mAb specific to β1 integrin (clone DE9) also blocked Eph-induced CD34+-derived DC adhesion to fibronectin (data not shown). Together these data showed that at least integrin β1 was involved in the increased adhesion to fibronectin induced through Eph engagement. Thus, these results strongly support that Eph/ephrin interactions in CD34+-derived DCs contribute to the regulation of cell adhesion through an integrin activation pathway.
Activation of Eph receptor by its ligand increases adhesion to fibronectin but not to PLL. (A) Ninety-six-well plates were coated with serial dilution of fibronectin or poly-l-lysine (PLL) (1-20μg/mL) overnight at 4°C. Nonspecific binding sites were blocked at rt for 1 hour using 1% BSA/PBS. Day-11 CD34+-derived DCs (1 × 105 cells in 100 μL serum-free medium) were plated in the presence of ephrin-A3-Fc (10 μg/mL; ▪) or control immunoglobulin (10 μg/mL; ▵) and centrifuged at 1200 rpm for 3 minutes. After washing with PBS and fixation with 0.5% paraformaldehyde/0.5% glutaraldehyde at rt for 30 minutes, adherent cells were stained with 0.5% crystal violet in 20% methanol at rt for 10 minutes. The cells were washed 3 times with water and were extracted with 50% ethanol/50 mM sodium citrate, pH 4.5. Values represent mean ± SD A570 absorbances from triplicate wells. Results are representative of 3 independent experiments. (B-D) Ninety-six-well plates were coated with 5 μg/mL fibronectin (B), PBS (C), or PLL (D) overnight at 4°C. Nonspecific binding sites were blocked at rt for 1 hour using 1% BSA/PBS. Day-11 CD34+-derived DCs (1 × 105 cells in 100 μL serum-free medium) were plated in the presence of PBS (control; light gray bars), Fas-Fc (white bars), ephrin-A3-Fc (hatched bars), ephrin-B3-Fc (dark gray bars), or ephrin-A4-Fc (10 μg/mL; black bars) and were centrifuged at 1200 rpm for 3 minutes. After washing and fixation, adherent cells were stained with crystal violet. Values represent mean ± SD A570 absorbances from triplicate wells. The results comparing the different ephrin-Fc chimera are representative of 3 independent experiments, and overall the results with ephrin-A3-Fc are representative of 13 experiments. O.D. = optical density.
Activation of Eph receptor by its ligand increases adhesion to fibronectin but not to PLL. (A) Ninety-six-well plates were coated with serial dilution of fibronectin or poly-l-lysine (PLL) (1-20μg/mL) overnight at 4°C. Nonspecific binding sites were blocked at rt for 1 hour using 1% BSA/PBS. Day-11 CD34+-derived DCs (1 × 105 cells in 100 μL serum-free medium) were plated in the presence of ephrin-A3-Fc (10 μg/mL; ▪) or control immunoglobulin (10 μg/mL; ▵) and centrifuged at 1200 rpm for 3 minutes. After washing with PBS and fixation with 0.5% paraformaldehyde/0.5% glutaraldehyde at rt for 30 minutes, adherent cells were stained with 0.5% crystal violet in 20% methanol at rt for 10 minutes. The cells were washed 3 times with water and were extracted with 50% ethanol/50 mM sodium citrate, pH 4.5. Values represent mean ± SD A570 absorbances from triplicate wells. Results are representative of 3 independent experiments. (B-D) Ninety-six-well plates were coated with 5 μg/mL fibronectin (B), PBS (C), or PLL (D) overnight at 4°C. Nonspecific binding sites were blocked at rt for 1 hour using 1% BSA/PBS. Day-11 CD34+-derived DCs (1 × 105 cells in 100 μL serum-free medium) were plated in the presence of PBS (control; light gray bars), Fas-Fc (white bars), ephrin-A3-Fc (hatched bars), ephrin-B3-Fc (dark gray bars), or ephrin-A4-Fc (10 μg/mL; black bars) and were centrifuged at 1200 rpm for 3 minutes. After washing and fixation, adherent cells were stained with crystal violet. Values represent mean ± SD A570 absorbances from triplicate wells. The results comparing the different ephrin-Fc chimera are representative of 3 independent experiments, and overall the results with ephrin-A3-Fc are representative of 13 experiments. O.D. = optical density.
β1 Integrin is involved in ephrin-A3-Fc inducing DC adhesion to fibronectin. Ninety-six-well plates were coated with 5 μg/mL fibronectin (left) or poly-l-lysine (PLL; right) overnight at 4°C. Nonspecific binding sites were blocked at rt for 1 hour using 1% BSA/PBS. Day-11 CD34+-derived DCs (1 × 105 cells in 100 μL serum-free medium) were plated in the presence of immunoglobulin (▵) or ephrin-A3-Fc (1-20μg/mL; □) and were centrifuged at 1200 rpm for 3 minutes. To test for the effect of the RGD peptide, cells were preincubated for 5 minutes with the peptide (10 μM; ○) before treatment with the fusion proteins. After washing and fixation, adherent cells were stained with crystal violet. Values represent mean ± SD A570 absorbances from triplicate wells. Results are representative of 3 independent experiments.
β1 Integrin is involved in ephrin-A3-Fc inducing DC adhesion to fibronectin. Ninety-six-well plates were coated with 5 μg/mL fibronectin (left) or poly-l-lysine (PLL; right) overnight at 4°C. Nonspecific binding sites were blocked at rt for 1 hour using 1% BSA/PBS. Day-11 CD34+-derived DCs (1 × 105 cells in 100 μL serum-free medium) were plated in the presence of immunoglobulin (▵) or ephrin-A3-Fc (1-20μg/mL; □) and were centrifuged at 1200 rpm for 3 minutes. To test for the effect of the RGD peptide, cells were preincubated for 5 minutes with the peptide (10 μM; ○) before treatment with the fusion proteins. After washing and fixation, adherent cells were stained with crystal violet. Values represent mean ± SD A570 absorbances from triplicate wells. Results are representative of 3 independent experiments.
Discussion
In this study, we present evidence that human DCs express several Eph receptors, originally described in the nervous system for their role in the control of axon guidance. We found that EphA2 and EphA7 are preferentially expressed by DCs generated in vitro obtained from CD34+ progenitors cultured with GM-CSF and TNF-α and that EphB3 is restricted to Langerhans cells. Furthermore, we demonstrate a role of ephrin-A3/EphA interaction in the modulation of integrin-mediated DC adhesion to fibronectin.
Despite the large expression of Eph and ephrins in many tissues during development,9,41 few studies to date have reported the presence of Eph receptors in cells of the immune system. Here, we found unexpectedly that DCs express mRNA for EphA and EphB members. Some of them—such as EphA4, EphB1, and EphB6—are widely expressed by hematopoietic cells, whereas others—such as EphA2, EphA7, and EphB3—are more specific to DCs. The restricted expression of EphA7 and EphB3 in DCs is strengthened by the presence of these transcripts in immune tissues, such as in lymph node and tonsil and in spleen and tonsil, respectively.42 EphA2 expression in DCs is in agreement with the presence of the transcript in spleen, lymph node, and bone marrow and confirms the suspected expression of EphA2 by DCs.26-28 The fact that different Eph members are expressed by CD34+-derived DCs suggests that a wide variety of ephrin ligands can interact with Eph expressed by DCs, probably in a tightly regulated mechanism, depending on the ligand-receptor affinity and the level of Eph receptor expression.11,12 The Eph/ephrin interaction may also vary according to the DC subtype localized in different organs in vivo. For instance, among the heterogeneous populations of DCs, EphB1 was abundantly found in human plasmacytoid DCs, suggesting a specific function of this molecule for this DC subset involved in viral innate immunity.23 Of interest, expression of most of the Eph receptors shown here is optimal in immature DCs (CD34+-derived DCs, days 11-12), a stage that could be associated with antigen capture,43 and it corresponds to resident cells found in vivo in the periphery, such as Langerhans cells in the epidermis.44 Indeed, Eph receptors (EphA2, EphA7, EphB3) are strongly expressed in Langerhans cells that reside in the epidermis for a considerable period as sentinels of the immune system45 and are down-regulated after stimulation of the cells by an inflammatory stimulus (eg, LPS). In line with these observations, we could not detect any significant Eph messenger in monocyte-derived DCs, which are more closely related to circulating DCs in vivo. Because both EphA2 and its cognate ligand are expressed by keratinocytes and Langerhans cells, the EphA2/ephrin-A3 interactions could be implicated in the localization and network organization of Langerhans cells in the epithelium of skin in normal conditions. Interestingly, it has been shown that E-cadherin, involved in the adhesion of Langerhans cells to keratinocytes,46 regulates EphA2 tyrosine phosphorylation and localization on E-cadherin-mediated adhesion in epithelial cells.47,48 Although E-cadherin stabilizes cell-cell contacts, the interactions between EphA2 and its ligand, active in its membrane-bound form only,49,50 are very likely facilitated, resulting in autophosphorylation of the Eph receptor that might underlie the inhibition of cell movement, probably reinforcing the network of Langerhans cells and the architecture of the skin. Indeed, Eph/ephrin interaction provokes a bidirectional signaling between adjacent cells that prevents cell intermingling between hindbrain segments.18,19 Other evidence for a role in controlling cell movement by complementary distribution of Eph and ephrin ligand has been observed in angiogenesis, in which ephrin-B2 is present in arteries and EphB4 in veins only.15
Interestingly, we found that in response to ephrin-A3-Fc, but not without stimuli, day-11 CD34+-derived DCs are induced to adhere to fibronectin through an integrin-dependent mechanism. Based on RT-PCR data and immunohistochemistry analysis with anti-EphA2 antibodies, it is likely that the observed effect of ephrin-A3-Fc is mediated through interaction with EphA2. Nevertheless, we cannot exclude the involvement of other Eph receptors, such as EphA7 and EphA4, that can bind to ephrin-A3-Fc but have a lower affinity than EphA2.11,12 Different studies have shown that Eph receptors regulate integrin-dependent cell adhesion either positively or negatively.22,39,51 In view of the current knowledge, at least 2 alternative mechanisms can be considered. First, specifically oligomerized forms of ephrin-A3-Fc could engage EphA2 on DCs, resulting in a positive regulation of integrin affinities on fibronectin. This hypothesis is consistent with reported B-ephrin properties in promoting the attachment of endothelial cells expressing endogenous EphB152 through integrin activation. On the other hand, given that several reports demonstrate that engaging Eph deactivates integrins,53,54 ephrin-A3-Fc may interrupt a constitutive Eph/ephrin interaction, resulting in integrin activation. Indeed, because ephrins are also expressed on DCs, ephrin-A3-Fc may compete with ephrin ligands expressed at the DC cell surface, which possibly results in a disengagement of the binding of EphA2 to its cognate ligand, ephrin-A3, resulting in integrin activation and adhesion to fibronectin. This last scenario is in agreement with reports showing that Eph engagement deactivates integrins; in particular, EphA2 and EphB2 activation in PC-3 prostate epithelial cells and transfected 293T cells, respectively, was shown to induce the inhibition of cell adhesion to fibronectin in an integrin-dependent manner.53,54 These 2 signaling scenarios are possible because EphA receptors and ephrin-A counterreceptors are involved in bidirectional signaling and seem to be supported by previous descriptions of the role of Eph receptor in the regulation of cell adhesion.20,21
According to the second scenario, one can propose the following hypothesis: after inflammation caused by injury or infection, Langerhans cells down-regulate E-cadherin55 and concomitantly decrease EphA2 expression, accounting for their emigration from the epithelium. Because of the down-regulation of Eph expression and the absence of ephrins in the surrounding tissue, Eph receptors are not engaged any longer, allowing for integrin activation and interaction with fibronectin, a component of the extracellular matrix observed in the connective tissue underlying the epithelium.56 This interaction with fibronectin is likely to be part of the sequential event for the emigration of Langerhans cells from the dermis en route to the draining lymph node.
To clarify the mechanism of action of ephrin-A3-Fc, future studies will be necessary to define the role of EphA2 as a positive or negative regulator of integrin functions and to determine whether these properties differ according to the extracellular matrix and the stage of DC differentiation. Because integrins, composed of 2 chains, α and β—together with other cell surface adhesion molecules—are required for the interactions of cells with other cell types (eg, endothelial cells) and with extracellular matrix components,57 it will be also of interest to identify the specific integrin regulated on Eph/ephrin interaction.
This study shows that Eph molecules, originally involved in axon guidance, are expressed by human DCs and regulate DC adhesion. They might also have the capacity to directly regulate responses to chemokines,58 as recently described for other neural guidance molecules.59,60 As such, these molecules might be part of important regulatory mechanisms of DC trafficking and might offer avenues to potently manipulate their recruitment in vivo. Finally, the present description of Eph molecules in DCs completes the restricted list of neuronal molecules expressed by DCs, such as neuropilin-1,61 and reinforces the similarities between the neuronal and the immune system.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-02-0500.
G.G. is a recipient of a grant from Fondation Marcel Mérieux, Lyon, France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Saeland for critically reading the manuscript; Dr C. Dezutter-Dambuyant for epithelial Langerhans cell samples; S. Aït-Yahia for preparing cDNA samples; I. Durand for cell sorting; C. Peronne for DNA sequencing; and physicians and colleagues from hospitals in Lyon who provided us with umbilical cord blood samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal